Abstract
The properties of woody debris (WD) vary across different forests under various soil conditions. Owing to the relatively shallow and low amounts of soils on karst terrains, it is necessary to determine the WD carbon inventory of karst forests. In this study, we recorded WD with a basal diameter for standing snags and the large-end diameter for fallen logs of ≥ 1 cm. The carbon density of WD in a secondary karst mixed evergreen and deciduous broad-leaved forest that had been clear-cut 55 years ago in southwestern China were inventoried in a 2 ha plot. Woody debris carbon density calculated using specific gravity and carbon concentration was 4.07 Mg C ha−1. Woody debris with diameters ≥ 10 cm (coarse WD) constituted 53.8% of total carbon storage whereas WD < 10 cm in diameters (fine WD) accounted for more pieces of WD (89.9%). Lithocarpus confinis contributed the most WD carbon (26.5%). Intermediate decayed WD was relatively more abundant, but WD with final decay contributed the least to the total pieces of WD (6.7%). The contribution of WD to carbon storage of karst forest was low compared to other forests worldwide. Significant positive correlations were found between WD carbon and biodiversity (R2 = 0.035, p < 0.01) and elevation (R2 = 0.047, p < 0.01) and negative correlations was found in outcrop coverage (R2 = 0.034, p < 0.01). Further studies are needed to elucidate the ecological functions of WD to better understand their roles in maintaining biodiversity, enhancing productivity, and controlling vegetation degradation in karst forest ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Senescence and death of trees during forest successions produce a large amount of woody residues referred to as woody debris (WD). It includes all forms of dead woody material above- and below-ground, and is an important component of the structure and function of forest ecosystems (Harmon et al. 1986; Harmon and Sexton 1996). Woody debris is mainly divided into coarse woody debris (CWD) and fine woody debris (FWD). The former is the main part of WD with pieces ≥ 10 cm diameter at the large end and ≥ 1.0 m in length, and is usually composed of snags, fallen logs, stumps, and large branches (Harmon et al. 1986; Harmon and Sexton 1996; Woodall and Nagel 2006). The latter has smaller pieces between 1 and 10 cm in diameter and consists mainly of suspended and downed twigs (Harmon and Sexton 1996). Many studies have indicated that, in general, dead wood is a key element in protecting and enhancing soil organic matter and nutrients, conserving water and soils (Harmon et al. 1986, 1994; Baldock and Nelson 1999), maintaining the biodiversity by providing habitats for small mammals, invertebrates and microbes, and refuge for plants and fungi (Kruys and Jonsson 1999; Martikainen et al. 1999), and facilitating biogeochemical processes by storing carbon and nitrogen (Crawford et al. 1997; Delaney et al. 1998; Carmona et al. 2002; Woodall and Liknes 2008).
Carbon in forest ecosystems is a key contributor to the global carbon budget (Dixon et al. 1994; Pan et al. 2011). In general, dead wood contributes a small amount (only 8%) to the forest carbon storage, but with 36% increment of sink in dead wood carbon storage over the last two decades, it has contributed more than 10% of the total carbon sink in the world’s forests (Pan et al. 2011) and as much as 20% in old-growth forests (Delaney et al. 1998). Patterns, processes and management of carbon in WD should, therefore, be given more attention in regional and global research of biogeochemical cycles. However, many studies have found that the composition and storage of WD and their contribution to ecosystem function varies greatly across different landscapes, depending on forest types, stand stages, climate conditions, regional topography, and disturbance regimes (Sturtevant et al. 1997; Carmona et al. 2002; Webster and Jenkins 2005; Woodall and Liknes 2008; Zeng et al. 2014). The carbon storage of WD also varies across different forest carbon pools (Muller and Liu 1991; Delaney et al. 1998; Baker et al. 2007; Weedon et al. 2009). Zonal forests in boreal, temperate, and tropical climate zones have frequently been studied for their patterns and dynamics of WD, but such studies have rarely been conducted in azonal forests, such as the ones growing on karst terrains (Liu et al. 2013).
