Abstract
Fusarium graminearum, a devastating fungal pathogen, is the main pathogen of Fusarium head blight (FHB) in wheat globally; it results in significant yield loss and mycotoxin contamination that severely threatens global wheat production and food safety. However, despite ongoing efforts, controlling this pathogen still remains a major challenge. Surfactin, primarily synthesized by Bacillus sp. via non-ribosomal peptide synthetases, exhibits potent surfactant and antibacterial properties, but its antifungal mechanism has yet to be fully elucidated. We found that the EC50 of surfactin against hyphal growth of F. graminearum was 102.1 µg/mL, and control efficacy against wheat FHB under field conditions achieved 86.38% in wheat cultivar Huaimai 40 and 81.60% in wheat cultivar Zhoumai 36, indicating that surfactin has potential antifungal activity against F. graminearum. Accumulated intracellular ROS, decreased mitochondrial membrane potential (MMP), activated metacaspase activity and condensed chromatin, were induced by surfactin in F. graminearum hyphae, suggesting that growth inhibition of fungus is mainly caused by apoptosis-like cell death. Furthermore, accumulated intracellular ROS was evidenced to act as a key mediator of surfactin-induced apoptosis. Broad-spectrum caspase inhibitor Z-VAD-FMK treatment indicated that surfactin induces caspase-independent apoptosis in F. graminearum. Collectively, this study provides evidence that surfactin induces a ROS-mediated mitochondrial apoptosis in F. graminearum hyphae, and may exert its antifungal activity against F. graminearum by activating apoptosis. This study demonstrates the potential of surfactin as an antifungal agent for FHB biocontrol, provides a new perspective on the antifungal mechanism of surfactin against filamentous fungi, and contributes to the application of surfactin-producing microbes in the biocontrol of plant diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium graminearum, a devastating fungal pathogen, is the main pathogen of Fusarium head blight (FHB) in wheat worldwide (Chen et al. 2018; Liu et al. 2019; Wachowska et al. 2022). This fungus not only can cause huge yield losses, but also can produce mycotoxins, such as deoxynivalenol (DON) and zearalenone (ZEN) (McMullen et al. 2012; Ntushelo et al. 2019; Ma et al. 2022). Even at the post-harvest stage, if not dried in time or stored in moist conditions, the fungus can immediately begin to grow, reproduce, and produce mycotoxins (Pei et al. 2022). Today, F. graminearum has already become one of the major threats to global cereal production, food safety, and human health. Unfortunately, no wheat cultivars that are fully resistant to F. graminearum have been reported, while existing chemical fungicides not only do not provide satisfactory control effects, but also bring a series of safety hazards, such as increased pathogen resistance, chemical residues, and environmental pollution (Liu et al. 2019).
To date, effective strategies to control F. graminearum are lacking, this has stimulated considerable research in developing eco-friendly control methods, including the use of biocontrol strains as well as metabolites, several of which have been reported as effective in suppress of plant diseases (Ongena and Jacques 2008; Snook et al. 2009; Chowdhury et al. 2015). Amongst, Bacillus sp., one of the most widely studied genera, is known for the synthesis of a series of antibiotics, such as Bacillus lipopeptides, surfactin, iturin and fengycin, which are known for their vast array of biological activities against a wide range of pathogens, considering versatile weapons for plant disease biocontrol (Ongena and Jacques 2008; Snook et al. 2009).
Surfactin, an amphipathic heptapeptide which was mainly synthesize by Bacillus sp. through non ribosomal peptide synthetases, is well-known for its strong surfactant and antibacterial activities (Ongena and Jacques 2008). However, its direct antifungal activity has been just reported in recent years, and the underlying mechanism of surfactin against filamentous fungi has not been fully elucidated (Sarwar et al. 2018; Krishnan et al. 2019; Andrić et al. 2020; Pang et al. 2021). Sarwar et al. reported that surfactin exhibited antifungal activity against F. moniliforme, F. solani, and Trichoderma atroviride, and significantly reduced rice bakanae disease caused by F. moniliforme by up to 80% (Sarwar et al. 2018). Krishnan et al. showed that surfactin inhibited the growth of F. moniliforme hyphae by causing DNA damage (Krishnan et al. 2019). Pang et al. found that antifungal activity of B. amyloliquefaciens M9 against Botryosphaeria dothidea was associated with the presence of surfactin by a postharvest storage experiment on kiwifruit (Pang et al. 2021). Interestingly, surfactin has been reported to induce apoptosis-like cell death in mammalian cells (Cao et al. 2010; Wu et al. 2017; Vo et al. 2020). And some compounds have also been reported to exert their antifungal activity by inducing apoptosis-like cell death, such as amphotericin B (Mousavi and Robson 2004), α-tomatine (Ito et al. 2007), farnesol (Wang et al. 2014) and thymol (Hu et al. 2018). Therefore, we hypothesized that surfactin may exert its antifungal activity against F. graminearum by inducing apoptosis-like cell death.
In the present study, we confirmed the antifungal activity of surfactin against F. graminearum by measuring the EC50 and a field trial, investigated the induction effect of surfactin on apoptosis in F. graminearum hyphae by detecting hallmarks occurrence of early and late apoptosis, and further clarified the role of ROS accumulation and metacaspase activation in surfactin-induced apoptosis. Therefore, this study provides evidence that surfactin may exert its antifungal activity on F. graminearum hyphae by activating a ROS-mediated apoptosis through a metacaspase-dependent mitochondrial pathway. The study demonstrates the potential of surfactin as an antifungal agent for FHB biocontrol, and provides a new perspective on the antifungal mechanism of surfactin against filamentous fungi.
Materials and methods
Chemicals
Hoechst 33342/PI double stain kit (CA1120), 2′,7′-Dichlorofluorescin diacetate (DCFH-DA), N-acetyl-l-cysteine (NAC), Mitochondrial membrane potential assay kit with JC-1 (M8650), Caspase 9 activity assay kit (BC3890) and Caspase 3 activity assay kit (BC3830) were purchased from Solarbio (Beijing, China). Pan caspase inhibitor Z-VAD-FMK was purchased from Beyotime (Shanghai, China). RNAiso Plus reagent (9108Q) was purchased from Takara (Dalian, China). NovoScript® Plus All-in-one 1st Strand cDNA Synthesis SuperMix Kit (E047-01 A) and NovoStart® SYBR qPCR SuperMix plus Kit (E096-01 A) were purchased from Novoprotein (Shanghai, China). Surfactin was extracted from Bacillus velezensis PH204 broth using HPLC, identified by UPLC-ESI-MS/MS, and dissolved in 0.1 M phosphate-buffered saline (PBS) for further experiments (Chen et al. 2018). All other chemicals were analytical grade.
