Abstract
Objectives
This study aimed to measure the palatal mucosal thickness and examine the location of the greater palatine foramen using cone-beam computerized tomography (CBCT).
Methods
In this study, cone-beam computed tomography (CBCT) images of the maxillary posterior region of 120 subjects were evaluated. The palatal mucosal thickness (PMT), palatal width and depth, and location of the greater palatine foramen (GPF) were determined on CBCT. The differences in the palatal mucosal thickness according to gender and palatal width/palatal depth were analyzed. The location of the GPF related to the maxillary molars was noted.
Results
The mean palatal mucosal thicknesses from the canine to the second molar teeth were 3.66, 3.90, 4.06, 3.76, and 3.92 mm, respectively. The mean PMT at the second premolar was statistically thicker than at other regions (p < 0,001). There was no relationship between PMT and gender. However, the palatal depth and width of the males were greater than females. (p = 0.004 and p = 0.014, respectively) PMT in the low palatal vault group had statistically higher compared to the high palatal vault group. (p = 0.023) Greater palatine foramen was mostly observed between second and third molar teeth. (48%).
Conclusions
According to our results, first and second premolar regions can be preferable in soft tissue grafting procedures for safe and successful treatment outcomes. The measurement of the thickness of the palatal mucosa and the evaluation of the greater palatine foramen location before the surgical procedures are essential steps to harvest from the ideal donor site and to achieve optimal surgical outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last decades, several periodontal plastic surgery techniques are widely performed for mucogingival deformities causing functional and aesthetic problems [1, 2]. Autologous soft tissue grafts [3], free gingival grafts [4] and subepithelial connective tissue grafts [5], are generally used for the root coverage, increasing keratinized tissue width, and soft tissue volume around natural teeth and implants [6]. In these therapies, the dimensions of the soft tissue graft can affect the success of the surgical procedures [7]. The prolonged process of revascularization and healing period may occur if the graft is too thick, on the other hand a graft that is too thin may be prone to excessive shrinkage and necrosis at the recipient site. In addition, the thick grafts could cause delayed wound healing and necrosis in the donor area and thus increased postoperative patient morbidity [8, 9]. In both situations, undesirable functional and aesthetic results can be obtained [10]. Therefore, the determination of palatal mucosal thickness (PMT) is of importance in harvesting optimal autogenous graft thickness [11].
The other critical issue for the clinicians is the knowledge of the anatomy of the palate and the course of the greater neurovascular bundle (GNB) [12]. Special care should be taken not to damage GNB to reduce intra/postoperative complications and patient morbidity [13]. Variations in the anatomy of the palatal vault should be evaluated in terms of the dimensions of the harvested tissue and the location of the greater palatine foramen (GPF) in order not to damage the neurovascular bundle in this area and cause complications, such as bleeding and paresthesia [14]. For the safety zone of the donor area, 2 mm below the cemento-enamel junction (CEJ) of the region between the distal of canine teeth and the palatal root of the first molar is recommended for avoiding hemorrhages, paresthesia, and the attachment loss [15].
The palatal masticatory mucosa is the main donor site for periodontal plastic surgeries. The clinical success of the soft tissue grafts is closely related to graft thickness and volume [6]. The evaluation of the masticatory mucosal thickness is described using direct and indirect methods in the literature. Cone beam computed tomography (CBCT) can be considered as a less invasive method compared to direct methods [11]. In addition, radiological evaluation could be useful for the clinician to determine both the palatal mucosal thickness and the locations of the anatomical structures in pre-surgical planning. Based on that, the aim of this retrospective study was to measure the palatal mucosal thickness and determine the greater palatine foramen location using cone beam computed tomography (CBCT) to suggest the ideal zone for soft tissue graft.
