Abstract
As lead molecules, cyclic lipopeptides with antibacterial, antifungal, and antiviral properties have garnered a lot of attention in recent years. Because of their potential, cyclic lipopeptides have earned recognition as a significant class of antimicrobial compounds with applications in pharmacology and biotechnology. These lipopeptides, often with biosurfactant properties, are amphiphilic, consisting of a hydrophilic moiety, like a carboxyl group, peptide backbone, or carbohydrates, and a hydrophobic moiety, mostly a fatty acid. Besides, several lipopeptides also have cationic groups that play an important role in biological activities. Antimicrobial lipopeptides can be considered as possible substitutes for antibiotics that are conventional to address the current drug-resistant issues as pharmaceutical industries modify the parent antibiotic molecules to render them more effective against antibiotic-resistant bacteria and fungi, leading to the development of more resistant microbial strains. Bacillus species produce lipopeptides, which are secondary metabolites that are amphiphilic and are typically synthesized by non-ribosomal peptide synthetases (NRPSs). They have been identified as potential biocontrol agents as they exhibit a broad spectrum of antimicrobial activity. A further benefit of lipopeptides is that they can be produced and purified biotechnologically or biochemically in a sustainable manner using readily available, affordable, renewable sources without harming the environment. In this review, we discuss the biochemical and functional characterization of antifungal lipopeptides, as well as their various modes of action, method of production and purification (in brief), and potential applications as novel antibiotic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence of multidrug resistance (MDR) among infectious microorganisms is a significant public health threat worldwide (Tanwar et al. 2014). Excessive use of antimicrobial agents results in the development of antibiotic-resistant pathogens and multi-resistance strains. This poses a public health emergency and could cause millions of deaths annually by 2050 (Colson et al. 2021). Methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacteriaceae (CRE), and vancomycin-resistant Enterococcus (VRE) are examples of significant superbugs that are combatted with a variety of treatment approaches, including antimicrobial polymeric biomaterials products, bio-nanotechnology approaches, combinatorial drug approaches. These superbugs are managed through pathogen-directed therapeutics, host-directed therapeutics, prudent antibiotic use, and the development of novel treatments (Parmanik et al. 2022). An effective antimicrobial therapy typically involves the selection of the most effective dosing regimens and combinations of antibiotics to treat bacterial infections (Rossolini et al. 2014).
To alleviate the effects of synthetic antimicrobial agents, natural synthesized antimicrobial peptides (AMPs) are discovered by researchers to serve as natural defense-effector molecules synthesized in bacteria, fungi, animals, and humans. These lipopeptides (LPs) are characterized by their surfactant, antibacterial, and antifungal properties, making them valuable candidates for various applications (Zaman and Toth 2013). The lipopeptides synthesized from the prevalent bacterial genus Bacillus have intrinsic properties, which makes them widely applicable in biotechnological and pharmaceutical industries, such as enhanced oil recovery, environmental bioremediation, and plant protection in agriculture (Santos et al. 2016; Shafi et al. 2017). These compounds have shown antimicrobial activity against bacteria and fungi, making them promising candidates for developing new antifungal therapeutics with new cellular targets for combating emerging multidrug-resistant pathogens (Barale et al. 2022; Parulekar & Sonawane 2018; Zhao et al. 2016) and less host toxicity (Karlapudi et al. 2018).In addition to it, microbial lipopeptides have exhibited anticancer activity, further expanding their pharmaceutical applications. Properties like broad-spectrum activity, multifaceted modes of action, rapid activity, and non-specificity of antimicrobial peptides (AMPs), including cyclic lipopeptides have been considered less prone to resistance development compared to traditional antibiotics. Despite these advantages, it's not accurate to say that resistance to AMPs is impossible. While the likelihood of resistance may be lower compared to traditional antibiotics, it is not zero. Factors such as high concentrations of AMPs, prolonged exposure, and specific environmental conditions could potentially lead to the emergence of resistance mechanisms (Tran et al. 2022). Lipopeptides are often considered biodegradable due to the presence of ester linkages in their structure. The ester bonds in the lipid portion of the molecule are prone to cleavage by esterases, which are enzymes commonly found in many biological systems, including microorganisms. The hydrolysis of ester bonds in lipopeptides results in the separation of the lipid and peptide components, facilitating the degradation of these molecules into smaller fragments. These smaller fragments are then more readily assimilated by microorganisms, leading to their ultimate conversion into simpler and environmentally benign substances. The biodegradability of lipopeptides can vary depending on their specific structure, the types of bonds present, and environmental conditions. Overall, the susceptibility of lipopeptides to enzymatic hydrolysis contributes to their relatively high biodegradability, making them less persistent in the environment compared to some other chemical compounds (Meena and Kanwar 2015; Santos et al. 2016). These benefits make lipopeptides more and more important in fields including pharmacology, agriculture, and food preservation. These findings suggest that lipopeptides may be developed as suitable substitutes for conventional antibiotics. This review discusses novel antibiotic agents and their modes of action, production strategies, and potential applications along with biochemical and functional characterization.
Lipopeptide’s potential to replace antibiotics
Lipopeptides have been deemed ‘the new class of therapeutic agents’ that have the potential to advance from the 'bench side to bedside’ (Schneider et al. 2014). The first ever lipopeptide antibiotic polymyxins were discovered in the 1940s (Benedict and Langlykke 1947; Stansly 1949), however, its development into a pharmaceutical product was limited by complex chemical structure and cytotoxicity. The polymyxins are characterized as amphiphilic due to their segregation of hydrophobic and hydrophilic domains. Different variants of polymyxins, Polymyxin B and Polymyxin E were reported to be produced from different Paenibacillus species (Carolin et al. 2021; Poursoleiman et al. 2019; Zhan et al. 2019) It demonstrated the best antibacterial activity through the mechanism of electrostatic and hydrophobic interaction with the lipopolysaccharide in the bacteria. Subsequently, about one and half decades later, a lipopeptide antibiotic amphomycin was discovered (Heinemann et al. 1953), whose applications were limited due to its adverse effects on cells. AMPs are a viable alternative to developing new antibiotics and offer several advantages over the challenges associated with conventional antibiotic development. AMPs frequently target a variety of Gram-positive and Gram-negative bacteria with their broad-spectrum antimicrobial action, even for certain fungi (Reddy et al. 2004). This characteristic enables AMPs to be effective against various microbial pathogens, potentially reducing the need for multiple antibiotics. AMPs typically act through unique mechanisms that differ from those of conventional antibiotics (Table 1). They are effective against pathogens that have evolved resistance to conventional antibiotics because of their capacity to rupture cell membranes, prevent the formation of biofilms, and obstruct vital cellular functions. This suggests that AMPs can be a potential weapon against certain bacteria that are resistant to antibiotics (Mba and Nweze 2022). The complex and multifaceted mechanisms of action exhibited by AMPs make it difficult for the microorganisms to develop resistance against these molecules. Their interactions with microbial cell membranes and essential cellular components can limit the emergence of resistance mechanisms, potentially extending their effectiveness as antimicrobial agents over time. AMPs often possess additional biological activities, such as surfactant properties, antiviral effects, and immunomodulatory functions (Talapko et al. 2022).
This multifunctional nature expands its potential applications beyond antimicrobial therapy, extending its uses in agricultural, pharmaceutical, and industrial settings. AMPs are typically biodegradable and exhibit lower toxicity toward mammalian cells compared to many conventional antibiotics (Bahar and Ren 2013; Tossi et al. 1997). These characteristics minimize the possibility of harmful effects on both human health and the environment, which is aligned with the principles of environmentally friendly and sustainable antibacterial strategies.
The discovery of AMPs dates back to the mid-twentieth century when researchers began to recognize the antimicrobial properties of certain natural peptides extracted from a soil bacterial strain of the Bacillus genus (Dubos 1939).. Microbial lipopeptides have demonstrated antiviral properties against vesicular stomatitis virus, feline calicivirus, and herpes simplex virus (HSV) in addition to their antagonistic action against fungi, oomycetes, and mycoplasmas (Meena and Kanwar 2015; Sreedharan et al. 2023). Antimicrobial peptides are classified according to their activity into groups such as antifungal, anti-HIV, anti-tumor, antibacterial, antiparasitic, and antiviral peptides.
One subclass of AMPs that targets fungal illnesses with increased medication resistance is called antifungal peptides or antifungal lipopeptides. Fungal cell walls or intracellular components can be targeted by antifungal lipopeptides to cause their death (De Lucca and Walsh 1999; Fernández de Ullivarri et al. 2020).
Actinomycetes, fungi, and bacteria are the three types of microbes that can produce antifungal peptides. The majority of these sources are bacteria, and the most widely used ones in biological control studies are Bacillus amyloliquefaciens, Bacillus subtilis, Bacillus cereus, (Anckaert et al. 2021). These antifungal lipopeptides are produced by one of the prevalent bacterial species Bacillus subtilis which has intrinsic properties that make them widely applicable in biotechnological and pharmaceutical industries, including enhanced oil recovery, environmental bioremediation, and plant protection in agriculture (Santos et al. 2016; Shafi et al. 2017). For example, the antifungal lipopeptide derived from B. subtilis RLID 12.1 inhibits various Candida spp. and Cryptococcus spp. (Ramachandran et al. 2014; Ramesh et al. 2023a). Quite interestingly, several recent reports suggest that biosurfactants can retain their microbicidal and biofilm eradicating activities even after exposure to harsh conditions like high temperature and extreme pH. Biosurfactant produced by Bacillus sp. retained its bactericidal and biofilm removing activity even in the hard water containing Mg2+ and Ca2+ ions which indicates that biosurfactants are effective against both planktonic and biofilm residing bacteria (Singh and Sharma 2020). Given the concerning rise in the emergence of drug-resistant microbial infections, both lipopeptides and AMPs are seen to offer potential as future broad-spectrum antibacterial medicines (De Zoysa et al. 2015) pathogenic fungal hyphae (Romero et al. 2007a).
Biosynthesis of lipopeptides
Lipopeptides are structurally diverse secondary metabolites possessing antimicrobial properties, mainly produced by Bacillus spp. via two major synthesis pathways, (1) non-ribosomally synthesized and (2) ribosomally synthesized (Li et al. 2012).
Biosynthesis of lipopeptides by non-ribosomal pathway
The non-ribosomal biosynthesis of lipopeptides involves the action of multifunctional enzymatic complexes known as non-ribosomal peptide synthetases (NRPSs) (100 to 1600 kDa) (Stachelhaus and Marahiel 1995). These large, modular enzyme complexes are capable of catalyzing the assembly of amino acids into peptides in a template-independent manner. NRPSs consist of several modules, each capable incorporating of a particular amino acid into the growing peptide chain. These modules typically include adenylation (A), thiolation (T), and condensation (C) domains, among others. These peptides can be either branched, cyclic, or linear and may be modified with acylation, hydroxylation, methylation, or glycosylation (Hancock and Chapple 1999). Gramicidin, bacitracin, iturins, surfactin, tyrocidine, and fengycins are a few peptide antibiotics produced by Bacillus spp (Sumi et al. 2015).
In the biosynthesis of lipopeptides, NRPSs not only incorporate amino acids but also incorporate fatty acids or lipids into the growing peptide chain. This process often involves the activation of the fatty acid by the adenylation domain of the NRPS, followed by the transfer of the activated fatty acid to the thiolation domain (Martínez-Núñez and López 2016). The fatty acid is then linked to the peptide chain during the elongation process. The specific combination of amino acids and fatty acids incorporated into the lipopeptide chain, as well as the order of their assembly, determines the final structure and function of the lipopeptide. Non-ribosomal biosynthesis of lipopeptides is a complex and highly regulated process that involves various enzymatic reactions and post-translational modifications. The resulting lipopeptides often exhibit diverse biological activities and have potential applications in the development of novel pharmaceuticals, agricultural products, and industrial compounds.
Ribosomal biosynthesis of lipopeptides
Lipopeptides synthesized through ribosomal pathway are typically spans around 12 and 50 amino acids in length with enormous structural diversity and often positively charged (Marx et al. 2001). Bacteriocins are typical lipopeptides synthesized through the ribosomal pathway and remain either amphiphilic or hydrophobic (Motta et al. 2008). Unlike non-ribosomal biosynthesis, this process primarily involves the ribosomal machinery and does not require the use of non-ribosomal peptide synthetases (NRPSs) or other complex enzymatic systems. The biosynthesis of ribosomally synthesized lipopeptides typically involves the translation of specific precursor peptides by ribosomes, resulting in the production of linear or cyclic peptides (Mcintosh et al. 2009; Liang et al. 2023). These precursor peptides often contain specific recognition sequences that are targeted by dedicated modifying enzymes responsible for lipidation. The lipidation process occurs post-translationally, where the lipid moieties are attached to the peptide chain to form the final lipopeptide product. This lipidation can occur through various mechanisms, such as enzymatic modifications or co-translational modifications, depending on the specific lipopeptide and the cellular context. The specific type of lipid attached to the peptide chain can vary and may include fatty acids, prenyl groups, or other lipid moieties, which can significantly impact the properties and functions of the resulting lipopeptide (Mcintosh et al. 2009).
