Abstract
Land snails are an important component of biodiversity but the information regarding the factors that influence their distribution is very incomplete or anecdotal in most geographic areas. In this article our aim was to uncover environmental factors that influence the distribution and diversity of a Gastropoda community in a Mediterranean Reserve (Collserola Natural Park, Barcelona). Fieldwork was conducted from 2001 to 2003, and we systematically sampled all 1 km2 UTM squares throughout the park by randomly selecting at least one 200 m2 plot within each square. We used a community-based approach to analyse the relationships between 61 Gastropod species distributions and environmental predictors by means of Redundancy Analysis (RDA). Our results highlighted that the land snail community was affected by the environmental predictors (even for short gradients), but their influence was low according to the explained variance (30 %). Climate and habitat predictors were more important than the spatial variables in determining the community composition and diversity. 48 out of 61 (78.7 %) land snail species showed significant responses to the environmental gradients with an association of specialist species with particular habitat types. Collserola is a reserve surrounded by urbanised areas and affected by multiple anthropogenic threats mostly related to habitat transformation. The high degree of specialisation within the Gastropoda community suggests that the restoration of heterogeneous landscapes would be useful to conserve and restore terrestrial mollusc diversity in Collserola. This study can help stakeholders to make decisions related to landscape planning and habitat transformation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge on the distribution patterns of species is a key to identifying biodiversity hotspots and prioritising management planning for conservation (Sarkar et al. 2006; Millspaugh and Thompson 2009; Naro-Maciel et al. 2009). In parallel, the environmental factors that influence species distribution are key indicators for understanding the processes that govern their distribution and for anticipating changes linked to natural or anthropological disturbances (Kimberling et al. 2001; Andersen et al. 2004; Douglas et al. 2013). For terrestrial ecosystems, this knowledge is unfortunately biased towards Vertebrate taxa and some Hexapoda groups (Solem 1984; Ward and Larivière 2004; McGeoch et al. 2011). For other taxa such as terrestrial molluscs, even though they are important components of biodiversity (Cameron and Killeen 2003; Holland and Cowie 2009), the information is very incomplete or anecdotal in most geographic areas (Triantis et al. 2008). It is probably for this reason that many terrestrial invertebrates are scarcely evaluated in local and global conservation Red Lists (IUCN 2013).
Land snails and slugs are organisms with a low active mobility adapted to very specialised ecological niches (Kappes 2005) and represent a significant fraction of the edaphic invertebrate community (Coleman et al. 2004; Harris 2008). Patterns of species richness and biogeography in this taxon respond to a wide variety of environmental factors (Hoffmann et al. 2011; Baur et al. 2014) with clear implications for the spatial distribution of populations (Snegin 2005; Menez 2007). For example, edaphic properties of the soil influence Gastropoda community composition (e.g.: Martin and Sommer 2004a, b; Kappes et al. 2006; Berg 2010). Although acid substrates are likely to support a lower species diversity and abundance of individuals than limestone areas, acidophilic communities can also have a varied and interesting wildlife (Nekola 2010). Likewise, landscape composition and heterogeneity (i.e., the number of different vegetation units) are amongst the most frequently referred environmental factors that influence the composition of Gastropoda communities (Ondina and Mato 2001; Torre et al. 2014). Elevation gradients also drive the distribution and composition of Gastropoda communities at a local scale, since these gradients can be considered as surrogates for many environmental variables that have covariates with elevation (e.g. temperature, rainfall, productivity, and habitat type; Liew et al. 2010; Baur et al. 2014).
Uncovering which environmental parameters drive the distribution of Gastropoda species at small spatial scales is important in terms of conservation management. This is more critical still when parameters other than those that are strictly environmental play a role to drive community composition and species distribution. For example, human-induced landscape changes (i.e. land abandonment and urbanisation) may be responsible for species extinction and the decline of diversity (Torre et al. 2014). In such cases, a correct assessment of the factors driving species diversity and composition of Gastropoda communities may be a necessary tool in terms of proceeding with active landscape conservation planning and management.