Materials and methods
Study population
This retrospective study included CBCT images of a total of 120 patients (61 female, 59 male) with a mean age of 26.39 ± 8.51 years (range; 18–50 years) attending the Baskent University Faculty of Dentistry, Department of Dentomaxillofacial Radiology. The study was conducted in accordance with the guidelines of the Declaration of Helsinki of 1975, as revised in 2013. This study was approved by Baskent University Institutional Review Board (Project no: D-KA21/08). The CBCT images were acquired between 2017 and 2021 for various purposes unrelated to this study, such as evaluations of impacted teeth, orthodontic treatment, and temporomandibular disorders. The patients aged ≥ 18 years of both genders with all teeth from the maxillary right and left canine to the second molars were included in this study. The exclusion criteria were as follows: (1) presence of syndrome or systemic disease affecting bone and soft tissue health; (2) CBCT scans that displayed pathology; (3) congenitally missing teeth, supernumerary teeth, dentigerous cyst, or an enlarged cystic follicle; (4) presence of malposition or malalignment of the maxillary posterior teeth, overhanging restorations in the palatal region of premolars and molars; (5) history of dental trauma or maxillary surgery; and (6) images of patients with motion or any significant artifact on the CBCT image. The demographic characteristics of all patients were obtained from the patients’ medical files.
CBCT Device
All CBCT images were acquired using Morita 3D Accuitomo 170 (J Morita, Kyoto, Japan) with the following parameters: 90 kVp, 5 mA, voxel size: 0.160, 0.250 mm, FOV size: 80 × 80 and 100 × 50 mm, respectively. The images were analyzed using the i-Dixel software (v.2.2.1.6, Morita, Kyoto, Japan) on the medical monitor (Eizo Radiforce MX270W, Eizo Corporation, Ishika, Japan).
Measurements on CBCT images
-
1.
On CBCT images, the palatal mucosal thickness was measured on coronal images from the mid-palatal region of each tooth. Four points were signed along the palatal mucosal surface from the beginning at the gingival margin to the mid-palatal suture at equal 3 mm intervals. A line perpendicular to a tangent line at each 3 mm interval point was drawn to mark the measurement areas from the soft tissue surface to the hard tissue. The thickness of palatal mucosa between the maxillary canine and second molar was measured at a distance of 3, 6, 9, and 12 mm from the gingival margin (Fig. 1).
-
2.
The width of palatine was defined as the horizontal distance between CEJ of the two maxillary first molars. The measurement of the palatal width was performed on mid-palatal point of maxillary first molars in the axial image (Fig. 2).
-
3.
The depth of the palate was determined by the vertical distance from the median palatine suture to the level of CEJ of the maxillary first molar on the coronal image (Fig. 3).
-
4.
After measuring all patients' palatal width and depth, the ratio of palatal width to palatal depth was calculated. The median value was obtained. The palatal vault was classified as high or low by the median value of the palatal width/palatal depth ratio. The palatal width/depth ratio that was lower than the median of the ratio was classified as high palatal vault, and greater values than median value were considered as low palate vault [11].
-
5.
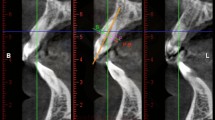
To determine the position of the GPF with respect to the upper molars, each image in axial, sagittal and coronal reconstructions were evaluated (Fig. 4).
The all measurements were obtained by the two researchers [the one radiologist (HK) and one periodontist (INK)] who had 5 and more years’ experience on CBCT images. To assess the inter and intra-examiner agreement, 20 cases were randomly selected, and all measurements were performed twice by the same two researchers. Prior to the measurements of the anatomical landmarks on the CBCT images, the periodontist (INK) was trained in the use of the software measurement tool (i-Dixel software). Inter- and intra-class correlation coefficient showed a high agreement (ICC = 0.89, ICC = 0.93 and 0.90, respectively). Therefore, the values measured by one radiologist (HK) were used for further statistical analysis.