Cyclic lipopeptides (CLPs)
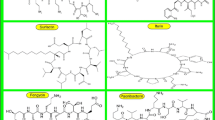
The most physiologically active class of LPs known to date is cyclic, whereas linear LPs have received little attention in the literature (Tran et al. 2019). In the case of cyclic lipopeptides, the positively charged residues, such as lysine or arginine, contribute to the overall positive charge of the molecule. These positively charged amino acids typically have basic side chains that can accept protons (H+) and thus carry a positive charge at physiological pH. The positively charged nature of cyclic lipopeptides can have several implications for their biological activity, including interactions with negatively charged components of microbial cell membranes. This positive charge is often involved in the mechanism of action of cyclic lipopeptides, as they may interact with and disrupt the negatively charged membranes of bacteria or fungi. It's important to note that the specific amino acid composition and sequence can vary among different cyclic lipopeptides, leading to variations in their overall charge. The net positive charge is just one aspect of their structure that contributes to their biological functions, such as antimicrobial activity (Patel et al. 2015). These compounds have a wide range of medicinal and biotechnological properties, making them of great interest in various fields. CLPs are characterized by their amphiphilic nature, possessing both hydrophilic peptide heads and hydrophobic fatty acid tails (Schneider et al. 2014). The cyclic lipopeptides can be classified into three main groups according to their amino acid groups: iturin, fengycin, and surfactin (Table 2) (Bionda et al. 2013). These are significantly produced by a bacterial species Bacillus subtilis. These lipopeptides exert their antimicrobial activities by disrupting the integrity and function of the plasma membrane. They achieve this through a pore-forming mechanism.
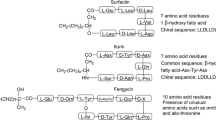
Surfactin is a cyclic lipopeptide that is produced by various strains of bacteria, primarily from the genus Bacillus, such as Bacillus subtilis (Ganesan and Rangarajan 2021). It is one of the most extensively studied and well-known lipopeptides, appreciated for its remarkable surface-active properties, biological activities, and potential industrial applications. Surfactin is a complex cyclic lipopeptide with a heptapeptide ring and a fatty acid tail (Fig. 1a). Its unique amphiphilic nature allows it to exhibit powerful surface-active properties. The peptide ring, consisting of seven amino acid residues, forms the hydrophilic portion of surfactin. The hydrophobic fatty acid tail, typically a β-hydroxy fatty acid chain, contributes to surfactin's amphiphilic properties, allowing it to interact with both hydrophobic and hydrophilic environments. The cyclic structure of surfactin is formed through the lactone linkage between the carboxyl group of the C-terminal amino acid and the amino group of the fourth amino acid in the peptide sequence. This unique structural configuration allows surfactin to form micelles and monolayers at interfaces, facilitating its surfactant and emulsifying activities. Surfactin's unique properties make it valuable in industrial applications, including detergents, cosmetics, and pharmaceuticals (Chen et al. 2022; Ganesan et al. 2023). Surfactin exhibits broad-spectrum antimicrobial activity against various Gram-positive (methicillin-resistant Staphylococcus aureus (MRSA) and Gram-negative bacteria and fungi (Candida spp.) (Sharma et al. 2020). It can disrupt the integrity of microbial cell membranes, leading to cell lysis and subsequent cell death (Arima et al. 1968). This antimicrobial activity has attracted attention for potential applications in the development of novel antimicrobial agents and the control of microbial infections. Surfactin has been found to possess anti-biofilm properties, inhibiting the formation and persistence of biofilms by interfering with microbial adhesion and biofilm matrix formation (Chtioui et al. 2010). This makes surfactin a promising candidate for combating biofilm-related infections and preventing the development of resistant microbial communities. Studies have indicated that surfactin can exhibit cytotoxic effects on certain cancer cell lines such as Ehrlich ascites, MCF-7, T47D, HCT15, HT29, K562, HepG2, HeLa, KB-3-1, SW1990, and B16 (Wu et al. 2017). Its ability to induce apoptosis and inhibit the proliferation of cancer cells has sparked interest in exploring its potential as an anticancer therapeutic agent.
The respirometric techniques and various biodegradability assays are used to determine the biodegradability of biosurfactants (Mulligan 2005); their low toxicity and environmentally friendly nature make them excellent candidates for environmental remediation, particularly in the context of soil bioremediation and oil spill cleanup (De Oliveira et al. 2017; Gayathiri et al. 2022; Tang et al. 2017). Biosurfactants (microbial lipopeptides) were claimed to exhibit better biodegradability and production from renewable sources than chemical surfactants (Carolin et al. 2021; Naughton et al. 2019). The diverse properties of surfactin underscore its potential applications in various industries, including biotechnology, pharmaceuticals, agriculture, and environmental science.
Iturin is a cyclic lipopeptide produced by various strains of Bacillus subtilis and closely related species. It is known for its potent antifungal and antibacterial properties, making it a valuable candidate for applications in agriculture, medicine, and biotechnology (Maget-Dana and Peypoux 1994). Iturin is a compound with a complex structure consisting of a cyclic heptapeptide and a β-amino fatty acid (Fig. 1b). Iturin's hydrophilic portion is a cyclic heptapeptide, while its hydrophobic fatty acid chain, typically 14–16 carbon atoms long, contributes to its amphiphilic nature. The peptide ring forms a stable cyclic structure through an amide bond between the carboxyl group of the C-terminal amino acid and the amino group of the fourth amino acid in the peptide sequence. This structure is crucial for iturin's diverse biological activities, including its antifungal and antibacterial properties. (Wan et al. 2022).
Iturin is highly effective against various plant pathogenic fungi such as Gaeumannomyces graminis var. tritici, Magnaporthe grisea, Fusarium solani, Fusarium oxysporum f.sp. lycopersici, Colletotrichum fioriniae, and Alternaria alternata (Zhou et al. 2020) (Xiao et al. 2021), including those causing diseases in crops. Iturins can disrupt both fungal and bacterial cell membranes. The lipophilic portion enables insertion into membranes leading to membrane disruption, leakage of cellular contents, and eventual cell death, making it a promising biocontrol agent for managing fungal diseases in agriculture (Yaraguppi et al. 2023).
Iturin's antimicrobial activity contributes to its potential use as an antimicrobial agent in various industrial and medical applications. Besides, its ability to suppress the growth of phytopathogenic fungi and bacteria makes it a promising candidate for the development of environmentally sustainable (Vigneshwaran et al. 2021) and robust crop protection strategies, reducing the reliance on chemical pesticides. It is worth mentioning that the most eclectic advantage of biosurfactants or lipopeptides, for example, iturins of microbial origins is their ecological acceptability. Biosurfactants being biodegradable as mentioned previously, the toxicity and accumulation in natural ecosystems are naturally avoided (Pacwa-Płociniczak et al. 2011; Soares da Silva et al. 2017). Similar to other lipopeptides, iturin exhibits surface-active properties, enabling it to reduce surface tension and stabilize emulsions. Iturin A has been observed to inhibit pathogenic spore germination or directly adsorb to the pathogenic fungal cell membrane, modify the permeability of the cell membrane, lead to an outflow of cell contents, and cause the pathogenic fungal hyphae to decompose and die (Thimon et al. 1995).
A cyclic lipopeptide known as fengycin is generated by several Bacillus subtilis strains and closely related species It has a well-established history of potent antifungal activity against a variety of plant-pathogenic fungi (Romero et al. 2007a). Fengycin consists of a cyclic decapeptide linked to a β-hydroxy fatty acid, giving it both hydrophilic and hydrophobic properties (Fig. 1c) (Pathak et al. 2012). Fengycin's peptide ring is composed of ten amino acid residues arranged in a cyclic structure. Fengycin is linked to a β-hydroxy fatty acid chain, which is attached to the peptide ring (Wei et al. 2010). The length of the fatty acid chain can vary, but it typically comprises 14 to 17 carbon atoms. This hydrophobic fatty acid chain contributes to the amphiphilic nature of fengycin, allowing it to interact with both hydrophilic and hydrophobic environments. The peptide ring of fengycin forms a stable cyclic structure via lactone linkage between the side-chain phenolic-OH group of Tyr3 and C-terminal –COOH group of Ile10 and the hydroxy group of the β-hydroxy fatty acid (Meena and Kanwar 2015). The complex structure of fengycin is crucial to its diverse biological activities, particularly its strong antifungal properties. Understanding the structural components of fengycin is essential for elucidating its mode of action and for the development of potential applications in areas such as agriculture, biotechnology, and pharmaceuticals.
Several fluorescent Pseudomonas species have been found to produce a variety of cyclic lipopeptides (CLPs) in recent years, which could be helpful for biological control. Among the numerous metabolites produced are CLPs, of which about 100 have been separately and to differing degrees reported. Because Ps-CLPs include a fatty acid tail, they share a distinguishing characteristic with Bacillus CLPs: they are biosurfactants because they lower surface tension (Fechtner et al. 2011). The cyclic LPs generated by Pseudomonas spp. were originally categorized into four primary groups: viscosin, amphisin (cyclic lipopundecapeptide), tolaasins I, II, A-E, and syringomycin, based on these distinctive structural characteristics (Nybroe and Sorensen 2004). The prominent cyclic lipopeptide produced by the Pseudomonas fluorescens BD5 is pseudofactin I and pseudofactin II (Janek et al. 2010). Pseudofactin II biosurfactant consists of an octapeptide chain linked to palmitic acid which has anti-biofilm and anti-adhesive properties (Fig. 1d). It is widely applied for therapeutic purposes (Janek et al. 2012).
Since the lipopeptides have lipid moieties added, which improve their membrane-disrupting abilities, they are frequently used in pharmaceutical manufacture to create medications that can replace some traditional antibiotics. Table 3 enlists a few of the medications that include lipopeptides or are modified with lipopeptides.
Daptomycin is a lipopeptide antibiotic that is used to treat serious infections caused by Gram-positive bacteria. It is derived from the soil bacterium Streptomyces roseosporus. Daptomycin is known for its efficacy against various multidrug-resistant Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) (Ehlert and Neu 1987). It is commonly used to treat skin and soft tissue infections, as well as bloodstream infections. Daptomycin is a cyclic lipopeptide antibiotic with a complex structure that contributes to its unique mechanism of action and its efficacy against Gram-positive bacteria. Daptomycin consists of a cyclic 13-amino acid peptide ring, which forms the hydrophilic portion of the molecule (Fig. 1e). The specific sequence of amino acids in the peptide ring contributes to the molecule's three-dimensional structure and its interactions with the bacterial cell membrane. This lipid tail is responsible for anchoring the antibiotic to the bacterial cell membrane, enabling the peptide portion to interact with the membrane surface and exert its antibacterial activity (Jung et al. 2004). The lipopeptide daptomycin was found to bind to membranes and cell walls but was unable to reach the cytoplasm. Although Ca2+ is necessary for binding to both of these structures, binding to the cytoplasmic membranes of bacteria seems to be of a different, unique nature. Ca2+ most likely acts as a bridge between daptomycin and the walls and membranes of cells (Canepari et al. 1990).
Echinocandins are antifungal compounds targeting cell wall synthesis by inhibiting β-(1,3)-d-glucan synthesis (Szymański et al. 2022). Echinocandin B, pneumocandin A0/B0, and FR901379 are natural echinocandins synthesized by filamentous fungi. Echinocandin B, the first among the class was discovered back in 1974 in Switzerland isolated from filamentous fungus Emericella (Benz 1974). The echinocandin, and pneumocandin A0 /B0 are naturally occurring hexapeptide from Zalerion arboricola and Glarea lozoyensis (Chen et al. 2015a, b). FR901379 was isolated from Spicellum roseum by Fujisawa Pharmaceutical in Japan (Hashimoto 2009).
