Abstract
Purpose
Mediastinal nodal (N)-staging done by integrated 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) positron emission tomography/x-ray computed tomography (PET/CT) in lung cancer patients is not always accurate. In order to reduce the need for invasive staging procedures, additional surrogate parameters for the detection of malignant lymph node infiltration would be helpful. The purpose of this study was to evaluate if radiomic semi-automated density profiling in mediastinal lymph nodes can improve preclinical N-staging, irrespective of the specific lung cancer entity.
Procedures
This retrospective study was approved by the institutional review board. Two hundred forty-eight histologically proven lymph nodes in 122 lung cancer patients were investigated. In malignantly infiltrated lymph nodes, the specific lung cancer entity was histologically classified; benign lymph nodes were histologically classified as benign. Non-contrast enhanced [18F]FDG-PET/CT was performed before surgery/biopsy. Lymph node analyses were performed on the basis of FDG uptake and volumetric CT histogram analysis for metric lymph node sampling.
Results
Of the 248 lymph nodes, 118 were benign, 130 malignant. Malignant lymph nodes had a significantly higher median CT density (32.4 Hounsfield units (HU) (min 5.4/max 77.5 HU)) compared to benign lymph nodes (9.3 HU (min −49.5/max 60.4 HU, p < 0.05), irrespective of the histological subtype. The discrimination between different malignant tumour subtypes by means of volumetric density analysis failed. Irrespective of the malignant subtype, a possible cutoff value of 20 HU may help differentiate between benign and malignant lymph nodes.
Conclusion
Density measurements in unclear mediastinal and hilar lymph nodes with equivocal FDG uptake in PET might serve as a possible surrogate parameter for N-staging in lung cancer patients, irrespective of the specific lung cancer subtype. This could also help to find possible high yield targets in cases where invasive lymph node staging is necessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the most widespread staging methodologies for lung cancer patients is 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) positron emission tomography/x-ray computed tomography PET/CT) [1, 2]. Since the degree of lymph node metastases plays an important role in the decision of therapy regimen, an exact evaluation of lymph node status prior to therapy planning is crucial. The involvement of contralateral or multiregional mediastinal lymph nodes might potentially exclude the patient from primary surgery [3, 4], which is often associated with a reduced life expectancy. False-positive findings are not uncommon in functional imaging with [18F]FDG-PET/CT, since the scan can mistakenly pick up on inflammation in the lung cancer patient due to acute and/or chronic infectious lung diseases such as chronic obstructive pulmonary disease (COPD) [5]. Therefore, invasive staging procedures with potential side effects have been recommended by different guidelines to verify PET-positive lymph nodes [6].
To further exhaust the information generated by non-invasive [18F]FDG-PET/CT, we evaluated in the concept of radiomics the metric and functional parameters, including volumetric histogram analysis, and set these findings in relation to the different lung cancer entities (i.e. adenocarcinoma, squamous cell carcinoma). Increased lymph node density was previously reported in metastatic lymph node involvement in a cohort of 45 patients, irrespective of the histological subtype [5], and in a cohort of 72 patients with non-small cell lung cancer (NSCLC)-patients [7]. Density analyses between various lung cancer subtypes had not yet been performed. It is essential to do so, since the finding that malignant lymph nodes have higher CT densities than benign ones might potentially play an important role in establishing the value of this method in clinical routine.
The hypothesis of our investigation was that metastases from different histological tumour entities have different CT densities. For this reason, lymph node density was defined as the primary endpoint. The metric parameters of short-axis diameter and lymph node volume, as well as the functional parameter standardized uptake value (SUV) uptake, were regarded as secondary endpoints.
Materials and Methods
Study Design and Patients
The study was conducted as a retrospective multicentre study. Lymph node density, as well as the metric parameters short-axis diameter (SAD) and volume of mediastinal and hilar lymph nodes, were examined in non-contrast CT using volumetric CT histogram analysis software for semi-automated lymph node segmentation (8). A total of 248 lymph nodes in 122 patients aged 39–77 years (68 male, median age 59 years) diagnosed with lung cancer were examined. [18F]FDG-PET/CTs were performed according to the clinical schedule prior to surgical resection with mediastinal lymph node dissection, mediastinoscopy or transbronchial biopsy. The study was approved by the institutional review board (IRB) and conducted according to the guidelines of the institutional review board (IRB) and to good clinical practice according to the ethical principles that have their origin in the Declaration of Helsinki. In this retrospective study, the requirement of informed consent was waived by the IRB.
