Abstract
Introduction
High quality data, based on reliable quantification and clear identification of the reported lipid species, are required for the clinical translation of human plasma lipidomic studies.
Objective
Lipid quantification can be efficiently performed on triple quadrupole (QqQ) mass spectrometers in targeted multiple reaction monitoring (MRM) mode. However, a series of issues can be encountered when aiming at unambiguous identification and accurate quantification, including (i) resolving peaks of polyunsaturated species, (ii) discriminating between plasmanyl-, plasmenyl- and odd chain species and (iii) resolving the isotopic overlap between co-eluting lipid species.
Methods
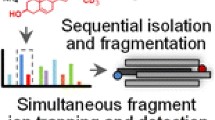
As a practical tool to improve the quality of targeted lipidomics studies, we applied a Dual MS platform by simultaneously coupling a reversed-phase liquid chromatography separation to a QqQ and a quadrupole-time of flight (Q-ToF) mass spectrometers. In one single experiment, this platform allows to correctly identify, by high-resolution MS and MS/MS, the peaks that are quantified by MRM.
Results
As proof of concept, we applied the platform on glycerophosphocholines (GPCs) and sphingomyelins (SMs), which are highly abundant in human plasma and play crucial roles in various physiological functions. Our results demonstrated that Dual MS could provide a higher level of confidence in the identification and quantification of GPCs and SMs in human plasma. The same approach can also be applied to improve the study of other lipid classes and expanded for the identification of novel lipid molecular species.
Conclusions
This methodology might have a great potential to achieve a better specificity in the quantification of lipids by targeted lipidomics in high-throughput studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plasma-based lipidomic studies are powerful tools to understand the molecular basis of patho-physiological processes and develop diagnosis or new therapeutic strategies for metabolic and cardiovascular diseases (Han 2016; Laaksonen et al. 2016; MaFadyen et al. 2017). Such clinical translation requires high quality data, as highlighted recently by the lipidomic research community in several publications (Teo et al. 2015; Bowden et al. 2017; Holčapek et al. 2018; Liebisch et al. 2017). The most important areas for improvements are reproducibility, accurate identification of the reported lipid species and reliable quantification. Liquid chromatography-tandem mass spectrometry (LC–MS/MS) is nowadays the most popular analytical technique for metabolomic, lipidomic and proteomic studies (Rochat 2016; Cajka et al. 2014). Using a similar analytical setup, different approaches can be taken: targeted methods can be more sensitive and give a more reliable quantitation of a selected list of molecules, while untargeted ones can deliver a more unbiased picture of the changes in the system under study and facilitate the discovery of novel molecules.
Triple quadrupole mass spectrometers (QqQ) are often preferred in quantitative targeted studies because of their high sensitivity when used in multiple reaction monitoring (MRM) mode. At the same time, a high-resolution instrument, such as a quadrupole-time of flight (Q-ToF) mass spectrometer, can provide high resolution and high mass accuracy at both MS and MS/MS levels, leading to unambiguous lipid identification and quantification, albeit at a lower sensitivity (Melnik et al. 2017). Combining the information generated by the two different instruments, by sequentially performing untargeted experiments followed by targeted ones, would be the most effective way to improve data quality. However, integrating data obtained by two different instrumental platforms and two independent experiments is not trivial, especially when considering alignment of retention times and sample properties. In order to solve this issue, a single chromatographic system can be coupled simultaneously to two different mass spectrometers, allowing for simultaneous analysis of a single sample with a targeted (at low resolution) and an untargeted (at high resolution) approach (Melnik et al. 2017). While such an experimental strategy is particularly useful for the discovery and quantification of novel molecules, we demonstrate here its relevance to improve data quality in targeted lipidomics and its contribution to advance the field. In particular, not only as a proof of concept of our methodology but also as a practical example of one of the common issues encountered by researchers when performing plasma lipidomic studies, we show how this new approach can help to correctly discriminate between plasmanyl-, plasmenyl- and odd chain glycerophosphocholines (GPCs) and sphingomyelins (SMs) signals, to allow for their correct annotation and quantification in human samples.