Caspofungin, micafungin, anidulafungin, and rezafungin are functionally effective semisynthetic derivatives of these natural cyclic lipopeptides (Fig. 2). These are acylated cyclic hexapeptides with fatty acids attached to the α-amino group of dihydroxy-ornithine, where the lipid residues support the anchoring of the molecule to the cell membrane (Hüttel 2021). Caspofungin is a derivative of pneumocandin B0 approved for clinical trial in 1992 (Balkovec et al. 2014). It was the first echinocandin drug for the clinical treatment of invasive aspergillosis and candidemia. Caspofungin inhibits the synthesis of beta-glucan, which is an essential component of the fungal cell wall (Bowman et al. 2002). Anidulafungin is another echinocandin B derivative with an alkoxy triphenyl side chain at the site of a naturally occurring fatty acid side chain (Pfaller et al. 2008). Micafungin or FK463 is another echinocandin antifungal agent derived from FR901379 with a sulphate moiety instead of a hydroxyl group in dihydroxy homo tyrosine targets the synthesis of beta-glucan, leading to the weakening and rupture of the fungal cell wall including Candida spp. and Aspergillus spp. (Hashimoto 2009). The structural analogue of anidulafungin (Fig. 2), a comparatively recent addition to the echinocandin class, is rezafungin (CD101), which has a choline amine ether at the hemiaminal region of C5 ornithine (Garcia-Effron 2020) in phase III clinical trial (Zhao and Perlin 2020) is being conducted on the molecule to treat invasive fungal infections brought on by species of Candida, Aspergillus, and Pneumocystis. Table 4 enlists the clinical trial phases of a few of the lipopeptides with clinical potential for therapeutic applications in industries.
Methods of purification
Purification of the lipopeptide is the most challenging multi-step process in the whole bioprocess.It takes around 60% of the total cost of the product (Chen et al. 2015a, b) to typically isolate and concentrate the desired compounds from the complex mixture of microbial biomass, fermentation broth, and other components. Various methods can be employed to purify lipopeptides, depending on the specific characteristics of the target compound and the nature of the fermentation process. Purification of the antifungal compound(s) to almost homogeneity was necessary to carry out the molecular characterization as Bacillus sp. coproduced a variety of lipopeptide homologues and isoforms with varying fungicidal effects (Pathak et al. 2012; Pathak and Keharia 2014). Typically, organic solvents or ammonium sulphate precipitation were used to extract antimicrobial peptides from Bacillus sp. from cell-free supernatant (CFS) followed by chromatography techniques (Yakimov et al. 1995). Since lipopeptide becomes insoluble at low pH levels and precipitates out, the acid precipitation approach provides a practical and affordable procedure. Lowering the pH (~2.0) of the cell-free supernatant containing these lipopeptides leads to the neutralization of the negative charges of the lipopeptides, reducing their solubility in the aqueous medium and resulting in their precipitation (Biniarz et al. 2016). Lipopeptides can be dissolved in organic solvents using the organic solvent approach because their hydrophobic ends are water-insoluble (Santos et al. 2016). The organic solvent has the capacity to selectively dissolve the lipopeptides from the aqueous phase, facilitating their enrichment with these compounds and their separation or isolation. Typically, a common approach for purifying lipopeptides from cell-free supernatant was the acid precipitation using 6 N to 12N HCl, reported by many (Barale et al. 2022; Nanjundan et al. 2019; Ramachandran et al. 2018; Varjani and Upasani 2017). Here for the partial purification of lipopeptides, the cell-free supernatant is acidified to final pH 2.0 and kept overnight at 4 °C with gentle stirring, and the precipitate is allowed to be collected by centrifugation at high speed at a low temperature, and subsequently dissolved in phosphate buffer at pH 8.0. Antifungal substances were extracted using n-butanol in a 1:1 ratio, and then the butanol was evaporated at 55 °C. In another report, the resultant precipitate is neutralized and extracted with methanol, evaporated, and re-dissolved in 70% methanol (v/v) to obtain crude lipopeptide (Geissler et al. 2017; Gond et al. 2015). The crude extract is loaded on a silica gel column of varying mesh size (60-120/250-325/260-430) and eluted stepwise with a linear gradient of chloroform: methanol solvent system in varying ratios (Sharma et al. 2014). Antifungal substances of extracted fractions were evaporated at 55 °C. The dried antifungal substance was reconstituted in phosphate buffer. To evaluate the antifungal activity of the collected fractions subjected to the cut-well agar method against fungal species. In another report, the UV absorbance of all collected fractions was recorded at 280 nm. However, a different elution gradient using methanol: water followed by absolute methanol for fractionation has been attempted (Korenblum et al. 2012). In a separate study, fengycin was eluted with chloroform/methanol/ water/ethanol after silica gel-based adsorption chromatography (Deleu et al. 2008). In a different strategy, silica gel-purified crude fengycin mix was purified by using a mixture composed of chloroform, methanol, and an aqueous solution of 28% (v/v) ammonium hydroxide with increasing polarities (De Faria et al. 2011). The strategy of purification of lipopeptides has also witnessed some amount of modifications. To purify lipopeptides from the cell-free supernatant of Pseudomonas sp. OXDC12, a column-based approach was followed. The purification steps included the concentration of lipopeptides in the cell-free broth using a multi-step purification process that involved ammonium sulphate fractionation, dialysis, and successive chromatographic methods (thin layer chromatography, DEAE-ion-exchange chromatography, and size exclusion (Sephadex-25) chromatography) (Chauhan et al. 2022).
The individual homologues and isoforms of the lipopeptide mixtures may not always be easily separated using chromatography methods. To obtain high purity of the antifungal homologues and isoforms without losing their biological action, reversed-phase HPLC (RP-HPLC) in particular was employed extensively (Sivapathasekaran et al. 2009). To achieve maximum purification of the bioactive fractions, the use of RP-HPLC using a semi-preparative C18 column and acetonitrile: water gradient as a mobile phase has been reported. Pooled fractions were concentrated by speed vacuum, dissolved in an appropriate buffer and screened for antimicrobial activity. In our study, the partially purified samples were purified further through reversed-phase high-performance liquid chromatography (RP-HPLC) at the semi-preparative scale, employing a C18 column (10 mm × 250 mm, particle size 5 μm). The solvent system utilized consisted of water and acetonitrile containing 0.1% trifluoroacetic acid (TFA). Verification of HPLC fractions corresponding to respective peaks was achieved by confirming their m/z values through electrospray ionization liquid chromatography-mass spectrometry (ESI/LC/MS) (Ramachandran et al. 2018). These purified samples can be used for further analysis of functional characterization or structural characterization, for example, NMR, CD spectra, FTIR, and GC–MS. The entire purification processes have limitations as these lengthy and labour-intensive steps involve acid, solvents, and skilled manpower (Singh et al. 2022). The general purification scheme of peptides and lipopeptides and their functional characterization have been encapsulated in the graphical abstract (Fig. 3).
The purification techniques vary for individual cyclic lipopeptides that are used commercially such as the purification process for daptomycin and polymyxin differ. The daptomycin is synthesized by cultivating Streptomyces roseosporus under submerged aerobic fermentation conditions followed by filtration and then purified by Diaon HP-20 chromatography and reverse phase silica gel/C18 chromatography (Kelleher et al. 2015). The process of purifying crude polymyxin, chosen from the class that includes polymyxin B and E, entails dissolving the crude polymyxin as an acid adding salt in water to form a solution, adding an alkaline solution to bring the pH to 8.5–11.0, removing the precipitated polymyxin base from the solution, and then adding a chelating agent chosen from the class that includes ethylene diamine–tetra-acetic acid, N,N′-dihydroxyethylglycine, and N-carboxymethyl-N-2-hydroxyethyl-N,N′-ethylenediglycine to the polymyxin base that has precipitated out of the solution (Weddle 1968).
Mode of action
At the molecular level, the antimicrobial activity of lipopeptide involves several key mechanisms. Lipopeptides exhibit antifungal activity through various mechanisms. Their amphiphilic nature allows for insertion into membranes, leading to loss of membrane structure and function which may lead to cell content leakage and loss of ion gradients. The lipid component of lipopeptides can alter the membrane fluidity compromising membrane integrity and cell function (Buda De Cesare et al. 2020). They can also weaken the fungal cell walls by inhibiting the enzymes involved in the cell wall assembly and disrupting components such as β-glucans and chitin (Ma et al. 2020; Tsai et al. 2011). Some lipopeptides cause oxidative damage to fungal cells by increasing the reactive oxygen species levels which can damage cellular components like the membranes and DNA (Li et al. 2015; Mello et al. 2011; Wang et al. 2015). Antifungal lipopeptides such as fengycin, plipastatin, and iturins can alter the cell membrane structure and permeability because they directly interact with the lipid bilayers and sterol molecules (Fan et al. 2017). Several lipopeptides may also interfere with the signaling pathways essential for normal cell function. Another unique mechanism is the ability of lipopeptides to trigger apoptotic pathways leading to cell death (Andrés et al. 2008; Redza-Dutordoir and Averill-Bates 2016).
Lipopeptides have a high affinity for cell membranes due to their amphiphilic nature (Epand 1997), and also is governed by several factors (Munusamy et al. 2020) allowing them to interact with hydrophilic and hydrophobic components of the membrane (Fig. 4). Upon contact with the microbial cell membrane, lipopeptides insert themselves into the lipid bilayer, leading to the formation of ion channels or pores (Schneider et al. 2014). Lipopeptides exhibit a preference for interacting with these specific lipids present in fungal or bacterial cell membranes (Mohid et al. 2022). The electrostatic attraction between the positively charged lipopeptides and the negatively charged fungal membrane can lead to strong interactions, disrupting membrane integrity (Juhaniewicz-Dębińska et al. 2020). The hydrophobic tail can anchor the lipopeptide into the fungal membrane, promoting membrane disruption. The balance between hydrophobic and hydrophilic properties in lipopeptides is essential for effective membrane interaction (Makovitzki et al. 2006; Malina and Shai 2005).
Additionally, certain amino acid residues may have a higher affinity for fungal membranes. The amphiphilic nature of many lipopeptides (having both hydrophobic and hydrophilic regions) is advantageous for membrane disruption (Hutchinson et al. 2017). Environmental conditions, such as pH, temperature, and ionic strength, can also impact the behaviour of lipopeptides in fungal membranes. The pH of the surrounding environment can also influence the protonation state of the cationic groups in lipopeptides, affecting their electrostatic interactions with fungal membranes (Sikora et al. 2020).
This perturbation interferes with essential cellular processes, including nutrient uptake, energy production, and cell signaling, ultimately leading to the inhibition of microbial growth and proliferation. Disruption of the membrane's structural integrity results in the leakage of cellular contents, and loss of membrane potential, ultimately leading to the destabilization of the cell and cell lysis.
Oppenheimer-Shaanan et al., (2013) postulated that small molecules like biosurfactants can be used as natural agents for dispersion and disassembly of biofilm by acting as cell envelope-modifying or anti-matrix molecules. Since hydrophobic interaction allows microbial colonization of substrata by eliminating the presence of water molecules between cells and interacting solid substratum, Rodrigues and Teixeira (2010) opined that biosurfactant treatment can reduce hydrophobic interactions between bacterial cell and solid surfaces, and consequently cell adhesion Rodrigues and Teixeira (2010). Iturin has been found to inhibit the formation of biofilms, which are communities of microorganisms encased within a self-produced extracellular matrix, by interacting with membrane-bound proteins and enzymes, affecting their conformation and function (Lei et al. 2019). This interaction can disrupt vital cellular processes and metabolic pathways. By disrupting the adhesion of microorganisms to surfaces and interfering with biofilm matrix formation, it also prevents the establishment and persistence of biofilm-related infections. Fengycin can alter the fluidity and stability of the fungal cell membrane (Liu et al. 2019). It also inhibits biofilm formation, a crucial mechanism for the persistence and virulence of various fungi. By interfering with the adhesion of fungal cells to surfaces and inhibiting biofilm matrix formation, fengycin prevents the establishment and growth of biofilm-related infections. Fengycin exhibits selective targeting of fungal cells while demonstrating relatively lower toxicity to mammalian cells. This selectivity enhances its potential for the development of novel antifungal agents with reduced harm to human cells, highlighting its significance in the treatment of fungal infections (Sur and Grossfield 2022). The potency of diverse types of lipopeptide homologues against a particular organism has been primarily influenced by its amino acid composition, and length of fatty acid chain (Nasir and Besson 2012; Ongena and Jacques 2008). The attachment of the acyl chains to peptides also promotes their aggregation (Makovitzki et al. 2008), rendering these lipopeptides excellent candidates for oligomerization. Coarse-grained molecular dynamics (MD) simulations of monomeric and oligomerized fengycins in various lipid bilayers suggested that whilst they bind to all membranes with their acyl chains inserted into the hydrophobic core (Horn et al. 2013), membrane composition can affect, for example, fengycin’s potential to aggregate in the membrane. It has been hypothesized that the presence of cholesterol rather than ergosterol in mammalian membranes increases the orderliness to guard mammalian membranes, though the mechanism is not yet fully understood and subject to future in-depth simulation studies (Fiedler and Heerklotz 2015). The coarse-grained MD simulation study also demonstrated that lipopeptide oligomer's binding to cytomembranes needs to overcome a significant free energy barrier that varies with membrane composition (Lin and Grossfield 2015) especially in mammalian cell membranes as compared to bacterial or fungal cell membranes. Makovitzki and Shai (2005) while delineating the membranolytic mode of action of lipopeptides found that almost all the lipopeptides in their study were non-hemolytic at respective MICs. Their observations were corroborated in our investigations by the AF3 and AF4 lipopeptides that were found nonhemolytic and minimally cytotoxic at respective MICs (Ramachandran et al. 2018; Ramesh et al. 2023a, b). Daptomycin binds to the cytoplasmic membrane of Gram-positive bacteria, causing a rapid depolarization of the membrane potential (Silverman et al. 2003). This depolarization leads to the formation of transmembrane ion channels, resulting in the efflux of potassium ions and the inhibition of essential cellular processes, ultimately leading to cell death. Daptomycin's mechanism of action is calcium-dependent, as it requires the presence of calcium ions for its binding to the bacterial cell membrane (Jung et al. 2004). This specific interaction with calcium facilitates its insertion into the lipid bilayer, leading to pore formation and membrane disruption, inducing the permeabilization of the bacterial cell membrane, causing the leakage of intracellular contents, and the disruption of membrane integrity. This permeabilization leads to the prevention of the synthesis of DNA, RNA, and protein that leads to bacterial cell death.