Fungal strain and culture condition
F. graminearum PH-1 was used in this study and maintained on a PDA slant at 4 °C. To produce conidia suspension, PH-1 was cultured on PDA plate for 5 d at 28 °C. Then five 6-mm-diameter plugs were taken from the edge of the colony and inoculated into a 250 mL flask containing 50 mL of CMC medium. After 48 h of incubation at 26 °C and 150 r/min, the conidia culture broth was filtered through two layers of sterile lens wiping paper and centrifuged at 4 °C and 7500×g for 10 min. The conidia were then harvested by centrifugation (4 °C, 7500×g, 10 min), and rinsed twice with sterile normal saline containing 0.01% Tween 80. The conidia suspension was therefore obtained by suspending with SNS and adjusting to 2 × 105 conidia per mL using a hemocytometer. To prepare the hyphae suspension, 1 mL of conidia suspension was inoculated into a 250 mL flask containing 50 mL of Czapek’s medium and cultured for 10 h at 26 °C and 50 r/min, and then the hyphae were harvested by centrifugation (4 °C, 7500×g, 10 min) and rinsed twice with sterile phosphate-buffered saline (PBS). The hyphae suspension was therefore obtained by suspending with 25 mL of PBS.
Effect of surfactin on radial growth of F. graminearum hyphae
Five 6-mm-diameter plugs of F. graminearum were inoculated into the center of a 90-mm-diameter PDA plate containing different concentrations of surfactin (0, 100, 200, 300, 400, and 500 µg/mL), respectively. All plates were then incubated at 28 °C, until the fungus in the control plate grew to full plate, the diameter of the fungal colony was measured to calculate the inhibition rate of hyphae growth, and the EC50 value was calculated from the relationship between surfactin concentration and inhibition rate.
Field trial
To evaluate control efficacy of surfactin against wheat FHB, field trial was carried out according to procedures in our previous work with some modifications (Chen et al. 2018). Briefly, field trial was conducted at Xuchang experimental Field of Henan Agricultural University, Xuchang, China (E112°75′, N34°17′) in 2021, and two wheat cultivars Zhoumai 36 and Huaimai 40 were used. Sterile water and surfactin served as untreated control and surfactin treatment, respectively. Each treatment was repeated at least four experimental plots. Plots (above 15 m2/plot, 400 heads/plot) were arranged in a randomized block design. At the first day (initial stage of wheat anthesis), 100 mL/m2 of sterile water and surfactin were sprayed towards wheat spikes and roots, respectively. Two days later, 200 mL of F. graminearum conidia (1 × 105 conidia/mL) were sprayed to wheat spikes of each plot. Ten days after first spraying, 100 mL/m2 of sterile water and surfactin were sprayed again as described above. Thirty days after second spraying, number of infected wheat spike, infected level of wheat spike, and spikes weight was investigated as described in NY/T 1464.15-2007, which is issued by Ministry of agriculture of the People’s Republic of China. Infected spike rate, disease index and control efficacy were respectively calculated according to the methods in NY/T 1464.15-2007.
DON content of different wheat samples was determined using the UPLC-MS/MS method described in GB 5009.111-2016, which is issued by National health commission of the People’s Republic of China. Each sample was conducted at least 3 times.
Surfactin treatment
1 mL of hyphae suspension was mixed with 4 mL of enzyme solution (10% snailase and 10% cellulose) at 37 °C. After exposure for 1 h, the hyphae were harvested, rinsed, and treated with 1 mL of 100 µg/mL surfactin at 26 °C for 4 h. Hyphae treated with 0 µg/mL surfactin served as the blank control group.
Intracellular ROS accumulation assay
Intracellular ROS accumulation in F. graminearum hyphae was assessed using a redox-sensitive fluorescent probe DCFH-DA. DCFH-DA is non-fluorescent, whereas in the presence of ROS, it can be oxidized to green-fluorescent 2,7-dichlorofluorescin (DCF). Briefly, different treatment groups of hyphae were harvested, rinsed, and incubated with 10 µM DCFH-DA for 30 min in the dark. The hyphae were then harvested, rinsed, and resuspended in PBS. Fluorescence observation and intensity measurement were performed using an inverted fluorescence microscope (Axio Observer 3, Zeiss, USA) and the corresponding fluorescence intensity was measured using a fluorescence spectrophotometer (F-7100, Hitachi, Japan) at 488/525 nm excitation/emission (DCF, green fluorescence).
In parallel, another group of hyphae was preloaded with 5 mM NAC (a ROS scavenger) prior to surfactin treatment, and then the assay for intracellular ROS accumulation was carried out as described above. Furthermore, changes in the typical hallmarks of early and late apoptosis were further evaluated.
Mitochondrial membrane potential (MMP) assay
The MMP in F. graminearum hyphae was determined using a commercial kit containing JC-1 dye (M8650, Solarbio, China). JC-1 dye can accumulate in mitochondria in a potential-dependent man. In normal cells, the MMP is high and JC-1 aggregates in the mitochondrial matrix, forming J-aggregates (red fluorescence). In early apoptotic cells, MMP decreases, and JC-1 cannot accumulate in the mitochondrial matrix and depolymerizes to J-monomers (green fluorescence). The change of MMP can be detected by the transition from red fluorescence to green fluorescence, and the increased G/R ratio (the ratio of green fluorescence intensity to red fluorescence intensity) is used to indicate the decrease of MMP. Briefly, different treatment groups of hyphae were harvested, rinsed, and suspended in 0.5 mL of JC-1 in the dark for 20 min. The hyphae were then harvested, rinsed, and resuspended in JC-1 dye buffer. Then fluorescence observation and intensity measurement were performed at 515/529 nm excitation/emission (J-monomers, green fluorescence) and 585/590 nm excitation/emission (J-aggregates, red fluorescence), respectively.
RNA extraction, cDNA synthesis, and qRT-PCR analysis
Different treatment groups of hyphae were collected, ground with a pestle in liquid nitrogen, and stored at − 80 °C, respectively. Total RNA was then extracted using RNAiso Plus reagent (9108Q, Takara, China) according to the manufacturer’s instructions. RNA concentration was measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA), and cDNA was reverse transcribed from total RNA using the NovoScript® Plus All-in-one 1st Strand cDNA Synthesis SuperMix kit (E047-01 A, Novoprotein, China) according to the manufacturer’s recommendations. The obtained cDNA was used for qRT-PCR analysis.
Measurement of metacaspase activation
Caspases, spartate-specific cysteine proteases, play a central role in the early stage of mammalian apoptosis, whereas metacaspases are caspase-like cysteine proteases in fungi (Uren et al. 2000). Activation of metacaspases is considered a biochemical hallmark of late apoptosis in fungi (Hwang et al. 2012; Shlezinger et al. 2012).
