Abstract
Purpose
Ga-68-labeled prostate-specific membrane antigen (PSMA) ligands have been used clinically for positron emission tomography (PET) imaging of prostate cancer. However, F-18-labeled compounds offer several advantages, including the potential for delayed imaging, high starting activities enabling multidose preparation, and improved spatial resolution in PET. For F-18 labeling of peptides conjugated with a suitable chelator, a fast and feasible method is the use of [Al18F]2+. In the present study, the radiofluorinations of a well-known PSMA ligand Glu-NH-CO-NH-Lys(Ahx)-HBED-CC (PSMA-HBED) via [Al18F]2+ were performed with respect to various reaction parameters, along with the biological evaluations in a cell experiment.
Procedures
[Al18F]PSMA-HBED was prepared by adding Na[18F]F into a vial containing 0.026 μmol peptide (in 0.05 M NaOAc buffer) and 0.03 μmol AlCl3⋅6H2O (in 0.05 M NaOAc buffer). Then, it was stirred at different temperatures from 1 to 30 min. Afterwards, purification was carried out by solid phase extraction. Biological evaluations were performed in PSMA-positive cell lines LNCaP C4-2, along with a negative control using PC-3 cell lines.
Results
The best labeling results (81 ± 0.5 %, n = 4) were observed with 0.026 μmol peptide (30 °C, 5 min). For preclinical experiments, the production of [Al18F]PSMA-HBED at 35 °C including purification by solid phase extraction (SPE) succeeded within 45 min, resulting in a radiochemical yield of 49 ± 1.2 % (decay-corrected, n = 6, radiochemical purity ≥98 %) at EOS. The labeled peptide revealed serum stability for 4 h as well as a promising binding coefficient (K D) value of 10.3 ± 2.2 nM in cell experiments with PSMA-positive LNCaP C4-2 cells.
Conclusion
An efficient and one-pot method for the radiosynthesis of [Al18F]PSMA-HBED was developed (0.26 μmol of precursor at 35 °C). In cell culture studies, the K D suggests [Al18F]PSMA-HBED as a potential PSMA ligand for future investigations in vivo and clinical applications afterwards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transmembrane glycoprotein glutamate carboxypeptidase II (GCPII), known as prostate-specific membrane antigen (PSMA), is a favored target for positron emission tomography (PET) imaging of prostate cancer (PCa) due to its overexpression in PCa tissue which is up to 100-folds more as compared to normal tissue [1–3]. Therefore, it is considered the most suitable protein for LNCaP-targeting drug delivery studies. The PSMA is internalized through clathrin-coated pits [4, 5]. The docking studies for various PSMA ligands bearing urea, phosphorous, or thiol moiety showed binding to the catalytic domain in a membrane pocket and, thus, exhibited high binding affinities. Because of the limited clinical application of phosphorous-based inhibitors as neuroprotective agents and the challenging synthesis to produce an optically pure form [6], many urea-based ligands [7–26] have been prepared and some of them have been applied successfully for clinical diagnosis.
Recently, another PSMA ligand (Glu-NH-CO-NH-Lys(Ahx)-HBED-CC: PSMA-HBED) has been prepared which bears a significant pharmacophoric group (Glu-CO-Lys, urea moiety) important for specific and direct coordination with two zinc atoms present in catalytic site S1 pocket of PSMA [27]. In this ligand, urea moiety is coupled to the chelator, i.e., HBED-CC (N,N′-bis[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid) and has been labeled with 68Ga efficiently at room temperature. PSMA-HBED showed excellent pharmacokinetics as well as stability in vivo, leading to its clinical applications worldwide [25, 26].
For PET imaging, fluorine-18 is one of the favored radionuclides because of its relatively long half-life (t 1/2 = 110 min) as compared to Ga-68 (t 1/2 = 68 min, E βmax = 1.9 MeV, 9.0 mm mean range in matter). In addition, the almost complete positron decay (97 %, by emission of β+) and the low energy of the positron (E βmax = 635 keV, 2.2 mm mean range in matter) are particularly advantageous in terms of better resolution of PET images and quantification of biochemical processes in vivo. This emphasizes the importance of development of radiofluorinated PSMA ligands.