Karst is a highly special landscape shaped by rainfall and groundwater acting on carbonate bedrocks, mainly limestone and dolomite. It is widely distributed in about 12% of the land area in the world, with the largest continuously distributed area of about 0.51 million km2 in southwestern China (Jiang et al. 2014). Vegetation in the karst regions of southwestern China mainly consists of mixed evergreen and deciduous broad-leaved forest as primary forest type, and shrubland, tussock, and grassland as degraded vegetation, which is significantly different from the zonal non-karst vegetation of evergreen broad-leaved forests in subtropical China (ECVC 1980). Extensive outcrops of carbonate rocks, shallow soils, and water leakage lead to low growth rate, low productivity, and high vulnerability of forest responding to environmental changes and human disturbances in this region (Zhu et al. 1995; Ni et al. 2015). The above-ground (Zhu et al. 1995; Liu et al. 2009, 2013) and belowground biomass (Tu and Yang 1995; Luo et al. 2010; Ni et al. 2015) of karst forests have already been found to be significantly lower than those of non-karst forests. However, the lack of WD survey has made estimates of the carbon budget of karst forests incomplete and inaccurate.
In addition, due to the high heterogeneity of karst rock-soil landscape, soils in the karst region are shallow (commonly 10–60 cm) and erosional both on the land surface and under the ground (Yang et al. 2014). The amount of soil on karst terrains, therefore, tends to be low. Woody debris can retain more water and nutrients for forest growth and also offer the benefit of preventing soil erosion (Gale 2000). It takes 4000–8500 years to form just 1 cm of soil on karst land (Wang et al. 1999). Biological residues such as WD and litter are important raw materials that contribute directly to and also accelerate soil formation (Scheu and Schulz 1996; Šamonil et al. 2010). Therefore, studies of WD in karst forests are ecologically and geologically significant.
To assess the accumulation of WD, volume, necromass, and carbon of WD in a subtropical karst forest were fully inventoried. We used a typical mixed-evergreen and deciduous broad-leaved forest that was growing in the plateau-surface type of karst terrain in central Guizhou province of southwestern China as an example. This study aims (1) to provide a relatively accurate WD background data on karst forest, (2) to determine which types and proportions of coarse and fine WD affect its carbon density, and (3) to quantify WD spatial patterns and factors potentially influencing them. Our findings provide useful information on managing and restoring degraded karst forests.
2 Materials and methods
2.1 Study site
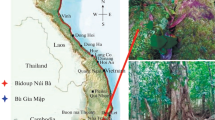
This study was conducted at the Tianlongshan permanent plot (105°45′50″E, 26°14′45″N and 1402–1512 m) of the Puding Karst Ecosystem Research Station in Puding county, central Guizhou province, southwestern China (Fig. 1a). The karst terrain in the study area has a plateau-surface type of karst morphology (Fig. 1b), which is one of the eight karst morphological types in southwestern China (Wang et al. 2013). This terrain is in a transitional area of north-subtropical and mid-subtropical humid monsoon climates. According to local meteorological records (Puding weather station, http://www.nmic.cn) from 1961 to 2008, the mean annual temperature is 15.1 °C, with mean January and July temperatures of 5.4 and 22.9 °C, respectively. The mean annual sunshine duration is 1177 h, which is relatively unfavorable to the growth of evergreen plants. The mean annual precipitation is 1367 mm, of which more than 70% falls between May and September.
The plot size was 2 ha (200 m × 100 m) and lied in a horizontally projected area in the middle hillside, facing south. The soil is black limestone soil (Rendzina in the Food and Agriculture Organization soil classification scheme) with a depth of 10–80 cm and pH of 6.6–6.8. The mean coverage of rock outcrops is 44.7 ± 25.8% (mean ± standard deviation based on visual estimates of 200 quadrats) with the highest one being 98%. The average slope is 31.0 ± 14.0°. The forest is a secondary mixed evergreen and deciduous broad-leaved forest recovered after clear-cutting in 1958, with light grazing by cows at the early stages. Cutting and grazing were both forbidden later by the local government. This forest is reasonably well protected compared with other secondary forests nearby, likely because a temple is located on the top of the hill.