The objective of this study is to uncover which environmental factors influence the distribution and diversity of a Gastropoda community in a Mediterranean Nature Reserve. The study area is the Collserola Natural Park, where active forestry and land abandonment during the last century, as well as proximity to a very populated area, have had a number of impacts for land snails. Previous studies were carried out in this reserve, aimed at discovering the composition and distribution of terrestrial snails (Bros 2004, 2009; Torre et al. 2014). Uncovering the ecological factors that are responsible for the species composition and distribution will be an important element for planning the conservation of Gastropoda biodiversity in this protected area.
Materials and methods
Study area
This study was conducted at Collserola Natural Park (Barcelona, Catalonia, Spain). This reserve covers 8295 ha (about 17 km long and 6 km wide) and is located in the middle of the metropolitan area of Barcelona. Collserola is dominated by a Palaeozoic base rock consisting essentially of silicates, with some isolated granite outcrops in the western area and some areas with limestone. The park has a mild, subhumid climate with an average yearly temperature of around 15 °C and annual rainfall of 650 mm (Raspall et al. 2004). The altitudinal range is 25–512 m.
Collserola has a rich mosaic of natural environments covering more than 40 habitats of interest for the European Community (Marull and Mallarach 2002). However, the park has undergone drastic modification in terms of land use. One hundred years ago, the park was dominated by a mosaic of vineyards with small forest patches in valleys and on hills and mountain tops (Raspall et al. 2004). Later on, pine plantations and natural reforestation modified the landscape to its present form. Thus, the woodland most commonly found (60 % of the park’s area) is a mixed pine forest of Aleppo pine (Pinus halepensis) and holm oak (Quercus ilex). Holm oak woodlands occupy 6.5 % of the park’s surface area. Other types of habitat in the park are scrublands (Quercus coccifera) (20 % of the surface area), water bodies and riparian vegetation (0.5 %), meadows and grassland (3 %), rocky spots (0.5 %), and crops and urbanised areas (14 %).
Sampling methodology
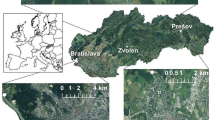
Fieldwork was conducted from 2001 to 2003. To cover all of the habitats we systematically sampled all 1 km2 UTM squares throughout the park (Fig. 1). Within each square, we randomly sampled at least one 200 m2 plot (14 × 14 m). The time taken to collect land snails and slugs in each plot was 90 min. Living terrestrial gastropods were sampled through a visual search for suitable microhabitats (under stones, dead wood, humus and litter, tree bark, scrubland and herbaceous vegetation and the aquatic surroundings). To sample micro-molluscs (<5 mm) in humus and litter, we examined a sample of 20 cm2 and 15 cm depth of ground-layer (20 × 20 × 15 cm = 6 L) at each plot. Specimens were identified at species level in the lab. Gastropod taxonomy was based on Bank (2011). To improve the total inventory of species, we also recorded shells of snails that had recently died, despite the inherent risk in including species that were not actually alive at the time of sampling (Cernohorsky et al. 2010). Our sampling approach was qualitative, so species were recorded but individuals were not counted in the field. This approach is useful when combining two species detection methods (timed search, litter/soil samples) of different sensitivity to snail body size (Durkan et al. 2013).
Statistical procedures
An incidence matrix (presence/absence) of 61 land snail species on 108 sampling plots was created. The association between the species matrix and the environmental variables (13 variables) was tested with the software CANOCO, version 4.5 for Windows (Ter Braak and Šmilauer 2002; Lepš and Šmilauer 2003). To explore which environmental factors determine land snail distribution at Collserola Natural Park, we used two types of variables: five continuous physical variables, namely elevation, mean maximum temperatures, mean minimum temperatures, radiation, and precipitation, and two categorical habitat variables (dummy variables): habitat type, divided into seven classes (anthropogenic, riparian, meadow, rocky, scrubland, holm oak forest, and pine-oak forest), and habitat heterogeneity, counting the number of habitats present within the sampling plots. The continuous variables were extracted from the environmental databases available in the mapping servers (Government of Catalonia: http://mediambient.gencat.cat/ca/05_ambits_dactuacio/patrimoni_natural/), while the habitat variables present at each location were recorded during fieldwork.
