Abstract
Coarse woody debris (CWD) plays an important role in long-term carbon storage in forest ecosystems. However, few studies have examined CWD in mangrove forests. A secondary mangrove forest on an estuary of the Trat River showed different structures along vegetation zones ranging from the river’s edge to inland parts of the forest (the Sonneratia–Avicennia, Avicennia, Rhizophora, and Xylocarpus zones, respectively). The mass distribution of CWD stock in downed wood and standing dead trees along these vegetation zones was evaluated. Most of the CWD stock in the Sonneratia–Avicennia and Avicennia zones was found in downed wood, while it mainly accumulated in standing dead trees in the Rhizophora and Xylocarpus zones. The total mass of CWD stock that accumulated in each zone ranged from 1.56–8.39 t ha−1, depending on the forest structure and inundation regimes. The annual woody debris flux in each zone was calculated by summing the necromass (excluding foliage) of dead trees and coarse litter from 2010 to 2013. The average woody debris flux was 5.4 t ha−1 year−1, and its zonal variation principally depended on the necromass production that resulted from forest succession, high tree-density, and lightning. Over all the zones, the above- and below-ground net primary production (ANPP and BNPP, respectively) was estimated at 18.0 and 3.6 t ha−1 year−1, respectively. The magnitude of BNPP and its contribution to the NPP was markedly increased when fine root production was taken into consideration. The contribution of the woody debris flux without root necromass to the ANPP ranged from 12 to 28%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coarse woody debris (CWD) is large, dead, wood material, which includes downed wood, stumps, standing dead trees (Jia-bing et al. 2005; Enrong et al. 2006), and coarse roots (Harmon et al. 1986). It is an important component of the long-term carbon storage in forest ecosystems. Over the last few decades, climatic changes have dramatically affected coastal vegetation through rising sea levels and changes in storm intensities and stream flows (Gilman et al. 2008), and will likely increase the amounts of CWD in coastal vegetation areas (Kauffman and Cole 2010).
Mangrove forests are outstanding examples of coastal vegetation in the tropics and subtropics. They are important ecosystems for carbon storage because of their high net primary production (NPP) (Komiyama et al. 2008; Poungparn et al. 2012). In general, NPP is usually estimated using the summation method, which is the summation of plant growth, litter production, and the plant mass grazed by herbivores (Kira and Shidei 1967; Clark et al. 2001a). Since the latter is usually assumed to be negligible (McNaughton et al. 1996), then NPP is typically expressed in terms of plant growth and litter production. Clark et al. (2001b) suggested that the growth of a stand consists of the biomass increment of living trees and the biomass of dead trees (necromass) in a defined time interval, while litter production refers to fine litter, including leaf litter and short-lived plant materials, such as flowers and twigs.
By considering the woody debris flux as the production of coarse litter and necromass in NPP estimation, Ohtsuka et al. (2007) reported that CWD flux accounted for 1.5 t C ha−1 year−1 of the 5.4 t C ha−1 year−1 of NPP in a temperate deciduous forest. However, CWD has rarely been studied in mangrove forests (i.e., Allen et al. 2000; Krauss et al. 2005; Kauffman and Cole 2010), despite their substantial contribution to coastal vegetation in the tropics and subtropics. Moreover, the woody debris flux has not been partitioned in NPP estimations of mangrove forests. Thus, a study of the carbon stock in woody debris should be conducted to clarify the carbon storage in an entire mangrove ecosystem.
Mangrove forests are usually influenced by the inundation time and depth, which is related to their distance from rivers, as well as their elevation. Different elevations and inundation patterns affect the dominant species in these forests, and this results in the formation of distinct vegetation zones (Poungparn et al. 2009). Vegetation zones may affect the magnitude of the woody debris flux (including both fine and coarse woody debris which are separated by a threshold of 4.5 cm) and the zonal distribution of CWD because of differences in environmental factors and forest structures. However, the zonal distribution of CWD in a mangrove forest has not been reported. Therefore, the distribution of CWD was evaluated by determining the number of pieces of CWD, their category (downed wood and standing dead trees), and mass along the different respective vegetation zones in a secondary mangrove forest. Variations in the annual woody debris flux in the respective zones were observed, and the NPP was estimated for each of the respective four zones to determine the contribution of the woody debris flux to NPP over a 4-years period (2010–2013). Accordingly, the patterns of the contribution of the woody debris flux to NPP are discussed by zone.