The dominant species in the forest are Platycarya strobilacea, Lithocarpus confinis, Itea yunnanensis, Machilus cavaleriei and Carpinus pubescens (Botanical nomenclature is based on Chen (1982–2004).) with a mean height of 5 m (3–12 m), whereas Pittosporum brevicalyx, Lindera communis and Celtis sinensis are found in the tree layer (about 3–10 m in height). The shrub layer, which is 1–3 m in height, is dominated by Ilex corallina, Rhamnella franguloides, Stachyurus obovatus, Rhamnus heterophylla and Zanthoxylum ovalifolium. Lianas are dominated by Rosa cymosa. The herb layer is generally dominated by Arthraxon prionodes, Senecio scandens and Woodwardia unigemmata. According to a previous study, the biomass of this plot is 158.1 Mg ha−1 with above- and belowground biomasses of 137.7 and 20.3 Mg ha−1, respectively. The necromass of the litter was 7.4 Mg ha−1 (Liu et al. 2016).
2.2 Woody debris inventory
The plot was divided into 200 contiguous 10 m × 10 m quadrats as the basic unit of vegetation survey. All plots have been established and censused following the plot protocols of the Centre for Tropical Forest Science (CTFS) (Condit 1998). In the summer of 2012, all woody plants with ≥ 1 cm diameter at breast height (DBH) were recorded. Plant species were identified (Chen 1982–2004), and relative coordinates of each individual to the lower left corner of the quadrat, DBH, height, crown width, as well as elevation and outcrop percentage were recorded.
Two kinds of WD were classified according to the angle between the stem and the ground, i.e., fallen logs and branches with angle < 45° and standing snags and very few stumps (formed by natural disturbances such as storms and thunders) with angle > 45°. In the summer of 2013, all WD longer than 0.5 m with basal diameter ≥ 1 cm for standing snags and large-end diameter ≥ 1 cm for fallen logs were inventoried. Meanwhile, the diameters of large (Dl) and small (Ds) ends of fallen logs, DBH (D) of snags, length or height, relative coordinates, and decay classes of WD were also recorded. Coarse and fine WD were separated according to their large-end diameter or DBH. The former was ≥ 10 cm and the latter was between 1 and 10 cm (Harmon and Sexton 1996). The species of WD were identified as much as possible based on the barks, leaves, and living trees nearby, except for a few kinds of dead woods with advanced decomposition. In total, 32 species forming WD belonging to 29 genera and 18 families were identified. All WD were divided into five diameter classes according to \(D_{l}\) or \(D\) based on Sturges’ formula (Sturges 1926): class 1 < 2.5, class 2 = 2.5–5.0, class 3 = 5.0–7.5, class 4 = 7.5–10.0 and class 5 ≥ 10 cm. The five decay classes described previously, namely incipient decay (I), intermediate decays (II, III and IV), and final decay (V), were also used (Sollins 1982; Spies et al. 1988).
2.3 Statistical analysis
The volumes of fallen logs (Vlogs) and standing snags (Vsnags) (m3) were calculated using Smalian’s formula (Eq. 1) and Huber’s formula (Eq. 2) (Figueiredo Filho et al. 2000), respectively:
where Dl is the diameter of the large end of fallen logs, Ds is the diameter of the small end of fallen logs, D is the DBH of snags, and l is the length or height of snags. Note that in Huber’s formula, DBH was used instead of diameter at mid-height.
We sampled WD of four dominant species in the tree layer with five decay classes (n = 60), measured the volume of each WD using the drainage method which uses the displacement of water due to a submerged object to determine the density of the object, and then oven-dried it to constant mass at 105 °C. The wood specific gravity (\(\rho\), g m−3) of each dominant species with different decay classes was calculated using Eq. 3:
where G and Vsample correspond to dry mass (g) and volume (m3) of sampled WD, respectively. The specific gravity of other species with different decay classes was calculated using the mean specific gravity of four dominant species corresponding to each decay class (Table 1).
Necromass of fallen logs (Wlogs, g) was calculated using Eq. 4:
Necromass of standing snags (Wsnags, g) was calculated using species-specific biomass models (Liu et al. 2009, 2013) applied to living woody plants.
The 60 samples of WD mentioned above for specific gravity measurements were further used to measure the organic carbon concentration (\(\rho_{c}\), g kg−1) of the four dominant species (Table 1) using the Degtjareff method (Walkley and Black 1934). Carbon storage of WD (C, g C) was finally calculated using Eq. 5:
where W corresponds to Wlogs or Wsnags, and \(\rho_{c}\) is the organic carbon concentration.