[18F]FDG-PET/CT and Volumetric CT Histogram Analysis
[18F]FDG-PET/CT was performed in a preoperative setting for staging reasons and therapeutic planning according to the clinical schedule within 1 week prior to surgery, mediastinoscopy or transbronchial biopsy. Patients with neoadjuvant radiation and/or chemotherapy were ruled out from the analysis.
After fasting for at least 8 h, [18F]FDG-PET/CT was performed at a blood glucose level below 150 mg/dl, administering 4 Mega Becquerel/kilogram (MBq/kg) body weight of [18F]FDG intravenously 1 h before the FDG-PET/CT scan. Patients were either examined with a Biograph 6 PET/CT-Scanner (Siemens Medical Solution, Knoxville, United States of America (USA)), or at a Discovery LS®-Scanner (GE Medical Systems, MI, USA) using comparable examination protocols and reconstruction parameters (slice thickness/reconstruction increment of 5.0/2.5 mm, standard soft-tissue reconstruction kernel B30).
Metric and volumetric CT histogram analyses were performed on the basis of the non-enhanced CT scans accompanying the integrated [18F]FDG-PET/CT examination; [18F]FDG-uptake was evaluated using the parameter SUVmax; PET and CT data sets were reconstructed at a slice thickness/reconstruction increment of 5.0/2.5 mm [7].
For the reconstruction of the CT images, a standard soft-tissue reconstruction kernel B30 was used.
Lymph node evaluations and post-processing including volumetric CT histogram analysis were performed by a radiologist with 5 years of experience in oncologic radiology, who was blinded to clinical information. Volumetric CT histogram analysis was performed semi-automatically (Fraunhofer MEVIS, Bremen, Germany, [8]); additional reading time for semi-automated lymph node analysis was measured. For volumetric CT histogram analysis and SUVmax evaluations, lymph nodes had to be clearly allocated in the histological report. Metric analyses, including histogram analysis, were initiated by providing a seed point in the investigated lymph node. Within an estimated region of interest, thresholds were generated automatically by the segmentation software [7]. Thereafter, spatial parameters were extracted and histogram analysis was performed automatically [7]. For reasons of post-procedural validation, results from semi-automated lymph node evaluation were verified by the conducting physician, who visually went through all three dimensions of each of the assessed lymph nodes using the integrated 3D viewer for multiplanar reconstruction [7]. If necessary, semi-automated segmentations could be corrected manually in all three dimensions. All imaging findings were correlated with the histological gold standard with respect to the histological tumour subtype (adenocarcinoma (ADC), adenosquamous cell carcinoma (ADC-SQCC), neuroendocrine tumours (carcinoid), squamous cell carcinoma (SQCC), non-small cell lung cancer not otherwise specified (NSCLC NOS), small cell lung cancer (SCLC), large cell carcinoma (LCC)) for each lymph node level, stated in the histopathological report.
Statistical Analysis
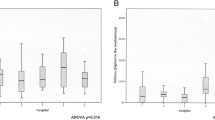
For statistical analysis, SigmaPlot (Systat Software GmbH, Erkrath) was used. Median values for density, SAD, volume and SUVmax +/− minimum and maximum were calculated and are illustrated in Box-Whisker plots. Differences were considered significant at p < 0.05 in a two-sided paired sample t test for the calculation of SAD and density and a two-sided Wilcoxon signed rank test for the calculation of SUVmax and volume.
Diagnostic ability of all four variables was investigated using receiver operating characteristic (ROC) analysis.
Results
Histological Findings and Patient Population
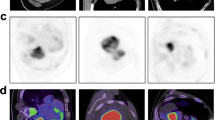
A total of 248 histologically assessed mediastinal and hilar lymph nodes in 122 patients could clearly be assigned to the respective [18F]FDG-PET/CT scan. Among the 248 lymph nodes, tissue acquisition was performed by means of surgical resection including mediastinal lymph node dissection in 212 lymph nodes (90 patients), mediastinoscopy in 10 lymph nodes (8 patients) and transbronchial biopsy in 28 lymph nodes (24 patients). Out of the 248 lymph nodes, 118 lymph nodes in 49 patients were of benign histology, 130 lymph nodes in 73 patients were malignant. The 130 malignant lymph nodes included the following tumour entities: ADC (n = 79), ADC-SQCC (n = 5), carcinoid, (n = 14), SQCC (n = 27), other tumour entities (n = 5, consisting of NSCLC NOS (n = 3), SCLC (n = 1), large cell CA (n = 1) (Table 1)). A correlation of CT, PET and PET/CT images with respective histograms and histopathological cross-sections is demonstrated in Fig. 1.