Statistical analysis
All continuous variables were analyzed by performing the Shapiro–Wilk tests were found to evaluate the normality of the data distribution. This test demonstrated that parametric tests were suitable for data analysis in this study. The differences in palatal mucosal thickness according to gender and palatal vault depth were analyzed using the Student’s t test. Both the relationship between gender and palate structure, and the relationship between age and measurements were analyzed by Pearson correlation. Intra and inter observer agreement was analyzed intraclass correlation coefficient (ICC). All analyzes were performed with SPSS 20.0 software (SPSS for Windows, version 12.1, SPSS Inc., Chicago, IL). Statistical significance was determined at p < 0.05.
Results
The mean thicknesses of the palatal mucosa (PMT) at each measurement point according to tooth side are presented in Table 1. Among twenty measurement sites, the first molar teeth had the thinnest mucosa at 3 mm and the second molar had the thickest mucosa at 12 mm. The overall mean thickness was increased from canine to the second premolar and decreased at first molar and increased at second molar. It was observed that the PMT of all teeth increased from the gingival margin to more apical parts (Table 1).
The overall mean thickness of the palatal masticatory mucosa according to gender at each tooth is shown in Table 2. The PMT was thicker in males than in females, but the difference was not significant. (3.88 mm and 3.84 mm, respectively; p > 0.05) An analysis of the palatal mucosal thickness at each measurement point (3 mm, 6 mm, 9 mm, and 12 mm) indicated that the PMT of the second molar at 3 mm showed a significant difference according to gender. Females had significantly thinner mucosa (2.90 ± 0.89 mm) than males (3.29 ± 1.25 mm; P = 0.049) in this measurement point. In addition, the mean values of palatal width and palatal depth were significantly higher in males than females. (p = 0.014 and 0.004, respectively; Table 2). Pearson correlation analysis found no correlation between age and palatal thickness at different measurements of all the tooth side. (p > 0.05).
In the present study, the median of the ratio (palatal width/palatal depth) was 1.99. The subjects were divided into two groups: high- and low-vault groups according to the median of the palatal width/palatal depth ratio (palatal width/palatal depth > 1.99: low palatal vault, palatal width/palatal depth < 1.99: high palatal vault). The low-vault group showed a significantly greater palatal mucosal thickness than the high-vault group (Table 3; p = 0.023).
Table 4 presents the prevalence of the location of greater palatine foramen in relation to maxillary molar teeth. The main location of greater palatine foramen was observed between the second and third molars. (40.8%).
Discussion
Soft tissue grafts are mainly harvested from the palatal masticatory mucosa in periodontal plastic procedures. The success of these surgeries is closely related to the soft tissue graft size and thickness [16]. In the literature, various techniques have been described to evaluate the palatal mucosal thickness, such as direct measurement [17, 18], ultrasonic devices [19], CBCT [20] or dental magnetic resonance imaging (MRI) [21]. The direct method, bone sounding, is an invasive technique, that may be uncomfortable for the patient, because it must be performed under local anesthesia and the measurement can be affected by inflammation and the volume of local anesthesia [17]. Ultrasonography is a noninvasive method; however, it has some disadvantages, including difficulty in measuring the same location and measurement errors due to anatomical features of the palate [22]. MRI provides reliable measurements but it has a low resolution, high cost and long examination time [23]. For these reasons, measuring the palatal mucosal thickness using CBCT is a noninvasive and practical method. CBCT has been recommended as a tool for examination of hard tissues but not for examination of soft tissues due to its limitations [24, 25]. However, the retraction of lips, cheeks, and tongue at the time of CBCT scanning makes the dentogingival unit and palatal mucosa visible and measurable [20, 26]. Ogawa et al. [27] and Gupta et al. [28] concluded that the measurement of palatal mucosal thickness using CBCT was an accurate method. CBCT images can be taken by retracting soft tissues away in patients who will get scans for any reason and these images should not be the first choice for the assessment of soft tissues. In this retrospective study, the thickness of palatal mucosa was evaluated at 20 measurement points using CBCT images.