In the genome of F. graminearum, two metacaspase genes (FGSG_12913 and FGSG_09204) have been found (Sikhakolli et al. 2012). To quantify the induction effect of surfactin on metacaspase activation in F. graminearum hyphae, the expression levels of two metacaspase genes under different treatment conditions were firstly measured using qRT-PCR analysis. qRT-PCR analysis was performed on a Mastercycler ep-realplex machine (Eppendorf, Germany) using the NovoStart® SYBR qPCR SuperMix Plus kit (E096-01 A, Novoprotein, China). Relative gene expression for each target gene was evaluated using the comparative 2−∆∆CT method and normalized against the F. graminearum β-tublin gene (FGSG_09530). The primers used for qRT-PCR are listed in Table S1. Furthermore, caspase 9 activity and caspase 3 activity in F. graminearum hyphae were measured using commercial assay kits (BC3890 and BC3830, Solarbio, China) with specific substrates according to the manufacturer’s instructions (Hu et al. 2018). The principle of assay kits is that caspase specifically cleavages its substrate to releases free p-nitroanilide (p-NA). Free p-NA is yellow with a maximum absorption peak at 405 nm, and can be measured using a spectrophotometer or a microtiter plate reader. From the standard curve of p-NA absorbance at 405 nm, the p-NA concentration released by caspase was calculated to obtain caspase activity. Briefly, equal volume of 100 µL hyphae from different treatment groups were harvested, rinsed, and suspended in 100 µL lysis buffer in an ice bath for 15 min. After centrifugation at 4 °C and 16,000×g for 15 min, the supernatant was transferred to the pre-cooling tube to determine the increase in absorbance at 405 nm. The release of p-nitroanilide (p-NA) was calculated based on a standard curve between its amount and its absorbance at 405 nm. 1 U of caspase activity is defined as the facilitation of 1 µM of pNA release (Hu et al. 2018).
In parallel, another group of hyphae was preloaded with 2 mM Z-VAD-FMK (a broad-spectrum pan caspase inhibitor) prior to the addition of surfactin. Subsequently, qRT-PCR analysis and measurement of caspase activity were performed as described above. We also further evaluated the changes of typical hallmarks of early and late apoptosis.
Hoechst 33342/PI double staining
Hoechst 33342/PI double staining was used to detect the late apoptotic phenotype by using a commercial kit (CA1120, Solarbio, China) (Hu et al. 2018). The Hoechst 33342/PI double stain kit contains two ready-to-use dyes (PI and Hoechst 33342) bound to DNA. PI, a membrane-impermeable and red-fluorescent nuclear dye, causes membrane-damaged necrotic cells to appear red fluorescence. Hoechst 33342, a membrane-permeant and blue fluorescent nuclear dye, provides a brighter blue fluorescence in apoptotic cells than that in normal cells, as there is much more condensed chromatin in apoptotic cells than that in normal cells. Importantly, chromatin condensation, one of the first morphological changes associated with apoptosis, is considered a well-established cytological hallmark of late apoptosis in fungi (Semighini and Harris 2010; Hwang et al. 2012). Thus, Hoechst 33342/PI double staining is widely used to distinguish normal (Hoechst 33342 negative and PI negative, weak blue), late apoptotic (Hoechst 33342 positive and PI negative, bright blue) and necrotic cells (Hoechst 33342 negative and PI positive, bright red and weak blue) and to detect condensed chromatin (Belloc et al. 1994; Hu et al. 2018). Briefly, different treatment groups of hyphae were harvested, rinsed, and resuspended with 1 mL of PBS, then incubated with 10 µL PI (1 mg/mL) and 200 µL Hoechst 33342 (0.1 µg/mL) for 20 min in the dark. The hyphae were then harvested, rinsed, and resuspended in PBS for further fluorescence observation and intensity measurement. Fluorescence observation was performed at 350/460 nm excitation/emission (Hoechst 33342, blue fluorescence) and 535/615 nm excitation/emission (PI, red fluorescence), respectively.
Statistical analysis
All experiments were performed in triplicate and all results are presented as mean ± standard deviation. Statistical differences between groups were analyzed by one-way ANOVA followed by Tukey’s test using GraphPad Prism 9.0 software (San Diego, CA, USA).
Results
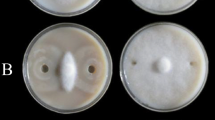
Surfactin inhibited the radial growth of F. graminearum hyphae
As shown in Fig. 1, under the action of 0, 100, 200, 300, 400, and 500 µg/mL of surfactin, the colony diameter of F. graminearum was 90.00 ± 0.00, 44.33 ± 1.07, 42.07 ± 0.45, 42.00 ± 0.28, 41.07 ± 0.29 and 39.17 ± 0.57 mm, respectively (Fig. 1B), and the value of EC50 was calculated to be 102.1 µg/mL, showing that surfactin can significantly inhibit the radial growth of F. graminearum hyphae. It is worth noting that within the 500 µg/mL concentration, the inhibition of surfactin on radial growth of F. graminearum hyphae was in a dose-independent manner, representing that a unique mechanism of action. And the concentration of 100 µg/mL surfactin was chosen for further experiments.
Surfactin reduced FHB incidence and severity under field conditions
As shown in Table 1, in relation to Zhoumai 36, compared to the untreated control, the infected rate of spikes was significantly reduced from 31.58% of the untreated control to 7.80% of the surfactin treatment (p < 0.01), a reduction of 64.01%, FHB disease index was significantly reduced from 37.19 to 6.94 (p < 0.01), a reduction of 72.27%, and grain DON content was significantly reduced from 12.89 to 5.49 µg/kg (p < 0.01), a reduction of 53.09%. Accordingly, control efficacy of surfactin against FHB in Zhoumai 36 was 81.60%. In trelation to Huaimai 40, the infected rate of spikes was significantly reduced from 33.24% of the untreated control to 7.35% of the surfactin treatment (p < 0.01), a reduction of 64.01%, FHB disease index was significantly reduced from 36.56 to 4.98 (p < 0.01), a reduction of 85.72%, and grain DON content significantly reduced from 14.61 to 5.02 µg/kg (p < 0.01), a reduction of 65.60%. Accordingly, control efficacy of surfactin against FHB in Huaimai 40 was 86.38%. Collectively, surfactin was effective in reducing FHB incidence and severity in wheat under field conditions.
In addition, spikes weight of Huaimai 40 increased from 135.3 g/50 heads of untreated control to 188.6 g/50 heads of surfactin treatment (p < 0.01), a significant increase of 39.39%, and spikes weight of Zhoumai 36 increased from 168.5 g/50 heads of untreated control to 224.8 g/50 heads of surfactin treatment (p < 0.01), a significant increase of 33.41%. Accordingly, surfactin was also effective in increasing wheat yield in wheat under field conditions.