Four environmental factors were considered to analyze the correlation between WD carbon and environmental factors. Biomass and diversity index of each quadrat were considered biotic factors, and the coverage of outcrop and elevation of each quadrat were regarded as topographical factors. Biomass of living trees in each quadrat was calculated by species-specific biomass models (Liu et al. 2016). The diversity index was measured by calculating the Shannon–Wiener index:
where ni is the number of individuals of each species (species i) and N is the total amount of individuals for the quadrat. Linear regressions were used to quantify the correlation between WD carbon and environmental factors. All calculations in this paper were conducted using the R statistical language (R Development Core Team).
3 Results
3.1 Necromass and carbon storage
Carbon storage of WD varied greatly from 0.35 to 538.93 kg C among the 200 quadrats. The necromass of all WD was 8.96 Mg ha−1 (Table 2), equivalent to only 6.5% of the above-ground biomass and 5.7% of the total forest biomass. Carbon density of WD was only 4.07 Mg C ha−1 based on the measured organic carbon concentrations (Table 1).
Coarse WD comprised only 11% of total WD, but it had higher volume, necromass and carbon density than fine WD. Carbon stored in CWD accounted for 53.8% of total WD carbon (Table 2). Logs had more pieces and stored more carbon (82.8%) than snags (Table 2).
The WD was mostly come from small trees and shrubs (Fig. 1c). There were 32 species of WD, 19 of them were deciduous species and the rest were evergreen species. Woody debris was mostly accumulated by major canopy tree species. L. confinis contributed to 26.5% of total carbon stored in WD, followed by P. strobilacea (19.4%), I. yunnanensis (13.5%), C. pubescens (8.4%), and M. cavaleriei (7.9%). Other tree, shrub and liana species only had 24.3% of WD carbon.
Woody debris with larger diameters at the large end or breast height stored more carbon (Fig. 2a). Woody debris with diameter ≥ 10 cm contributed more than half of the total carbon compared to the sum of WD at the other four diameter classes (Fig. 2a). From a decay class point of view, more carbon was stored (72.5%) in WD with intermediate decay classes II, III, and IV (72.5%), and with the incipient decay class I (20.7%). The WD with decay class V only had 6.7% of total WD carbon (Fig. 2b).
3.2 Influential factors
Linear regression analyses showed that there was no significant correlation between the carbon of WD and the biomass of living trees in each quadrat (Fig. 3a). By contrast, biodiversity, coverage of outcrop and elevation of each quadrat was significantly correlated with WD carbon (Fig. 3b–d). Woody debris was positively correlated with biodiversity (R2 = 0.035, p < 0.01) and elevation (R2 = 0.047, p < 0.01), while WD was negatively correlated with the coverage of outcrop (R2 = 0.034, p < 0.01). Thus, dead wood carbon storage tended to be higher in quadrats with higher plant diversity and elevation, but lower coverage of outcrop.
4 Discussion
The plot-based inventory method adopted in this study is a widely used standard method in WD survey, which works well both for snags and logs (Ståhl et al. 2001). Using the same method, the estimation of Liu et al. (2013) showed that the necromass of WD of a parallel forest nearby was 1.7 Mg ha−1, which is only 19% of the present estimation. It has been suggested that estimates of WD storage can vary based on the investigation method and sampling size (Ståhl et al. 2001; Chave et al. 2004). The observed discrepancy is probably due to the very small sample size of only 100 m2 used by Liu et al. (2013). The sample plot method with a large inventory area performed in this study is likely to yield more accurate data. However, only four dominant species were sampled and measured for their wood specific gravity and carbon density of WD. For the remaining species, an average of values from these four species was used, which might have led to uncertainties of carbon density of WD in this forest. The Huber’s formulation was applied to calculate the volume of snags by using DBH rather than the diameter at mid-height, which might cause overestimation of the volume.