The use of [Al18F]2+ enables an easy and direct access to the radiofluorination of peptides conjugated with well-suited metal chelators [12, 18–24]. Almost all trivalent radiometals such as 68Ga3+ or 111In3+ form very stable octahedral complexes with hexadentate chelators like derivatives of tricycloazanonane. Due to the similarity in ionic radius of Ga3+ (0.062 nm) to that of Al3+ (0.054 nm) [27–30], it can be assumed that [Al18F]2+ may show excellent stability with macrocyclic chelators which have N2O3 donor set such as 1,4,7-triazacyclononane-triacetic acid (NOTA) [27–30].
In present study, the 18F-labeling of PSMA-HBED via [Al18F]2+ approach is the primary focus with respect to reaction parameters like precursor content, pH value, reaction time, and reaction temperature. Moreover, an efficient one-pot synthesis involving simple solid phase extraction (SPE) purification will be developed. The stabilization experiments in human serum as well as binding assays with PSMA-positive LNCaP C4-2 cells will also be performed.
Materials and Methods
General
All chemicals and solvents for syntheses and analyses were purchased commercially and were of >98 % purity. All were directly used in the syntheses without further purification. The precursor Glu-NH-CO-NH-Lys(Ahx)-HBED-CC (commercial name: PSMA-HBED) was purchased from ABX (Radeberg, Germany) with 99 % purity, prepared previously by Eder et al. [25, 26]. Silica gel 60 plates (RP-18, F254 S, alumina sheets of 5 × 7.5 cm) were obtained from Merck, Germany. A UV lamp was used for visualization of compounds for cold synthesis, whereas radio-TLC scans were performed with a phosphorimager (Raytest, FLA3000). In addition, radio high pressure liquid chromatography (radio-HPLC, P680, Dionex, Germany) equipped with NaI (TI)-scintillation detector (GABI, Raytest, Germany) and a UV detector (GY-400, 254 nm) was used for further identification of 18F-labeled products. A reversed-phase column (Gemini, C18, 250 × 4.6 mm, Phenomenex) was used for HPLC analyses. For additional control of radiochemical yield (RCY), a γ-counter (1480 Wallac WIZARD 3″, PerkinElmer, USA) was applied. For elution of Na[18F]F, QMA light cartridge (Sep-Pak©, Accell plus) was used, whereas for purification of 18F-labeled peptide, different cartridges such as C18 light (Sep-Pak©, Waters), Strata-X (30 mg/ml, Phenomenex), Oasis plus (Sep-Pak©, MCX, Waters), Alumina N light (Sep-Pak©, Waters), and Oasis HLB (Sep-Pak©, 1 cc, Waters) were applied. Matrix-assisted laser ionization (MALDI) experiments were performed using a Fourier transform-ion cyclotron resonance (FT-ICR) mass spectrometer Solarix (Bruker Daltonik GmbH, Bremen, Germany) equipped with a 7.0-T superconducting magnet and interfaced to an Apollo II Dual ESI/MALDI source. α-Cyano-4-hydroxy-cinnamic acid was used as matrix.
Organic Synthesis of [Al19F]PSMA-HBED
To a solution of 25 μg (0.026 μmol) PSMA-HBED in 20 μl of 0.05 M NaOAc, 3 μl of 0.01 M AlCl3⋅6H2O (7.24 μg, 0.03 μmol) and 50 μl of 0.01 M of NaF (21 μg, 0.5 μmol; in 0.05 M NaOAc buffer of pH 5) were added and the reaction mixture was stirred at 30 °C for 30 min. The aliquots for HPLC analysis were taken after 30 min (HPLC: gradient elution; stationary phase: Gemini, C18, 250 × 4.6 mm, Phenomenex; mobile phase: A: acetonitrile (MeCN)/0.1 % trifluoroacetic acid (TFA), B: water/0.1 % TFA, 2 ml/min) (Fig. 1). MS (MALDI): [Al19F]PSMA-HBED: C44H60N6O17Al19F, calc. 990.30; found [M+H]+ 991.30 (see Supporting Information).