GPCs are composed of a glycerol backbone bearing a phosphocholine head group at the sn-3 position and two hydrophobic moieties at the sn-1 and sn-2 position. The hydrophobic substituents can consist of: (i) two acyl esters at the sn-1 and sn-2 positions (phosphatidylcholine, PC); (ii) an alkyl ether at the sn-1 position and an acyl ester and the sn-2 position (plasmanylcholine, PC-O); or (iii) a vinyl ether at the sn-1 position and an acyl ester and the sn-2 position (plasmenylcholine, PC-P) (Fig. 1a–c). PCs, which are mainly synthesized in endoplasmic reticulum (ER), are one of the most abundant lipids in eukaryotic cell membranes and key players in membrane-mediated cell signalling (Van Meer et al. 2008; Walter et al. 2004). PC-O and PC-P are a unique class of membrane glycerophospholipids, whose particular structure provides them with specific properties. They can represent up to 20% of the total phospholipid mass and they play a role in the development of tissues such as brain, kidney and heart. Their biosynthesis starts in peroxisomes and it has been shown that plasmanylcholines and plasmenylcholines are significantly lower in tissues and erythrocytes of humans suffering from Zellweger syndrome or rhizomelic type of chondrodysplasia punctate, two diseases in which the peroxisome biogenesis is disturbed (Harjra 1995; Honsho et al. 2017; Braverman et al. 2012; Heymans et al. 1983; Van den Bosch et al. 1992). Besides, serum plasmenylcholines may also serve as a sensitive biomarker for atherosclerosis and the atherogenic state (Nishimukai et al. 2014a, b).
SM consists of a phosphocholine head group, a sphingoid base and a fatty acyl chain (Fig. 1d). Although the sphingosine backbone (i.e. a sphingoid base with 18 carbon atoms and 1 double bond) is the most prevalent, other sphingoid bases containing 14–22 carbon atoms and 0–2 double bonds can also be present (Acharya et al. 2003; Rietveld et al. 1999). SMs are specifically enriched in the plasma membrane, the endocytic recycling compartment, and the trans Golgi network (Slotte 2013). SMs play important roles in various cellular functions and processes, including regulation of endocytosis and receptor-mediated ligand uptake, protein sorting and ion channel and G-protein coupled receptor function (Ortegren et al. 2007; Shakor et al. 2011; Xu et al. 2008; Singh et al. 2012). Sphingomyelin metabolism has been linked to several diseases including coronary artery disease, ovarian endometriosis, and cancer, and also with longevity (Jiang et al. 2000; Vouk et al. 2012; Kim et al. 2013; Petersen et al. 2013; Gonzalez-Covarrubias et al. 2013).
Quantification of GPCs and SMs is usually based on MRM transitions using the common dominant phosphocholine product ion at m/z 184.0733. However, a series of issues can be encountered with these head group-based MRM transitions when aiming at accurate quantification and identification of these lipid species, including (i) resolving multiple peaks of polyunsaturated species, (ii) discrimination of GPC isobars and isomers, and (iii) isotopic overlap from co-eluting species with close m/z values. In this study we used a reversed-phase liquid chromatography (RPLC) separation, simultaneously coupled to a QqQ and a Q-ToF platform (Dual mass spectrometry), in order to solve the issues described above and show, more in general, the utility of a method that can achieve a comprehensive coverage with high specificity for the lipid quantification in human plasma.
2 Materials and methods
2.1 Annotation of lipid species
Species of GPCs were annotated by their total number of carbon atoms and double bonds in their fatty acyl/alkyl/alkenyl moieties. The molecular species were annotated by their fatty acyl/alkyl/alkenyl moieties separated by underscore (_) or slash symbols (/) if their sn-1 and sn-2 positioning was ambiguous or certain, respectively. For example, PC 32:1 stands for phosphatidylcholine molecules comprising a total of 32 carbon atoms and one double bond in the two hydrophobic moieties; PC 16:0_16:1 indicates that this PC includes 16:0 and 16:1 fatty acyl moieties located at unspecified positions on the glycerol backbone; PC 16:0/16:1 indicates that 16:0 and 16:1 fatty acyl moieties are located at the sn-1 and sn-2 positions, respectively. SM species were annotated by the total number of carbon atoms and double bonds in the sphingoid base and fatty acyl moieties.
2.2 Chemicals and lipid standards
Chemicals and reagents were obtained from the following sources: ammonium formate and butanol from Sigma-Aldrich or Merck (Darmstadt, Germany); MS-grade acetonitrile, methanol and isopropanol from Fisher Scientific (Waltham, MA); PC 14:0/14:0, PC-P 18:0/18:1 and SM d18:1/12:0 from Avanti Polar Lipids (Alabaster, AL). Ultrapure water (18 MΩ cm at 25 °C) was obtained from an Elga Labwater system (Lane End, U.K.). Commercial human plasma, as a pooled sample representative of 20 females and 30 males between the ages of 18 and 75 and different ethnicities, was purchased from Sera Laboratories International Ltd (Haywards Heath, U.K.).