Firstly, a detrended correspondence analysis (DCA) was performed with the species incidence matrix. The result of this analysis is expressed in the length of gradients measuring beta diversity, i.e. the turnover or rate of change of the species. When gradient lengths were less than four (as in our case), we used linear instead of unimodal ordination methods (Lepš and Šmilauer 2003). We used redundancy analysis (RDA) to assess whether changes in community composition in plots could be explained by a matrix of environmental variables. Associations of each species with the environmental axes extracted were calculated after fitting Generalized Linear Models with the logit link function for binomial data. The log link function for Poisson distributed data was used to fit the environmental axes to the species density, that is, the number of species recorded by sampling unit disregarding the number of individuals (Gotelli and Colwell 2001).
Apart from these environmental variables, we included a set of nine spatial predictors, i.e. a third-degree polynomial function of the geographic coordinates (cubic trend surface regression), to control spatial autocorrelation in the community composition of the sampled plots (Borcard et al. 1992; Boone and Krohn 2000). The MS-DOS program SpaceMaker2 (Borcard et al. 2004; available at http://www.bio.umontreal.ca/casgrain/en/labo/spacemaker.html) was used to obtain the polynomial terms from the coordinates of the plots sampled. Then, partial constrained ordination (was used to remove the effects of: 1) spatial structure on species-environment associations by using the geographic coordinates of sampling plots as covariates, and 2) environment on spatial patterns in the species data by using the environmental variables as covariates (see Borcard et al. 1992 for details). With this procedure we were able to partition the variance that every group of variables had on the species matrix, obtaining the non-spatial environmental variation for the species data, the spatial patterns in species data not shared with the environment, the spatial-environmental fraction and the unexplained fraction of the variance (see Borcard et al. 1992 for details). Since some of the species were considered as rare, we used the down-weight algorithm available in CANOCO (see Labaune and Magnin 2001). The significant level for all the statistical tests was set at P < 0.05.
Results
Land community level
We recorded 61 species of terrestrial gastropods in the 108 plots sampled (Table 1).
The DCA performed with the species matrix explained 27.8 % of the variance in the land snail community, and the first axis explained 12.6 %. The gradient length along the axis was lower than 4, so linear ordination methods were used in further analyses. The first RDA with the species matrix constrained by environmental variables showed that these variables explained 26.3 % of the variance of the land snail community, and the first axis was significant (F ratio = 13.58, P = 0.002), as well as the four axes altogether (F ratio = 2.70, P = 0.002). The first axis can be considered as a gradient for elevation and climate, with negative values associated with holm oak and pine-oak forests located at the highest elevations, and positive values associated with open habitats in the temperate lowlands. The second axis was mainly a gradient from higher elevation and areas with more precipitation to areas situated at low elevation and covered by streams, ponds and lakes (Fig. 2).
Species responses to the environmental variables in the space generated by the two first axes extracted from the redundancy analysis with the species matrix constrained by the environmental variables in Collserola Natural Park. Codes of species as in Table 1, and codes of environmental variables as in Table 2. Only species showing axis coordinates larger than ±0.2 are plotted
The stepwise procedure for selecting environmental variables after the first RDA (Table 2) showed that the mean maximum temperature was the most important variable influencing the land snail community (6 %), followed by annual radiation (3 %), the mean minimum temperature and rainfall (1 % for both). The presence of meadow (4 %), anthropogenic (3 %), riparian, rocky and shrubby habitats (2 % each) also influenced the land snail community. Climate and habitat had similar influences on the community (11 vs. 13 %, respectively).
Species density (number of species recorded at every sampling plot) showed a strong and positive association with the first axis (F = 111.9, P < 0.0001, Fig. 3). The habitats with the lowest mean species density were pine-oak forests (7–8 species), and holm oak forests (8–9 species), whereas rocky, meadow (grasslands/crops) and anthropogenic habitats contained the richest communities at the sampling plot level, with 11–13 species (Fig. 3). The highest species densities were found in the peripheral areas of the Natural Park (covered by open and anthropogenic habitats), with there being lower densities in central areas almost covered by continuous forests.