Methods
Study site
The study site was located in a secondary mangrove forest along the Trat River in Trat Province, eastern Thailand. This forest is managed by the Mangrove Research Station at Trat (Department of Marine and Coastal Resources). The average (mean ± SD) annual precipitation and air temperature from 2010 to 2013 was 5164 ± 673 mm and 28.1 ± 0.2 °C, respectively (Department of Meteorology, Thailand). The southwest monsoon usually occurs during the rainy season (May–October), causing a high level of precipitation. The average precipitation during the rainy season in this 4-years study period was 4492 ± 632 mm, accounting for 87% of the annual average. The strong, southwest monsoon prevails over Trat Province from June to September (in the rainy season) with average maximum-recorded wind speeds of 22–24 knots. The summer monsoon occurs in April (in the dry season) with an average maximum wind speed of 20 knots (Department of Meteorology, Thailand).
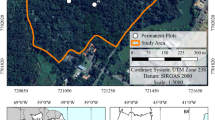
A permanent study plot (50 m × 120 m) was established in 2009. The study plot clearly showed four vegetation zones, based on the dominant tree species, from the river to the inland parts of the forest (Fig. 1). At the river’s edge, Sonneratia caseolaris (L.) Engl. and Avicennia alba Blume were dominant in a narrow belt (0–10 m from the river) forming the so-called Sonneratia–Avicennia zone. The S. caseolaris and A. alba are pioneer species, which dominated on open areas of river and seaward edges or tidal flat (Bird 1986; Friess et al. 2012). The second zone (10–40 m from the river) was the Avicennia zone, which was primarily dominated by Avicennia alba. Rhizophora apiculata Blume and Rhizophora mucronata Poir. were also found in both zones because they replaced after succession of S. caseolaris and A. alba. The third zone (40–100 m from the river) was the Rhizophora zone, which was primarily dominated by R. apiculata and R. mucronata, although there were other species in this zone, including A. alba, Bruguiera gymnorrhiza (L.) Savigny, Ceriops tagal (Perr.) C. B. Rob., and Xylocarpus granatum Koenig. The last zone (100–120 m from the river) was the Xylocarpus zone, which was dominated by X. granatum, with R. apiculata, B. gymnorrhiza, and C. tagal as minor constituents.
The zonation occurred because of differences in the elevation from the river to inland portions of the plot, which caused variations in the inundation period (Poungparn et al. 2009), where the inundation period decreased with increasing distance from the river. The average relative elevation compared to a datum point located at the lowest point of the study plot was 23 ± 14, 33 ± 8, 48 ± 12 and 65 ± 12 cm for the Sonneratia–Avicennia, Avicennia, Rhizophora and Xylocarpus zones, respectively (Fig. 1). The inundation period was investigated six times during August to October (rainy season) and February to April (dry season) by recording the time when the tide moving from the river to each part of the study plot as described in Poungparn et al. (2009). The Sonneratia–Avicennia and Avicennia zones located on the river’s edge had a relatively long inundation period of more than 12 h per day. In the inland zones (the Rhizophora and Xylocarpus zones), the inundation period was shorter (5–11 h) because of the relatively high elevation of the forest.
Forest structure, biomass, and necromass increment
The tree density (diameter at breast height; DBH ≥4.5 cm), average DBH, and height (H) in each zone were calculated annually from 2010 to 2013. The total living-tree biomass was obtained by summing the aboveground biomass (W Top) and root biomass (W R), where W Top and W R were calculated using common allometric equations for mangrove species (Komiyama et al. 2005) of W Top = 0.247ρD 2.46 and W R = 0.196ρ 0.899 D 2.22 (where ρ is the wood density of stems and D is the DBH). Growth, which is an increment of total living-tree biomass, was calculated in separate 1-year intervals for each zone. The growth of new trees was calculated by the difference of the biomass of new tree and the biomass of the minimum tree (diameter = 4.5 cm), according to Clark et al. (2001a). Dead trees having a DBH of ≥4.5 cm were recorded annually. The aboveground and root necromass were estimated by using the allometric equation for W Top and W R, respectively. Mortality and recruitment rate were calculated using a logarithmic model as reported by Miura et al. (2001). The biomass increment, which is the change in living-tree biomass (Ohtsuka et al. 2007), was calculated as the difference between growth and the necromass production.