Surfactin triggered intracellular ROS accumulation in F. graminearum hyphae
Intracellular ROS accumulation is closely associated with most instances apoptosis (Cheng et al. 2003; Ito et al. 2007; Robson 2006; Bugeda et al. 2020), and is considered an early event in apoptotic cell death (Hwang et al. 2012). To evaluate whether surfactin induces intracellular ROS accumulation in F. graminearum hyphae, a redox-sensitive fluorescent probe DCFH-DA was employed. As shown in Fig. 2A, control hyphae appeared with faint green fluorescence, while surfactin-treated hyphae showed strong green fluorescence. And the intensity of green fluorescence in surfactin-treated hyphae was up to 4925.00 ± 154.41 Au/ug, significantly higher than 707.33 ± 98.49 Au/ug of control hyphae (p ≤ 0.0001) (Fig. 2B). The results demonstrated that surfactin triggered intracellular ROS accumulation in F. graminearum hyphae.
Surfactin induced intracellular ROS accumulation in F. Graminearum hyphae. A Control hyphae (CK) appeared faint green fluorescence, while surfactin-treated hyphae (Surfactin) showed strong green fluorescence. B The intensity of green fluorescence was significantly increased from 707.33 ± 98.49 Au/ug of control hyphae to 4925.00 ± 154.41 Au/ug of surfactin-treated hyphae (p ≤ 0.0001). BF, bright field. Bar = 50 μm. ****p ≤ 0.0001
Surfactin leads to MMP decrease in F. graminearum hyphae
The mitochondrial membrane potential (MMP) is essential for normal mitochondrial function, the decrease of MMP would initiate a series of cellular events, and trigger an irrevocable apoptotic process, therefore, the decrease of MMP has been considered as an important hallmark of early apoptosis (Hwang et al. 2012).
As shown in Fig. 3A, after exposure to surfactin for 4 h, control hyphae showed aggregated red fluorescence with no blue fluorescence, indicating high MMP, whereas in hyphae treated by 100 µg/mLsurfactin, strong green fluorescence appeared and aggregated red fluorescence was significantly reduced, indicating the transition from high MMP to low MMP. And the G/R ratio increased significantly from 0.71 ± 0.09 of control hyphae to 3.47 ± 0.24 of surfactin-treated hyphae (p ≤ 0.0001) (Fig. 3B). The results indicated that surfactin caused a decrease in MMP in F. graminearum hyphae.
Surfactin caused MMP decrease in F. Graminearum hyphae. A Control hyphae (CK) showed strong aggregated red fluorescence, indicative of high MMP, while surfactin-treated hyphae (Surfactin) showed strong green fluorescence, indicating the transition from high MMP to low MMP. B The G/R ratio was significantly increased from 0.71 ± 0.09 of control hyphae to 3.47 ± 0.24 of surfactin-treated hyphae (p ≤ 0.0001). BF bright field. Bar = 50 μm. ****p ≤ 0.0001
Surfactin activated metacaspase activity in F. graminearum hyphae
Caspases, spartate-specific cysteine proteases, play a central role in the early stage of mammalian apoptosis, while metacaspases are caspase-like cysteine proteases in fungi (Uren et al. 2000). metacaspase activation is considered a biochemical hallmark of early apoptosis in fungi (Hwang et al. 2012; Shlezinger et al. 2012). Two metacaspase genes (FGSG_12913 and FGSG_09204) have been found in the F. graminearum genome (Sikhakolli et al. 2012).
qRT-PCR analysis showed that under the treatment of 100 µg/mL surfactin, the relative expression levels of FGSG_12913 and FGSG_09204 in surfactin-treated hyphae were up-regulated more than 2.1-fold (Fig. 4A) and 1.4-fold (Fig. 4B) compared to control hyphae, respectively. Similar results were also obtained when we measured the activity of caspases. Both the activity caspase 9 and caspase 3 were significantly activated in surfactin-treated hyphae, caspase 9 activity of surfactin-treated hyphae increased from 3.30 ± 0.62 U of control hyphae to 13.69 ± 0.59 U (p ≤ 0.0001) (Fig. 4C), and caspase 3 activity increased from 8.63 ± 0.10 to 12.11 ± 0.22 U (p ≤ 0.0001) (Fig. 4D), resulting in a 4.2- and 1.4-fold increase, respectively. Although caspases have not been identified in fungi, we detected the activity of caspases with specific substrates in vitro, which could indicate that there should be homologues of caspase 9 and 3 in F. graminearum with low sequence similarity to the known caspase 9 and 3 sequences. A similar case was also present in thymol-mediated apoptosis in A. flavus conidia (Hu et al. 2018). These results indicated that surfactin could activate gene expression of metacaspases and then activate corresponding pathways to induce apoptosis in F. graminearum hyphae.
Surfactin activated metacaspase activity in F. Graminearum hyphae. A Compared to control hyphae (CK), the relative expression level of metacaspase gene FGSG_12913 in surfactin-treated hyphae was up-regulated more than 2.1-fold. B Compared to control hyphae (CK), the relative expression level of metacaspase gene FGSG_09204 in surfactin-treated hyphae was up-regulated more than 1.4-fold. C Caspase 9 activity increased from 3.30 ± 0.62 U of control hyphae (CK) to 13.69 ± 0.59 U of surfactin-treated hyphae (p ≤ 0.0001). D Caspase 3 activity increased from 8.63 ± 0.10 to 12.11 ± 0.22 U (p ≤ 0.0001). CK control hyphae. Surfactin, hyphae treated with 100 µg/mL surfactin
Surfactin induced late apoptotic phenotype in F. graminearum hyphae
Chromatin condensation is one of the first morphological changes associated with apoptosis (Semighini and Harris 2010), and is considered a well-established cytological hallmark of late apoptosis in fungi (Hwang et al. 2012). To obtain further evidence of surfactin-induced late apoptosis in F. graminearum hyphae, we performed Hoechst 33342/PI double staining to detect condensed chromatin.
As shown in Fig. 5, under 535 nm excitation (PI), control hyphae and hyphae treated with 100 µg/mL surfactin did not exhibit red fluorescence, indicating that the hyphae did not suffer from membrane-damaged necrosis, whereas hyphae treated with 1000 µg/mL surfactin showed strong red fluorescence, indicating that the hyphae suffered membrane-damaged necrosis. Under 350 nm excitation (Hoechst 33342), control hyphae showed faint blue fluorescence, while hyphae treated with 100 µg/mL sufactin showed more intense blue fluorescence than control hyphae, indicating chromatin condensation. These results indicated that a high concentration of surfactin (1000 µg/mL) could disrupt the hyphal membrane of F. graminearum, showing pro-necrotic activity, while a low concentration of surfactin (100 µg/mL) was not sufficient to destroy the hyphal membrane, but induced chromatin condensation, showing pro-apoptotic activity.