Specific responses of Cepaea nemoralis (a), Montserratina martorelli (b), Cernuella virgata (c), Cornu aspersum (d) and Deroceras leave (e), and species density (f) to the environmental variables in the space generated by the two first axes extracted from the redundancy analysis with the species matrix constrained by the environmental variables in Collserola Natural Park. Contour lines represent the changes in species abundance/density along the environmental axes. All species presented fitted GLZ models with the environmental axes. Codes of environmental variables as in Table 2
The second RDA with species constrained by the spatial variables showed that these variables only explained 4.5 % of the variance of the land snail community. Nonetheless, the first axis was significant (F ratio = 2.55, P = 0.04), as well as the four axes altogether (F ratio = 1.62, P = 0.03). Only the linear terms (bx and by) were selected in the stepwise procedure, explaining 3 % of the variance altogether, a fact which meant that there was low spatial autocorrelation and the nearest plots did not have similar gastropod communities.
Lastly, we performed two partial constrained RDA, the first one with species data constrained using the environmental data, adding the spatial variables as covariates (22.0 % of variance), and the second one with species data constrained using the spatial variables, adding the environmental variables as covariates (3.7 % of variance). In both cases, the four axes extracted were significant (P = 0.002 for both). Our results indicated that the non-spatial environmental fraction of the variance was the most important in determining the land snail community (22.0 %), followed by spatial structuring in species data shared by the environment (4.3 %) and spatial patterns not shared with the environment (3.7 %), with a high fraction of unexplained variance (70 %).
Gastropod species level
48 out of 61 (78.7 %) land snail species showed significant responses to the environmental gradients extracted from RDA. From the total land snails recorded in the park, 57.3 % of the species showed significant associations with axis 1, and 40.9 % of them showed significant associations with axis 2. Twelve species showed a negative association with axis 1 (Table 1), but only two showed negative associations with both axes. These species were associated with holm oak woodland on mountain tops; in contrast, almost none of the species were associated with pine-oak forests (Fig. 3) despite their extensive presence throughout the park. 23 species showed positive associations with axis 1, and five showed positive associations with both axes (Table 1).
Specific land snail species responses varied according to their biogeographic affinity: species with European distribution such as Cepaea nemoralis selected forest (holm oak and pine-oak) habitats, whereas species with Mediterranean distribution such as Cernuella virgata and Cornu aspersum showed their preference for open lowland habitats (Fig. 3). Iberian endemics such as Montserratina martorelli showed a negative association with axis 2 and a preference for highland forests (Fig. 3). Lastly, species with a wide distribution range in Europe and the Palaearctic, such as Deroceras laeve, although relatively scarce in the study area, showed a positive association with axis 2 and were associated with riparian environments (Fig. 3).
Discussion
Our results suggested that environmental variables (climate and habitat) were more important than spatial variables in determining the composition of the land snail communities of Collserola Natural Park. Spatial structure may affect the distribution of communities from local to continental scales (Borcard et al. 1992; Diniz-Filho et al. 2003), as confirmed for land snail communities along elevation gradients (Labaune and Magnin 2001). However, the lower values of the length gradients extracted from the RDA suggested a low turnover rate and high species similarity at sampling stations (Diniz-Filho et al. 2003), without evidence of relevant spatial structure influences on land snail communities.