HPLC chromatogram for the synthesis of [Al19F]PSMA-HBED by injecting aliquot from a reaction mixture after 30 min of reaction time (conditions: reversed-phase column, Gemini, C18, 5 μ, 250 × 4.6 mm, Phenomenex; gradient elution of solvent A (MeCN/0.1 % TFA) and solvent B (water/0.1 % TFA) at a flow rate of 2 ml/min; first peak being the dead volume of column).
Radiochemistry
Production of [18F]Fluoride
No-carrier-added (n.c.a.) [18F]fluoride was produced on a PETtrace cyclotron (General Electric Medical Systems, Uppsala, Sweden) via the 18O(p,n)18F reaction by irradiating 2 ml of enriched [18O]water (>95 %) with 16.5 MeV protons.
[Al18F]PSMA-HBED—Radiolabeling Optimization Experiments
The study for kinetics of radiofluorination was carried out using various parameters such as different concentrations of precursor and halides (AlCl3⋅6H2O and AlBr3), high and low temperatures, and time points ranging from 1 to 30 min. The purpose of using such broad range of parameters was to obtain the best labeling results under mild experimental conditions.
[18F]fluoride (1–2 GBq) was loaded on a QMA light cartridge (preconditioned—10 ml of 0.5 M NaOAc, 10 ml water) and Na[18F]F was eluted by 700 μl of 0.9 % NaCl solution (first fraction: up to 60 MBq/200 μl, second fraction: up to 760 MBq/500 μl). One hundred microliters of Na[18F]F (up to 150 MBq) of F-18 activity was added to each vial containing 20 μl of peptide solution with different peptide amounts [10 μg (0.01 μmol), 25 μg (0.026 μmol), 50 μg (0.05 μmol), and 100 μg (0.1 μmol) in 0.05 M NaOAc of pH 5] and 3 μl (7.26 μg) of AlCl3⋅6H2O in 0.05 M NaOAc. Total reaction volume was 123–150 μl.
Experiments were carried out at 30, 60, and 100 °C. Aliquots were taken after 1, 5, 10, 20, and 30 min and analyzed by radio-TLC and radio-HPLC.
Dependence on AlX3 Concentration
Additionally, the dependence of the RCY on the molar range of AlX3 and on the type of the corresponding anion (X: −Cl, 6H2O, or –Br) was investigated. Labeling experiments were carried out for [Al18F]PSMA-HBED (10 min) at 30 °C using 25 μg (0.026 μmol) of the peptide in 20 μl of 0.05 M NaOAc along with three different contents of AlX3 in 0.05 M NaOAc—0.03 μmol (3 μl), 0.06 μmol (6 μl), and 0.09 μmol (9 μl)—For AlCl3⋅6H2O (7.26, 14.5, and 21.7 μg) or AlBr3 (8, 16, and 24 μg).
Dependence on pH
For determination of optimal pH value, experiments between pH 2 and 7 were performed by using 25 μg (0.026 μmol) of precursor in 20 μl of 0.05 M NaOAc buffer (pH 2, 3, 4, 5, 6, or 7; adjusted with AcOH) and 3 μl (0.03 μmol, 7.26 μg) of 0.01 M AlCl3⋅6H2O solution in 0.05 M NaOAc at 30 °C for 10 min.
Radiosyntheses for Biological Experiments
In order to purify the radiolabeled product for biological investigations, several SPE cartridges were tested such as Strata-X, C18 light, Oasis plus (MCX), Alumina N light, and Oasis HLB (1 cc) to remove the remaining free F-18 fluoride and [Al18F]2+. All cartridges, except Alumina N light, were preconditioned with 10 ml ethanol (EtOH) and 10 ml trace-select water, followed by 10 ml air.