Surfactin triggered chromatin condensation in F. graminearum hyphae. Control hyphae (CK) showed PI negative and Hoechst 33342 negative (weak blue fluorescence), hyphae treated by 100 µg/mL surfactin (Surfactin) showed PI negative and Hoechst 33342 positive (brightly blue fluorescence), indicative of chromatin condensation, and hyphae treated by 1000 µg/mL surfactin (S1000) showed PI positive and Hoechst 33342 positive (brightly red fluorescence and weak blue fluorescence), indicating membrane-damaged necrosis. BF bright field. Bar = 50 μm
Surfactin-induced apoptosis in F. graminearum hyphae depends on ROS mediated
To further clarify whether ROS play a pivotal mediating role in surfactin-induced apoptosis, comparative experiments with NAC pretreatment were carried out to examine the effect of ROS accumulation on surfactin-induced early and late apoptosis in F. graminearum hyphae. As shown in Fig. 6, NAC pretreatment resulted in very few hyphae in NAC-pretreated hyphae showing green fluorescence, indicating of accumulated ROS (Fig. 6A), and the intensity of green fluorescence was significantly reduced (p ≤ 0.0001) compared to surfactin-treated hyphae (Fig. 6B). The data suggested that NAC effectively scavenged surfactin-induced ROS accumulation in F. graminearum hyphae.
Surfactin-induced apoptosis in F. graminearum hyphae depends on ROS mediated. A Compared to surfactin-treated hyphae, the number of hyphae showing green fluorescence significantly decreased, which was similar to that of control hyphae. B The intensity of green fluorescence in NAC-pretreated hyphae was significantly reduced compared to surfactin-treated hyphae (p ≤ 0.0001). C The relative expression levels of FGSG_12913 in NAC-pretreated hyphae were found to significantly reduce compared to surfactin-treated hyphae (p ≤ 0.0001). D The relative expression levels of FGSG_09204 in NAC-pretreated hyphae were found to significantly reduce compared to surfactin-treated hyphae (p ≤ 0.0001), and had no significant difference with that of control hyphae (p > 0.05). E Compared to surfactin-treated hyphae, NAC-pretreated hyphae appeared strong aggregated red fluorescence, which was similar to that of the control. F The G/R ratio of NAC-pretreated hyphae was significantly decreased than that of surfactin-treated hyphae (p ≤ 0.0001), and had no significant difference with that of control hyphae (p > 0.05). G Compared to surfactin-treated hyphae, the number of hyphae showing blue fluorescence was significantly reduced in NAC-pretreated hyphae. H The intensity of blue fluorescence was significantly reduced compared to surfactin-treated hyphae (p ≤ 0.0001). CK, control hyphae. Surfactin, hyphae treated with 100 µg/mL surfactin. S + NAC, hyphae treated with NAC and 100 µg/mL surfactin. BF bright field. Bar = 50 μm. ****p ≤ 0.0001. ns statistical insignificance (p > 0.05)
Meanwhile, we also evaluated the effects of NAC pretreatment on surfactin-induced early and late apoptosis hallmarks in F. graminearum hyphae, respectively. The relative expression levels of FGSG_12913 (Fig. 6C) and FGSG_09204 (Fig. 6D) in NAC-pretreated hyphae were significantly reduced compared to surfactin-treated hyphae (p ≤ 0.0001), especially for FGSG_09204, whose expression level was reduced to have no significant difference with that of control hyphae (p > 0.05). Furthermore, caspase 9 activity was found to decrease from 9.68 ± 0.80 µM/mL of surfactin-treated hyphae to 6.35 ± 0.38 µM/mL (p ≤ 0.001) in NAC-pretreated hyphae, and caspase 3 activity was found to decrease from 9.11 ± 0.30 to 7.17 ± 0.30 µM/mL (p ≤ 0.001), which was not significantly different from control hyphae (6.62 ± 0.20 µM/mL) (p > 0.05).
Interestingly, this change in caspase 3 activity coincided with the change in gene expression change of FGSG_09204. These data suggest that once the surfactin-induced ROS accumulation was scavenged, the surfactin-activated metacaspase activity was consequently suppressed. On the other hand, NAC-pretreated hyphae showed strong aggregated red fluorescence similar to that of control hyphae (Fig. 6E), and the G/R ratio was also significantly decreased compared to surfactin-treated hyphae (p ≤ 0.0001) and not significantly different from control hyphae (p > 0.05) (Fig. 6F), indicating that once the surfactin-induced ROS accumulation was scavenged, the surfactin-induced MMP decrease was consequently prevented. Furthermore, compared to surfactin-treated hyphae, the number of hyphae showing blue fluorescence was significantly reduced in NAC-pretreated hyphae (Fig. 6G), and the intensity of blue fluorescence was significantly reduced (p ≤ 0.0001) (Fig. 6H), indicating that surfactin-induced chromatin condensation was also consequently suppressed. Taken together, these results confirmed that ROS played a pivotal mediating role in surfactin-induced apoptosis in F. graminearum hyphae.
Surfactin-induced apoptosis in F. graminearum hyphae involved in metacaspase activation
To determine whether surfactin-induced apoptosis in F. graminearum hyphae occurs through a metacaspase-dependent mechanism, the effects of a broad-spectrum pan caspase inhibitor Z-VAD-FMK on surfactin-induced early and late apoptosis were examined, respectively.
As shown in Fig. 7, pretreatment with Z-VAD-FMK significantly reduced the relative expression levels of FGSG_12913 (Fig. 7A) and FGSG_09204 (Fig. 7B) compared to surfactin-treated hyphae (p ≤ 0.0001). Furthermore, the activity of caspase 9 and 3 in Z-VAD-FMK-pretreated hyphae was also significantly reduced compared to surfactin-treated hyphae, with caspase 9 activity decreasing from 13.69 ± 0.59 to 8.43 ± 0.68 U (p ≤ 0.001) and caspase 3 activity decreased from 12.11 ± 0.22 to 10.73 ± 0.27 µM/mL (p ≤ 0.001), indicating that surfactin-activated metacaspase activity can be blocked by Z-VAD-FMK.