Very recently, we have demonstrated the broad spectrum of antifungal potential of the three promising lipopeptides AF3, AF4, and AF5 purified to homogeneity from the B. subtilis RLID 12.1 cell-free supernatant, acid precipitation, solvent extraction, and reversed-phase HPLC. Out of these AF3 and AF4 are isomers and three lipopeptides (AF3, AF4, and AF5) had the m/z ratios of (AF3/AF4) 1071.4 and AF5 1085.6 determined by LC–MS and constituted of seven amino acid residues (Ramachandran et al. 2018; Ramachandran et al. 2018; Ramesh et al. 2023a). The lipopeptides AF3 and AF4 demonstrated antifungal activities against 115-plus clinical isolates and strains of Candida albicans, non-albicans Candida (including Candida glabrata, Candida tropicalis and Candida cauris), Cryptococcus neoformans, Cryptococcus gattii, and mycelial isolates including Aspergillus niger, Aspergillus fumigatus, Scedosporium sp. Sporothrix sp., Bipolaris, and others (Ramachandran et al. 2018; Ramachandran et al. 2020; Ramesh et al. 2023a). The AF4 lipopeptide demonstrated antifungal activities against 20 dermatophytes all of the clinical origins (Ramesh et al. 2023a); some of these were terbinafine-resistant as well. The encouraging fact is that the minimum inhibitory concentrations (MICs) for all these lipopeptides against such a wide variety of fungal strains ranged between 2 and 8 µg/ml; the ratio of MICs and minimum fungicidal concentrations (MFCs) never exceeded 1:2.
The mainstays for treating systemic fungal infections are currently amphotericin B (AMB) and 5-flucytosine (5-FC); the long-term use of these antifungals causes nephrotoxicity of AMB and resistance to 5-FC is a serious concern. This is because Candida tropicalis has remained a major concern for candidiasis in India and Candida auris has emerged as resistant yeast causing difficult-to-treat infections (Ray et al. 2022). To reinforce the arsenal's pipeline, antifungal lead compounds with minimal side effects must be developed immediately. The antifungal potentials of AF3 and AF4 lipopeptides as well as their mode of action were determined by a variety of methods, including binding assays with fluorescent probes, scanning electron microscopy (Fig. 5), transmission electron microscopy, other fluorescence-based assays, and flow cytometry.
Scanning electron micrographs exhibiting ultrastructure alterations of the cells with a MIC of 8 mg/L. (i) C. auris IL-3331 cells a untreated, b treated (ii) C. tropicalis ATCC 750 cells, a untreated, b treated (iii) C. albicans SC5314 a untreated with, b treated. The scale bar is 5 μm. (Unpublished data from BITS Pilani KK Birla Goa Campus)
With the membrane probe di-8-ANEPPS, the effects of the lipopeptides AF3 and AF4 on plasma membrane dipole potential have been studied. The di-8-ANEPPS, a potentiometric probe, is utilized to observe membrane depolarization by being integrated into the outer leaflet of plasma membranes (Hollmann et al. 2013). When the peptides bind by adsorption or insertion into the membrane, dipole potential in cells labeled with di-8-ANEPPS is predicted to decrease (Hollmann et al. 2013). With increasing concentration of lipopeptide AF4 and AF3, we found in our work that the normalized ratio of fluorescence intensities in lipopeptide-treated samples decreased (Ramesh et al. 2023a). The increasing concentration of lipopeptides correlates with the decrease in membrane potentials. Our results unambiguously proved the binding of the lipopeptides to the cell surface because we found that the lipopeptide-plasma membrane interaction resulted in a greater reduction in initial dipole potential with high initial dipole potential. These results corresponded with the observation and inference made in a study of peptide-bio membrane interaction using dipole potential (Matos et al. 2008).
The SEM images in (Fig. 5) indicate morphological changes of three different Candida spp. The experimental shreds of evidence that include indicate a multipronged antifungal action of the lipopeptide AF4. Scanning (Fig. 5) and transmission electron microscopy images of the lipopeptide-treated cells revealed severe cell surface damage and cytoplasmic organization disruption respectively (Ramesh et al. 2023a). The lipopeptides AF3 and AF4 mainly target the fungal cell membrane, perhaps by binding the ergosterol present in the plasma membrane to cause severe membrane perturbations and permeabilization. Using acridine orange (AO)/propidium iodide (PI)-based and FUN-1-based confocal microscopy and PI-based flow cytometry followed by the plate (viability assessment) assays, the permeabilizing and cidal effect of lipopeptides on C. albicans, C. tropicalis, C. auris, and C. glabrata membranes and cells were established (Ramesh et al. 2023a).
According to membrane dipole potential and ergosterol binding assays, lipopeptide interaction, by means of adsorption or insertion, can modify the structural properties of the cell membranes (Hollmann et al. 2013). These changes in the physical or chemical characteristics of the membrane can lead to membrane stress, which can cause reactive oxygen species (ROS) to be generated and accumulate inside the cells (Wang et al. 2022).
These findings were further supported by flow cytometry (FC), which demonstrated increased production of reactive oxygen species (ROS) and loss of membrane integrity in cells treated with AF4 from the increased uptake of 2′–7′ dichloro-dihydro-fluorescein diacetate (DCFH-DA) and propidium iodide (Ramesh et al. 2023a) The ergosterol binding assay and a 1,6-diphenyl-1,3,5-hexatriene (DPH) fluorescence investigation, revealed membrane disruption and the interaction with ergosterol. Increased fluorescence was seen in DAPI-stained yeast cells treated with lipopeptides AF3 and AF4 at varying dosages. The cells were observed to have damaged aberrant nuclei, showing teardrop-shaped, tube-like, or distorted atypical morphology (Ramesh et al. 2023a). According to several studies, (Ludovico et al. 2001; Hao et al. 2013; Jia et al. 2019), DNA damage is typically thought to be an indicator of late apoptosis. Thus, the investigational lipopeptide used target plasma membranes to exert its antifungal action. This resulted in membrane permeabilization and depolarization, endogenous ROS accumulation that led to mitochondrial dysfunction, and damage to nuclear DNA. The studies on the investigational lipopeptides AF3 and AF4 at our laboratory re-establish the battery of previous observations that lipopeptides have the attributes, potentials and prospects for multidirectional uses for the benefit of mankind.
Applications
From the perspective of biological control of fungal-caused plant diseases, putative antagonistic effects of plipastatins (fengycins), iturins, and surfactins—three families of lipopeptides against different phytopathogens were investigated (Bonmatin et al. 2003; Ongena and Jacques 2008). These benefits render lipopeptides more and more attractive and important in fields including pharmacology, agriculture (cost-effective extensive antifungal and antibacterial agents), and food preservation. The typical lipopeptide such as iturin A was proposed to be used as animal feed for decreasing mortality and enhancing immunity, as biopesticides and sprayed on the leaves to control phytopathogens (Jin et al. 2015). When treated with synthetic short antifungal lipopeptides that consist of L- and D- amino acids attached to an aliphatic acid in varying lengths, induce membrane disruption mechanisms and lysis of the pathogens. It also explains that varying the length of the aliphatic acid the activity differs on the plant pathogens. It was discovered that the phytopathogens Botrytis cinerea, which infects cucumber fruits and leaves, and Cochliobolus heterostrophus, which infects corn leaves cause less damage and increase plant protection. Makovitzki et al. (2007), Yan et al., (2020) demonstrated the potential of iturin A purified from B. amyloliquifaciens MG3 can prevent the spore germination and mycelial growth of Colletotrichum gloeosporioides in loquat fruits. It claimed the post-harvesting potential of such iturin A. Additionally, lipopeptides are applied to preserve fruits after harvest, such as oranges or lemons (Arrebola et al. 2010), apples (Arroyave-Toro et al. 2017), avocados (Guardado-Valdivia et al. 2018), and litchis (Jiang et al. 2001). CLPs, being a specialized type of lipopeptide, have shown promise in a variety of therapeutic applications. Extensively researched for their antimicrobial (Mba and Nweze 2022), antifungal (Baindara and Korpole 2016), antiviral (Chowdhury et al. 2021), anticancer (Wu et al. 2017), immunomodulatory (Sajid et al. 2020), biofilm disruption (Englerová et al. 2021), and neuroprotective properties (Yaraguppi et al. 2023), these compounds have significant potential for improving human health. Lipopeptides, a type of drug, have potential therapeutic applications in antimicrobial and anticancer treatments. However, they face several challenges, including low bioavailability due to their lipophilic nature, which can affect their absorption and distribution. They may also have a broad range of cell membranes, making it difficult to achieve high specificity and selectivity. Lipopeptides may also be immunogenic, causing antibodies against them, and reducing their efficacy over time (Hamley 2021). Several surfactins and iturinic compounds were also found to exhibit cytotoxic effects on mammalian cells at high concentrations, potentially leading to off-target effects and toxicity in host cells (Rodríguez-López et al. 2020). The purification and synthesis of lipopeptides can be complex and costly, hindering large-scale production and commercial viability (Singh et al. 2022; Jha et al. 2016). Resistance development from bacteria can limit their long-term effectiveness in treating infections. Storage stability is also a concern, as maintaining the integrity and efficacy of lipopeptides is crucial for practical use. Regulatory hurdles may also pose challenges in the long-term approval process.
Conclusion and the way forward
The discovery and advancement of drug delivery mechanisms and the application of novel combinations of drugs provide essential areas for research when it comes to treating fungal diseases. Certain structural homologues in each family appear to be more active than others. Thus, some Bacillus strains' greater efficacy in lowering infectious illnesses than others can be explained by the range of lipopeptides that are naturally produced. The distribution of microbial lipopeptides has been limited because of its higher production cost, complexity of purification and low yield. These problems can be addressed by using appropriate statistical methods, renewable substrates and alternative purification strategies involving sustainable chemicals and genetic engineering tools directed towards a manifold increased yield of lipopeptides (Cai et al. 2014; Ebadipour et al. 2016; X. Zhao and Kuipers 2016). Therefore, future studies should be more focused on screening antifungal-producing microorganisms with higher activity and low toxicity as well as maximizing the production and purification of novel antimicrobials including antifungal lipopeptides. It would be essential to identify the structure–activity correlations of the antifungal peptides, and identify methods to synthetically produce them. These lipopeptides that exist naturally can have their molecular structures modified by genetic engineering methods and bioinformatics, resulting in more stable, minimally cytotoxic and pharmacodynamically and pharmacokinetically effective leads.
Data availability
Data will be made available upon reason request from the corresponding author.