The palatal mucosal thickness of the maxillary canine, premolar, and molar teeth measurements were performed at distances of 3, 6, 9, and 12 mm from the beginning of the gingival margin. In a recent review, Tavelli et al. [15] described the safety zone for palatal harvesting to avoid damaging the neurovascular bundle based on the distance from the greater palatine artery (GPA) to the CEJ of the maxillary teeth. The authors used the distance of 2 mm for the gingival margin. The biological width is defined as the distance between the bottom of the gingival pocket and the crest of the alveolar bone, reported as approximately 2 mm [29]. Therefore, in this study, we determined the first measurement point at 3 mm from the gingival margin. It can be considered a safety zone for soft tissue graft procedures to prevent violation of biological width.
The results of this study showed that the mean palatal mucosal thickness ranged from 3.66 to 4.06 mm at different tooth sites. The thickness was minimum in the canine regions, and the maximum in the second premolars. Based on these measurements, the first and second premolar regions are suitable for soft tissue graft procedures. Song et al. [11] measured the palatal mucosal thickness of 100 subjects using computerized tomography. The authors found that the thickest mucosa was in the second premolar area, and the mean values were from 3.13 to 3.81 mm. Yilmaz et al. [30] determined the thickness of the palatal mucosa using CBCT and reported mean values that ranged between 3.0 and 3.7 mm. The same investigators also observed that the second premolars and second molars had statistically thicker mucosa than the other regions. Barriviera et al. [20] measured the palatal mucosal thickness using CBCT, and the mean values ranged between 2.92 and 3.28 mm. The mean palatal mucosal thickness shown in the previously mentioned studies was thinner than in our study. This difference may be due to age, gender, ethnicity and different measurement points. The use of different methods, such as bone-sounding, ultrasonic devices, MRI or CBCT, to measure palatal mucosal thickness may also explain the differences between the studies.
Similar to other studies in the literature, PMT increased with greater distance from the gingival margin in the current study [11, 20, 31]. In addition, PMT increased from the canine to the second premolar (4.06 mm), decreased in the first molar (3.76 mm) and increased again in the second molar (3.92 mm). This may be due to the prominence of the palatal roots of first molars. In previous studies, the palatal root of the first molar was reported as the limit for soft tissue grafts, and it was suggested that it should not be exceeded due to the risk of damaging neurovascular structures [17, 32].
The findings of this study investigating the relationship between gender and mean palatal mucosal thickness of all teeth regions showed that there was no significant difference between males and females regarding mean palatal mucosal thickness (3.88 mm and 3.84 mm, respectively). Similar to our findings, several studies found that gender did not affect palatal mucosal thickness [17, 18, 20, 33]. In this study, males had slightly thicker mucosa than females. Müller et al. [31] who measured the thickness of masticatory mucosa by ultrasonic device reported that females had significantly thinner mucosa than males. Stipetić et al. [34] found that the mucosa was significantly thicker in males than in females using the bone sounding technique. This variation may be explained by differences in the sites and methods chosen for measurement. Overall, age, ethnicity, genetics, body mass index, and hormonal changes in female subjects are factors that may influence the thickness of the palatal mucosa [18, 33].
The correlation analysis found no correlation between age and palatal mucosal thickness at different measurements of all teeth sides in the current study. This study was conducted using CBCT images of subjects ranging from 18 to 50 years of age. This finding corresponds with the data from Karadağ and Yilmaz [35] who evaluated the palatal mucosal thickness of the 400 subjects aged 18–57 years using CBCT. However, our findings are inconsistent with studies that suggest that the palatal mucosal thickness increases with age [11, 18, 20, 23]. In these studies, the researchers found a significant effect of age by dividing the cohort into a younger age group and an older age group. When we divided the subjects into age groups in our study, we realized that some age groups had an insufficient number of patients, so the statistical analysis was not performed. In this regard, the two major limitations of our study are the relatively small sample size and unequal distribution of age.