The collection and use of all human plasma samples was approved by the Institutional Review Board of the National University of Singapore (approval numbers NUS-IRB N-17-082E).
2.3 Sample preparation
Aliquots of plasma were thawed on ice for 60 min. Twenty μL of plasma were mixed with 180 μL of 1-butanol:methanol (1:1, v/v) spiked with PC 14:0/14:0 (200 ng/mL) and SM d18:1/12:0 (100 ng/mL) as internal standards (IS) (Alshehry et al. 2015). The mixture was vortexed for 3 min, sonicated for 30 min and then centrifuged twice at 4 °C (14,000 rcf for 10 min). The supernatant fraction was collected for LC–MS/MS analysis.
2.4 Dual LC–MS/MS analysis
The Dual LC–MS/MS analysis was performed on an Agilent UHPLC 1290 liquid chromatography system simultaneously connected to an Agilent QqQ 6490 and an Agilent Q-ToF 6550 via a tee and two PEEK tubings with same inner diameter (0.13 mm) and length (120 cm) (Fig. 2). Serial cables from the QqQ 6490 and the Q-ToF 6550 were connected to the pump and the autosampler of the LC system respectively, to trigger data acquisition. Computer 1 and 2 were used for the control of the LC 1290-QqQ 6490 and the Q-ToF 6550, respectively.
2.4.1 LC
An Agilent rapid resolution HD Zorbax Eclipse-C18 column (2.1 × 50 mm, 1.8 µm) was used for the RPLC separation. The mobile phases A (60% water and 40% acetonitrile with 10 mmol/L ammonium formate) and B (10% acetonitrile and 90% isopropanol with 10 mmol/L ammonium formate) were employed for both positive and negative ionization. The following gradient was applied: 0–2 min, 20–60% B; 2–12 min, 60–100% B; 12–14 min, 100% B; 14.01–15.8 min, 20% B. The oven temperature was maintained at 40 °C. Flow rate was set at 0.4 mL/min and the sample injection volume was 2 µL.
2.4.2 QqQ
The positive ionization spray voltage and nozzle voltage were set at 3000 V and 1000 V, respectively. The drying gas and sheath gas temperatures were both maintained at 250 °C. The drying gas and sheath gas flow rates were 14 L/min and 11 L/min, respectively. The nebulizer nitrogen gas flow rate was set at 35 psi. The iFunnel high and low pressure RF were 150 V and 60 V, respectively. The MRM list for GPCs and SMs is provided in the Supplemental Information (Supplemental Table S1).
2.4.3 Q-ToF
The instrument was operated in either MS-only or targeted MS/MS mode under positive or negative ionization during independent analyses. The ion spray and nozzle voltage were set at 3500 V and 1000 V, respectively. The drying gas and sheath gas temperatures were maintained at 200 °C and 350 °C, respectively. The drying gas and sheath gas flow rates were 14 L/min and 11 L/min, respectively. The nebulizer nitrogen gas flow rate was set at 35 psi. The iFunnel high and low pressure RF were 150 V and 60 V, respectively. Full scan MS and targeted MS/MS spectra, acquired from 100 to 1500 m/z at 2 spectra/sec, were stored in centroid mode.
3 Results
3.1 Dual MS for simultaneous QqQ- and Q-ToF-based lipidomic analyses
QqQ mass spectrometers are widely used in lipidomic studies since they can provide high sensitivity for quantitative analysis through MRM mode because of its two-step ion filtration procedure. However, due to the intrinsic disadvantage of the quadrupole mass analyzers, they cannot provide high mass resolution measurements. In lipidomics, many analytes show similar physicochemical properties and many isobaric and isomeric lipids cannot be differentiated at a unit mass resolution. Due to the structural heterogeneity and the high number of lipid molecules in biological samples, it is not possible to use specific labelled standards for each lipid measured. The lipidomics community then agrees to use one or two standards for each lipid class in most of the experimental efforts in the field. For the same reason (limiting the number of concurrent transitions) lipidomic MRM targeted analyses are also often undertaken with a single transition, compromising the unambiguous identification of specific molecules using qualifier ions.
On the other hand, Q-ToF instruments are capable of providing accurate mass at both MS and MS/MS levels, which benefits the structural elucidation and identification of lipids. These considerations suggest that these two mass spectrometric platforms can be highly complementary and that integrating results obtained with both instruments will increase the quality of lipidomic analyses. The potential of this approach can be fully expressed when the untargeted side (Q-ToF) also allows the identification of new lipid species that were not originally included in the targeted list (QqQ). In that case, a new expanded targeted list can then be created including new molecules. However, in this manuscript we only focus on the application of Dual MS for the improvement of data annotation in targeted lipidomics, while a new discovery approach will be covered in future publications.