References
Anckaert A, Arias AA, Hoff G, Calonne-Salmon M, Declerck S, Ongena M (2021) The use of Bacillus spp. as bacterial biocontrol agents to control plant diseases. pp 247–300 Doi: https://doi.org/10.19103/as.2021.0093.10
Andrés MT, Viejo-Díaz M, Fierro JF (2008) Human lactoferrin induces apoptosis-like cell death in Candida albicans: critical role of K + -channel-mediated K + efflux. Antimicrob Agents Chemother 52(11):4081–4088. https://doi.org/10.1128/AAC.01597-07
Aranda FJ, Teruel JA, Ortiz A (2005) Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim Biophys Acta (BBA) 1713(1):51–56. https://doi.org/10.1016/j.bbamem.2005.05.003
Arima K, Kakinuma A, Tamura G (1968) Surfactin, a crystalline peptide lipid 509 surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin 510 clot formation. Biochem Biophys Res Commun 31(3):488–494
Arrebola E, Sivakumar D, Korsten L (2010) Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol Control 53(1):122–128. https://doi.org/10.1016/j.biocontrol.2009.11.010
Arroyave-Toro JJ, Mosquera S, Villegas-Escobar V (2017) Biocontrol activity of Bacillus subtilis EA-CB0015 cells and lipopeptides against postharvest fungal pathogens. Biol Control 114:195–200. https://doi.org/10.1016/j.biocontrol.2017.08.014
Bahar AA, Ren D (2013) Antimicrobial peptides. Pharmaceuticals 6(12):1543–1575. https://doi.org/10.3390/ph6121543
Baindara P, Korpole S (2016) Lipopeptides: Status and strategies to control fungal infection. In: Recent trends in antifungal agents and antifungal therapy. pp 97–121. Springer India. Doi:https://doi.org/10.1007/978-81-322-2782-3_4
Balkovec JM, Hughes DL, Masurekar PS, Sable CA, Schwartz RE, Singh SB (2014) Discovery and development of first in class antifungal caspofungin (CANCIDAS®): A case study. Nat Prod Rep 31(1):15–34. https://doi.org/10.1039/c3np70070d
Barale SS, Ghane SG, Sonawane KD (2022) Purification and characterization of antibacterial surfactin isoforms produced by Bacillus velezensis SK. AMB Express 12(1):89. https://doi.org/10.1186/s13568-022-01348-3
Benedict RG, Langlykke AF (1947) Antibiotic activity of Bacillus polymyxa. J Bacteriol 54(1):24
Benz VF (1974) Echinocandin B, ein neuartiges Polipeptid-Antibiotikum aus Aspergillus nidulans var. echinatus: Isolierung und Bausteine. Heiv Chim Acta 57:2459–2477. https://doi.org/10.1002/hlca.19740570818
Biniarz P, Łukaszewicz M, Janek T (2016) Screening concepts, characterization and structural analysis of microbial-derived bioactive lipopeptides: a review. Critical Rev Biotechnol 37(3):393–410. https://doi.org/10.3109/07388551.2016.1163324
Bionda N, Pitteloud JP, Cudic P (2013) Cyclic lipodepsipeptides: a new class of antibacterial agents in the battle against resistant bacteria. Fut Med Chem 5(11):1121–1330. https://doi.org/10.4155/fmc.13.86
Bonmatin J-M, Laprévote O, Peypoux F (2003) Diversity among microbial cyclic lipopeptides: iturins and surfactins activity-structure relationships to design new bioactive agents. Combin Chem High Throughput Screen 6(6):541–556
Bowman JC, Hicks PS, Kurtz MB, Rosen H, Schmatz DM, Liberator PA, Douglas CM (2002) The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother 46(9):3001–3012. https://doi.org/10.1128/AAC.46.9.3001-3012.2002
Buda De Cesare G, Cristy SA, Garsin DA, Lorenz MC (2020) Antimicrobial peptides: a new frontier in antifungal therapy. Mbio 11(6):10–1128. https://doi.org/10.1128/mBio.02123-20
Cai Q, Zhang B, Chen B, Zhu Z, Lin W, Cao T (2014) Screening of biosurfactant producers from petroleum hydrocarbon contaminated sources in cold marine environments. Mar Pollut Bull 86(1–2):402–410. https://doi.org/10.1016/j.marpolbul.2014.06.039
Canepari P, Boaretti M, Lleo MM, Satta G (1990) Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin (LY146032). Antimicrob Agents Chemother 34(6):1220–1226. https://doi.org/10.1128/aac.34.6.1220
Cappelletty D, Eiselstein-McKitrick K (2007) The echinocandins. Pharmacother 27(3):369–388. https://doi.org/10.1592/phco.27.3.369
Carolin CF, Kumar PS, Ngueagni PT (2021) A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.124827
Chander CS, Lohitnath T, Kumar DM, Kalaichelvan PT (2012) Production and characterization of biosurfactant from Bacillus subtilis MTCC441 and its evaluation to use as bioemulsifier for food bio-preservative. Adv Appl Sci Res 3:1827–1831
Chauhan V, Dhiman VK, Kanwar SS (2022) Purification and characterization of a novel bacterial Lipopeptide(s) biosurfactant and determining its antimicrobial and cytotoxic properties. Process Biochem 120:114–125. https://doi.org/10.1016/j.procbio.2022.06.005
Chen L, Yue Q, Li Y, Niu X, Xiang M, Wang W, Bills GF, Liu X, An Z (2015a) Engineering of Glarea lozoyensis for exclusive production of the pneumocandin B0 precursor of the antifungal drug caspofungin acetate. Appl Environ Microbiol 81(5):1550–1558. https://doi.org/10.1128/AEM.03256-14
Chen WC, Juang RS, Wei YH (2015b) Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms. Biochem Eng J 103:158–169. https://doi.org/10.1016/j.bej.2015.07.009
Chen X, Lu Y, Shan M, Zhao H, Lu Z, Lu Y (2022) A mini-review: mechanism of antimicrobial action and application of surfactin. World J Microbiol Biotechnol 38(8):143. https://doi.org/10.1007/s11274-022-03323-3
Chowdhury T, Baindara P, Mandal SM (2021) LPD-12: a promising lipopeptide to control COVID-19. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.106218
Chtioui O, Dimitrov K, Gancel F, Nikov I (2010) Biosurfactants production by immobilized cells of Bacillus subtilis ATCC 21332 and their recovery by pertraction. Process Biochem 45(11):1795–1799. https://doi.org/10.1016/j.procbio.2010.05.012
Colson AR, Morton A, Årdal C, Chalkidou K, Davies SC, Garrison LP, Xiao Y (2021) Antimicrobial resistance: is health technology assessment part of the solution or part of the problem? Value in Health 24(12):1828–1834. https://doi.org/10.1016/j.jval.2021.06.002
De Faria AF, Stéfani D, Vaz BG, Silva ÍS, Garcia JS, Eberlin MN, Grossman MJ, Alves OL, Durrant LR (2011) Purification and structural characterization of fengycin homologues produced by Bacillus subtilis LSFM-05 grown on raw glycerol. J Ind Microbiol Biotechnol 38(7):863–871. https://doi.org/10.1007/s10295-011-0980-1
De Lucca AJ, Walsh TJ (1999) Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother 43(1):1–11. https://doi.org/10.1128/aac.43.1.1
De Oliveira DWF, Cara AB, Lechuga-Villena M, García-Román M, Melo VMM, Gonçalves LRB, Vaz DA (2017) Aquatic toxicity and biodegradability of a surfactant produced by Bacillus subtilis ICA56. J Environ Sci Health Part A 52(2):174–181. https://doi.org/10.1080/10934529.2016.1240491
De Vuyst L, Vandamme EJ (1992) Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. J Gen Microbiol 138(3):571–578. https://doi.org/10.1099/00221287-138-3-571
De Zoysa GH, Cameron AJ, Hegde VV, Raghothama S, Sarojini V (2015) Antimicrobial peptides with potential for biofilm eradication: synthesis and structure activity relationship studies of battacin peptides. J Med Chem 58(2):625–639. https://doi.org/10.1021/jm501084q
Deleu M, Paquot M, Nylander T (2008) Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes. Biophys J 94(7):2667–2679. https://doi.org/10.1529/biophysj.107.114090
Dijksteel GS, Ulrich MM, Middelkoop E, Boekema BK (2021) Lessons learned from clinical trials using antimicrobial peptides (AMPs). Front Microbiol 12:616979. https://doi.org/10.3389/fmicb.2021.616979
Dubos RJ (1939) Studies on a bactericidal agent extracted from a soil bacillus: i preparation of the agent: its activity in vitro. J Exp Med 70(1):1
Ebadipour N, Lotfabad TB, Yaghmaei S, RoostAazad R (2016) Optimization of low-cost biosurfactant production from agricultural residues through response surface methodology. Prep Biochem Biotechnol 46(1):30–38. https://doi.org/10.1080/10826068.2014.979204
Ehlert F, Neu HC (1987) In vitro activity of LY146032 (daptomycin), a new peptolide. Eur J Clin Microbiol 6:84–90. https://doi.org/10.1007/BF02097208
Englerová K, Bedlovičová Z, Nemcová R, Király J, Maďar M, Hajdučková V, Reiffová K (2021) Bacillus amyloliquefaciens—derived lipopeptide biosurfactants inhibit biofilm formation and expression of biofilm-related genes of Staphylococcus aureus. Antibiotics 10(10):1252. https://doi.org/10.3390/antibiotics10101252
Epand RM (1997) Biophysical studies of lipopeptide-membrane interactions. Pept Sci 43(1):15–24. https://doi.org/10.1002/(SICI)1097-0282(1997)43:1%3c15::AID-BIP3%3e3.0.CO;2-3
Fan H, Ru J, Zhang Y, Wang Q, Li Y (2017) Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol Res 199:89–97. https://doi.org/10.1016/j.micres.2017.03.004
Fechtner J, Koza A, Sterpaio PD, Hapca SM, Spiers AJ (2011) Surfactants expressed by soil pseudomonads alter local soil–water distribution, suggesting a hydrological role for these compounds. FEMS Microbiol Ecol 78(1):50–58. https://doi.org/10.1111/j.1574-6941.2011.01141.x
Fernández de Ullivarri M, Arbulu S, Garcia-Gutierrez E, Cotter PD (2020) Antifungal peptides as therapeutic agents. Front Cell Infect Microbiol 10:105. https://doi.org/10.3389/fcimb.2020.00105
Fiedler S, Heerklotz H (2015) Vesicle leakage reflects the target selectivity of antimicrobial lipopeptides from Bacillus subtilis. Biophys J 109(10):2079–2089. https://doi.org/10.1016/j.bpj.2015.09.021
Gálvez A, Abriouel H, López RL, Omar NB (2011) Biological control of pathogens and post-processing spoilage microorganisms in fresh and processed fruit and vegetables. In: Protective cultures, antimicrobial metabolites and bacteriophages for food and beverage biopreservation. pp 403–432. Woodhead Publishing. https://doi.org/10.1533/9780857090522.3.403
Gálvez A, Maqueda M, Martínez-Bueno M, Valdivia E (1989) Bactericidal and bacteriolytic action of peptide antibiotic AS-48 against Gram-positive and Gram-negative bacteria and other organisms. Res Microbiol 140(1):57–68. https://doi.org/10.1016/0923-2508(89)90060-0
Ganesan NG, Rangarajan V (2021) A kinetics study on surfactin production from Bacillus subtilis MTCC 2415 for application in green cosmetics. Biocatal Agric Biotechnol 33:102001. https://doi.org/10.1016/j.bcab.2021.102001
Ganesan NG, Singh RD, Rangarajan V (2023) Optimization of processing parameters for the improved production of surfactin from B. subtilis using a statistical approach and demonstration of its imminent potential in cosmetic applications. Mater Today 76:70–80. https://doi.org/10.1016/j.matpr.2022.09.042
Garcia-Effron G (2020) Rezafungin—mechanisms of action, susceptibility and resistance: similarities and differences with the other echinocandins. J Fungi 6(4):262. https://doi.org/10.3390/jof6040262
Gayathiri E, Prakash P, Karmegam N, Varjani S, Awasthi MK, Ravindran B (2022) Biosurfactants: potential and eco-friendly material for sustainable agriculture and environmental safety—a review. Agronomy 12(3):662. https://doi.org/10.3390/agronomy12030662
Geissler M, Oellig C, Moss K, Schwack W, Henkel M, Hausmann R (2017) High-performance thin-layer chromatography (HPTLC) for the simultaneous quantification of the cyclic lipopeptides Surfactin, Iturin A and Fengycin in culture samples of Bacillus species. J Chromatogr B 1044:214–224. https://doi.org/10.1016/j.jchromb.2016.11.013
Ghazala I, Charfeddine S, Charfeddine M, Gargouri-Bouzid R, Ellouz-Chaabouni S, Haddar A (2022) Antimicrobial and antioxidant activities of Bacillus mojavensis I4 lipopeptides and their potential application against the potato dry rot causative Fusarium solani. Arch Microbiol 204(8):484. https://doi.org/10.1007/s00203-022-03098-z
Gond SK, Bergen MS, Torres MS, White JF (2015) Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol Res 172:79–87. https://doi.org/10.1016/j.micres.2014.11.004
Guardado-Valdivia L, Tovar-Pérez E, Chacón-López A, López-García U, Gutiérrez-Martínez P, Stoll A, Aguilera S (2018) Identification and characterization of a new Bacillus atrophaeus strain B5 as biocontrol agent of postharvest anthracnose disease in soursop (Annona muricata) and avocado (Persea americana). Microbiol Res 210:26–32. https://doi.org/10.1016/j.micres.2018.01.007
Hamley IW (2021) Lipopeptides for vaccine development. Bioconjug Chem 32(8):1472–1490. https://doi.org/10.1021/acs.bioconjchem.1c00258
Hancock RE, Chapple DS (1999) Peptide antibiotics. Antimicrob Agents Chemother 43(6):1317–1323. https://doi.org/10.1128/aac.43.6.1317
Hao B, Cheng S, Clancy CJ, Nguyen MH (2013) Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob Agents Chemother 57(1):326–332. https://doi.org/10.1128/AAC.01366-12
Hashimoto S (2009) Micafungin: a sulfated echinocandin. J Antibiot 62(1):27–35. https://doi.org/10.1038/ja.2008.3