CT, PET and PET/CT images of lymph nodes with respective histograms and histopathological cross-sections for different tumour subtypes. Native CT with magnified region of interest (ROI) for density analysis, corresponding [18F]FDG-PET image, fused image of [18F]FDG-PET/CT (orange arrow indicates the examined lymph node), corresponding histogram derived by density analysis and histopathological cross-section (histo, 100 times and 200 times magnified, white arrow indicating malignant lymph node infiltration).
Metric and Functional Lymph Node Analysis
Less than 5 min of additional reading time had to be invested for a complete evaluation of the mediastinal and hilar lymph nodes per patient, including semi-automated size, volume and density measurements.
Regarding lymph node density, a median density of 9.3 ± 13.2 HU (min −49.5/max 60.4 HU) was found in benign lymph nodes, which was significantly lower compared to malignant lymph nodes with a median density of 32.4 ± 6.7 HU (min 5.4/max 77.5 HU), irrespective of the specific tumour entity (p ≤ 0.05; Table 2; Fig. 2). Between the different malignant subgroups, no statistically significant differences were found in respect of lymph node density (Table 2; Fig. 2).
In terms of SAD, benign lymph nodes had a median SAD of 11.7 ± 2.9 mm (min 4.2/max 25.6 mm), which was significantly lower than malignant lymph nodes with 16 ± 5.2 mm (min 3.4/max 38.8 mm; p ≤ 0.05), irrespective of the histological subtype (Table 2; Fig. 3). Lymph node volume was significantly lower in benign lymph nodes than in malignant ones (p ≤ 0.05; Table 2; Fig. 4) with a median volume of 0.6 ± 0.6 ml (min 0.4/max 4.6 mm) in benign lymph nodes, compared to 1.5 ± 2.1 ml (min 0.1/max 18.4 ml; p ≤ 0.05) in malignant nodes.
Regarding the functional parameter of glucose uptake, benign lymph nodes showed significantly lower SUVmax values than malignant ones. Benign lymph nodes had a median SUVmax of 1.4 ± 0.5 (min 0.6/max 5), which was significantly lower than malignant lymph nodes with 2.9 ± 3.1 (min 0.6/max 19.3; p ≤ 0.05). Between the different histological tumour entities, no statistically significant differences in SUVmax were found (Table 2, Fig. 5).
Density Cutoff Value, Area Under the Curve and ROC Analyses for Metric (Density, SAD, Volume) and Functional (SUVmax) Lymph Node Parameters
Ninety-two percent of the malignant lymph nodes had a mean density of more than 20 HU (sensitivity of 92 %, specificity 77 %). In the synopsis of the 248 examined lymph nodes, the most reliable density cutoff value was 20 HU with 82 % of the lymph nodes with a density of more than 20 HU being malignant (positive predictive value), while 90 % of lymph nodes with a density of less than 20 HU were benign (negative predictive value, Table 3).
ROC analyses of LN density, SAD, volume and SUVmax showed that LN density reached the highest AUC of 0.89, followed by SUVmax with an AUC of 0.82, followed by the metric parameters SAD and LN volume with an AUC of 0.65 and 0.64, respectively (Fig. 6).
Discussion
We found that it was not possible, by means of semi-automated, density-based CT histogram profiling, to differentiate between the different histological subtypes among the malignant lymph node metastases in the concept of radiomics ; our hypothesis was therefore rejected. Nevertheless, it was feasible to differentiate benign from malignant mediastinal and hilar lymph nodes. Based on these findings, a possible cutoff value of 20 HU might help to differentiate between malignant and benign mediastinal and hilar lymph nodes in all cases of malignant lung tumours, without exceptions for specific lung cancer entities.