Litter production
Litter production was estimated using litter traps (1 m × 1 m). Twelve litter traps were placed throughout the study plot. Litter fall in the traps was collected monthly and oven dried at 80 °C to a constant mass. The dried litter fall was then separated into fine litter (leaf and reproductive organs) and coarse litter (woody organs), and weighed. The annual litter production in each zone was calculated by summing the monthly litter fall from 2010 to 2013.
Woody debris flux and NPP
Annual woody debris flux was obtained by summing the necromass excluding foliage, and coarse litter production from 2010 to 2013. This necromass production was calculated by the difference between the aboveground and leaf necromass. Leaf necromass was given by using the allometric equation for leaf biomass; W L = 0.135ρD 1.696B , where D B is the diameter of the lowest living branch (Komiyama et al. 2005). The aboveground NPP (ANPP) in each zone was calculated for each year by summing the aboveground biomass increment, aboveground necromass production, coarse and fine litter production. The belowground NPP (BNPP) was likewise calculated by summing the root biomass increment and root necromass production. The NPP was then derived as the summation product of the ANPP and BNPP estimates.
CWD stock
In October 2013, all the CWD was surveyed and divided into downed wood (fallen branches and trees) and standing dead trees by a modified procedure from Enrong et al. (2006). The positions of the CWD were plotted on a tree map (Fig. 1). The CWD in each category was categorized into decay classes, according to the decomposition status and morphological characteristics of the wood (Enrong et al. 2006; modified from Maser et al. 1979). The decay classes were (I) bark present and wood intact, (II) decayed wood found in an area from 0 to 50% of the radius of the CWD, and (III) decayed wood found in an area greater than 50% of the radius of the CWD. The CWD in each decay class was then identified to the species level using the morphological characteristics of the wood (i.e., bark, wood, and wood color), except Rhizophora spp. were only identified to the genus level due to the similarity of their wood characteristics between species following visual assessments. The diameter along the downed wood and length of each CWD was measured using a diameter tape and a ruler, respectively. For a standing dead tree, the DBH and buttress diameter were measured. Upper diameter was estimated by using a taper function of Chambers et al. (2001), according to the study of Baker et al. (2007). The CWD volume of both downed wood and standing dead tree were calculated from the diameters and length using Smalian’s formula (Baker et al. 2007). The CWD mass was calculated from the CWD volume and the specific wood density.
To determine the specific wood density, small pieces (length 5–10 cm) of CWD samples (n ≥ 5) of each decay class and species were cut using a handsaw. The fresh volume of the CWD samples was measured by water displacement (Chao et al. 2008), and they were oven dried at 80 °C to a constant dry weight. The CWD density was calculated from the ratio of dry weight to fresh volume for each decay class and species. The CWD stock in 2013 of the respective zones was given by the CWD mass in terms of downed wood and standing dead trees.
Statistical analysis of the data
The data are presented as the mean ± one standard deviation (SD). Statistically significant differences were analyzed by one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests using the SPSS 20 for Windows software (IBM Corp., Armonk, NY, USA). Statistical significance was accepted at the P < 0.05 level.
Results
Forest structure
From 2010 to 2013, the highest tree density and lowest average DBH were found in the Xylocarpus zone. The Avicennia zone had the lowest tree density, but the highest average DBH (Table 1). Overall, the average DBH and H ranged from 9.5–13.6 cm, and 9.6–13.3 m, respectively, across the vegetative zones.
More than 80% of the dead trees in each zone belonged to the dominant species of the respective zone, but the dead trees in the Xylocarpus zone were distributed among X. granatum (50%), R. apiculata (30%), and other species (20%). The number of dead trees in the Sonneratia–Avicennia zone was stable from 2010 to 2013 (40 stems ha−1 in each year), with a mortality rate of 2% throughout the period (Table 1), while the recruitment rate increased from 0 to 2% during 2010 to 2013. The diameters of the dead trees (8.4–14.2 cm DBH) were large (Table 1). The number of dead trees in the Avicennia zone from 2010 to 2012 was lower than that in the Sonneratia-Avicennia zone (20, 13, and 13 stems ha−1 from 2010 to 2012, respectively), but the number of dead trees in 2013 was similar in the two zones (40 vs. 47 stems ha−1 for the Sonneratia-Avicennia and Avicennia zones, respectively). The mortality rate of the Avicennia zone increased from 2 to 5% during 2010 to 2013. In the same period, the recruitment rate of Rhizophora trees in this zone also increased from 0 to 10%. However, the recruitment rate in the Rhizophora and Xylocarpus zones was very low (0–1%), while the numbers of dead trees varied from 2010 to 2013. The mortality rate of these zones ranged from 1–3%. In the Rhizophora zone, the numbers of dead trees from 2010 to 2013 was 17, 20, 47, and 57 stems ha−1, respectively. The Xylocarpus zone had a large number of dead trees in 2010, but this decreased in successive years (100, 90, 50, and 60 stems ha−1 from 2010 to 2013, respectively). The high mortality rate found in 2010 (3%) in the Xylocarpus zone was the result of lightning, which covered an area of an approximately 2.5 m radius (Fig. 1).