We established an RPLC-based separation, coupled simultaneously with a QqQ and a Q-ToF platform. The LC flow was equally split between the two mass spectrometers via a tee, to provide simultaneous quantitative and qualitative information. When lipids having close mass-to-charge ratios and targeted MRM transitions elute next to each other during a chromatographic separation, a wrong assignment and quantification of the species measured could be avoided by high-resolution MS and/or MS/MS spectra performed with the Q-ToF. With this experimental setup, the high-resolution platform can be used to verify and correct the targeted data. All the LC and MS instruments used in this study were from the same vendor (Agilent), facilitating seamless connection, communication and synchronization. Since the LC flow was directed to the MS from a unique chromatographic system, the retention times of the lipids measured on the QqQ and the Q-ToF could be overlapped after subtracting the systematic acquisition delay caused by the sample injection (Supplemental Fig. S1). This experimental design decreases the identification uncertainty caused by possible retention time shifts, often present when comparing independent untargeted analyses either before or after targeted analyses, even when using the same separation method. The correct alignment of retention times was also verified by spiked internal standards and endogenous lipid species in the study samples (Supplemental Fig. S1).
In our platform, the QqQ was operated in MRM mode to provide quantitative results with high sensitivity while the Q-ToF was operated in either MS-only or targeted MS/MS mode, to provide the accurate mass at both precursor and product ion levels for an improved lipid identification. Targeted MS/MS was performed under positive ionization for SM and plasmanyl- and plasmenyl- glycerophospholipids and negative ionization for other glycerophospholipids, respectively. Lipid extracts from a commercially available human plasma pooled sample were used for our proof of concept study, to show how the QqQ and Q-ToF data were integrated to provide accurate lipid quantification and identification for the improvement of human plasma lipidomic studies.
3.2 Improved identification of polyunsaturated GPC and SM species
Reversed-phase separation of lipids is driven by the hydrophobicity of the fatty acid chains (Cajka et al. 2014). For this reason, most SM and GPC species with the same total carbon and double bond numbers will have a very similar retention time on an RPLC system. Under RPLC-MRM experimental conditions however, m/z values corresponding to polyunsaturated SMs and GPCs may show multiple (although not baseline-separated) chromatographic peaks (Fig. 3a–d). MS/MS results from the Q-ToF analysis in negative ionization revealed that there was a variety of possible fatty acyl/alkyl/alkenyl chain combinations for GPCs (Fig. 3e–g, i–k). Similarly, different combinations of sphingoid bases and fatty acyl chains for SMs were shown to be present by high-resolution MS/MS in positive ionization (Fig. 3h, l). Therefore, if the quantitative results were obtained exclusively by a QqQ-based MRM measurement (using the m/z 184.1 ion as a sole fragment) and reported at lipid species level, peak areas from these multiple peaks should be summed up and described by different possible lipid IDs, such as PC 38:6, SM 42:2 etc. However, quantitative (after improving peak separation) and qualitative information at fatty acyl/alkyl/alkenyl and/or sphingoid base level could be integrated if obtained simultaneously by Dual MS analysis and the lipid species represented in Fig. 3 correctly assigned to PC 18:2_20:4, PC 16:0_22:6, SM d18:1/24:1, SM d18:2/24:0 etc., in one single experiment.
Lipid species from the same lipid class with shorter carbon chain lengths and more double bonds are less hydrophobic and hence elute earlier from the RP column. In our experimental conditions, there is a linear correlation between retention time and carbon chain length or double bond number (Supplemental Fig. S2), which can be used as a reference for peak annotation. However, special attention was required when the lipid species with three and four double bonds were analysed. The chromatographic separation between them is poorer than for other lipid pairs differing by one double bond (Fig. 4). We used the Dual MS approach to clarify the reason for this behaviour. MS/MS results obtained by Q-ToF analysis in negative ionization revealed that, for example, the two peaks of PC 36:3 were identified as 18:1_18:2 and 16:0_20:3 (Fig. 4b, c) while PC 36:4 contained two fatty acyl chains as 16:0 and 20:4 (Fig. 4d), respectively. PC 16:0_20:3 eluted later that PC 18:1_18:2, suggesting that the higher heterogeneity of fatty acyl chain composition or the different distribution of double bonds between the two chains made the molecule more hydrophobic. Therefore, PC 16:0_20:4 elutes much closer to PC 18:1_18:2. The same approach represents a solution that can be applied to other lipid classes containing polyunsaturated fatty acids, as similar results could be observed for phosphatidylethanolamines (PE) (Supplemental Fig. S3), and phosphatidylinositols (PI) (Supplemental Fig. S4), etc.