All experiments were done with higher starting activities up to 2.5 GBq (ca. 140 μl) of Na[18F]F fraction. These experiments were performed with 25 μg of peptide in 20 μl 0.05 M NaOAc (pH 5) and 3 μl (7.26 μg) of 0.01 M AlCl3⋅6H2O solution (in 0.05 M NaOAc) at 35 °C for 15 min. The reaction mixture was diluted with 5 ml of water, passed through preconditioned Oasis HLB (1 cc) or Sep-Pak C18 light. After washing with 5 ml of water, the product was eluted with 1 ml of EtOH in a vial containing 10 ml of PBS (Fig. 2). Total time for EOS was 45 min.
Analytical Assays
Radio-TLCs
Analyses by radio-TLC were performed with silica gel 60 plates (RP-18, F254 S) as stationary phase and 75 % MeCN in water (MeCN/H2O = 3:1) as a mobile phase. One microliter aliquots were taken from the reaction mixture or final product solution. The radiochemical yields were measured by radio-TLC using a phosphorimager and were additionally proven by radio-HPLC.
Radio-HPLC
The radio-HPLC analyses were done by injecting 20 μl from the reaction mixture or final product solution. As stationary phase, a reversed-phase column (Gemini, C18, 250 × 4.6 mm, Phenomenex) was used. The gradient elution was performed by the use of MeCN/0.1 % TFA as mobile phase A and water/0.1 % TFA as mobile phase B (from 0 to 4 min, 15 % A; from 4 to 11 min, increase to 70 % of A; 11 to 14 min, decrease to 15 % of A up to 16 min). The flow rate was 2 ml/min. A retention time (R t ) for [Al18F]PSMA-HBED of 7.5 min was observed. The precursor, PSMA-HBED, showed a R t of 7.2 min.
Free F-18 and [Al18F]2+ were observed between 1.5 and 2 min. For radioactive balance purposes and to observe the total free fluoride residing on column, same volumes (20 μl) of aliquots were injected with and without column (see “Analytical Assays”).
Biological Experiments
Serum Stability Studies
For the serum stability assay, aliquots of 200 μl (total activity of final product: up to 780 MBq of [Al18F]PSMA-HBED in ca. 11 ml of EtOH/PBS solution) were diluted with 950 μl of serum and incubated at 37 °C for 4 h. Afterwards, 350 μl out of the samples (ca. 270 kBq) were taken and 400 μl of cold EtOH was added. After mixing, proteins were precipitated. The samples were then centrifuged at 10,000 rpm for 10 min to settle down the proteins. The supernatants were taken and added to other Eppendorf vials having prewashed ultrafilters for centrifugation at 10,000 rpm for 15 min (Millipore, 3 kDa NMW, cleaned by adding 500 μl of PBS and centrifuging at 10,000 rpm for 10 min). The radioactivity of both vials, one having a supernatant (ca. 253 kBq) and the other one with protein pellets (ca. 10 kBq), was measured. The filtrates from supernatant vials were analyzed by radio-TLC (see “Analytical Assays”).
Cell Culture Analysis for In Vitro Binding Specificity
PSMA-positive cell lines LNCaP C4-2 were obtained from ViroMed Laboratories (Minnetonka, MN, USA) and grown in T-media (Dulbecco’s modified Eagle’s medium (DMEM, high glucose, Gibco) and 20 % Ham’s F-12 (Biochrom) supplemented with 5 % fetal bovine serum, 5 μg/ml insulin (Sigma), 13.65 pg/ml triiodothyronine (Sigma), 5 μg/ml apo-transferrin (Sigma), 0.244 μg/ml d-biotin (Sigma), and 25 μg/ml adenine (Sigma). As a negative control, PC-3 cells (DSMZ, ACC 465) were maintained in DMEM and 10 % Ham’s F12 with 10 % FBS, 1 % penicillin/streptomycin, and 2 mM glutamine. All cell lines were incubated at 37 °C under constant humidity and an atmosphere of 5 % CO2.