Biomass, necromass, and litter production
The average aboveground biomass for the 4-years study period from 2010 to 2013 ranged from 156.8 to 257.7 t ha−1 among the four zones (Table 2). The root biomass in the four zones was roughly equal to 40% of the aboveground biomass, and it showed the same pattern of zonal distribution as the aboveground biomass. The average total biomasses ranged from 216.5 to 361.4 t ha−1 year−1 (Table 2), but was significantly different among the zones (one-way ANOVA, P < 0.0001). The average total biomass was the highest in the Rhizophora zone, followed by that in the Xylocarpus, Sonneratia–Avicennia, and Avicennia zones. Although the total biomass of the Sonneratia–Avicennia zone was lower than in the Rhizophora and Xylocarpus zones, relatively high growth was observed. The average growth of the Sonneratia–Avicennia zone was the highest, followed by the Rhizophora, Avicennia, and Xylocarpus zones.
The average necromass production ranged widely (1.9–8.7 t ha−1 year−1) because of the variation in dead tree diameters among the zones. The highest average necromass production was recorded in the Sonneratia–Avicennia zone (Table 2) owing to the large size of the dead trees (Table 1). Although the numbers of dead trees in the Xylocarpus zone was the highest, the average necromass production was lower than that in the Sonneratia-Avicennia zone because of the small size of the dead trees. Furthermore, the average necromasses production in the Sonneratia-Avicennia (8.7 ± 10.7 t ha−1 year−1) and Avicennia (1.9 ± 2.0 t ha−1 year−1) zones had a high degree of variation.
The average litter production did not significantly differ by zone (one-way ANOVA, P = 0.147), being comparable in the Sonneratia–Avicennia and Rhizophora zones but relatively low in the Avicennia and Xylocarpus zones (Table 2). Fine litter accounted for the highest proportion of the litter production in all of the zones (84–90% of the litter production). The average woody litter production showed a narrow range (0.6–1.0 t ha−1 year−1), and did not significantly differ by zone (one-way ANOVA, P = 0.541). However, a high variation in the woody litter production was clearly seen in the Avicennia and Rhizophora zones during the experiment period (Table 2).
Woody debris flux and NPP
As with the average necromass (except for foliage) and coarse litter production, the average woody debris flux (Table 2) did not significantly differ by zone (one-way ANOVA, P = 0.461). Regardless of the zone, the average woody debris flux was 5.4 t ha−1 year−1, which was composed of 3.3 t ha−1 year−1 of aboveground necromass production, 1.4 t ha−1 year−1 of root necromass production and 0.7 t ha−1 year−1 of coarse litter production. The highest woody debris flux was recorded in the Sonneratia–Avicennia zone (but with a very large variation), followed by the Xylocarpus, Rhizophora, and Avicennia zones, respectively. The woody debris flux in the Sonneratia–Avicennia and Avicennia zones also greatly fluctuated over the 4-year, mainly due to their high variation in annual necromass. Indeed, necromass production (except for foliage) was the highest component of the woody debris flux in all zones. The proportion of necromass production to the woody debris flux was obviously high in the Xylocarpus (81 ± 15%) and Sonneratia-Avicennia (80 ± 19%) zones, while it was 77 ± 21 and 55 ± 29%, for the Rhizophora and Avicennia zones, respectively.
The average biomass increment ranged widely from 2.5–14.2 t ha−1 year−1 across the zones, but was not significantly different among the zones (one-way ANOVA, P = 0.252). The highest value was recorded in the Sonneratia–Avicennia zone but with a very large variance, while the lowest value was observed in the Xylocarpus zone, also with a very large variance (Table 2). The Rhizophora and Avicennia zones showed comparable average biomass increment values.