Hawser S, Borgonovi, M, Markus A, Isert D (1999) Mulundocandm, an echinocandin-like lipopeptide antifungal agent: biological activities in vitro. J Antibiot 52(3):305–310. https://doi.org/10.7164/antibiotics.52.305
Heinemann B, RD M, IR H (1953) Amphomycin, a new antibiotic. Antibiot Chemother (northfield, Ill) 3(12):1239–1242
Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, Jenks JD (2021) The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81:1703–1729. https://doi.org/10.1007/s40265-021-01611-0
Hoffmann M, Mück D, Grossmann L, Greiner L, Klausmann P, Henkel M, Hausmann R (2021) Surfactin from Bacillus subtilis displays promising characteristics as O/W-emulsifier for food formulations. Colloids Surf B 203:111749. https://doi.org/10.1016/j.colsurfb.2021.111749
Hollmann A, Matos PM, Augusto MT, Castanho MA, Santos NC (2013) Conjugation of cholesterol to HIV-1 fusion inhibitor C34 increases peptide-membrane interactions potentiating its action. PLoS ONE 8(4):e60302. https://doi.org/10.1371/journal.pone.0060302
Horn JN, Romo TD, Grossfield A (2013) Simulating the mechanism of antimicrobial lipopeptides with all-atom molecular dynamics. Biochemistry 52(33):5604–5610. https://doi.org/10.1021/bi400773q
Hutchinson JA, Burholt S, Hamley IW (2017) Peptide hormones and lipopeptides: from self-assembly to therapeutic applications. J Pept Sci 23(2):82–94. https://doi.org/10.1002/psc.2954
Hüttel W (2021) Echinocandins: structural diversity, biosynthesis, and development of antimycotics. Appl Microbiol Biotechnol 105:55–66. https://doi.org/10.1007/s00253-020-11022-y
Janek T, Łukaszewicz M, Krasowska A (2012) Antiadhesive activity of the biosurfactant pseudofactin II secreted by the Arctic bacterium Pseudomonas fluorescens BD5. BMC Microbiol. https://doi.org/10.1186/1471-2180-12-24
Janek T, Łukaszewicz M, Rezanka T, Krasowska A (2010) Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Biores Technol 101(15):6118–6123. https://doi.org/10.1016/j.biortech.2010.02.109
Jha SS, Joshi SJ, SJ G (2016) Lipopeptide production by Bacillus subtilis R1 and its possible applications. Braz J Microbiol 47:955–964. https://doi.org/10.1016/j.bjm.2016.07.006
Jia C, Zhang J, Yu L, Wang C, Yang Y, Rong X, Xu K, Chu M (2019) Antifungal activity of coumarin against Candida albicans is related to apoptosis. Front Cell Infect Microbiol 8:445. https://doi.org/10.3389/fcimb.2018.00445
Jiang YM, Zhu XR, Li YB (2001) Postharvest control of litchi fruit rot by Bacillus subtilis. LWT- Food Sci Technol 34(7):430–436. https://doi.org/10.1006/fstl.2001.0758
Jin H, Li K, Niu Y, Guo M, Hu C, Chen S, Huang F (2015) Continuous enhancement of iturin A production by Bacillus subtilis with a stepwise two-stage glucose feeding strategy. BMC Biotechnol. https://doi.org/10.1186/s12896-015-0172-6
Jin P, Wang H, Tan Z, Xuan Z, Dahar GY, Li QX, Liu W (2020) Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pesticide Biochem Physiol 163:102–107. https://doi.org/10.1016/j.pestbp.2019.11.004
Juhaniewicz-Dębińska J, Lasek R, Tymecka D, Burdach K, Bartosik D, Sęk S (2020) Physicochemical and biological characterization of novel membrane-active cationic lipopeptides with antimicrobial properties. Langmuir 36(43):12900–12910. https://doi.org/10.1021/acs.langmuir.0c02135
Jung D, Rozek A, Okon M, Hancock RE (2004) Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem Biol 11(7):949–957. https://doi.org/10.1016/j.chembiol.2004.04.020
Kakinuma A, Sugino H, Isono M, Tamura G, Arima K (1969) Determination of fatty acid in surfactin and elucidation of the total structure of surfactin. Agric Biol Chem 33(6):973–976. https://doi.org/10.1080/00021369.1969.10859409
Karlapudi AP, Venkateswarulu TC, Tammineedi J, Kanumuri L, Ravuru BK, Ramu Dirisala V, Kodali VP (2018) Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 4(3):241–249. https://doi.org/10.1016/j.petlm.2018.03.007
Kelleher TJ, Lai J-J, Decourcey JP, Zenoni M, Tagliani AR (2015) Process for the purification of daptomycin. Eur Patent Appl Bull 5:89
Khelissa S, Chihib NE, Gharsallaoui A (2021) Conditions of nisin production by Lactococcus lactis subsp. lactis and its main uses as a food preservative. Arch Microbiol 203:465–480. https://doi.org/10.1007/s00203-020-02054-z
Korenblum E, de Araujo LV, Guimarães CR, De Souza LM, Sassaki G, Abreu F, Seldin L (2012) Purification and characterization of a surfactin-like molecule produced by Bacillus sp H2O–1 and its antagonistic effect against sulfate reducing bacteria. BMC Microbiol 12(1):1–13. https://doi.org/10.1186/1471-2180-12-252
Lamoth F (2023) Novel therapeutic approaches to invasive candidiasis: considerations for the clinician. Infect Drug Resist 1087–1097. https://doi.org/10.2147/IDR.S375625
Latoud C, Peypoux F, Michel G, Genet R, Morgat JL (1986) Interactions of antibiotics of the iturin group with human erythrocytes. Biochim Biophys Acta (BBA) 856(3):526–535. https://doi.org/10.1016/0005-2736(86)90144-6
Lei S, Zhao H, Pang B, Qu R, Lian Z, Jiang C, Shao D, Huang Q, Jin M, Shi J (2019) Capability of iturin from Bacillus subtilis to inhibit Candida albicans in vitro and in vivo. Appl Microbiol Biotechnol 103(11):4377–4392. https://doi.org/10.1007/s00253-019-09805-z
Li G, Liu B, Shang Y, Yu Z, Zhang R (2012) Novel activity evaluation and subsequent partial purification of antimicrobial peptides produced by Bacillus subtilis LFB112. Ann Microbiol 62(2):667–674. https://doi.org/10.1007/s13213-011-0303-9
Li Y, Chang W, Zhang M, Li X, Jiao Y, Lou H (2015) Diorcinol D exerts fungicidal action against Candida albicans through cytoplasm membrane destruction and ROS accumulation. PLoS ONE 10(6):e0128693. https://doi.org/10.1371/journal.pone.0128693
Liang C, Xi-Xi X, Yun-Xiang S, Qiu-Hua X, Yang-Yong L, Yuan-Sen H, Ke B (2023) Surfactin inhibits Fusarium graminearum by accumulating intracellular ROS and inducing apoptosis mechanisms. World J Microbiol Biotechnol 39(12):340. https://doi.org/10.1007/s11274-023-03790-2
Lin D, Grossfield A (2015) Thermodynamics of micelle formation and membrane fusion modulate antimicrobial lipopeptide activity. Biophys J 109(4):750–759. https://doi.org/10.1016/j.bpj.2015.07.011
Lin LZ, Zheng QW, Wei T, Zhang ZQ, Zhao CF, Zhong H, Guo LQ (2020) Isolation and characterization of fengycins produced by Bacillus amyloliquefaciens JFL21 and its broad-spectrum antimicrobial potential against multidrug-resistant foodborne pathogens. Front Microbiol 11:579621. https://doi.org/10.3389/fmicb.2020.579621
Liu Y, Lu J, Sun J, Lu F, Bie X, Lu Z (2019) Membrane disruption and DNA binding of Fusarium graminearum cell induced by C16-Fengycin A produced by Bacillus amyloliquefaciens. Food Control 102:206–213. https://doi.org/10.1016/j.foodcont.2019.03.031
Ludovico P, Sansonetty F, Côrte-Real M (2001) Assessment of mitochondrial membrane potential in yeast cell populations by flow cytometry. Microbiology 147(12):3335–3343. https://doi.org/10.1099/00221287-147-12-3335
Luo C, Liu X, Zhou X, Guo J, Truong J, Wang X, Chen Z (2015) Unusual biosynthesis and structure of locillomycins from Bacillus subtilis 916. Appl Environ Microbiol 81(19):6601–6609. https://doi.org/10.1128/AEM.01639-15
Ma Z, Hu J (2014) Production and characterization of iturinic lipopeptides as antifungal agents and biosurfactants produced by a marine Pinctada martensii-derived Bacillus mojavensis B0621A. Appl Biochem Biotechnol 173:705–715. https://doi.org/10.1007/s12010-014-0879-1
Ma H, Zhao X, Yang L, Su P, Fu P, Peng J, Guo G (2020) Antimicrobial peptide AMP-17 affects Candida albicans by disrupting its cell wall and cell membrane integrity. Infect Drug Resis 8:2509–2520. https://doi.org/10.2147/IDR.S250278
Maget-Dana R, Peypoux F (1994) Iturins, a special class of lipopeptides: biological and properties pore-forming physicochemical. Toxicology 87:151–174. https://doi.org/10.1016/0300-483X(94)90159-7
Makovitzki A, Shai Y (2005) pH-dependent antifungal lipopeptides and their plausible mode of action. Biochemistry 44(28):9775–9784. https://doi.org/10.1021/bi0502386
Makovitzki A, Avrahami D, Shai Y (2006) Ultrashort antibacterial and antifungal lipopeptides. Proc Natl Acad Sci 103(43):15997–16002. https://doi.org/10.1073/pnas.0606129103
Makovitzki A, Baram J, Shai Y (2008) Antimicrobial lipopolypeptides composed of palmitoyl di- and tricationic peptides: in vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 47(40):10630–10636. https://doi.org/10.1021/bi8011675
Makovitzki A, Viterbo A, Brotman Y, Chet I, Shai Y (2007) Inhibition of fungal and bacterial plant pathogens in vitro and in planta with ultrashort cationic lipopeptides. Appl Environ Microbiol 73(20):6629–6636. https://doi.org/10.1128/AEM.01334-07
Malina A, Shai Y (2005) Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide. Biochem J 390(3):695–702. https://doi.org/10.1042/BJ20050520
Martínez-Núñez MA, López VEL (2016) Nonribosomal peptides synthetases and their applications in industry. Sustain Chem Processes. https://doi.org/10.1186/s40508-016-0057-6
Marx R, Stein T, Entian KD, Glaser SJ (2001) Structure of the Bacillus subtilis peptide antibiotic subtilosin A determined by 1H-NMR and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Protein Chem 20:501–506. https://doi.org/10.1023/A:1012562631268
Matos PM, Gonçalves S, Santos NC (2008) Interaction of peptides with biomembranes assessed by potential-sensitive fluorescent probes. J Pept Sci 14(4):407–415. https://doi.org/10.1002/psc.1005
Mba EI, Nweze IE (2022) Antimicrobial peptides therapy: an emerging alternative for treating drug-resistant bacteria. Yale J Biol Med 95(4):445
McIntosh JA, Donia MS, Schmidt EW (2009) Ribosomal peptide natural products: bridging the ribosomal and nonribosomal worlds. Nat Prod Rep 26(4):537–559. https://doi.org/10.1039/B714132G
Meena KR, Kanwar SS (2015) Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. BioMed Res Int. https://doi.org/10.1155/2015/473050
Meleney FL, Johnson BA (1949) Bacitracin. Am J Med 7(6):794–806. https://doi.org/10.1016/0002-9343(49)90418-0
Mello EO, Ribeiro SFF, Carvalho AO, Santos IS, Da Cunha M, Santa-Catarina C, Gomes VM (2011) Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification, and induction of ROS in fungi cells. Curr Microbiol 62(4):1209–1217. https://doi.org/10.1007/s00284-010-9847-3
Melander RJ, Zurawski DV, Melander C (2018) Narrow-spectrum antibacterial agents. Medchemcomm 9(1):12–21. https://doi.org/10.1039/C7MD00528H
Mohid SA, Biswas K, Won T, Mallela LS, Gucchait A, Butzke L, Sarkar R, Barkham T, Reif B, Leipold E, Roy S, Misra AK, Lakshminarayanan R, Lee D, Bhunia A (2022) Structural insights into the interaction of antifungal peptides and ergosterol containing fungal membrane. Biochim Biophys Acta (BBA) 1864(10):183996. https://doi.org/10.1016/j.bbamem.2022.183996
Morrison DC, Jacobs DM (1976) Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry 13(10):813–818. https://doi.org/10.1016/0019-2791(76)90181-6
Motta AS, Flores FS, Souto AA, Brandelli A (2008) Antibacterial activity of a bacteriocin-like substance produced by Bacillus sp P34 that targets the bacterial cell envelope: antonie van Leeuwenhoek. Int J General Mol Microbiol 93(3):275–284. https://doi.org/10.1007/s10482-007-9202-2
Moyne AL, Cleveland TE, Tuzun S (2004) Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol Lett 234(1):43–49. https://doi.org/10.1111/j.1574-6968.2004.tb09511.x
Mulligan CN (2005) Environmental applications for biosurfactants. Environ Pollut 133(2):183–198. https://doi.org/10.1016/j.envpol.2004.06.009
Munusamy S, Conde R, Bertrand B, Munoz-Garay C (2020) Biophysical approaches for exploring lipopeptide-lipid interactions. Biochimie 170:173–202. https://doi.org/10.1016/j.biochi.2020.01.009
Nanjundan J, Ramasamy R, Uthandi S, Ponnusamy M (2019) Antimicrobial activity and spectroscopic characterization of surfactin class of lipopeptides from Bacillus amyloliquefaciens SR1. Microb Pathog 128:374–380. https://doi.org/10.1016/j.micpath.2019.01.037
Naruse N, Tenmyo O, Kobaru S, Kamei H, Miyaki T, Konishi M, Oki T (1990) Pumilacidin, a complex of new antiviral antibiotics production, isolation, chemical properties, structure and biological activity. J Antibiot 43(3):267–280. https://doi.org/10.7164/antibiotics.43.267
Nasir MN, Besson F (2012) Interactions of the antifungal mycosubtilin with ergosterol-containing interfacial monolayers. Biochim Biophys Acta (BBA) 1818(5):1302–1308. https://doi.org/10.1016/j.bbamem.2012.01.020
Nation RL, Li J (2009) Colistin in the 21st century. Curr Opin Infect Dis 22(6):535.