The participants were divided into two groups based on the median palatal width/depth and the low vault group had significantly thicker palatal mucosa than the high vault group. Similarly, Song et al. [11] reported that the low vault group showed thicker palatal mucosal thickness compared to the high vault group, but there was no significant difference between groups. On the other hand, Reiser et al. [32] proposed to subdivide the palatal vault into three groups high, average, and shallow, and they reported that there was greater tissue availability in the high palate vaults compared to the shallow palate vaults, due to the course of the greater palatine nerves and blood vessels. Hormdee et al. [36] categorized the images into shallow, moderate, and high according to palatal vault angle and reported a significantly negative correlation between palatal vault angle and thickness of palatal mucosa. Karadağ and Yilmaz observed a significant negative correlation between the palatal mucosal thickness and palatal depth [35].
Determining the localization of the greater palatine foramen is important for clinicians in terms of reducing the bleeding complication and ensuring a successful nerve block during the harvesting the soft tissue grafts [37]. In our study, greater palatine foramen was mostly observed in the region between the third and second molar (40.8%). Similar to our study, Wang et al. [38] found that the GPF was most frequently located in the region between the 2nd and 3rd molar (48%) in Chinese subjects. In other study evaluating the location of the GPF using the CBCT, it was similarly found that 37.5% of GPFs positioned in the region near the second and third molar apices [14]. In another study evaluating cadaver samples, it was found that 66.6% of GPFs were between the second and third molar, 19.1% at the second molar and 14.3% at the third molar regions [39]. To analyse the dry skulls may be better than CBCT to evaluate the location of the GPF anatomically, but CBCT images ensures a larger number of exams for anatomical analysis of GPF characteristics [40]. Compared to our study, Betgee et al. [41] who used micro-CT to determine the location of the GPF reported that the most frequent location was between the second and third molar (83.5%). In addition, Yilmaz et al. [30] mentioned that GPF was located at level of third molar in 63% of men subjects and 56% female subjects. Ikuta et al. [37] concluded that the in Brazilian population, the GPF location was more closely related to third molar.
In conclusion, the safety zone determined by evaluating palatal mucosal thickness and anatomical structures prior to periodontal plastic surgery provides benefits in the success of the surgical procedure and reduced patient morbidity. In the present study, the first and second premolar regions may be the most suitable for harvesting soft tissue grafts regarding palatal mucosal thickness. In addition, the shape of the palatal vault was determined using CBCT and the thickness of the palatal mucosa was found thinner in the high vault depth group than in the low vault depth group. In CBCT images that allow clear visibility of intraoral soft tissues, clinicians can easily identify dimensions of soft and hard tissues and important anatomical landmarks in the pre-surgical examination.
Data availability
Data is available upon request from the corresponding author.
References
Chambrone L, Chambrone D, Pustiglioni FE, Chambrone LA, Lima LA. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of miller class I and II recession-type defects? J Dent. 2008;36(9):659–71.
Bouchard P, Malet J, Borghetti A. Decision-making in aesthetics: root coverage revisited. Periodontol. 2001;27(1):97–120.
Miller PD Jr. Root coverage using the free soft tissue autograft following citric acid application III. A successful and predictable procedure in areas of deep-wide recession. Int J Periodontics Restorative Dent. 1985;5(2):14–37.
Hangorsky U, Bissada NF. Clinical assessment of free gingival graft effectiveness on the maintenance of periodontal health. J Periodontol. 1980;51(5):274–8.
Tözüm TF, Dini FM. Treatment of adjacent gingival recessions with subepithelial connective tissue grafts and the modified tunnel technique. Quintessence Int. 2003;34(1):7–13.
Zucchelli G, Tavelli L, McGuire MK, Rasperini G, Feinberg SE, Wang HL, et al. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J Periodontol. 2020;91(1):9–16.
Zuhr O, Bäumer D, Hürzeler M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: critical elements in design and execution. J Clin Periodontol. 2014;41(Suppl 1):S123–42.
Yu SK, Lee BH, Lee MH, Cho KH, Kim DK, Kim HJ. Histomorphometric analysis of the palatal mucosa associated with periodontal plastic surgery on cadavers. Surg Radiol Anat. 2013;35(6):463–9.