Finally, the average ANPP, BNPP, and NPP (Table 2) differed significantly among the zones (one-way ANOVA, P < 0.0001). With the ANPP contributing 80–85% of the NPP, it showed a similar trend of zonal variation as the NPP. The highest average NPP was found in the Sonneratia-Avicennia zone (32.1 t ha−1 year−1), while the lowest was seen in the Xylocarpus zone (15.1 t ha−1 year−1). The average NPP of the Rhizophora and Avicennia zones was 21.2 and 18.2 t ha−1 year−1, respectively.
The contributions of the average biomass increment, woody debris flux, and fine litter production to the average NPP is shown in Fig. 2, where the Rhizophora and Avicennia zones showed a similar pattern of contribution (χ2, P = 0. 232). The biomass increment made the high contribution in the Sonneratia–Avicennia, Avicennia, and Rhizophora zones, but the woody debris flux and fine litter production made the largest contributions in the Xylocarpus zone.
Percentage of the biomass increment, necromass, fine litter, and coarse litter to the net primary production in each mangrove forest zone. The woody debris flux is shown by combined portions among the aboveground necromass (except for foliage), root necromass and coarse litter production. Shown are the a Sonneratia–Avicennia, b Avicennia, c Rhizophora, and d Xylocarpus zones
Distribution of CWD stock
The distribution of the number of CWD pieces in the Sonneratia–Avicennia and Avicennia zones showed a similar trend, with downed wood being the more prevalent type, and accounting for approximately 50% of the total number of pieces of CWD (Table 3). The downed wood in the Sonneratia–Avicennia zone was large, with an average diameter and length of 21.6 cm and 3.3 m, respectively. Most of the CWD in the Sonneratia–Avicennia zone was categorized as decay class I, while the CWD in the Avicennia zone was smaller than that in the Sonneratia–Avicennia zone (average diameter and length of 10.5 cm and 3.2 m, respectively), and was categorized as decay class II. In the Rhizophora zone, pieces of downed wood accounted for 63% of the total CWD (Table 3). We found small pieces of downed wood (average diameter of 9.1 cm and length range of 0.5–5.7 m) in this zone. The distribution of the CWD number in the Xylocarpus zone (72% of the total number of pieces of CWD) differed from those in the other zones because most of the CWD was standing dead trees (Table 3). Most of the CWD in each zone belonged to the dominant tree species in that zone.
The density of wood that constituted the CWD from the dominant species, namely, S. caseolaris, A. alba, Rhizophora spp., and X. granatum, is shown in Table 4. The specific density of CWD decreased when the wood was highly decomposed for all tree species. Within a respective species, the densities of fresh wood and CWD of decay class I were similar but differed significantly from those of decay classes II and III (one-way ANOVA, P < 0.0001). Rhizophora spp. had the highest density, while S. caseolaris had the lowest density. However, the density of S. caseolaris CWD in decay classes II and III was not measured because no CWD belonging to these decay classes was found in the plot.
The mass distribution pattern of the CWD stock in the Sonneratia–Avicennia and Avicennia zones showed the same trend as the CWD number distribution, and accounted for approximately 55% of the total CWD mass (Table 3). In contrast, the Rhizophora and Xylocarpus zones of the inland areas showed a different CWD mass distribution pattern, with a high proportion of the mass residing in standing dead trees at 72 and 93% of the total CWD masses in the Rhizophora and Xylocarpus zones, respectively (Table 3).
Discussion
Growth, necromass, and biomass increment
During the 4-year study period (2010–2013), the high growth in the Sonneratia–Avicennia zone was due to fast-growing S. caseolaris, a pioneer species, and the high necromass production was due to the deaths of the large pioneer trees in 2012 and 2013. In the Avicennia zone, a moderately high growth was obtained, mostly due to the high biomass increment of Avicennia trees. We found that both the mortality and recruitment rates in the Sonneratia–Avicennia and Avicennia zones increased during 2010–2013. Thus, the dead trees in both zones were probably caused by forest succession, as the mortality of pioneer species (S. caseolaris and A. alba) increased, and Rhizophora spp. subsequently became dominant.
The growth in the Rhizophora zone was less than half of that in the Sonneratia–Avicennia zone, which might be caused by the slower-growing Rhizophora spp. and the lower necromass production in the Rhizophora zone. The small Rhizophora trees died because of a dense crown, which resulted in competition for light. Despite the high necromass production, the lowest growth was recorded in the Xylocarpus zone that had a very high density of small trees, which resulted in the smallest biomass increment. The high necromass production in the Xylocarpus zone resulted from the high number of dead trees following a lightning strike in 2010 (Fig. 1).