Naughton PJ, Marchant R, Naughton V, Banat IM (2019) Microbial biosurfactants: current trends and applications in agricultural and biomedical industries. J Appl Microbiol 127(1):12–28. https://doi.org/10.1111/jam.14243
Neu TR, Härtner T, Poralla K (1990) Surface active properties of viscosin: a peptidolipid antibiotic. Appl Microbiol Biotechnol 32:518–520. https://doi.org/10.1007/BF00173720
Nybroe O, Sørensen J (2004) Production of Cyclic Lipopeptides by Fluorescent Pseudomonads: in Pseudomonas: Biosynthesis of Macromolecules and Molecular Metabolism, pp 147–172. Boston, MA: Springer US. Doi:https://doi.org/10.1007/978-1-4419-9088-4_5
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16(3):115–125. https://doi.org/10.1016/j.tim.2007.12.009
Oppenheimer-Shaanan Y, Steinberg N, Kolodkin-Gal I (2013) Small molecules are natural triggers for the disassembly of biofilms. Trends Microbiol 21(11):594–601. https://doi.org/10.1016/j.tim.2013.08.005
Pacwa-Płociniczak M, Płaza GA, Piotrowska-Seget Z, Cameotra SS (2011) Environmental applications of biosurfactants: recent advances. Int J Mol Sci 12(1):633–654. https://doi.org/10.3390/ijms12010633
Parmanik A, Das S, Kar B, Bose A, Dwivedi GR, Pandey MM (2022) Current treatment strategies against multidrug-resistant bacteria: a review. Curr Microbiol 79(12):388. https://doi.org/10.1007/s00284-022-03061-7
Parulekar RS, Sonawane KD (2018) Insights into the antibiotic resistance and inhibition mechanism of aminoglycoside phosphotransferase from Bacillus cereus: In silico and in vitro perspective. J Cell Biochem 119(11):9444–9461. https://doi.org/10.1002/jcb.27261
Patel S, Ahmed S, Eswari JS (2015) Therapeutic cyclic lipopeptides mining from microbes: latest strides and hurdles. World J Microbiol Biotechnol 31:1177–1193. https://doi.org/10.1007/s11274-015-1880-8
Pathak KV, Keharia H (2014) Identification of surfactins and iturins produced by potent fungal antagonist, Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) tree using mass spectrometry. 3 Biotech 4(3):283–295. https://doi.org/10.1007/s13205-013-0151-3
Pathak KV, Keharia H, Gupta K, Thakur SS, Balaram P (2012) Lipopeptides from the banyan endophyte, Bacillus subtilis K1: mass spectrometric characterization of a library of fengycins. J Am Soc Mass Spectrom 23(10):1716–1728. https://doi.org/10.1007/s13361-012-0437-4
Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ (2008) In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol 46(1):150–156. https://doi.org/10.1128/JCM.01901-07
Poursoleiman A, Karimi-Jafari MH, Zolmajd-Haghighi Z, Bagheri M, Haertlé T, Behbehani GR, Ghasemi A, Stroylova YY, Muronetz VI, Saboury AA (2019) Polymyxins interaction to the human serum albumin: a thermodynamic and computational study. Spectrochim Acta Part A 217:155–163. https://doi.org/10.1016/j.saa.2019.03.077
Ramachandran R, Chalasani AG, Lal R, Roy U (2014) A broad-spectrum antimicrobial activity of Bacillus subtilis RLID 12.1. Sci World J 194:1. https://doi.org/10.1155/2014/968487
Ramachandran R, Shrivastava M, Narayanan NN, Thakur RL, Chakrabarti A, Roy U (2018) Evaluation of antifungal efficacy of three new cyclic lipopeptides of the class bacillomycin from Bacillus subtilis RLID 12.1. Antimicrob Agents Chemother 62(1):10–1128. https://doi.org/10.1128/AAC.01457-17
Ramchandran R, Ramesh SAA, Thakur R, Chakrabarti A, Roy U (2020) Improved production of two anti candida lipopeptide homologues co- produced by the wild-type Bacillus subtilis RLID 12.1 under optimized conditions. Curr Pharm Biotechnol 21(5):438–450. https://doi.org/10.2174/1389201020666191205115008
Ramesh S, Madduri M, Rudramurthy SM, Roy U (2023a) Functional characterization of a Bacillus-derived novel broad-spectrum antifungal lipopeptide variant against Candida tropicalis and Candida auris and unravelling its mode of action. Microbiol Spectr 11(2):e01583-e1622. https://doi.org/10.1128/spectrum.01583-22
Ramesh S, Shaw D, Madhuri M, Rudramurthy SM, Roy U (2023b) In vitro evaluation of novel Bacillus-derived lipopeptide on clinically prevalent Trichophyton spp. in dermatophytosis. Acta microbiol. Bulg 39(1):83–86
Ramesh S, Roy U, Sushashish R, Rudramurthy SM (2024) A natural lipopeptide from Bacillus subtilis RLID12.1 with promising antifungal activity: its partial characterization and insight into the mode of action. Appl Microbiol Biotechnol 108:161. https://doi.org/10.1007/s00253-023-12976-5
Ray A, Aayilliath KA, Banerjee S, Chakrabarti A, Denning DW (2022) Burden of serious fungal infections in India. Open Forum Infect Dis 9(12):603. https://doi.org/10.1093/ofid/ofac603
Reddy KVR, Yedery RD, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24(6):536–547. https://doi.org/10.1016/j.ijantimicag.2004.09.005
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta (BBA) 1863(12):2977–2992. https://doi.org/10.1016/j.bbamcr.2016.09.012
Roberts KD, Zhu Y, Azad MA, Han ML, Wang J, Wang L, Li J (2022) A synthetic lipopeptide targeting top-priority multidrug-resistant Gram-negative pathogens. Nat Commun 13(1):1625. https://doi.org/10.1038/s41467-022-29234-3
Rodrigues LR, Teixeira JA (2010) Biomedical and therapeutic applications of biosurfactants. Biosurfactants 5:75–87. https://doi.org/10.1007/978-1-4419-5979-9_6
Rodríguez-López L, López-Prieto A, Lopez-Álvarez M, Pérez-Davila S, Serra J, González P, Moldes AB (2020) Characterization and cytotoxic effect of biosurfactants obtained from different sources. ACS Omega 5(48):31381–31390. https://doi.org/10.1021/acsomega.0c04933
Romero D, De Vicente A, Olmos JL, Dávila JC, Pérez-García A (2007a) Effect of lipopeptides of antagonistic strains of Bacillus subtilis on the morphology and ultrastructure of the cucurbit fungal pathogen Podosphaera fusca. J Appl Microbiol 103(4):969–976. https://doi.org/10.1111/j.1365-2672.2007.03323.x
Romero D, De Vicente A, Rakotoaly RH, Dufour SE, Veening JW, Arrebola E, Pérez-García A (2007b) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant-Microbe Int 20(4):430–440. https://doi.org/10.1094/MPMI-20-4-0430
Rossolini GM, Arena F, Pecile P, Pollini S (2014) Update on the antibiotic resistance crisis. Curr Opin Pharmacol 18:56–60. https://doi.org/10.1016/j.coph.2014.09.006
Roy K, Mukhopadhyay, Reddy T, GCS, Desikan, KR, Ganguli BN (1987) Mulundocandin, a new lipopeptide antibiotic I. Taxonomy, fermentation, isolation, and characterization. J Antibiot 40(3):275–280. https://doi.org/10.7164/antibiotics.40.275
Sabnis A, Hagart KL, Klöckner A, Becce M, Evans LE, Furniss RCD, Edwards AM (2021) Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. Elife 10:e65836. https://doi.org/10.7554/eLife.65836
Saini HS, Barragán-Huerta BE, Lebrón-Paler A, Pemberton JE, Vázquez RR, Burns AM, Maier RM (2008) Efficient purification of the biosurfactant viscosin from Pseudomonas libanensis strain M9–3 and its physicochemical and biological properties. J Nat Products 71(6):1011–1015. https://doi.org/10.1021/np800069u
Sajid M, Khan MSA, Cameotra SS, Al-Thubiani AS (2020) Biosurfactants: potential applications as immunomodulator drugs. Immunol Lett 223:71–77. https://doi.org/10.1016/j.imlet.2020.04.003
Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA (2016) Biosurfactants: multifunctional biomolecules of the 21st century. Int J Mol Sci 17(3):401. https://doi.org/10.3390/ijms17030401
Settanni L, Corsetti A (2008) Application of bacteriocins in vegetable food biopreservation. Int J Food Microbiol 121(2):123–138. https://doi.org/10.1016/j.ijfoodmicro.2007.09.001
Schneider T, Müller A, Miess H, Gross H (2014) Cyclic lipopeptides as antibacterial agents–potent antibiotic activity mediated by intriguing mode of actions. Int J Med Microbiol 304(1):37–43. https://doi.org/10.1016/j.ijmm.2013.08.009
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31(3):446–459. https://doi.org/10.1080/13102818.2017.1286950
Sharma D, Mandal SM, Manhas RK (2014) Purification and characterization of a novel lipopeptide from Streptomyces amritsarensis sp. nov. active against methicillin-resistant Staphylococcus aureus. AMB Express 4(1):1–9. https://doi.org/10.1186/s13568-014-0050-y
Sharma D, Singh SS, Baindara P, Sharma S, Khatri N, Grover V, Korpole S (2020) Surfactin like broad spectrum antimicrobial lipopeptide co-produced with sublancin from Bacillus subtilis strain A52: dual reservoir of bioactives. Front Microbiol 11:1167. https://doi.org/10.3389/fmicb.2020.01167
Sikora K, Jaśkiewicz M, Neubauer D, Migoń D, Kamysz W (2020) The role of counter-ions in peptides: an overview. Pharmaceuticals 13(12):442. https://doi.org/10.3390/ph13120442
Silverman JA, Perlmutter NG, Shapiro HM (2003) Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 47(8):2538–2544. https://doi.org/10.1128/AAC.47.8.2538-2544.2003
Singh AK, Sharma P (2020) Disinfectant-like activity of lipopeptide biosurfactant produced by Bacillus tequilensis strain SDS21. Colloids Surf B 185:110514. https://doi.org/10.1016/j.colsurfb.2019.110514
Singh S, Sequeira RA, Kumar P, Ghadge VA, Vaghela P, Mohanty KA, Ghosh A, Prasad K, Shinde PB (2022) Selective partition of lipopeptides from fermentation broth: a green and sustainable approach. ACS Omega 7(50):46646–46652. https://doi.org/10.1021/acsomega.2c05587
Sivapathasekaran C, Mukherjee S, Samanta R, Sen R (2009) High-performance liquid chromatography purification of biosurfactant isoforms produced by a marine bacterium. Anal Bioanal Chem 395(3):845–854. https://doi.org/10.1007/s00216-009-3023-2
Soares da Silva R, de C. F., Almeida, D. G., Meira, H. M., Silva, E. J., Farias, C. B. B., Rufino, R. D., Luna, J. M., & Sarubbo, L. A. (2017) Production and characterization of a new biosurfactant from Pseudomonas cepacia grown in low-cost fermentative medium and its application in the oil industry. Biocatal Agric Biotechnol 12:206–215. https://doi.org/10.1016/j.bcab.2017.09.004
Soares GMS, Figueiredo LC, Faveri M, Cortelli SC, Duarte PM, Feres M (2012) Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J Appl Oral Sci 20:295–309. https://doi.org/10.1590/S1678-77572012000300002