The WD in the present secondary forest dominated by P. strobilacea, L. confinis and I. yunnanensis was a result of comprehensive influences from the forest developmental stage, which were caused by past selective logging and more recent natural and anthropogenic interferences. However, the necromass and carbon density of WD in this karst forest, both in CWD and FWD, were lower than global records from boreal coniferous forests (Harmon et al. 1986; Spies et al. 1988; Sturtevant et al. 1997; Webster and Jenkins 2005), temperate deciduous broad-leaved forests (Harmon et al. 1986; Muller and Liu 1991; Carmona et al. 2002; Muller 2003), to moist tropical rainforests (Delaney et al. 1998; Chambers et al. 2000; Baker et al. 2007). Even compared with forests in very dry or cool habitats (Harmon et al. 1995; Delaney et al. 1998), the karst forest still had low WD necromass and carbon density, and a low ratio of necromass to biomass. This is also true when compared with non-karst forests in the same bioclimatic zone. The karst forest was found to have lower WD necromass and carbon density than primary and secondary evergreen broad-leaved forests in subtropical China (Table 3). As mentioned above, multiple factors such as forest type, stand stage, climate condition, regional topography and disturbance regime is likely to have contributed to the magnitude of WD we observed (Sturtevant et al. 1997; Carmona et al. 2002; Webster and Jenkins 2005; Woodall and Liknes 2008; Zeng et al. 2014). The secondary forest, young stand age, very harsh and rocky habitat and steep slope likely account for the low living biomass in karst forests (Liu et al. 2009, 2013; Ni et al. 2015) and also the low necromass and carbon density of dead woods observed in this study.
WD composition in the present karst forest, fallen logs, followed by snags, were a principal input source of WD, this finding was consistent with the results from previous studies (Delaney et al. 1998; Carmona et al. 2002). Generally, most of the WD in natural forests was derived from gradual accumulation after severe disturbances (Chen and Harmon 1992). Thus, we believed that the substantial WD in the karst forest should primarily result from the most recent local pulses in mortality, which were driven by a combination of strong winds, steep topography and thin soil.
The karst forest had slightly higher carbon (or necromass) stored in CWD than in FWD, which is quite different from the estimate in the primary evergreen broad-leaved forest in Ailao Mountains with very high CWD and very low FWD necromass. However, in a secondary evergreen forest in the same mountain, CWD carbon was similar to and FWD carbon was slightly higher than those of karst forest (Table 3, Yang et al. 2007). In most cases, CWD snags in subtropical forests including the karst forest were much higher than logs, but the ratios were quite different. Ratios of total WD carbon, especially CWD carbon to AGB was very low in the karst forest (Table 3).
Dead wood carbon tended to be higher in quadrats with higher plant diversity, higher elevation, but lower coverage of outcrop. However, there was no relationship between WD carbon and living biomass in each quadrat. Previous studies showed that the amount of WD depended significantly on the number of living trees in European beech forests (Christensen et al. 2005), and WD might also have both positive and negative effects on living biomass (productivity) (Brais et al. 2005). Thus, the relationship between the amount (necromass or carbon) of WD and living biomass in karst forests is complex. The specific karst topography and very deep slope might have been factors leading to position changes of some dead woods, which might, in turn, have masked the relationship between dead woods and living trees. In places with more rocky outcrops, it is likely that dead woods could not remain on the rocks, resulting in less WD carbon in quadrats of higher outcrop coverage. However, increased WD carbon with elevation should be due to the existence of some large dead woods at the top of the plot; however, we note that the positive relationship observed in this study between WD and elevation is not very robust. Mechanisms behind such phenomena need to be investigated further.
As mentioned before, dead wood plays key roles in conserving water and soils, maintaining biodiversity, and facilitating biogeochemical processes in forests all over the world. Unfavorable karst habitats have been reported to result in low biomass in karst forests (Zhu et al. 1995; Ni et al. 2015; Liu et al. 2016) and thus low carbon storage of dead woods, which is unsuitable for protecting water, soils and organisms to sustainably manage karst forests. However, the existence of karst forests and even shrublands with low biomass and low WD necromass ensures lower soil erosion on karst terrains and slows the undesirable process of rocky desertification.
Compared to virgin forests, the amounts and structures of WD with artificial interference were decreasing, secondary karst forests required centuries of non-interference cycles for development to be close to the state of virgin forests. However, there were few data for virgin karst forests. Therefore, a comprehensive understanding of the natural background of WD in secondary karst forests is important to better manage the forests and to more effectively protect organisms sheltered by the WD. Features of WD reported in this study can serve as a preliminary reference for the management of karst forests around southwestern China. Future researches will focus on succession progression based on the long-term monitoring of WD in dedicated permanent sample plots.