3.3 Differentiation between isobaric and isomeric GPC species
Since only unit mass resolution can be achieved on a quadrupole mass analyser, isobaric and isomeric compounds with the same unit mass cannot be differentiated. The odd chain phosphatidylcholine species such as PC 33:2 has the same unit mass (m/z 744.6) as the plasmanylcholine species PC-O 34:2 and the plasmenylcholine species PC-P 34:1. Such isobaric compounds, which are measured by the same transition 744.6 > 184.1 in MRM mode, showed multiple peaks (I, II and III in Fig. 5a) in the reversed-phase-based chromatogram, leading to difficulties when an unambiguous peak annotation is the goal.
a Chromatographic peaks (I), (II), (III) for the MRM transition 744.6 > 184.1. b–d MS spectra of peak (I)–(III) in a from Q-ToF measurements in positive ionization. e–g MS/MS spectra of peak (I)–(III) in a from Q-ToF measurements in negative ionization. h–j MS/MS spectra of peak (I)–(III) in a from Q-ToF measurements in positive ionization
For the corresponding peaks, the accurate mass could be obtained from the Q-ToF analysis for the above-mentioned isobaric and isomeric compounds. First, odd chain PC 33:2 was annotated to peak (I) by its accurate m/z 744.5538 for [M+H]+, which was further confirmed by fragmentation of [M+HCOO]− in negative ionization, yielding fragments at m/z 241.2173 and m/z 279.2330, corresponding to fatty acyl chains 15:0 and 18:2, respectively(Fig. 5b, e). The isomeric PC-O 34:2 and PC-P 34:1, only differing by the presence of a vinyl ether bond in PC-P 34:1, have the same accurate mass at m/z 744.5902 (Fig. 5c, d). Therefore, only the fatty acyl chain fragments from sn-2 position in peak (II) (Fig. 5f) and peak (III) (Fig. 5g) in negative ionization are not specific enough to distinguish a vinyl ether bond (PC-P) from a distant double bond (PC-O) at sn-1 position and characteristic fragments from sn-1 position are still needed to differentiate these two isomers.
Although the phosphocholine fragment at m/z 184.0733 dominates the MS/MS spectrum in positive ionization, other ions of relative low abundance can still provide certain structural information. Fragments at m/z 482.3241 (neutral loss of C16H29CHC=O as a ketene) and 502.3292 (neutral loss of C14H29COOH as an acid) in peak (I) (Fig. 5h) added more evidence for its annotation as PC 33:2 (15:0_18:2). A specific fragment at m/z 504.3449 in peak (III) (Fig. 5j), arising from neutral loss of C14H29CH=CHOH as an alcohol at sn-1 position, identified the compound as a plasmenylcholine. The fragment at m/z 480.3449 (Fig. 5j), corresponding to a neutral loss of the fatty acyl chain 18:1 as a ketene C16H31CHC=O at sn-2 position, further confirmed that the compound can be identified as PC-P 34:1 (16:0/18:1). In contrast, the fragment at m/z 502.3292 was absent in peak (II) (Fig. 5i), suggesting that the compound is a plasmanylcholine, as it has been shown that it is much more difficult to cleave the 1-O-alkyl residue from a plasmanylcholine than the 1-O-alkenyl residue from a plasmenylcholine after collision-induced dissociation (Hsu et al. 2007).
Furthermore, a commercially available standard PC-P 18:0/18:1 could confirm, according to retention time, accurate mass and MS/MS spectrum under both positive and negative ionizations, that peak (III) from the above-mentioned peak profiling was a plasmenylcholine (Supplemental Fig. S5).
By this approach, the 97 MRM transitions for measuring GPCs and SMs in human plasma as listed in Supplemental Table S1 can be further split into 129 individual transitions at different retention times (dynamic or scheduled MRM) with a narrower detection window, which will lead to clearer identification and more accurate quantification, as well as improved analytical throughput.