Thus, we confirmed that forest succession and natural disturbance resulted in tree mortality and, consequently, the accumulation of CWD mass in this forest. The average mortality rate of this forest was 2%, which was lower than that reported in a Micronesian mangrove forest (Kauffman and Cole 2010), where the mortality rates ranged from 6–32% were the highest in the sites that had been impacted by a typhoon.
Distribution of CWD stock
The distribution of CWD stock in 2013 showed that the downed wood in the Sonneratia–Avicennia zone had the highest mass, which may result from a long period of inundation in the zone adjacent to the river that softened the dead wood and subsequently broke into downed wood. The large tree size and low degree of wood decay resulted in a relatively large CWD mass that remained on the forest floor of this zone. The differences in specific wood density among the decay classes (Table 4) resulted in differences in the CWD stock distribution. However, the mass stock of CWD classes II and III in this zone was very low or not found. We assumed that the small size and low wood density of downed wood after decomposition might have led to the removal of CWD classes II and III from the Sonneratia-Avicennia zone by daily tides.
That number of pieces of downed wood in the Rhizophora zone was much higher than in the other three zones (Table 3) is because the forest floor had a high density of prop roots. When a large branch or dead tree falls, it may hit the prop roots and break into smaller pieces of downed wood that then remained in the area because they are trapped by the prop roots. However, a high proportion of mass in standing dead trees was found in the Rhizophora and Xylocarpus zones, which implies that the remaining standing dead trees in this zone resulted from a relatively low frequency of inundation.
The average mass of the CWD stock in this study was 5.2 t ha−1, equivalent to 3% of the aboveground biomass. Kauffman and Cole (2010) studied the effects of typhoons on the forest structure of Micronesian mangroves, and reported that the mass of downed wood (diameter >2.5 cm) ranged from 25.5–30.5 t ha−1 and accounted for 7–8% of the aboveground biomass. It is remarkable that the mass of downed wood in the present study is much lower than that in the Micronesian mangroves, but this may have occurred for two reasons. Firstly, the large tree size and high mortality rate in the study by Kauffman and Cole (2010) may have been caused by typhoons. Secondly, a lower CWD diameter threshold may have resulted in the higher mass of the CWD stock in the Micronesian mangroves. Nevertheless, coarse root debris was excluded from the CWD mass in this study because of the difficulties in observing the belowground parts under high-water table conditions, even though the biomass of the roots was substantial when compared with the aboveground biomass.
Woody debris flux and NPP
The proportion of necromass production (except for foliage) to woody debris flux was high, and variations in the annual necromass in the respective zones were high, with the annual woody debris flux of each zone fluctuating greatly over the 4-year study period. Consequently, the average woody debris flux did not differ among the zones, being calculated as 2.7 t C ha−1 year−1 when assuming an approximate 50% of carbon content. This was comprised of 2.35 t C ha−1 year−1 of necromass production and 0.35 t C ha−1 year−1 of coarse litter production. The necromass production (except for foliage) obtained in this study was higher than that previously reported in a temperate deciduous forest in Japan at 1.0 and 0.5 t C ha−1 year−1 for the necromass and coarse litter production, respectively (Ohtsuka et al. 2007). It was notable that the proportion of the necromass production (except for foliage) to woody debris flux in the present study was as high as 87%, while this proportion in the temperate deciduous forest in Japan was only 67% (Ohtsuka et al. 2007). Differences in stand structure, as well as the character of the coastal vegetation of the mangrove forest, caused the relatively high woody debris flux. Thus, the long-term carbon input resulting from the woody debris flux is remarkable for the coastal vegetation of a mangrove forest.
The zonal difference in NPP was due to variations in growth among the four zones (Table 2). The BNPP magnitudes in this study ranged from 2.2 to 6.4 t ha−1 year−1, with an average of 3.6 ± 1.9 t ha−1 year−1, that contributed approximately 16% of the NPP. However, note that the estimation of BNPP in the present study excluded fine root production, whereas fine root production in Rhizophora stands is generally equivalent to the ANPP (Robertson and Phillips 1995) and so is not negligible in any estimation of the mangrove productivity. Using the same mangrove forest examined in the present study, Poungparn et al. (2016) recently gave a range of fine root production as 3.4–4.1 t ha−1 year−1 using ingrowth cores. When the fine root production rates were incorporated into the BNPP estimation here, the BNPP increased to 5.6–7.2 t ha−1 year−1, and then contributed up to 35% of the average NPP. Thus, reliable estimates of fine root production are important for NPP estimation in mangrove forests. The ANPP magnitude in the present study ranged from 12.9 to 25.7 t ha−1 year−1 (average of 18.0 ± 5.5 t ha−1 year−1). Komiyama et al. (2008) reviewed that the ANPP in mangrove forests in subtropical and tropical regions ranged from 4.0 to 26.7 t ha−1 y−1, and so the estimated ANPP of this study lies within this range and demonstrates the high ANPP of mangrove forests.