The explained variance of environmental and spatial variables on land snail communities was low (30 %). This result suggests that other important factors acting at the microhabitat level such as edaphic conditions, chemical composition, and microhabitat structure may influence land snail species composition and richness (Kappes et al. 2006; Cejka et al. 2008; Moreno-Rueda et al. 2009). Nonetheless, our results highlighted that several physical variables influenced the distribution of land snail species, and hence, the composition and diversity of gastropod communities in the park. In fact, 75 % of species showed significant linear responses to the environmental gradients extracted from RDA. For example, species density decreased with elevation (range 43–463 m.a.s.l.), as observed for land snail communities in other mountain ranges (Aubry et al. 2005; Liew et al. 2010), even in short elevation gradients (Labaune and Magnin 2002). Maximum temperature was the variable with the highest explained variance on Collserola land snail community structure (see Aubry et al. 2005; Hoffmann et al. 2011 for similar results), and this variable showed a negative association with elevation. Nonetheless, and owing to the short elevation gradient studied, we might expect there to be an influence of other variables that are correlated with temperature in relation to elevation. As such, the decreasing pattern could also be interpreted in terms of changes in landscape composition and structure in relation to elevation, bearing in mind that patterns of covariation between climatic and habitat variables prevented detailed interpretations of causal factors (Liew et al. 2010).
As small ectothermic animals, land molluscs are expected to be more directly influenced by abiotic soil and climate gradients rather than indirectly by the effects of climate and soil on vegetation (Tattersfield et al. 2001). Forest canopy, presumably because of solar radiation, moisture and temperature, as well as the proximity to water courses, affect the Gastropoda community composition (Kappes 2006; Bros et al. 2011; Rancka et al. 2015). We also found a clear association between the presence of snails and particular habitat types. This finding suggests that habitat structure, with woodland and open habitats being the two extremes, probably to some extent govern the relationship between species richness and environmental variables (Chiba 2007).
Snails, like other taxonomic groups, show a gradient between generalist and specialist species in habitat selection (Hoffmann et al. 2011). In Collserola, some snails and slugs are considered to be habitat generalists (e.g. Arion lusitanicus autoct. non Mabille, 1868, Cornu aspersum and Euomphalia strigella) and are able to occupy different plant communities in specific geographic areas. In contrast, we found associations between several species and particular habitat types such as holm-oak forests (Acanthinula aculeata and Hypnophila boissii), open dry habitats (Caracollina lenticula, Ferussacia folliculus, Jaminia quadridens and Xerocrassa penchinati) and aquatic environments (Oxyloma elegans, Deroceras laeve and Zonitoides nitidus). In summary, habitat structure, floristic composition and other environmental variables can play key roles in shaping mollusc community composition. As these factors interact, it is difficult to distinguish between the influence of climatic factors, historical events and/or biotic factors that shape snail spatial composition within environmental gradients (Labaune and Magnin 2001).
In addition to this complexity, historical anthropogenic land uses and disturbance regimes (i.e. fire, Raspall et al. 2004; Sala 2012) can also generate patterns on a local scale (Labaune and Magnin 2001). For example, human historical buildings may function as refuges for some species of snails, particularly in landscapes with poor limestone substrate (Juricková and Kucera 2005). The effect of fire seems unimportant in Collserola when compared to close natural reserves (Santos et al. 2009, 2014; Santos and Poquet 2010). In contrast, habitat-mediated uses such as pine plantations has negatively affected snail composition and has probably caused local extinction of some mollusc species (Torre et al. 2014).
Conservation implications
Natural parks located near urban areas such as Collserola are strongly influenced by anthropogenic processes that occur outside their limits. In fact, they are islands that consist of natural vegetation and are affected by landscape processes such as habitat uses in the surrounding edges (Pino and Marull 2012). In Collserola, urbanisation and forestry are the main pressures on biodiversity (Basnou et al. 2013) with there being emphasis on the loss of open habitats for the terrestrial mollusc community (Torre et al. 2014). Studies that uncover environmental factors that influence diversity patterns are useful tools for biodiversity conservation, especially for species with low mobility patterns such as snails. In the metropolitan area of Barcelona, landscape processes are transforming agroforestry mosaics in urbanised areas (Basnou et al. 2013) causing a human impact on biodiversity that is particularly negative for wild communities such as Gastropoda, which are very sensitive to land-use changes (Douglas et al. 2013). This trend is even more dangerous for habitat specialists and can even result in local extinction (Dahirel et al. 2014). With regard to a more trivial and simple biocenosis, we propose the re-establishment of heterogeneous landscapes as a measure to restore terrestrial mollusc diversity in Collserola. Studies such as the present can help stakeholders make decisions related to landscape planning and habitat transformation.