3.4 Isotopic overlap of GPC and SM species
Since lipid species differing by one double bond can be chromatographically separated on RPLC, usually there is no isotopic overlap, an issue typically observed in hydrophilic interaction liquid chromatography (HILIC) even with high resolution instruments. However, coelution was observed for SM species and PC species differing by + 3 amu (Table 1). For example, SM 36:1 with formula C41H83N2O6P and m/z 731.6 showed the same retention time as PC 32:0 with C40H80NO8P and m/z 734.6 (Fig. 6a, b), as also confirmed by the Q-ToF results with high mass accuracy (Fig. 6c). The total effects on polarity given by different moieties in different molecules, such as the amide bond and free hydroxyl groups in SM m:n and both ester bonds at sn-1 and sn-2 positions in corresponding PC (m-4):(n-1), might be similar, and causes a highly similar chromatographic behaviour in a reversed-phase system.
Since the M + 3 isotopologue of SM m:n overlapped with PC (m-4):(n-1), its abundance was calculated based on natural isotopic distributions and subtracted from the peak area of PC (m-4):(n-1). This isotopic correction method was implemented to avoid a wrong quantification or even misidentification for the PC species, especially when the signal intensity of SM m:n is much higher than the signal of PC (m-4):(n-1). In the commercial plasma sample used in this study, twenty PC species would be wrongly quantified without using a proper isotopic correction, while PC 29:0 would even be erroneously reported above the limit of detection (Table 1).
Another case showing isotopic overlap occurs when measuring PC-O and PC-P. As above-mentioned, PC-P m:(n-1) elutes later than its isomer PC-O m:n, while PC-P m:n, which is less hydrophobic due to one more double bond, almost coelutes with PC-O m:n. It means that adding a vinyl ether bond to PC-O m:n has a small effect on the hydrophobicity of the molecule. This can be explained if we consider that one more double bond makes the molecule more hydrophilic while the vinyl ether bond increases its hydrophobicity. Therefore, the M + 2 isotopologue of PC-P m:n overlaps with PC-O m:n and isotopic correction is needed (supplemental Table S2).
4 Discussion
The Dual MS approach presented here aims to combine the advantages of targeted and untargeted MS approaches, to improve data quality in lipidomics. As low-resolution MRM-based measurements are prone to misannotation when applied to molecules with similar m/z and elution times, high-resolution MS and MS/MS can be used to confirm molecular compositions. At the same time, the higher sensitivity of MRM measurements is fundamental for the quantification of low abundant lipids, which is difficult to achieve with other techniques. We show here the application of Dual MS to solve ambiguities when measuring GPCs and SMs in human plasma. However, its applications can be broader as similar issues are encountered when measuring other lipid classes in human plasma and other samples. An example, similar to the one described above, is the annotation of isobaric and isomeric glycerophosphoethanolamines (GPEs) species (odd chain, plasmanyl- and plasmenyl-PE) with similar mass-to-charge ratios and elution times. Another useful application of the Dual MS platform in lipidomics is the validation of peak identities after changing chromatographic conditions (new columns, different solvents or gradients), after targeted methods transfer to other instruments or after changing the sample type. In these cases, the separation of chromatographic peaks previously measured with well-established methods might change considerably, requiring tedious new annotations. The Dual MS approach facilitates this operation, encouraging the evolution and improvement of existing routine methods. Although this manuscript describes in detail the application of this platform to plasma lipidomics, the same advantages would be present when analysing different tissues and organisms. As reported previously (Melnik et al. 2017), one of the main uses of Dual MS will also be the discovery and quantification of novel molecules, made possible by the capacity to join untargeted and targeted detection modes. This will also be applicable to lipidomics and will be the goal of our future studies.
A possible limitation of Dual MS might be found in its moderate throughput and high cost, due to the fact that it uses two MS systems at the same time, a situation that could not be compatible with laboratory setups that deal with hundreds or thousands of samples per study and need to achieve the maximum level of productivity on each instrument. However, the preferential application of Dual MS measurements to the analysis of pooled Quality Control (QC) samples, generated by pooling aliquots from all (or a representative portion) of the original samples included in large studies, would be enough to correctly assign chromatographic peaks that then could be confidently quantified by targeted MS, in all the remaining study samples, on a single instrument. More concentrated QC samples or larger injection volumes could be used in case low-abundance lipid species could not be detected selectively by one of the two systems. We are also aware that, currently, high-resolution analysis of pooled samples for lipidomic studies, before (or after) setting up the MRM method for quantification on another instrument, is commonly performed. In this context, using the Dual MS workflow might only sound as a limited improvement. However, when high-throughput, fast chromatographic methods are used, a large number of isobaric, isomeric and isotopic peaks are present in the targeted MRM chromatogram. Hence, even a minor retention time shift from an independent LC-HRMS system will make it difficult to assign the peak correctly, especially when the same method is used with a different sample matrix. The proposed dual system will decrease the identification uncertainty because the LC flow is directed to the MS from a unique chromatographic system.