Sofjan AK, Mitchell A, Shah DN, Nguyen T, Sim M, Trojcak A, Garey KW (2018) Rezafungin (CD101), a next-generation echinocandin: A systematic literature review and assessment of possible place in therapy. J Glob Antimicrob Resist 14:58–64. https://doi.org/10.1016/j.jgar.2018.02.013
Sreedharan SM, Rishi N, Singh R (2023) Microbial lipopeptides: Properties, mechanics, and engineering for novel lipopeptides. Microbiol Res. https://doi.org/10.1016/j.micres.2023.127363
Stachelhaus T, Marahiel MA (1995) Modular structure of genes encoding multifunctional peptide synthetases required for non-ribosomal peptide synthesis. FEMS Microbiol Lett 125(1):3–14. https://doi.org/10.1111/j.1574-6968.1995.tb07328.x
Stansly PG (1949) The polymyxins; a review and assessment. Am J Med 7(6):807–818. https://doi.org/10.1016/0002-9343(49)90419-2
Sucher AJ, Chahine EB, Balcer HE (2009) Echinocandins: the newest class of antifungals. Ann Pharmacother 43(10):1647–1657. https://doi.org/10.1345/aph.1M237
Sumi CD, Yang BW, Yeo IC, Hahm YT (2015) Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can J Microbiol 61(2):93–103. https://doi.org/10.1139/cjm-2014-0613
Sur S, Grossfield A (2022) Effects of cholesterol on the mechanism of fengycin, a biofungicide. Biophys J 121(10):1963–1974. https://doi.org/10.1016/j.bpj.2022.04.006
Szymański M, Chmielewska S, Czyżewska U, Malinowska M, Tylicki A (2022) Echinocandins–structure, mechanism of action and use in antifungal therapy. J Enzyme Inhib Med Chem 37(1):876–894. https://doi.org/10.1080/14756366.2022.2050224
Talapko J, Meštrović T, Juzbašić M, Tomas M, Erić S, Horvat Aleksijević L, Škrlec I (2022) Antimicrobial peptides Mechanisms of action, antimicrobial effects and clinical applications. Antibiotics 11(10):1417. https://doi.org/10.3390/antibiotics11101417
Tang J, He J, Liu T, Xin X, Hu H (2017) Removal of heavy metal from sludge by the combined application of a biodegradable biosurfactant and complexing agent in enhanced electrokinetic treatment. Chemosphere 189:599–608. https://doi.org/10.1016/j.chemosphere.2017.09.104
Tanwar J, Das S, Fatima Z, Hameed S (2014) Multidrug resistance: an emerging crisis. Interdiscip Perspect Infect Dis. https://doi.org/10.1155/2014/541340
Thimon L, Peypoux F, Wallach J, Michel G (1995) Effect of the lipopeptide antibiotic, iturin A, on morphology and membrane ultrastructure of yeast cells. FEMS Microbiol Lett 128(2):101–106. https://doi.org/10.1111/j.1574-6968.1995.tb07507.x
Thompson GR, Soriano A, Honore PM, Bassetti M, Cornely OA, Kollef M, Pappas PG (2023) Efficacy and safety of rezafungin and caspofungin in candidaemia and invasive candidiasis: pooled data from two prospective randomised controlled trials. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(23)00551-0
Tossi A, Tarantino C, Romeo D (1997) Design of synthetic antimicrobial peptides based on sequence analogy and amphipathicity. Eur J Biochem 250(2):549–558. https://doi.org/10.1111/j.1432-1033.1997.0549a.x
Tran C, Cock IE, Chen X, Feng Y (2022) Antimicrobial Bacillus: metabolites and their mode of action. Antibiotics 11(1):88. https://doi.org/10.3390/antibiotics11010088
Tran PN, Yen MR, Chiang CY, Lin HC, Chen PY (2019) Detecting and prioritizing biosynthetic gene clusters for bioactive compounds in bacteria and fungi. Appl Microbiol Biotechnol 103:3277–3287. https://doi.org/10.1007/s00253-019-09708-z
Tsai PW, Yang CY, Chang HT, Lan CY (2011) Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 6(3):e17755. https://doi.org/10.1371/journal.pone.0017755
Tsuge K, Akiyama T, Shoda M (2001) Cloning, sequencing, and characterization of the iturin A operon. J Bacteriol 183(21):6265–6273. https://doi.org/10.1128/jb.183.21.6265-6273.2001
Varjani SJ, Upasani VN (2017) Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Biores Technol 232:389–397. https://doi.org/10.1016/j.biortech.2017.02.047
Velkov T, Roberts KD, Nation RL, Thompson PE, Li J (2013) Pharmacology of polymyxins: new insights into an ‘old class’ of antibiotics. Future Microbiol 8(6):711–724. https://doi.org/10.2217/fmb.13.39
Vertesy L, Ehlers E, Kogler H, Kurz M, Meiwes J, Seibert G, Hammann P (2000) Friulimicins: novel lipopeptide antibiotics with peptidoglycan synthesis inhibiting activity from Actinoplanes friuliensis sp. nov. II: Isolation and structural characterization. J Antibiot 53(8):816–827. https://doi.org/10.7164/antibiotics.53.816
Vigneshwaran C, Vasantharaj K, Krishnanand N, Sivasubramanian V (2021) Production optimization, purification and characterization of lipopeptide biosurfactant obtained from Brevibacillus sp. AVN13. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.104867
Wan C, Fan X, Lou Z, Wang H, Olatunde A, Rengasamy KR (2022) Iturin: cyclic lipopeptide with multifunction biological potential. Crit Rev Food Sci Nutr 62(29):7976–7988. https://doi.org/10.1080/10408398.2021.1922355
Wang K, Dang W, Xie J, Zhu R, Sun M, Jia F, Wang R (2015) Antimicrobial peptide protonectin disturbs the membrane integrity and induces ROS production in yeast cells. Biochim Biophys Acta (BBA) 1848(10):2365–2373. https://doi.org/10.1016/j.bbamem.2015.07.008
Wang Q, Pan L, Han Y, Zhou Z (2022) Antimicrobial mechanisms of enterocin CHQS against Candida albicans. Curr Microbiol 79(7):191. https://doi.org/10.1007/s00284-022-02878-6
Weddle R (1968) Purification of polymyxin application great britain. Claims 78:424–123
Wei YH, Wang LC, Chen WC, Chen SY (2010) Production and characterization of fengycin by indigenous Bacillus subtilis F29–3 originating from a potato farm. Int J Mol Sci 11(11):426–4538. https://doi.org/10.3390/ijms11114526
Woodworth JR, Nyhart EH Jr, Brier GL, Wolny JD, Black HR (1992) Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob Agents Chemother 36(2):318–325. https://doi.org/10.1128/aac.36.2.318
Wu YS, Ngai SC, Goh BH, Chan KG, Lee LH, Chuah LH (2017) Anticancer activities of surfactin and potential application of nanotechnology assisted surfactin delivery. Front Pharmacol 8:761. https://doi.org/10.3389/fphar.2017.00761
Xiao J, Guo X, Qiao X, Zhang X, Chen X, Zhang D (2021) Activity of fengycin and iturin a isolated from Bacillus subtilis Z-14 on gaeumannomyces graminis var tritici and soil microbial diversity. Front Microbiol 12:682437. https://doi.org/10.3389/fmicb.2021.682437
Yakimov MM, Timmis KN, Wray V, Fredrickson HL (1995) Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl Environ Microbiol 61(5):1706–1713. https://doi.org/10.1128/aem.61.5.1706-1713.1995
Yamasaki K, Sakurama K, Nish K, Watanabe H, Maruyama T, Seo H, Taguchi K (2020) Characterization of the interaction of daptomycin with site II on human serum albumin. J Pharm Sci 109(9):2919–2924. https://doi.org/10.1016/j.xphs.2020.06.011
Yan F, Li C, Ye X, Lian Y, Wu Y, Wang X (2020) Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against Colletotrichum gloeosporioides in loquat fruits. Biol Control 146:104281. https://doi.org/10.1016/j.biocontrol.2020.104281
Yaraguppi DA, Bagewadi ZK, Patil NR, Mantri N (2023) Iturin: a promising cyclic lipopeptide with diverse applications. Biomolecules 13(10):1515. https://doi.org/10.3390/biom13101515
Yocum RR, Rasmussen JR, Strominger JL (1980) The mechanism of action of penicillin: Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. J Biol Chem 255(9):3977–3986. https://doi.org/10.1016/S0021-9258(19)85621-1
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395. https://doi.org/10.1038/415389a
Zaman M, Toth I (2013) Immunostimulation by synthetic lipopeptide-based vaccine candidates: structure-activity relationships. Front Immunol 4:318. https://doi.org/10.3389/fimmu.2013.00318
Zhan Y, Ma N, Liu R, Wang N, Zhang T, He L (2019) Polymyxin B and polymyxin E induce anaphylactoid response through mediation of Mas-related G protein–coupled receptor X2. Chem Biol Interact 308:304–311. https://doi.org/10.1016/j.cbi.2019.05.014
Zhao Q, Mei X, Xu Y (2016) Isolation and Identification of antifungal compounds produced by Bacillus Y-IVI for suppressing Fusarium wilt of muskmelon. Plant Protect Sci 52(3):167–175. https://doi.org/10.17221/70/2015-PPS
Zhao X, Kuipers OP (2016) Identification and classification of known and putative antimicrobial compounds produced by a wide variety of Bacillales species. BMC Genomics 17(1):1–8. https://doi.org/10.1186/s12864-016-3224-y
Zhao Y, Perlin DS (2020) Review of the novel echinocandin antifungal rezafungin: animal studies and clinical data. J Fungi 6(4):192. https://doi.org/10.3390/jof6040192
Zhou S, Liu G, Zheng R, Sun C, Wu S (2020) Structural and functional insights into iturin W, a novel lipopeptide produced by the deep-sea bacterium Bacillus sp. strain wsm-1. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01597-20
Acknowledgements
First authors (S.K.P. & M.M.) sincerely acknowledge the funding agencies Department of Biotechnology (DBT)-Builder File no. BT/INF/22/SP2543/2021), and Science & Engineering Research Board (SERB) (File no. EMR/2017/000572), Govt. of India for the fellowships. S.R. acknowledges the Indian Council of Medical Research (ICMR), Govt. of India, New Delhi for the senior research fellowship. V.R. and U.R. sincerely acknowledge the DBT-Builder.
Funding
Authors sicerely acknowledge the funding from the DBT-Builder (File no.BT/INF/22/SP2543/2021). First co-author and the second author acknowledge the funding agencies SERB (File no. EMR/2017/000572) and ICMR for the fellowships respectively.
Author information
Authors and Affiliations
Contributions
SKP and MM drafted the main manuscript text and SKP, MM, and SR prepared figures and graphical abstracts, VR and UR reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sreelakshmi, K.P., Madhuri, M., Swetha, R. et al. Microbial lipopeptides: their pharmaceutical and biotechnological potential, applications, and way forward. World J Microbiol Biotechnol 40, 135 (2024). https://doi.org/10.1007/s11274-024-03908-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-03908-0