References
Andersen AN, Fisher A, Hoffmann BD, Read JL, Richards R (2004) Use of terrestrial invertebrates for biodiversity monitoring in Australian rangelands, with particular reference to ants. Austral Ecol 29:87–92
Aubry S, Magnin F, Bonnet V, Preece RC (2005) Multi-scale altitudinal patterns in species richness of land snail communities in south-eastern France. J Biogeogr 32:985–998
Bank R (2011) Fauna europaea project. Checklist of the land and freshwater Gastropoda of the Iberian peninsula (Spain, Portugal, Andorra, Gibraltar). [http://www.nmbe.ch/sites/default/files/uploads/pubinv/fauna_europaea_-_gastropoda_of_iberian_peninsula.pdf]
Basnou C, Álvarez E, Bagaria G, Guardiola M, Isern R, Vicente P, Pino J (2013) Spatial patterns of land use changes across a Mediterranean metropolitan landscape: implications for biodiversity management. Environ Manag 52:971–980
Baur B, Meier T, Baur A, Schmera D (2014) Terrestrial gastropod diversity in an alpine region: disentangling effects of elevation, area, geometric constraints, habitat type and land-use intensity. Ecography 37:390–401
Berg MP (2010) Spatio-temporal structure in soil communities and ecosystem processes. In: Verhoef HA, Morin PJ (eds) Community ecology processes, models, and applications. Oxford University Press Inc., New York, pp 69–80
Boone RB, Krohn WB (2000) Partitioning sources of variation in vertebrate species richness. J Biogeogr 27:457–470
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H (2004) Dissecting the spatial structure of ecological data at multiple scales. Ecology 85:1826–1832
Bros V (2004) Mol·luscs terrestres i d’aigua dolça de la serra de Collserola (Barcelona, NE península Ibèrica). Arxius de Miscel·lània Zoològica 2:7–44
Bros V (2009) Inventari faunístic dels mol·luscs continentals de la serra de Collserola (Barcelona, NE de la península Ibérica): resultat d’una revisió bibliogràfica. Arxius de Miscel·lània Zoològica 7:1–45
Bros V, Moreno-Rueda G, Santos X (2011) Post-fire management affects response of Mediterranean animal communities: the case study of terrestrial gastropods. For Ecol Manag 261:611–619
Cameron RAD, Killeen IJ (2003) Land slugs and snails. In: David L. Hawksworth (ed) The changing wildlife of Great Britain and Ireland. The Systematics Association special volume series, 62
Cejka T, Horsak M, Nemethova D (2008) The composition and richness of Danubian floodplain forest land snail faunas in relation to forest type and flood frequency. J Mollus Stud 74:37–45
Cernohorsky NH, Horsak M, Cameron RAD (2010) Land snail species richness and abundance at small scales: the effects of distinguishing between live individuals and empty shells. J Conchol 40:233–241
Coleman DC, Crossley DA, Hendrix PF (2004) Fundamentals of soil ecology, 2nd edn. Elsevier, Burlington
Chiba S (2007) Species richness patterns along environmental gradients in island land molluscan fauna. Ecology 88:1738–1746
Dahirel M, Olivier E, Guiller A, Martin MC, Madec L, Ansart A (2014) Movement propensity and ability to correlate with ecological specialization in European land snails: comparative analysis of a dispersal syndrome. J Anim Ecol 84:228–238
Diniz-Filho JAF, Bini LM, Hawkins BA (2003) Spatial autocorrelation and red herrings in geographical ecology. Global Ecol Biogeogr 12:53–64
Douglas DD, Brown D R, Pederson N (2013) Land snail diversity can reflect degrees of anthropogenic disturbance. Ecosphere 4: art 28
Durkan TH, Yeung NW, Meyer WM III, Hayes KA, Cowie RH (2013) Evaluating the efficacy of land snail survey techniques in Hawaii: implications for conservation throughout the Pacific. Biodiv Conserv 22:3223–3232