Surfactin-induced apoptosis in F. Graminearum hyphae requires metacaspase activity. A The relative expression levels of FGSG_12913 in Z-VAD-FMK-pretreated hyphae were found to significantly reduce compared to surfactin-treated hyphae (p ≤ 0.0001). B The relative expression levels of FGSG_09204 in Z-VAD-FMK-pretreated hyphae were found to significantly reduce compared to surfactin-treated hyphae (p ≤ 0.0001). C Compared to surfactin-treated hyphae, the number of hyphae showing green fluorescence significantly decreased, which was similar to that of control hyphae. D The intensity of green fluorescence in Z-VAD-FMK-pretreated hyphae was significantly reduced compared to surfactin-treated hyphae (p ≤ 0.0001). E Compared to surfactin-treated hyphae, the number of hyphae showing blue fluorescence was significantly reduced in Z-VAD-FMK-pretreated hyphae. F The intensity of blue fluorescence was significantly reduced compared to surfactin-treated hyphae (p ≤ 0.0001). G Compared to surfactin-treated hyphae, Z-VAD-FMK-pretreated hyphae appeared strong aggregated red fluorescence. H The G/R ratio of Z-VAD-FMK-pretreated hyphae was significantly decreased than that of surfactin-treated hyphae (p ≤ 0.0001). CK control hyphae. Surfactin, hyphae treated with 100 µg/mL surfactin. S + Z, hyphae treated with Z-VAD-FMK and 100 µg/mL surfactin. BF bright field. Bar = 50 μm. ****p ≤ 0.0001. ns statistical insignificance (p > 0.05)
Meanwhile, when surfactin-activated metacaspase activity was blocked by Z-VAD-FMK, the surfactin-induced apoptosis was also significantly alleviated. In detail, pretreatment with Z-VAD-FMK resulted in a significant reduction of green fluorescence (Fig. 7C) and blue fluorescence (Fig. 7E) than those of surfactin-treated hyphae, which are signs of intracellular ROS accumulation and chromatin condensation, and the intensity of green and blue fluorescence was reduced by 71.68% (Fig. 7D) and 48.65% (Fig. 7F), respectively, compared to surfactin-treated hyphae, indicating that once the surfactin-activated metacaspase activity was blocked, surfactin-induced ROS accumulation and chromatin condensation were consequently suppressed. Furthermore, in hyphae pretreated by Z-VAD-FMK, the aggregated red fluorescence increased and became stronger compared to surfactin-treated hyphae, and the green fluorescence decreased and became weaker (Fig. 7G), with the G/R ratio decreased by 33.43% (p ≤ 0.001) (Fig. 7H), suggesting that surfactin-triggered MMP decrease could be suppressed by Z-VAD-FMK. Collectively, we concluded that once surfactin-activated metacaspase activity was blocked, the metacaspase-activated apoptosis cascade would not occur, thus the surfactin-induced apoptosis in F. graminearum hyphae was significantly suppressed. Thus, the results clearly indicated that surfactin-induced apoptosis in F. graminearum hyphae involves in metacaspase activation.
Discussion
Surfactin, an amphipathic heptapeptide, was linked to a β-hydroxy fatty acid to form a cyclic lactone ring structure. Because of its amphiphilic nature, surfactin can easily associate and tightly anchor into membrane phospholipid layer, destroying membrane integrity (Ongena and Jacques 2008). However, the presence of cholesterol in the phospholipid layer attenuates the destabilizing effect of surfactin, suggesting that the susceptibility of membranes may vary in a specific way depending on the sterol content of the target organisms and the dose of surfactin (Carrillo et al. 2003; Ongena and Jacques 2008). Coincidentally, filamentous fungi have higher levels of cholesterol (ergosterol) than bacteria, which explains why surfactin has strong antibacterial activity but no significant direct antifungal activity. However, recent studies have shown that surfactin plays a vital role in the actual suppression of plant fungal diseases. For example, Chowdhury et al. reported that compared to wild-type B. velezensis FZB42, the mutants deficient in surfactin production failed to reduce disease incidence in lettuce, indicating that surfactin plays a role in the actual disease suppression (Chowdhury et al. 2015). More interesting, in the presence of fungal pathogen Rhizoctonia solani, B. velezensis FZB42 overproduced surfactin in the lettuce rhizosphere, which was also identified as the main metabolite (Chowdhury et al. 2015). Pang et al. showed that the antifungal activity of B. amyloliquefaciens M9 against Botryosphaeria dothidea was associated with the presence of surfactin in a post-harvest storage experiment on kiwifruit (Pang et al. 2021). In this study, we confirmed the antifungal activity of surfactin against F. graminearum by measuring the EC50 and a field trial, the EC50 against hyphal growth was 102.1 µg/mL, and the control efficacy against FHB under field conditions was 86.38% in Huaimai 40 and 81.60% in Zhoumai 36, indicating that surfactin has potential antifungal activity against F. graminearum.
We therefore speculated that in addition to direct antibiotic action, surfactin may have other modes of action. Existing studies have also discussed such case, for example, Chowdhury et al. reported that until now, it has not demonstrated that the concentration of secondary metabolites secreted by FZB42 or other representative bacilli is sufficient for inhibition of the pathogen by direct antibiotic action (Chowdhury et al. 2015), while Raaijmakers and Mazzola highlighted that antibiotics at subinhibitory concentrations can elicit other effects on microorganisms (Raaijmakers and Mazzola 2012). Furthermore, of the large number of metabolites secreted by microbes to date, only a small fraction has been shown to possess useful therapeutic antibiotic activity, most metabolites may play roles as cell-signalling molecules, and the antagonistic potential of metabolites produced by Bacillus sp. is also not their only function (Yim et al. 2007; Schoenborn et al. 2021). In this study, we confirmed that surfactin can induce apoptosis in F. graminearum hyphae. To determine whether surfactin can induce apoptosis in F. graminearum hyphae, both early and late apoptosis hallmarks, involved in morphological, biochemical, and biological changes, were detected by using multiple methods. In detail, surfactin induced intracellular ROS accumulation (biochemical changes of early apoptosis), MMP decrease (biological change of early apoptosis), metacaspase activation (biochemical changes of early apoptosis) and chromatin condensation (morphological change of late apoptosis) in F. graminearum hyphae. These results clearly indicated that surfactin induces apoptosis in F. graminearum hyphae. To our knowledge, this is the first report describing apoptosis in F. graminearum hyphae induced by surfactin. Furthermore, we found that at high dose (1000 µg/mL), surfactin caused membrane damage in F. graminearum hyphae (PI positive, Fig. 2), showing a pro-necrotic effect, whereas at low dose (100 µg/mL), surfactin failed to disrupt membrane integrity, but showed a pro-apoptotic effect. More importantly, under natural field conditions, Bacillus sp. could not synthesize enough surfactin to cause membrane-damaged necrosis in fungal cells (Chowdhury et al. 2015). Therefore, we suggested that the induction of apoptosis is more likely to be the actual action mechanism of action of surfactin against F. graminearum in nature.
Apoptosis, a form of programmed cell death (PCD), is critical for the development and homeostasis in all organisms, in which cells commit suicide by activating the intracellular death machinery (Muzaffar et al. 2016). In filamentous fungi, apoptosis was discovered late, but to date, apoptosis has been observed in many filamentous fungi, such as Mucor racemosus (Roze and Linz 1998), Aspergillus nidulans (Cheng et al. 2003), A. flavus (Wang et al. 2014; Hu et al. 2018), Botrytis cinerea (Wei et al. 2021), Neurospora crassa (Marek et al. 2003), and Penicillium digitatum (Bugeda et al. 2020), and are recognized to play an essential role in the growth and development of filamentous fungi (Robson 2006; Wang et al. 2014; Hu et al. 2018; Bugeda et al. 2020). Nowadays, filamentous fungi pose an increasing threat to global food production, food security, and public health, thus novel control strategies are urgently needed to combat these dangerous fungi (Ramsdale 2008; Kulkarni et al. 2019). The strategy of activating the intrinsic cell death pathways encoded by the fungi themselves, which is analogous to new anticancer therapeutics now entering the clinic, has been considered a promising strategy to control filamentous fungi (Tian et al. 2016; Kulkarni et al. 2019). Predictably, this strategy would provide new pathway for the control of filamentous fungi and new targets for the development of antifungal agents with broad application potential.