In summary, we established a reversed-phase separation method coupled simultaneously to a QqQ and a Q-ToF platform, in order to provide both quantitative and qualitative information for lipidomic analyses in a single experiment. As shown by the high-accuracy MS and MS/MS spectra generated by the Q-ToF, we could discriminate between the isobars and isomers from GPC and SM species, whose quantitation was based on the MRM transitions from the dominant product ion (phosphocholine group). Furthermore, we also showed the presence of an isotopic overlap between M + 3 of SMs and the monoisotopic peak of PCs and between M + 2 of plasmenylcholines and the monoisotopic peak of plasmanylcholines which should be corrected. Our platform provides a higher level of confidence in the targeted measurement of lipids in human plasma. As this approach can be expanded to other lipid classes whose analyses suffer from issues similar to those described here for GPCs and SMs, we think that this new methodology might have a great potential in comprehensive studies that want to achieve an acceptable specificity in the quantification of lipids. Our work is aligned with several new initiatives in the lipidomics field with the aim to establish better methodologies and standard procedures to generate high quality lipidomic data.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author.
Abbreviations
- QqQ:
-
Triple quadrupole
- Q-ToF:
-
Quadrupole-time of flight
- GPCs:
-
Glycerophosphocholines
- MRM:
-
Multiple reaction monitoring
- PC:
-
Phosphatidylcholine
- PC-O:
-
Plasmanylcholine
- PC-P:
-
Plasmenylcholine
- IS:
-
Internal standards
- PE:
-
Phosphatidylethanolamines
- PI:
-
Phosphatidylinositols
- HILIC:
-
Hydrophilic interaction liquid chromatography
- QC:
-
Quality control
References
Acharya, U., Patel, S., Koundakjian, E., Nagashima, K., Han, X., & Acharya, J. K. (2003). Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science,299, 1740–1743.
Alshehry, Z. H., Barlow, C. K., Weir, J. M., Zhou, Y., McConville, M. J., & Meikle, P. J. (2015). An efficient single phase method for the extraction of plasma lipids. Metabolites.,5, 389–403.
Bowden, J. A., Heckert, A., Ulmer, C. Z., Jones, C. M., Koelmel, J. P., Abdullah, L., et al. (2017). Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950-Metabolites in Frozen Human Plasma. Journal of Lipid Research,58, 2275–2288.
Braverman, N. E., & Moser, A. B. (2012). Functions of plasmalogen lipids in health and disease. Biochimica et Biophysica Acta,1822, 1442–1452.
Cajka, T., & Fiehn, O. (2014). Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends in Analytical Chemistry,61, 192–206.
Gonzalez-Covarrubias, V., Beekman, M., Uh, H. W., Dane, A., Troost, J., Paliukhovich, I., et al. (2013). Lipidomics of familial longevity. Aging Cell,12, 426–434.
Hajra, A. K. (1995). Glycerolipid biosynthesis in peroxisomes (microbodies). Progress in Lipid Research,34, 343–364.
Han, X. (2016). Lipidomics for studying metabolism. Nature Reviews Endocrinology,12, 668–679.
Heymans, H. S., Schutgens, R. B., Tan, R., van den Bosch, H., & Borst, P. (1983). Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature,306, 69–70.
Holčapek, M., Liebisch, G., & Ekroos, K. (2018). Lipidomic analysis. Analytical Chemistry,90, 4249–4257.
Honsho, M., & Fujiki, Y. (2017). Plasmalogen homeostasis—Regulation of plasmalogen biosynthesis and its physiological consequence in mammals. FEBS Letters,591, 2720–2729.
Hsu, F. F., & Turk, J. (2007). Differentiation of 1-O-alk-1'-enyl-2-acyl and 1-O-alkyl-2-acyl glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. Journal of the American Society for Mass Spectrometry,18, 2065–2073.
Jiang, X. C., Paultre, F., Pearson, T. A., Reed, R. G., Francis, C. K., Lin, M., et al. (2000). Plasma sphingomyelin level as a risk factor for coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology,20, 2614–2618.
Kim, I. C., Lee, J. H., Bang, G., Choi, S. H., Kim, Y. H., Kim, K. P., et al. (2013). Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Anticancer Research,33, 2467–2472.
Laaksonen, R., Ekroos, K., Sysi-Aho, M., Hilvo, M., Vihervaara, T., Kauhanen, D., et al. (2016). Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. European Heart Journal,37, 1967–1976.
Liebisch, G., Ekroos, K., Hermansson, M., & Ejsing, C. S. (2017). Reporting of lipidomics data should be standardized. Biochimica et Biophysica Acta,1862, 747–751.