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Harris SA (2008) Terrestrial snails as indicators of the health of the decomposer part of the ecosystem in Parks in Alberta. Contributed paper for the Canadian Parks for Tomorrow: 40th Anniversary Conference, May 8–11, 2008, University of Calgary, Calgary
Hoffmann MH, Meng S, Kosachev PA, Terechina TA, Silanteva MM (2011) Land snail faunas along an environmental gradient in the Altai Mountains (Russia). J Mollus Stud 77:76–86
Holland BS, Cowie RH (2009) Land snail models in island biogeography: a tale of two snails. Amer Malac Bull 27:59–68
IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2. www.iucnredlist.org. Downloaded on 18 December 2014
Juřičková L, Kučera T (2005) Ruins of medieval castles as refuges of interesting land snails in the landscape. In: Tajovský K, Schlaghamerský J, Pižl V (eds.) Contributions to Soil Zoology in Central Europe I, 41–46. ISB AS CR, České Budějovice
Kappes H (2005) Influence of coarse woody debris on the gastropod community of a managed calcareous beech forest in Western Europe. J Mollus Stud 71:85–91
Kappes H (2006) Relations between forest management and slug assemblages (Gastropoda) of deciduous regrowth forests. For Ecol Manag 237:450–457
Kappes H, Topp W, Zach P, Kulfan J (2006) Coarse woody debris, soil properties and snails (Mollusca: Gastropoda) in European primeval forests of different environmental conditions. Eur J Soil Biol 42:139–146
Kimberling DN, Karr JR, Fore LS (2001) Measuring human disturbance using terrestrial invertebrates in the shrub-steppe of eastern Washington (USA). Ecol Ind 1:63–81
Labaune C, Magnin F (2001) Land snail communities in Mediterranean upland grasslands: the relative importance of four sets of environmental and spatial variables. J Mollus Stud 67:463–474
Labaune C, Magnin F (2002) Pastoral management vs. land abandonment in Mediterranean uplands: Impact on land snail communities. Global Ecol Biogeogr 11:237–245
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, New York
Liew TS, Schilthuizen M, bin Lakim M (2010) The determinants of land snail diversity along a tropical elevational gradient: insularity, geometry and niches. J Biogeogr 37:1071–1078
Martin K, Sommer M (2004a) Effects of soil properties and land management on the structure of grassland snail assemblages in SW Germany. Pedobiologia 48:193–203
Martin K, Sommer M (2004b) Relationships between land snail assemblage patterns and soil properties in temperate–humid forests. J Biogeogr 31:531–545
Marull J, Mallarach JM (2002) La conectividad ecológica en el Área Metropolitana de Barcelona. Ecosistemas 2002/2
McGeoch M, Sithole H, Samways MJ, Pryke JS, Picker M, Uys C, Armstrong AJ, Dippenaar-Schoeman AS, Engelbrecht IA, Braschler B, Hamer M (2011) Conservation and monitoring of invertebrates in terrestrial protected areas. Koedoe 5, 53 doi:10.4102/koedoe.v53i2.1000
Menez A (2007) A new approach to studying and sampling land molluscs: habitat structure and the effects of scale on land molluscs. J Conch 39:321–328
Millspaugh JJ, Thompson FR (2009) Models for planning wildlife conservation in large landscapes. Elsevier Science, San Diego
Moreno-Rueda G, Ruiz-Ruiz A, Collantes-Martín E, Arrebola JR (2009) Relative importance of humidity and temperature on microhabitat use by land snails in arid versus humid environments. In: Fernandez-Bernal A, De la Rosa MA (eds) Arid environments and wind erosion. Nova Science Publishers Inc, New York
Naro-Maciel E, Sterling E, Rao M (2009) Protected areas and biodiversity conservation I: Reserve planning and design-synthesis. Lessons Conserv 2:18–48
Nekola JC (2010) Acidophilic terrestrial gastropod communities of North America. J Mollus Stud 76:144–156