Analysis of the contribution of the woody debris flux to the NPP of the four respective zones (Fig. 2) showed that the woody debris flux made a significant contribution to the NPP estimation in the secondary mangrove forest. Large woody debris fluxes, which were caused by severe lightning, which occurred once in 3 years in average, and forest succession, accounted for 39 and 31% of the NPP in the Xylocarpus and Sonneratia–Avicennia zones, respectively, and illustrated the necessity for including the woody debris flux in NPP estimates of mangrove forests. However, as discussed above, our NPP estimates were obtained by excluding the fine root production to the BNPP. The contribution of the woody debris flux without root necromass was 26, 14, 16 and 31% of the ANPP in the Sonneratia–Avicennia, Avicennia, Rhizophora and Xylocarpus zones, respectively. These were lower than that of a temperate deciduous forest, where the woody debris flux without root necromass production was reported to contribute 38% to the ANPP (Ohtsuka et al. 2007). This may be caused by the higher aboveground biomass increment in the mangrove forest (32% of ANPP) compared to the temperate deciduous forest (9% of ANPP).
The accumulation of the annual aboveground woody debris flux from 2010 to 2013 was highest in the Sonneratia-Avicennia zone (26.6 t ha−1), followed by the Xylocarpus (16.0 t ha−1), Rhizophora (11.6 t ha−1), and Avicennia (8.9 t ha−1) zones. However, the highest proportion of CWD stock in 2013 to the accumulation of the annual aboveground woody debris flux in the same period was found in the Rhizophora zone (41%), followed by the Xylocarpus (37%), Sonneratia–Avicennia (32%), and Avicennia (18%) zones. These results demonstrate that the high CWD storage in the Rhizophora and Xylocarpus forest resulted from its prop root system and inundation frequency, as mentioned above.
Conclusions
Tree growth differed among the four zones of this tropical mangrove forest, with a trend of high growth in the zone adjacent to the river’s edge, while a large variation of necromass production occurred because of forest succession, high tree-density, and lightning. The distribution patterns of the CWD stock differed among the four zones, which was largely because of the transportation of CWD by water currents, as well as inundation regimes that affected CWD fragmentation. In the zones adjacent to the river’s edge, the mass of downed wood was greater than that of standing dead trees, while the standing dead trees made the largest contribution to the CWD mass in the inland zones. The annual woody debris flux, which mainly resulted from necromass production, varied greatly over the 4-year period in each zone. A high ANPP was confirmed for the mangrove forest. The magnitude of the BNPP, and so its contribution to the NPP, was markedly increased when fine root production was taken into consideration, and so fine root production is a significant component for estimation of the NPP in mangrove forests. A contribution of the woody debris flux without root necromass to ANPP was also shown. These results demonstrate a prominent contribution of the woody debris flux to the carbon dynamics in a mangrove forest. This study revealed unique characteristics of the woody debris flux and the distribution of CWD stock in mangrove forests, which is beneficial for the study of carbon storage in mangrove forests.