References
Baker TR, Coronado ENH, Phillips OL, Martin J, van der Heijden GMF, Garcia M, Espejo JS (2007) Low stocks of coarse woody debris in a southwest Amazonian forest. Oecologia 152:495–504. https://doi.org/10.1007/s00442-007-0667-5
Baldock JA, Nelson PN (1999) Soil organic matter. In: Sumner ME (ed) Handbook of soil science. CRC Press, New York, pp 25–84
Brais S, Sadi F, Bergeron Y, Grenier Y (2005) Coarse woody debris dynamics in a post-fire jack pine chronosequence and its relation with site productivity. For Ecol Manag 220(1–3):216–226. https://doi.org/10.1016/j.foreco.2005.08.013
Carmona MR, Armesto JJ, Aravena JC, Perez CA (2002) Coarse woody debris biomass in successional and primary temperate forests in Chiloé Island, Chile. For Ecol Manag 164:265–275. https://doi.org/10.1016/S0378-1127(01)00602-8
Chambers JQ, Higuchi N, Schimel JP, Ferreira LV, Melack JM (2000) Decomposition and carbon cycling of dead trees in tropical forests of the Central Amazon. Oecologia 122(3):380–388. https://doi.org/10.1007/s004420050044
Chave J, Condit R, Aguilar S, Hernandez A, Lao S, Perez R (2004) Error propagation and scaling for tropical forest biomass estimates. Philos Trans R Soc Lond B Biol Sci 359(1443):409–420. https://doi.org/10.1098/rstb.2003.1425
Chen QH (1982–2004) Flora Guizhouensis. Guizhou Science and Technology Publishing House, Guiyang. (in Chinese)
Chen H, Harmon ME (1992) Dynamic study of coarse woody debris in temperate forest ecosystems. Chin J Appl Ecol 3(2):99–104 (in Chinese)
Christensen M, Hahn K, Mountford EP, Ódor P, Standovár T, Rozenbergar D, Diaci J, Wijdeven S, Meyer P, Winter S, Vrska T (2005) Dead wood in European beech (fagus sylvatica) forest reserves. For Ecol Manag 210(1–3):267–282. https://doi.org/10.1016/j.foreco.2005.02.032
Condit R (1998) Tropical forest census plots: methods and results from Barro Colorado Island, Panama and comparison with other plots. Springer, Berlin
Crawford RH, Li CY, Floyd M (1997) Nitrogen fixation in root-colonized large woody residue of Oregon coastal forests. For Ecol Manag 92:229–234. https://doi.org/10.1016/S0378-1127(96)03923-0
Delaney M, Brown S, Lugo AE, Torres-Lezama A, Quintero NB (1998) The quantity and turnover of dead wood in permanent forest plots in six life zones of Venezuela. Biotropica 30:2–11. https://doi.org/10.1111/j.1744-7429.1998.tb00364.x
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystem. Science 263:185–190. https://doi.org/10.1126/science.263.5144.185
Editorial Committee for Vegetation of China (ECVC) (1980) Vegetation of China. Science Press, Beijing, p 1375 (in Chinese)
Figueiredo Filho A, Machado SA, Carneiro MRA (2000) Testing accuracy of log volume calculation procedures against water displacement techniques (xylometer). Can J For Res 30(6):990–997. https://doi.org/10.1139/cjfr-30-6-990
Gale N (2000) The aftermath of tree death: coarse woody debris and the topography in four tropical rain forests. Can J For Res 30(9):1489–1493. https://doi.org/10.1139/x00-071
Harmon ME, Sexton J (1996). Guidelines for measurements of woody detritus in forest ecosystems. US Long-Term Ecological Research Network Office, University of Washington. Washington Publication, Seattle, p 73
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell JR, Lienkaemper GW, Cromack KJ, Cummins KW (1986) Ecology of coarse woody debris in temperate ecosystems. Adv Ecol Res 15:133–302. https://doi.org/10.1016/S0065-2504(03)34002-4
Harmon ME, Caldwell BA, Sexton J, Caldwell BA, Carpenter SE (1994) Fungal sporocarp mediated losses of Ca, Fe, K, Mg, Mn, N, P, and Zn from conifer logs in the early stages of decomposition. Can J For Res 24:1883–1893. https://doi.org/10.1139/x94-243