Determination of Binding Coefficient
To determine the binding coefficient (K D) of the radiolabeled peptide, 5 × 105 cells/well were grown in coated 12-well plates in 1 ml medium for 48 h. The cells were washed twice with PBS and 900 μl fresh media were administered. The radiolabeled peptide was added in a serial dilution resulting in final concentrations of 802, 401, 200, 100, 50, 25, and 12.5 nM. In parallel, a PSMA-specific inhibitor (2-phosphonomethyl pentanedioic acid—2-PMPA) was applied to a final concentration of 30 μM to determine the unspecific binding. All samples were prepared in triplicate. Following 60 min of incubation at 37 °C, cells were washed twice to remove unbound activity and afterwards lysed in 1 ml of 0.5 M NaOH. Activity was measured in a gamma counter (COBRA™ II, Packard Instrument). Aliquots of the solution administered to the cells were also measured for calculation of the cellular uptake as %ID. Data were analyzed using GraphPad Prism 5.02 (one site—total and nonspecific binding evaluation). Similar experiments were performed using [68Ga]PSMA-HBED for comparison with radiofluorinated analogue.
Results and Discussions
[Al18F]PSMA-HBED—Radiolabeling Optimization Experiments
Dependence on Precursor Concentration, Reaction Time, and Temperature
For [Al18F]PSMA-HBED, the best RCY was observed at 30 °C by using 25 μg (0.026 μmol) of peptide and 3 μl of 0.01 M AlCl3⋅6H2O (0.03 μmol). At 100 °C, different concentrations such as 100 μg (0.1 μmol), 50 μg (0.05 μmol), 25 μg (0.026 μmol), and 10 μg (0.01 μmol), each in 20 μl of 0.05 M NaOAc (pH 5), were used. For 100 μg, the highest RCY (79 ± 0.7 %) was observed within 5 min. However, when the concentration of the peptide was lowered to 0.05 μmol, maximum RCY was 83 ± 0.7 % at 5 min which decreased to ca. 7.9 ± 0.9 % after 30 min of reaction time (Fig. 3a). With lower concentrations such as 0.026 and 0.01 μmol, high RCYs (ca. 75–55 %) were obtained after 1 min that decreased to ca. 1–5 % after 30 min due to strong decomposition at high temperatures.
The kinetics of labeling at lower temperatures (60 and 30 °C) was also studied. At 60 °C, with 50 μg (0.05 μmol) of PSMA-HBED, RCY of 85 ± 1 % was obtained after 30 min of reaction time (Fig. 3b). However, when decreased to 25 μg (0.026 μmol) at 60 °C, the highest RCY (84 ± 1.4 %, 5 min) was observed and remained stable over 30 min of reaction time (88 ± 1 %). By lowering the temperature to 30 °C, RCYs were excellent for 100 μg (0.1 μmol), 50 μg (0.05 μmol), and 25 μg (0.026 μmol)] within 1 min (ca. 79 %) that remained constant to ca. 85 % after 30 min (Fig. 3c).
Dependence on AlX3 Concentration
The best results with RCY of 90 % were obtained by the use of 0.03 μmol (3 μl of stock solution) of AlCl3⋅6H2O (Fig. 4a). With 0.06 μmol (6 μl of stock solution) and 0.09 μmol (9 μl of stock solution) of AlCl3⋅6H2O, RCYs ranged from ca. 80 to 50 %, respectively. For AlBr3, maximum yields ranged between 70 and 80 % with 0.03 μmol (3 μl stock solution), whereas by the use of 0.09 μmol (9 μl of stock solution), the lowest RCY was observed (ca. 2 %) (Fig. 4a).
Dependence of RCYs for [Al18F]PSMA-HBED on different concentrations of a AlX3 solution (0.01 M AlX3 in 0.05 M NaOAc buffer): 3 μl (0.03 μmol; AlCl3 7.24 μg; AlBr3 8 μg), 6 μl (0.06 μmol; AlCl3 14.5 μg; AlBr3 16 μg), and 9 μl (0.09 μmol; AlCl3 21.7 μg; AlBr3 24 μg). b Dependence of RCYs for [Al18F]PSMA-HBED on reaction pH.