References
Allen JA, Ewel KC, Keeland BD, Tara T, Smith TJ (2000) Downed wood in Micronesian mangrove forests. Wetlands 20:169–176
Baker TR, Honorio Coronado EN, Phillips OL, Martin J, Heijden GMF, Garcia M, Espejo JS (2007) Low stocks of coarse woody debris in a southwest Amazonian forest. Oecologia. doi:10.1007/s00442-007-0667-5
Bird ECF (1986) Mangroves and intertidal morphology in Westernport Bay, Victoria, Australia. Mar Geol 69:251–271
Chambers JQ, dos Santos J, Ribeiro RJ, Higuchi N (2001) Tree damage, allometric relationships, and above-ground net primary production in a central Amazon forest. For Ecol Manage 152:73–84
Chao K, Phillips OL, Baker TR (2008) Wood density and stocks of coarse woody debris in a Northwestern Amazonian landscape. Can J For Res 38:795–805
Clark DA, Brown S, Kicklighter DW, Chambers JQ, Thomlinson JR, Ni J (2001a) Measuring net primary production in forest: concepts and field methods. J Appl Ecol 11:356–370
Clark DA, Brown S, Kicklighter DW, Chambers JQ, Thomlinson JR, Ni J, Holland EA (2001b) Net primary production in tropical forests: an evaluation and synthesis of existing field data. J Appl Ecol 11:371–384
Enrong Y, Xihua W, Jianjun H (2006) Concept and classification of coarse woody debris in forest ecosystems. Front Biol China 1:76–84
Friess DA, Krauss KW, Horstman EM, Balke T, Bouma TJ, Galli D, Webb EL (2012) Are all intertidal wetlands naturally created equal? Bottlenecks, thresholds and knowledge gaps to mangrove and saltmarsh ecosystems. Biol Rev 87:346–366
Gilman EL, Ellison J, Duke NC, Field C (2008) Threats to mangroves from climate change and adaptation options. Aquat Bot 89:237–250
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell JR, Lienkaemper GW, Cromack JR, Cummins KW (1986) Ecology of coarse woody debris in temperate ecosystems. Adv Ecol Res 15:133–302
Jia-bing W, De-xin G, Shi-jie H, Mi Z, Chang-jie J (2005) Ecological functions of coarse woody debris in forest ecosystem. J For Res 16:247–252
Kauffman JB, Cole TG (2010) Micronesian mangrove forest structure and tree responses to a severe typhoon. Wetlands 30:1077–1084
Kira T, Shidei T (1967) Primary production and turnover of organic matter in different forest ecosystems of the Western Pacific. Jpn J Ecol 17:70–87
Komiyama A, Poungparn S, Kato S (2005) Common allometric equations for estimating the tree weight of mangroves. J Trop Ecol 21:471–477
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass, and productivity of mangrove forests: a review. Aquat Bot 89:128–137
Krauss KW, Doyle TW, Twilley RR, Smith TJ, Whelan KRT, Sullivan JK (2005) Woody debris in the mangrove forests of South Florida. Biotropica 37:9–15
Maser C, Anderson RG, Cromack JrK, Williams JT, Martin RE (1979) Dead and down woody material. In: Thomas JW (ed) Wildlife habitats in managed forests: the Blue Mountains of Oregon and Washington. USDA Forest Service Agricultural Handbook, Washington DC, pp 78–95
McNaughton SJ, Milchunas DG, Frank DA (1996) How can net primary productivity be measured in grazing ecosystem? Ecology 77:974–977
Miura M, Manabe T, Nishimura N, Yamamoto S (2001) Forest canopy and community dynamics in a temperate old-growth evergreen broad-leaved forest, south-western Japan: a 7-year study of a 4-ha plot. J Ecol 89:841–849
Ohtsuka T, Mo W, Satomura T, Inatomi M, Koizumi H (2007) Biometric based carbon flux measurements and net ecosystem production (NEP) in a temperate deciduous broad-leaved forest beneath a flux tower. Ecosystems 10:324–334
Poungparn S, Komiyama A, Tanaka A, Sangtiean T, Maknual C, Kato S, Tanapermpool P, Patanaponpaiboon P (2009) Carbon dioxide emission through soil respiration in a secondary mangrove forest of eastern Thailand. J Trop Ecol 25:393–400
Poungparn S, Komiyama A, Sangteian T, Maknual C, Patanaponpaiboon P, Suchewaboripont V (2012) High primary productivity under submerged soil raises the net ecosystem productivity of a secondary mangrove forest in eastern Thailand. J Trop Ecology 28:303–306
Poungparn S, Charoenphonphakdi T, Sangtiean T, Patanaponpaiboon P (2016) Fine root production in three zones of secondary mangrove forest in eastern Thailand. Trees 30:467–474
Robertson AI, Phillips MJ (1995) Mangroves as filters of shrimp pond effluent: predictions and biogeochemical research needs. Hydrobiologia 295:311–321
Acknowledgements
We thank the Thailand Research Fund for financial support (Grant No. RSA5680017) and the staff at the Mangrove Research Station at Trat Province, Thailand, for field assistance. We are also grateful to the anonymous reviewers for constructive comments on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Umnouysin, S., Sangtiean, T. & Poungparn, S. Zonal distribution of coarse woody debris and its contribution to net primary production in a secondary mangrove forest. Ecol Res 32, 51–60 (2017). https://doi.org/10.1007/s11284-016-1414-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-016-1414-3