Harmon ME, Whigham DF, Sexton J, Olmsted I (1995) Decomposition and mass of woody detritus in the dry tropical forests of the Northeastern Yucatan Peninsula, Mexico. Biotropica 27:305–316. https://doi.org/10.2307/2388916
Jiang ZC, Lian YQ, Qin XQ (2014) Rocky desertification in Southwest China: impacts, causes, and restoration. Earth Sci Rev 132:1–12. https://doi.org/10.1016/j.earscirev.2014.01.005
Kruys N, Jonsson BG (1999) Fine woody debris is important for species richness on logs in managed boreal spruce forests of Northern Sweden. Can J For Res 29:1259–1299. https://doi.org/10.1139/x99-106
Li LH, Xing XR, Huang DM, Liu CD, He JY (1996) Storage and dynamics of coarse woody debris in Castanopsis eyrei forest of Wuyi Mountain, with some considerations for its ecological effects. Acta Phytoecolocica Sinica 20:132–143 (in Chinese)
Liu CC, Wei YF, Liu YG, Guo K (2009) Biomass of canopy and shrub layers of Karst forest in Puding, Guizhou, China. Chin J Plant Ecol 33:698–705. https://doi.org/10.3773/j.issn.1005-264x.2009.04.008 (in Chinese)
Liu YG, Liu CC, Wang SJ, Guo K, Yang J, Zhang XS, Li GQ (2013) Organic carbon storage in four ecosystem types in the karst region of southwestern China. PLoS ONE 8:e56443. https://doi.org/10.1371/journal.pone.0056443
Liu LB, Wu YY, Hu G, Zhang ZH, Cheng AY, Wang SJ, Ni J (2016) Biomass of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China: a comprehensive inventory of a 2 ha plot. Silva Fennica. https://doi.org/10.14214/sf.1492
Luo DH, Xia J, Yuan JW, Zhang ZH, Zhu JD, Ni J (2010) Root biomass of karst vegetation in a mountainous area of southwestern China. Chin J Plant Ecol 34:611–618. https://doi.org/10.3773/j.issn.1005-264x.2010.05.015 (in Chinese)
Martikainen P, Siitonen J, Kaila L, Punttila P, Rauh J (1999) Bark beetles (Coleoptera, Scolytidae) and associated beetle species in mature managed and old-growth boreal forests in southern Finland. For Ecol Manag 116:233–245. https://doi.org/10.1016/S0378-1127(98)00462-9
Muller RN (2003) Landscape patterns of change in coarse woody debris accumulation in an old-growth deciduous forest on the Cumberland Plateau, southeastern Kentucky. Can J For Res 33:763–769. https://doi.org/10.1139/x02-210
Muller RN, Liu Y (1991) Coarse woody debris in an old growth deciduous forest on the Cumberland Plateau, southeastern Kentucky. Can J For Res 21:1567–1572. https://doi.org/10.1139/x91-218
Ni J, Luo DH, Xia J, Zhang ZH, Hu G (2015) Vegetation in karst terrain of southwestern china allocates more biomass to roots. Solid Earth 6:799–810. https://doi.org/10.5194/se-6-799-2015
Pan YD, Birdsey RA, Fang JY, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao SL, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993. https://doi.org/10.1126/science.1201609
Šamonil P, Král K, Hort L (2010) The role of tree uprooting in soil formation: a critical literature review. Geoderma 157(3):65–79. https://doi.org/10.1016/j.geoderma.2010.03.018
Scheu S, Schulz E (1996) Secondary succession, soil formation and development of a diverse community of oribatids and saprophagous soil macro-invertebrates. Biodivers Conserv 5(2):235–250. https://doi.org/10.1007/BF00055833
Sollins P (1982) Input and decay of coarse woody debris in coniferous stands in western Oregon and Washington. Can J For Res 12:18–28. https://doi.org/10.1139/x82-003
Spies TA, Franklin JF, Thomas TB (1988) Coarse woody debris in Douglas-fir forests of western Oregon and Washington. Ecology 69:1689–1702. https://doi.org/10.2307/1941147
Ståhl G, Ringvall A, Fridman J (2001) Assessment of coarse woody debris: a methodological overview. Ecol Bull 49:57–70. https://doi.org/10.2307/20113264