Mörmann W, Schaer F, Firestone AR. The Relationship between success of free gingival grafts and transplant thickness: revascularization and shrinkage—a one year clinical study. J Periodontol. 1981;52(2):74–80.
Baker P. The management of gingival recession. Dent Update. 2002;29(3):114.
Song J-E, Um Y-J, Kim C-S, Choi S-H, Cho K-S, Kim C-K, et al. Thickness of posterior palatal masticatory mucosa: the use of computerized tomography. J Periodontol. 2008;79(3):406–12.
Chackartchi T, Romanos GE, Sculean A. Soft tissue-related complications and management around dental implants. Periodontol. 2019;81(1):124–38.
Griffin TJ, Cheung WS, Zavras AI, Damoulis PD. Postoperative complications following gingival augmentation procedures. J Periodontol. 2006;77(12):2070–9.
Klosek SK, Rungruang T. Anatomical study of the greater palatine artery and related structures of the palatal vault: considerations for palate as the subepithelial connective tissue graft donor site. Surg Radiol Anat. 2009;31(4):245–50.
Tavelli L, Barootchi S, Ravidà A, Oh TJ, Wang HL. What Is the safety zone for palatal soft tissue graft harvesting based on the locations of the greater palatine artery and foramen? a systematic review. J Oral Maxillofac Surg. 2019;77(2):271.e1-271.e9.
Zucchelli G, De SM. Treatment of multiple recession-type defects in patients with esthetic demands. J Periodontol. 2000;71(9):1506–14.
Studer SP, Allen EP, Rees TC, Kouba A. The thickness of masticatory mucosa in the human hard palate and tuberosity as potential donor sites for ridge augmentation procedures. J Periodontol. 1997;68(2):145–51.
Wara-Aswapati N, Pitiphat W, Chandrapho N, Rattanayatikul C, Karimbux N. Thickness of palatal masticatory mucosa associated with age. J Periodontol. 2001;72(10):1407–12.
Eger T, Muller HP, Heinecke A. Ultrasonic determination of gingival thickness. Subject variation and influence of tooth type and clinical features. J Clin Periodontol. 1996;23(9):839–45.
Barriviera M, Duarte WR, Januário AL, Faber J, Bezerra ACB. A new method to assess and measure palatal masticatory mucosa by cone-beam computerized tomography. J Clin Periodontol. 2009;36(7):564–8.
Hilgenfeld T, Kästel T, Heil A, Rammelsberg P, Heiland S, Bendszus M, et al. High-resolution dental magnetic resonance imaging for planning palatal graft surgery—a clinical pilot study. J Clin Periodontol. 2018;45(4):462–70.
Müller HP, Schaller N, Eger T. Ultrasonic determination of thickness of masticatory mucosa: A methodologic study. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology. 1999;88(2):248–53.
Heil A, Schwindling FS, Jelinek C, Fischer M, Prager M, Gonzalez EL, et al. Determination of the palatal masticatory mucosa thickness by dental MRi: a prospective study analysing age and gender effects. Dentomaxillofac Radiol. 2018;47(2):20170282.
Guerrero ME, Jacobs R, Loubele M, Schutyser F, Suetens P, van Steenberghe D. State-of-the-art on cone beam CT imaging for preoperative planning of implant placement. Clin Oral Investig. 2006;10(1):1–7.
Suomalainen A, Pakbaznejad Esmaeili E, Robinson S. Dentomaxillofacial imaging with panoramic views and cone beam CT. Insights Imaging. 2015;6(1):1–16.
Januário AL, Barriviera M, Duarte WR. Soft tissue cone-beam computed tomography: a novel method for the measurement of gingival tissue and the dimensions of the dentogingival unit. J Esthet Restor Dent. 2008;20(6):366–73.
Ogawa M, Katagiri S, Koyanagi T, Maekawa S, Shiba T, Ohsugi Y, et al. Accuracy of cone beam computed tomography in evaluation of palatal mucosa thickness. J Clin Periodontol. 2020;47(4):479–88.