ROS have been shown to induce various biological processes, including apoptosis. In this study, compared to control hyphae, a significant increase of intracellular ROS accumulation was observed in surfactin-treated hyphae (p ≤ 0.0001), indicating that surfactin triggered intracellular ROS accumulation. As reported, ROS are detrimental to cell viability, causing membrane disruption, enzyme inactivation, and highly reactive and modify proteins, lipids, and nucleic acids, and ROS-induced cell damage is a frequent event (Hwang et al. 2012). Therefore, we wondered whether surfactin-induced ROS accumulation would cause oxidative stress and mediate apoptosis-like PCD in F. graminearum hyphae. Therefore, we performed comparative experiments with NAC to investigate the effects of ROS accumulation on early and late apoptosis in F. graminearum hyphae. The results showed that through NAC pretreatment, the decreased MMP and activated metacaspase activity induced by surfactin were significantly blocked or suppressed (p ≤ 0.0001) compared to surfactin-treated hyphae (Fig. 6), especially for the expression level of FGSG_09204, caspase 3 activity, and MMP decrease, which were reduced to have no significant difference with those of control hyphae. In addition, chromatin condensation was consistently suppressed in NAC-pretreated hyphae compared to surfactin-treated hyphae. Considering that ROS accumulation can damage nucleic acids in cells, it is reasonable to assume that it also causes chromatin condensation. In summary, since MMP decrease and metacaspase activation are both typical hallmarks of early apoptosis in fungi, and chromatin condensation is a typical cytological hallmark of late apoptosis in fungi (Semighini and Harris 2010; Hwang et al. 2012), thus we concluded that once surfactin-induced ROS accumulation was scavenged, either early or late apoptosis in F. graminearum hyphae induced by surfactin was significantly blocked or suppressed. These results suggest that ROS accumulation has a critical mediating effect on surfactin-induced early and late apoptosis of F. graminearum hyphae. Similarly, α-tomatine was proved to induce apoptosis in F. oxysporum mediated by ROS (Ito et al. 2007).
Apoptosis-like cell death can be induced by environmental stresses and exposure to toxic metabolites through the extrinsic death receptor pathway or the intrinsic mitochondrial pathway in mammals (Robson 2006; Bugeda et al. 2020). In fungi, the core machinery is similar to that of mammals, but the network of apoptosis is less complex and of more ancient origin (Sharon et al. 2009). Among them, the mitochondrion plays a central role in apoptosis, and mitochondrial dysfunctions are always associated with apoptosis (Sharon et al. 2009; Cao et al. 2010). Previous studies have shown that generated ROS can directly activate the mitochondrial permeability transition and lead to a decrease in MMP. Normal MMP is essential for normal mitochondrial functions, MMP decrease, indicating mitochondrial dysfunction, sequentially triggers the release of apoptotic factors, including cytochrome c (Cyt c) and apoptosis-inducing factor (AIF), from mitochondria into the cytoplasm, sequentially activating caspases and inducing the propagation of the apoptotic cascade and the execution of apoptosis-like PCD (Kroemer et al. 2007; Cao et al. 2010; Hwang et al. 2012). In this study, both MMP decrease and metacaspase activation were observed in surfactin-treated hyphae, and these events were blocked or suppressed by NAC, suggesting that surfactin-induced apoptosis in F. graminearum hyphae is mediated by a ROS-dependent mitochondrial pathway.
In summary, based on the above results, we proposed a preliminary model to elucidate the action of surfactin-induced apoptosis in F. graminearum hyphae. Under the influence of low concentrations of surfactin, the hyphae membrane of F. graminearum suffered a certain degree of damage that was insufficient to induce necrosis. Nevertheless, the plasma membrane-embedded NADPH oxidase (NOX) complex, which is known as one of the main sources for producing ROS (Yaakoub et al. 2022), was activated under the treatment of surfactin (data not shown). Consequently, the NOX-produced ROS accumulated in cells and acted as an early signaling mediator of apoptosis, triggering a cascade of cellular events including mitochondrial membrane damage. During this process, mitochondria damaged by ROS tend to generate even more ROS, leading to increased oxidative stress and subsequent dysfunction characterized by decreased MMP. As a result, pro-apoptotic factors are released from mitochondria and activate metacaspases to initiate metacaspase-dependent mitochondria-mediated apoptosis. The findings have enhanced our understanding of the antifungal mode of action of surfactin, thereby expanding its potential as an antifungal agent for controlling fungal and mycotoxin contamination in agricultural products and food. Additionally, these results provide valuable guidance for the application of surfactin and Bacillus sp. strains in disease control. However, to develop surfactin as a novel antifungal agent against F. graminearum, there is still a lot of work to be done. Future studies will focus on identifying key pathways or factors that stimulate and/or enhance apoptosis to facilitate the induction of apoptosis in harmful fungi.
Data availability
The datasets generated during and/or analysed during this study are available from the corresponding author upon reasonable request.