McFadyen, J. D., Meikle, P. J., & Peter, K. (2017). Platelet lipidomics: a window of opportunity to assess cardiovascular risk? European Heart Journal,38, 2006–2008.
Melnik, A. V., da Silva, R. R., Hyde, E. R., Aksenov, A. A., Vargas, F., Bouslimani, A., et al. (2017). Coupling targeted and untargeted mass spectrometry for metabolome-microbiome-wide association studies of human fecal samples. Analytical Chemistry,89, 7549–7559.
Nishimukai, M., Maeba, R., Ikuta, A., Asakawa, N., Kamiya, K., Yamada, S., et al. (2014a). Serum choline plasmalogens-those with oleic acid in sn-2-are biomarkers for coronary artery disease. Clinica Chimica Acta,437, 147–154.
Nishimukai, M., Maeba, R., Yamazaki, Y., Nezu, T., Sakurai, T., Takahashi, Y., et al. (2014b). Serum choline plasmalogens, particularly those with oleic acid in sn-2, are associated with proatherogenic state. Journal of Lipid Research,55, 956–965.
Ortegren, U., Aboulaich, N., Ost, A., & Stralfors, P. (2007). A new role for caveolae as metabolic platforms. Trends in Endocrinology and Metabolism,18, 344–349.
Petersen, N. H., Olsen, O. D., Groth-Pedersen, L., Ellegaard, A. M., Bilgin, M., Redmer, S., et al. (2013). Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell,24, 379–393.
Rietveld, A., Neutz, S., Simons, K., & Eaton, S. (1999). Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. Journal of Biological Chemistry,274, 12049–12054.
Rochat, B. (2016). From targeted quantification to untargeted metabolomics: Why LC-high-resolution-MS will become a key instrument in clinical labs. Trends in Analytical Chemistry,84, 151–164.
Shakor, A. B., Taniguchi, M., Kitatani, K., Hashimoto, M., Asano, S., Hayashi, A., et al. (2011). Sphingomyelin synthase 1-generated sphingomyelin plays an important role in transferrin trafficking and cell proliferation. Journal of Biological Chemistry,286, 36053–36062.
Singh, P., & Chattopadhyay, A. (2012). Removal of sphingomyelin headgroup inhibits the ligand binding function of hippocampal serotonin1A receptors. Biochemical and Biophysical Research Communications,419, 321–325.
Slotte, J. P. (2013). Biological functions of sphingomyelins. Progress in Lipid Research,52, 424–437.
Teo, C. C., Chong, W. P. K., Tan, E. N., Basri, B., Low, Z. J., & Ho, Y. S. (2015). Advances in sample preparation and analytical techniques for lipidomics study of clinical samples. Trends in Analytical Chemistry,66, 1–18.
Van den Bosch, H., Schutgens, R. B., Wanders, R. J., & Tager, J. M. (1992). Biochemistry of peroxisomes. Annual Review of Biochemistry,61, 157–197.
Van Meer, G., Voelker, D. R., & Feigenson, G. W. (2008). Membrane lipids: Where they are and how they behave. Nature Reviews Molecular Cell Biology,9, 112–124.
Vouk, K., Hevir, N., Ribic-Pucelj, M., Haarpaintner, G., Scherb, H., Osredkar, J., et al. (2012). Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Human Reproduction,27, 2955–2965.
Walter, A., Korth, U., Hilgert, M., Hartmann, J., Weichel, O., Hilgert, M., et al. (2004). Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiology of Aging,25, 1299–1303.
Xu, Y., Ramu, Y., & Lu, Z. (2008). Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature,451, 826–829.
Disclaimer
A very preliminary version of some of the results showed in this work is available as a poster abstract that can be accessed at https://www.imsc2018.it/images/Abstract_book_proofs_2.pdf.
Funding
This study was supported by grants from the National University of Singapore via the Life Sciences Institute (LSI), the National Research Foundation (NRFI2015-05) and Human Frontier Science Program (RGP0055/2015).
Author information
Authors and Affiliations
Contributions
LG and FTT conceived and designed the project. MRW coordinated the project. LG performed samples preparation and LC–MS analysis. LG, ACG, BB and FTT analyzed the data. LG wrote the first draft of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, L., Cazenave-Gassiot, A., Burla, B. et al. Dual mass spectrometry as a tool to improve annotation and quantification in targeted plasma lipidomics. Metabolomics 16, 53 (2020). https://doi.org/10.1007/s11306-020-01677-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-020-01677-z