Gupta P, Jan SM, Behal R, Mir RA, Shafi M. Accuracy of cone-beam computerized tomography in determining the thickness of palatal masticatory mucosa. J Indian Soc Periodontol. 2015;19(4):396–400.
Gargiulo AW, Wentz FM, Orban B. Dimensions and relations of the dentogingival junction in humans. J Periodontol. 1961;32(3):261–7.
Yilmaz HG, Boke F, Ayali A. Cone-beam computed tomography evaluation of the soft tissue thickness and greater palatine foramen location in the palate. J Clin Periodontol. 2015;42(5):458–61.
Müller HP, Schaller N, Eger T, Heinecke A. Thickness of masticatory mucosa. J Clin Periodontol. 2000;27(6):431–6.
Reiser GM, Bruno JF, Mahan PE, Larkin LH. The subepithelial connective tissue graft palatal donor site: anatomic considerations for surgeons. Int J Periodontics Restorative Dent. 1996;16(2):130–7.
Said KN, Khalid ASA, Fathima I, Farook F. Anatomic factors influencing dimensions of soft tissue graft from the hard palate a clinical study. Clin Exp Dent Res. 2020;6(4):462–9.
Stipetic J, Hrala Z, Celebic A. Thickness of masticatory mucosa in the human hard palate and tuberosity dependent on gender and body mass index. Coll Antropol. 2005;29:243–7.
Karadag I, Yilmaz HG. Palatal mucosa thickness and palatal neurovascular bundle position evaluation by cone-beam computed tomography-retrospective study on relationships with palatal vault anatomy. PeerJ. 2021;9: e12699.
Hormdee D, Yamsuk T, Sutthiprapaporn P. Palatal soft tissue thickness on maxillary posterior teeth and its relation to palatal vault angle measured by cone-beam computed tomography. Int J Dent. 2020;2020:8844236.
Ikuta CRS, Cardoso CL, Ferreira O, Lauris JRP, Souza PHC, Rubira-Bullen IRF. Position of the greater palatine foramen: an anatomical study through cone beam computed tomography images. Surg Radiol Anat. 2013;35(9):837–42.
Wang TM, Kuo KJ, Shih C, Ho LL, Liu JC. Assessment of the relative locations of the greater palatine foramen in adult chinese skulls. Cells Tissues Organs. 1988;132(3):182–6.
Fu J-H, Hasso DG, Yeh C-Y, Leong DJM, Chan H-L, Wang H-L. The accuracy of identifying the greater palatine neurovascular bundle: a cadaver study. J Periodontol. 2011;82:1000–6.
Gibelli D, Borlando A, Dolci C, Pucciarelli V, Cattaneo C, Sforza C. Anatomical characteristics of greater palatine foramen: a novel point of view. Surg Radiol Anat. 2017;39:1359–68.
Beetge MM, Todorovic VS, Oettlé A, Hoffman J, Van Zyl AW. A micro-ct study of the greater palatine foramen in human skulls. J Oral Sci. 2018;60(1):51–6.
Funding
The study was supported by Baskent University Research Fund.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to data collection, conception and design, acquisition of data, interpretation of data and initial and final drafting of the manuscript and were accountable for all aspects of the work. BFO, MNNY, Eİ and İNK contributed to data analyses and critically revised the manuscript. HK contributed to data collection and prepared figures. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Ethical approval
This study was approved by Baskent University Institutional Review Board (Project no: D-KA21/08). The study was performed in accordance with the ethical standards of the Helsinki Declaration.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oduncuoğlu, B.F., Karslioğlu, H., Karasu, I.N. et al. Assessment of palatal mucosal thickness and location of the greater palatine foramen using cone-beam computed tomography: a retrospective study. Oral Radiol 39, 784–791 (2023). https://doi.org/10.1007/s11282-023-00699-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-023-00699-2