Dependence on pH
For radiofluorinations with [Al18F]2+, the pH of the reaction mixture is a very crucial parameter [18–24]. In order to optimize the pH for labeling, experiments using 25 μg of precursor in 20 μl of 0.05 M NaOAc buffer (pH 2, 3, 4, 5, 6, or 7) were carried out at 30 °C. Under proven conditions, the best pH was 5 with a RCY ≥90 % (Fig. 4b).
Radiosyntheses for Biological Experiments
The radiosynthesis of [Al18F]PSMA-HBED for biological evaluations succeeded in RCY of 49 % (decay-corrected) within a total time of 45 min. The observed radiochemical purity was ≥98 %. Concerning cartridge purification, the best results were observed with Oasis HLB cartridge (1 cc) and C18 light. However, with Oasis and Strata-X cartridges, the RCYs for [Al18F]PSMA-HBED were lower than 50 % (Strata-X 7 %; Oasis 50 %) because of reduced retention on these cartridges.
Analytical Assays
In order to control the RCYs of reactions and radiochemical purities of [Al18F]PSMA-HBED after cartridge purification, radio-TLC and radio-HPLC methods were applied. For this purpose, reaction times (R t) and retention factors (R f) of the precursor, radiofluorinated product, and free fluoride were determined by radio-HPLC and radio-TLC, respectively (see “Analytical Assays”). The R f for [Al18F]PSMA-HBED was 0.81, whereas [Al18F]2+ and [18F]fluoride retained on baseline. Although the free fluoride showed R t ranging from 1 to 2.5 min on radio-HPLC, yet, unfortunately, most of the ions like [18F]fluoride or [Al18F]2+ retained on the column (Gemini, C18, Phenomenex). To prove this, radioactivity balance analyses were carried out by injecting the identical volumes of aliquots of free fluoride to radio-HPLC with and without column. Radio-HPLC chromatogram showed the clear difference for the observed free fluoride content between both injections.
However, the radio-TLC method proved to be precise by showing the correct amount of free fluoride as well as product in samples and can, therefore, be used for activity balance. Under the applied conditions, the radio-HPLC method can be used for the control of product identity and product purity based on organic peptides. For proving this analytical evaluation, two methods were applied on radio-HPLC. In the first one, after injection (20 μl) of the purified product sample, the peak of [Al18F]PSMA-HBED (R t 7.5 min) was collected and measured in γ-counter in comparison to total injected activity (20 μl). In the second method, injections (20 μl each) from the product fraction were carried out with and without column (Fig. 5a: radioactive channel, b: UV channel) which evidenced the product identity and proved the radiochemical purity (≥98 %). All results of radio-HPLC analyses of the product were cross-checked by radio-TLCs (Fig. 5c). In summary, the application of different radioanalytical methods proved that the labeling yields were free of background activities and other artifacts, and therefore, the yields are considered to be correct.
Radio-HPLC chromatograms showing peaks with and without column for [Al18F]PSMA-HBED product fraction (R t 7.5 min) eluted from cartridge (conditions: Gemini, C18, 5 μ, 250 × 4.6 mm, Phenomenex; gradient elution of solvent A (MeCN/0.1 % TFA) and solvent B (water/0.1 % TFA) at a flow rate of 2 ml/min). a Radioactive channel, b UV channel, and c radio-TLCs (1 μl aliquot on each spot, eluent 75 % MeCN in water) showing both waste (free fluoride with little bit product) and purified [Al18F]PSMA-HBED (R f 0.81).
Biological Experiments
Stability Studies and In Vitro Binding Specificity
The stability studies were carried out in human serum (1, 2, and 4 h for [Al18F]HBED complex). The radioactivity balance between precipitated proteins and supernatant during serum stability studies showed negligible amounts of free fluoride bound with proteins. Analyses by radio-TLC showed no decomposition (Fig. 6a). From 1 to 4 h, serum stability is shown by the graph (Fig. 6b).