Sturges HA (1926) The choice of a class interval. J Am Stat Assoc 21(153):65–66. https://doi.org/10.1080/01621459.1926.10502161
Sturtevant BR, Bissonette JA, Long JN, Roberts DW (1997) Coarse woody debris as a function of age, stand structure, and disturbance in boreal Newfoundland. Ecol Appl 7:702–712. https://doi.org/10.1890/1051-0761(1997)007%5b0702:CWDAAF%5d2.0.CO;2
Tang XL, Zhou GY (2005) Coarse woody debris biomass and its potential contribution to the carbon cycle in successional subtropical forests of southern China. Chin J Plant Ecol 29:559–568. https://doi.org/10.3321/j.issn:1005-264X.2005.04.006 (in Chinese)
Tu YL, Yang J (1995) Study on biomass of the karst scrub communities in central region of Guizhou Province. Carsologica Sinica 14:199–208 [in Chinese]
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38. https://doi.org/10.1097/00010694-193401000-00003
Wang SJ, Ji HB, Ouyang ZY, Zhou DQ, Zheng LP, Li TY (1999) Preliminary study on weathering and pedogenesis of carbonate rock. Sci China 42:572–581. https://doi.org/10.1007/BF02877784
Wang SJ, Zhang XB, Bai XY (2013) Discussion on nomenclature of the karst desertification regions and illustration for their environment characteristics in southwest China. J Mt Sci 31:18–24. https://doi.org/10.3969/j.issn.1008-2786.2013.01.003 (in Chinese)
Webster CR, Jenkins MA (2005) Coarse woody debris dynamics in the southern Appalachians as affected by topographic position and anthropogenic disturbance history. For Ecol Manag 217:319–330. https://doi.org/10.1016/j.foreco.2005.06.011
Weedon JT, Cornwell WK, Cornelissen JHC, Zanne AE, Wirth C, Coomes DA (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species. Ecol Lett 12:45–56. https://doi.org/10.1111/j.1461-0248.2008.01259.x
Woodall CW, Liknes GC (2008) Climatic regions as an indicator of forest coarse and fine woody debris carbon stocks in the United States. Carbon Balance Manag 3:1–8. https://doi.org/10.1186/1750-0680-3-5
Woodall CW, Nagel LM (2006) Coarse woody type: a new method for analyzing coarse woody debris and forest change. For Ecol Manag 227:115–121. https://doi.org/10.1016/j.foreco.2006.02.032
Yang LP, Liu WY, Yang GP, Ma WZ, Li DW (2007) Composition and carbon storage of woody debris in moist evergreen broad-leaved forest and its secondary forests in Ailao Mountains of Yunnan Province. Chin J Appl Ecol 18:2153–2159. https://doi.org/10.13287/j.1001-9332.2007.0376 (in Chinese)
Yang Q, Zhang F, Jiang Z, Li W, Zhang J, Zeng F, Li H (2014) Relationship between soil depth and terrain attributes in karst region in southwest china. J Soils Sediments 14(9):1568–1576. https://doi.org/10.1007/s11368-014-0904-6
Zeng ZQ, Wang SL, Zhang CM, Wu ZJ, Li XQ, Luo J (2014) Carbon storage of woody debris of evergreen broad-leaf forests in mid-subtropical region of China at three succession stages. Forest Resources Management 2:66–72. https://doi.org/10.13466/j.cnki.lyzygl.2014.02.013 (in Chinese)
Zhu SQ, Wei LM, Chen ZR, Zhang CG (1995) A preliminary study on biomass components of karst forest in Maolan of Guizhou Province, China. Acta Phytoecologica Sinica 19:358–367. https://doi.org/10.3321/j.issn:1005-264X.1995.04.011 (in Chinese)
Acknowledgements
This study was financially supported by National Natural Science Foundation of China (31870462), National Key Research & Development Program of China (2016YFC0502304 and 2016YFC0502101).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Y., Liu, L., Guo, C. et al. Low carbon storage of woody debris in a karst forest in southwestern China. Acta Geochim 38, 576–586 (2019). https://doi.org/10.1007/s11631-019-00339-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-019-00339-9