Ondina P, Mato S (2001) Influence of vegetation type on the constitution of terrestrial gastropod communities in Northwest Spain. Veliger 44:8–19
Pino J, Marull J (2012) Ecological networks: are they enough for connectivity conservation? A case study in the Barcelona Metropolitan Region (NE Spain). Land Use Policy 29:684–690
Rancka B, von Proschwitz T, Hylander K, Götmark F (2015) Conservation thinning in secondary forest: negative but mild effect on Land Molluscs in closed-canopy mixed Oak Forest in Sweden. PLoS ONE 10:e0120085
Raspall A, Llimona F, Navarro M, Tenés A (2004) Guía de Natura del Parc de Collserola. Consorci del Parc de Collserola, Barcelona
Sala M (2012) Mediterranean landscapes. In: Slaymaker O, Spencer T, Embleton-Hamann C (eds) Geomorphology and global environmental change. Cambridge University Press, New York
Santos X, Bros V, Miño A (2009) Recolonization of a burned Mediterranean area by terrestrial gastropods. Biodiver Conserv 18:3153–3165
Santos X, Poquet JM (2010) Ecological succession and habitat attributes affect the post-fire response of a Mediterranean reptile community. Eur J Wildlife Res 56:895–905
Santos X, Mateos E, Bros V, Brotons L, de Mas E, Herraiz JA, Herrando S, Miño A, Olmo-Vidal JM, Quesada J, Ribes J, Sabaté S, Sauras-Yera T, Serra A, Vallejo VR, Viñolas A (2014) Is response to fire influenced by dietary specialization and mobility? A comparative study with multiple animal assemblages. PLoS ONE 9:e88224
Sarkar S, Pressey RL, Faith DP, Margules CR, Fuller T, Stoms DM, Moffett A, Wilson KA, Williams KJ, Williams PH, Andelman S (2006) Biodiversity conservation planning tools: present status and challenges for the future. Annu Rev Environ Resour 31:123–159
Snegin EA (2005) Ecological and genetic characteristics of the distribution of Bradybaena fruticum Mull. (Mollusca, Gastropoda, Pulmonata) in a Forest-Steppe Landscape. Russ J Ecol 36:33–40
Solem A (1984) A world model of land snail diversity and abundance. In: Solem A, van Bruggen AC (eds) Worldwide snails: biogeographical studies on Non-Marine Mollusca. E.J. Brill/Dr W, Backhuys
Tattersfield P, Waruj CM, Seddon MB, Kiringe JW (2001) Land-snails faunas of Afromontane forest of Mount Kenya, Kenya: ecology, diversity and distribution patterns. J Biog 28:843–861
Ter Braak CJ, Šmilauer P (2002) CANOCO reference manual and CanoDraw for Windows. User’s Guide: Software for canonical community ordination (version 4.5), Microcomputer Power, NY
Torre I, Bros V, Santos X (2014) Assessing the impact of reforestation on the diversity of Mediterranean terrestrial Gastropoda. Biodiver Conserv 23(10):2579–2589
Triantis KA, Vardinoyannis K, Mylonas M (2008) Biogeography, land snails and incomplete data sets: the case of three island groups in the Aegean Sea. J Nat His 42:467–490
Ward DF, Larivière MC (2004) Terrestrial invertebrate surveys and rapid biodiversity assessment in New Zealand: lessons from Australia. New Zeal J Ecol 28:151–159
Acknowledgments
This study was carried out with funding from the Consorci del Parc de Collserola. We acknowledge the logistic support provided by Francesc Llimona, Alfons Raspall and Sean Cahill. Xavier Santos was supported by a postdoctoral grant (SFRH/BPD/73176/2010) from Fundaçaõ para a Ciência e a Tecnologia (FCT, Portugal).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bros, V., Torre, I. & Santos, X. Uncovering the environmental factors that influence diversity patterns of Mediterranean terrestrial Gastropod communities: a useful tool for conservation. Ecol Res 31, 39–47 (2016). https://doi.org/10.1007/s11284-015-1310-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-015-1310-2