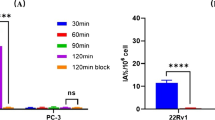
In PSMA-positive cell line LNCaP C4-2 (Fig. 7a), the K D values of 10.3 ± 2.2 nM were determined and a cellular uptake of 3.53 ± 0.78 % ID was obtained. No specific binding was observed for the PSMA-negative cell line PC-3 (Fig. 7b). For comparison, the parallel cell culture experiments using [68Ga]PSMA-HBED resulted in K D values of 12.58 ± 1.09 nM. However, uptake was not studied for the Ga-68-labeled analogue.
Determination of the binding coefficient K D of [Al18F]PSMA-HBED. The binding curve was determined for a the PSMA-expressing cell line LNCaP C4-2 and b the PSMA-negative control PC-3. The specific binding was blocked by additional application of 2-PMPA to a final concentration of 30 μM. Background was observed measuring empty test tubes in the γ-counter. Data are expressed as mean ± SEM (n = 3) and evaluated using GraphPad Prism 5.02 (cpm counts per minute).
Conclusion
The radiosynthesis of [Al18F]PSMA-HBED was systematically investigated, particularly focusing on reaction parameters such as reaction time, temperature, amount of precursor, pH value, and the content and type of the corresponding anion in AlX3. The best results (RCY 81 ± 0.5 %) were observed with 25 μg (0.026 μmol in 20 μl of 0.05 M NaOAc buffer) of peptide at 30 °C within 5 min of reaction time using 3 μl (0.03 μmol) of 0.01 M AlCl3⋅6H2O in 0.05 M NaOAc buffer in a total volume of ca. 123 μl at pH 5.
An efficient one-pot radiofluorination method using 25 μg of precursor (0.026 μmol), at 35 °C (15 min reaction time), including product purification via SPE was developed for application of [Al18F]PSMA-HBED in first preclinical evaluations (RCY 49 %, decay-corrected, ≥98 % radiochemical purity). The ligand showed serum stability for 4 h as well as a promising binding coefficient (K D = 10.3 ± 2.2 nM) in cell experiments with PSMA-positive LNCaP C4-2 cells.
References
Ghosh A, Heston WD (2004) Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem 91:528–539
Elsasser-B U, Reischl G, Wiehr S et al (2009) PET imaging of prostate cancer xenografts prostate-specific membrane antigen. J Nucl Med 50:606–611
Reske SN, Winter G, Baur B et al (2013) Comment on Afshar-Oromieh et al.: PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 40:969–970
Goodman OB Jr, Barwe SP, Ritter B et al (2007) Interaction of prostate specific membrane antigen with a highly specific antibody against the clathrin and the adaptor protein complex-2. Int J Oncol 31:1199–1203
Ikeda M, Ochi R, Wada A et al (2010) Supramolecular hydrogel capsule showing prostate specific antigen-responsive function for sensing and targeting prostate cancer cells. Chem Sci 1:491–498
Blank BR, Alayoglu P, Engen W et al (2011) N-substituted glutamyl sulfonamides as inhibitors of glutamate carboxypeptidase II (GCP2). Chem Biol Drug Des 77:241–247
Olson WC, Heston WD, Rajasekaran AK (2007) Clinical trials of cancer therapies targeting prostate-specific membrane antigen. Rev Recent Clin Trials 2:182–190
Kularatne AS, Zhou Z, Yang J et al (2009) Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted 99mTc-radioimaging agents. Mol Pharm 6:790–800
Kularatne SA, Wang K, Santhapuram H-KR, Low PS (2009) Prostate-specific membrane antigen targeted imaging and therapy of prostate cancer using a PSMA inhibitor as a homing ligand. Mol Pharm 6:780–789
Becaud J, Mu L, Karramkam M et al (2009) Direct one-step 18F-labeling of peptides via nucleophilic aromatic substitution. Bioconjug Chem 20:2254–2261