The density cutoff value of 20 HU for N-staging in lung cancer patients has been proposed earlier [5], which however did not comment on the limitation that some tumour entities might cause lymph node infiltrates with density values below 20 HU. This was due to the relatively small sample size of that study (45 patients), which did not allow for further subgroup analyses. Shao et al. also reported the high diagnostic value of density analyses in their study with 72 NSCLC patients, but did not mention a possible cutoff value in a per lymph node analysis for a clinically feasible density measurement in daily routine. Data from animal studies also indicate possible cutoff values between benign and malignant mediastinal lymph nodes, but specific cutoff values from the animal experiment could not be applied on human tumours [9]. Based on an analysis of 248 histologically proven lymph nodes, this study allows us to generalize that a possible cutoff value of 20 HU could serve as a surrogate parameter in daily routine for all of the tumour subtypes. This is crucial, since mediastinal lymph node analysis is integral to diagnostic tumour staging, therapy stratification and post-surgical follow-up.
The use of x-ray phase contrast microtomography for density evaluation of lymph nodes has been suggested in a preclinical setting by Jensen et al. in patients with breast cancer [10]. The authors stated its high potential for non-invasive lymph node staging of the axilla. For lymph node staging in lung cancer patients, the combination of anatomical and functional imaging by integrated [18F]FDG-PET/CT allows for a high diagnostic accuracy [11–17], with some limitations, especially in very small mediastinal lymph nodes that still need invasive intrathoracic lymph node staging [18]. Nevertheless, the combination of metric SAD analysis and functional [18F]FDG uptake in integrated PET/CT is still the most reliable modality among the non-invasive staging techniques [19–21]. Based on the anatomical parameter SAD, response evaluation criteria in solid tumours (RECIST) 1.1 summarizes criteria for lymph node staging and therapy response evaluation in clinical routine [21].
Besides SAD measurements, volumetric lymph node analyses can help to improve nodal staging [22–24]. Especially in patients with malignant lymphomas, volumetric lymph node analysis showed significantly superior results compared to the more commonly used measurement of long-axis diameter (LAD) [25].
To improve the clinical value of integrated [18F]FDG-PET/CT, it is also important to further validate the information provided by the PET component. The fact that advanced tumour stages of the primary tumour are often associated with higher SUVmax values has been shown previously [26], but similar to density evaluations, a reliable discrimination between the different lung cancer subtypes is still not possible by SUVmax measurements alone [18]. According to Cuaron et al., primary tumours of specific tumour entities present with different characteristics in respect of [18F]FDG uptake, with squamous cell carcinomas being more avid to FDG as compared to adenocarcinomas [27]. This finding was mainly attributed to differences in the expression of the glucose transporter [28].
Functional analyses of FDG uptake in the respective lymph node metastases in this study could not verify these findings for mediastinal and hilar lymph node metastases.
As stated by the current guidelines for the management of NSCLC patients [29], non-invasive lymph node staging by [18F]FDG-PET/CT is often complemented by invasive staging techniques, especially due to the abovementioned limitations in non-invasive imaging. The additional information derived from semi-automated lymph node density measurement might potentially help to find high yield targets for core biopsies, especially in cases of non-specific findings in functional imaging. Thus, the number of futile biopsies by means of transbronchial ultrasound, mediastinoscopy, video-assisted thoracoscopy (VATS) or thoracotomy can possibly be reduced in the future. In the long term, semi-automated lymph node density measurements might even help to make invasive staging techniques unnecessary, especially in cases of positive FDG-PET findings in suspected mediastinal lymph nodes.
A limitation of this study is the retrospective design. Also, the recently introduced technique of respiratory and cardiac gated-PET can possibly improve SUVmax measurements, which might reduce the number of lymph nodes that need further evaluation by density measurements, or even by invasive staging procedures [29].
Conclusion
In conclusion, semi-automated density profiling in mediastinal and hilar lymph nodes in lung cancer patients might potentially create a promising imaging surrogate for N-staging, irrespective of malignant tumour subtype, which is of clinical relevance in patients with unclear PET findings in integrated [18F]FDG-PET/CT, prior to invasive lymph node sampling.