References
Andrić S, Meyer T, Ongena M (2020) Bacillus responses to plant-associated fungal and bacterial communities. Front Microbiol 11:1350
Belloc F, Dumain P, Boisseau MR, Jalloustre C, Reiffers J, Bernard P, Lacombe F (1994) A flow cytometric method using Hoechst 33342 and propidium iodide for simultaneous cell cycle analysis and apoptosis determination in unfixed cells. Cytometry 17: 59-65
Bugeda A, Garrigues S, Gandia M, Manzanares P, Marcos JF, Coca M (2020) The antifungal protein afpb induces regulated cell death in its parental fungus Penicillium digitatum. Msphere 5:e00520–e00595
Cao XH, Wang AH, Wang CL, Mao DZ, Lu MF, Cui YQ, Jiao RZ (2010) Surfactin induces apoptosis in human breast cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase pathway. Chem Biol Interact 183:357–362
Carrillo C, Teruel JA, Aranda FJ, Ortiz A (2003) Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta 1611:91–97
Chen L, Heng JY, Qin SY, Bian K (2018) A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS ONE 13:e0198560
Cheng J, Park TS, Chio LC, Fischl AS, Ye XS (2003) Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Mol Cell Biol 23:163–177
Chowdhury SP et al (2015) Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol Plant-Microbe Interact 28:984–995
Hu LB et al (2018) Thymol induces conidial apoptosis in Aspergillus flavus via stimulating k+ eruption. J Agric Food Chem 66:8530–8536
Hwang JH, Hwang IS, Liu QH, Woo ER, Lee DG (2012) (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie 94:1784–1793
Ito SI et al (2007) α-Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysporum. FEBS Lett 581:3217–3222
Krishnan N, Velramar B, Velu RK (2019) Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic Biochem Physiol 155:101–107
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163
Kulkarni M, Stolp ZD, Hardwick JM (2019) Targeting intrinsic cell death pathways to control fungal pathogens. Biochem Pharmacol 162:71–78
Liu YN, Lu J, Sun J, Lu FX, Bie XM, Lu ZX (2019) Membrane disruption and DNA binding of Fusarium graminearum cell induced by C16-Fengycin A produced by Bacillus amyloliquefaciens. Food Control 102:206–213
Ma D, Wang G, Zhu J, Mu W, Dou D, Liu F (2022) Green leaf volatile trans-2-hexenal inhibits the growth of Fusarium graminearum by inducing membrane damage, ROS accumulation, and cell dysfunction. J Agric Food Chem 70:5646–5657
Marek SM, Wu J, Louise Glass N, Gilchrist DG, Bostock RM (2003) Nuclear DNA degradation during heterokaryon incompatibility in Neurospora crassa. Fungal Genet Biol 40:126–137
McMullen M, Bergstrom G, De Wolf E, Dill-Macky R, Hershman D, Shaner G, Van Sanford D (2012) A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis 96:1712–1728
Mousavi SA, Robson GD (2004) Oxidative and amphotericin B-mediated cell death in the opportunistic pathogen aspergillus fumigatus is associated with an apoptotic-like phenotype. Microbiology 150:1937–1945
Muzaffar S, Bose C, Banerji A, Nair BG, Chattoo BB (2016) Anacardic acid induces apoptosis-like cell death in the rice blast fungus Magnaporthe oryzae. Appl Microbiol Biotechnol 100:323–335
Ntushelo K, Ledwaba LK, Rauwane ME, Adebo OA, Njobeh PB (2019) The mode of action of Bacillus species against Fusarium graminearum, tools for investigation, and future prospects. Toxins 11:606
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Pang L et al (2021) Improvement of antifungal activity of a culture filtrate of endophytic Bacillus amyloliquefaciens isolated from kiwifruit and its effect on postharvest quality of kiwifruit. J Food Biochem 45:e13551
Pei P, Xiong K, Wang X, Sun B, Zhao Z, Zhang X, Yu J (2022) Predictive growth kinetic parameters and modelled probabilities of deoxynivalenol production by Fusarium graminearum on wheat during simulated storing conditions. J Appl Microbiol 133:349–361
Raaijmakers JM, Mazzola M (2012) Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol 50:403–424
Ramsdale M (2008) Programmed cell death in pathogenic fungi. Biochim Biophys Acta 1783:1369–1380
Robson GD (2006) Programmed cell death in the aspergilli and other filamentous fungi. Med Mycol 44:109–114
Roze LV, Linz JE (1998) Lovastatin triggers an apoptosis-like cell death process in the fungus Mucor racemosus. Fungal Genet Biol 25:119–133
Sarwar A et al (2018) Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol Res 209:1–13
Schoenborn AA, Yannarell SM, Wallace ED, Clapper H, Weinstein IC, Shank EA (2021) Defining the expression, production, and signaling roles of specialized metabolites during Bacillus subtilis differentiation. J Bacteriol 203:e0033721
Semighini CP, Harris SD (2010) Methods to detect apoptotic-like cell death in filamentous fungi. Methods Mol Biol 638:269–279
Sharon A, Finkelstein A, Shlezinger N, Hatam I (2009) Fungal apoptosis: function, genes and gene function. FEMS Microbiol Rev 33:833–854
Shlezinger N, Goldfinger N, Sharon A (2012) Apoptotic-like programed cell death in fungi: the benefits in filamentous species. Front Oncol 2:97
Sikhakolli UR, López-Giráldez F, Li N, Common R, Townsend JP, Trail F (2012) Transcriptome analyses during fruiting body formation in Fusarium graminearum and Fusarium verticillioides reflect species life history and ecology. Fungal Genet Biol 49:663–673
Snook ME, Mitchell T, Hinton DM, Bacon CW (2009) Isolation and characterization of leu7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J Agric Food Chem 57:4287–4292
Tian J, Wang Y, Lu Z, Sun C, Zhang M, Zhu A, Peng X (2016) Perillaldehyde, a promising antifungal agent used in food preservation, triggers apoptosis through a metacaspase-dependent pathway in Aspergillus flavus. J Agric Food Chem 64:7404–7413
Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM (2000) Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 6:961–967
Vo TTT, Liu JF, Wu CZ, Lin WN, Chen YL, Lee IT (2020) Surfactin from Bacillus subtilis induces apoptosis in human oral squamous cell carcinoma through ROS-regulated mitochondrial pathway. J Cancer 11:7253–7263
Wachowska U, Sulyok M, Wiwart M, Suchowilska E, Kandler W, Krska R (2022) The application of antagonistic yeasts and bacteria: an assessment of in vivo and under field conditions pattern of Fusarium mycotoxins in winter wheat grain. Food Control 138:109039
Wang X, Wang Y, Zhou Y, Wei X (2014) Farnesol induces apoptosis-like cell death in the pathogenic fungus Aspergillus flavus. Mycologia 106:881–888
Wei CL, Zhang F, Song LL, Chen XF, Meng XH (2021) Photosensitization effect of curcumin for controlling plant pathogen Botrytis cinerea in postharvest apple. Food Control 123:107683
Wu YS, Ngai S, Goh BH, Chan KG, Lee LH, Chuah LH (2017) Anticancer activities of surfactin and potential application of nanotechnology assisted surfactin delivery. Front Pharmacol 8:761
Yaakoub H, Mina S, Calenda A, Bouchara JP, Papon N (2022) Oxidative stress response pathways in fungi. Cell Mol Life Sci 79:333
Yim G, Wang HH, Davies J (2007) Antibiotics as signalling molecules. Philos Trans R Soc Lond B Biol Sci 362:1195–1200
Acknowledgements
This work was supported by National natural science foundation of China (32172280), Natural science foundation of Henan province (182300410042) and the earmarked fund for CARS-13.
Funding
This study was funded by National natural science foundation project of China (32172280), Natural science foundation of Henan province (182300410042) and the earmarked fund for CARS-13.
Author information
Authors and Affiliations
Contributions
CL: Investigation, Conceptualization, Data curation, Formal analysis, Methodology, Writing-original draft, Writing-review & editing, Supervision. XX: Data curation, Formal analysis, Methodology, Writing-original draft, Writing-review & editing. SY: Data curation, Formal analysis, Methodology. XQ: Data curation, Formal analysis, Methodology. LY: Investigation, Formal analysis, Methodology. HY: Resources, Supervision, Project administration. BK: Conceptualization, Resources, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, C., Xi-xi, X., Yun-xiang, S. et al. Surfactin inhibits Fusarium graminearum by accumulating intracellular ROS and inducing apoptosis mechanisms. World J Microbiol Biotechnol 39, 340 (2023). https://doi.org/10.1007/s11274-023-03790-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03790-2