Zhang AX, Murelli RP, Barinka C et al (2010) A remote arene-binding site on prostate specific membrane antigen revealed by antibody-recruiting small molecules. J Am Chem Soc 132:12711–12716
Laverman P, McBride WJ, Sharkey RM et al (2010) A novel facile method of labelling octreotide with 18F-fluorine. J Nucl Med 51:454–461
Kularatne SA, Venkatesh C, Santhapuram H-KR et al (2010) Synthesis and biological analysis of prostate-specific membrane antigen-targeted anticancer prodrugs. J Med Chem 53:7767–7777
Chen Y, Pullambhatla M, Foss CA et al (2011) 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res 17:7645–7653
Al-Momani E, Malik N, Machulla H-J et al (2013) Radiosynthesis of [18F]FEt-Tyr-urea-Glu ([18F]FEtTUG) as a new PSMA ligand. J Radioanal Nucl Chem 295:2289–2294
Olberg DE, Arukwe JM, Grace D et al (2010) One step radiosynthesis of 6-[18F]fluoronicotinic acid 2,3,5,6-tetrafluorophenyl ester ([18F]F-Py-TFP): a new prosthetic group for efficient labelling of biomolecules with fluorine-18. J Med Chem 53:1732–1740
Malik N, Machulla H-J, Solbach C et al (2011) Radiosynthesis of a new PSMA targeting ligand ([18F]FPy-DUPA-Pep). Appl Radiat Isot 69:1014–1018
McBride WJ, D’Souza CA, Sharkey RM et al (2010) Improved 18F-labeling of peptides with a fluoride-aluminum-chelate complex. Bioconjug Chem 21:1331–1340
D’Souza CA, McBride WJ, Sharkey RM et al (2011) High-yielding aqueous 18F-labeling of peptides via Al18F chelation. Bioconjug Chem 22:1793–1803
McBride WJ, D’Souza CA, Sharkey RM, Goldenberg DM (2012) The radiolabeling of proteins by the [18F]AlF method. Appl Radiat Isot 70:200–204
McBride WJ, D’Souza CA, Karacay H et al (2013) New lyophilized kit for rapid radiofluorination of peptides. Bioconjug Chem 23:538–547
McBride WJ, Sharkey RM, Goldenberg DM (2013) Radiofluorination using aluminum-fluoride (Al18F). EJNMMI Res 3:36
Malik N, Zlatopolskiy B, Reske SN et al (2012) One pot radiofluorination of a new potential PSMA ligand [Al18F]NOTA-DUPA-Pep. J Label Compd Radiopharm 55:320–325
Wan W, Guo N, Pan D et al (2013) First experience of 18F-Alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med 54:691–698
Eder M, Wängler B, Knackmuss S et al (2008) Tetrafluorophenolate of HBED-CC: a versatile conjugation agent for 68Ga-labeled small recombinant antibodies. Eur J Nucl Med Mol Imaging 35:1878–1886
Afshar-Oromieh A, Malcher A, Eder M et al (2013) PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 40:486–495
Rajasekaran AK, Anilkumar G, Christiansen JJ (2005) Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol 288:975–981
Martin RB (1988) Ternary hydroxide complexes in neutral solutions of Al3+ and F−. Biochem Biophys Res Commun 155:1194–1200
Delgado R, Sun Y, Motekaitis RJ, Martel AE (1993) Stabilities of divalent and trivalent metal ion complexes of macrocyclic triazatriacetic acids. Inorg Chem 32:3320–3326
Kodama K, Kimura E (1995) Complexation reactions of aluminum ions with polyamino polycarboxylic macrocycles in an aqueous solution. Bull Chem Soc Jpn 68:852–857
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 327 kb)
Rights and permissions
About this article
Cite this article
Malik, N., Baur, B., Winter, G. et al. Radiofluorination of PSMA-HBED via Al18F2+ Chelation and Biological Evaluations In Vitro . Mol Imaging Biol 17, 777–785 (2015). https://doi.org/10.1007/s11307-015-0844-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0844-6