References
Kratochwil C, Haberkorn U, Giesel FL (2010) PET/CT for diagnostics and therapy stratification of lung cancer. Radiologe 50:684–691
Abramyuk A, Appold S, Zöphel K et al (2012) Quantitative modifications of TNM staging, clinical staging and therapeutic intent by FDG-PET/CT in patients with non small cell lung cancer scheduled for radiotherapy—a retrospective study. Lung Cancer 78:148–152
Tournoy KG, Keller SM Annema JT (2012) Mediastinal staging of lung cancer: novel concepts. Lancet Oncol 13:e221–e229
Rami-Porta R, Call S (2012) Invasive staging of mediastinal lymph nodes: mediastinoscopy and remediastinoscopy. Thorac Surg Clin 22:177–189
Flechsig P, Kratochwil C, Schwartz LH et al (2014) Quantitative volumetric CT-histogram analysis in N-staging of [18F]FDG-equivocal patients with lung cancer. J Nucl Med 55:559–564
Goeckenjan G, Sitter H, Thomas M et al (2011) Prevention, diagnosis, therapy, and follow-up of lung cancer. Interdisciplinary guideline of the German Respiratory Society and the German Cancer Society—abridged version. Pneumologie 65:e51–e75
Shao T, Yu L, Li Y, Chen M (2015) Density and SUV ratios from PET/CT in the detection of mediastinal lymph node metastasis in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 18:155–160
Flechsig P, Dadrich M, Bickelhaupt S et al (2012) LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res 18:3616–3627
Flechsig P, Choyke P, Kratochwil C et al (2016) Increased x-ray attenuation in malignant vs. benign mediastinal nodes in an orthotopic model of lung cancer. Diagn Interv Radiol 22:35–39
Jensen TH, Bech M, Binderup T et al (2013) Imaging of metastatic lymph nodes by X-ray phase-contrast micro-tomography. PLoS One 8:e54047
Toloza EM, Harpole L, McCrory DC (2003) Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 123:137S–146S
Birim O, Kappetein AP, Stijnen T, Bogers AJ (2005) Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 79:375–382
Schaefer NG, Hany TF, Taverna C et al (2004) Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging—do we need contrast-enhanced CT? Radiology 232:823–829
Gould MK, Kuschner WG, Rydzak CE et al (2003) Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 139:879–892
Hellwig D, Baum RP, Kirsch C (2009) FDG-PET, PET/CT and conventional nuclear medicine procedures in the evaluation of lung cancer: a systematic review. Nuklearmedizin 48:59–69, quiz N8-9
Silvestri GA, Gould MK, Margolis ML et al (2007) Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 132:178S–201S
Beyer F, Buerke B, Gerss J et al (2010) Prediction of lymph node metastases in NSCLC. Three dimensional anatomical parameters do not substitute FDG-PET-CT. Nuklearmedizin 49:41–48, quiz N1
Tournoy KG, Maddens S, Gosselin R et al (2007) Integrated FDG-PET/CT does not make invasive staging of the intrathoracic lymph nodes in non-small cell lung cancer redundant: a prospective study. Thorax 62:696–701
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Yankelevitz DF, Reeves AP, Kostis WJ et al (2000) Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 217:251–256
Heussel CP, Meier S, Wittelsberger S et al (2007) Follow-up CT measurement of liver malignoma according to RECIST and WHO vs. volumetry. Röfo 179:958–964
Fabel M, Bolte H, von Tengg-Kobligk H et al (2011) Semi-automated volumetric analysis of lymph node metastases during follow-up—initial results. Eur Radiol 21:683–692
Puesken M, Buerke B, Gerss J et al (2010) Prediction of lymph node manifestations in malignant lymphoma: significant role of volumetric compared with established metric lymph node analysis in multislice computed tomography. J Comput Assist Tomogr 34:564–569
Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA (2005) The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg 130:151–159
Cuaron J, Dunphy M, Rimner A (2013) Role of FDG-PET scans in staging, response assessment, and follow-up care for non-small cell lung cancer. Front Oncol 2:208
Brown RS, Leung JY, Kison PV et al (1999) Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer. J Nucl Med 40:556–565
Goeckenjan G, Sitter H, Thomas M et al (2011) Prevention, diagnosis, therapy, and follow-up of lung cancer: interdisciplinary guideline of the German Respiratory Society and the German Cancer Society. Pneumologie 65:39–59
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the institutional review board (IRB) and conducted according to the guidelines of the institutional review board (IRB) and to good clinical practice according to the ethical principles that have their origin in the Declaration of Helsinki. In this retrospective study, the requirement of informed consent was waived by the IRB.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Flechsig, P., Frank, P., Kratochwil, C. et al. Radiomic Analysis using Density Threshold for FDG-PET/CT-Based N-Staging in Lung Cancer Patients. Mol Imaging Biol 19, 315–322 (2017). https://doi.org/10.1007/s11307-016-0996-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0996-z