Abstract
Phaffia rhodozyma is a basidiomycetous yeast characterized by its production of the carotenoid pigment astaxanthin, which holds high commercial value for its significance in aquaculture, cosmetics and as nutraceutics, and the UV-B-absorbing compound mycosporine-glutaminol-glucoside (MGG), which is of great biotechnological relevance for its incorporation into natural sunscreens. However, the industrial exploitation has been limited to the production of astaxanthin in small quantities. On the other hand, the accumulation of MGG in P. rhodozyma was recently reported and could add value to the simultaneous production of both metabolites. In this work, we obtain a mutant strain that overproduces both compounds, furthermore we determined how the accumulation of each is affected by the carbon-to-nitrogen ratio and six biotic and abiotic factors. The mutant obtained produces 159% more astaxanthin (470.1 μg g−1) and 220% more MGG (57.9 mg g−1) than the parental strain (295.8 μg g−1 and 26.2 mg g−1 respectively). Furthermore, we establish that the carotenoids accumulate during the exponential growth phase while MGG accumulates during the stationary phase. The carbon-to-nitrogen ratio affects each metabolite differently, high ratios favoring carotenoid accumulation while low ratios favoring MGG accumulation. Finally, the accumulation of both metabolites is stimulated only by photosynthetically active radiation and low concentrations of hydrogen peroxide. The mutant strain obtained is the first hyper-productive mutant capable of accumulating high concentrations of MGG and astaxanthin described to date. The characterization of how both compounds accumulate during growth and the factors that stimulate their accumulation, are the first steps toward the future commercial exploitation of strains for the simultaneous production of two biotechnologically important metabolites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phaffia rhodozyma is a basidiomycetous yeast species initially isolated in 1970 by Herman Phaff from tree exudates in the mountainous regions of Japan and Alaska. This species has the unique ability to synthesize astaxanthin (Andrewes et al. 1976), an economically relevant carotenoid for the aquaculture (Johnson et al. 1980; Lim et al. 2018). Astaxanthin (3,3′-dihydroxy–β,β′-carotene-4,4′-dione) is a carotenoid pigment responsible for the characteristic coloration of crustaceans, birds and salmonids (Johnson and An 1991) but also is a pigment of enormous value for the food and nutraceutical industries due to their positive effect on human health (Hussein et al. 2006; Bjørklund et al. 2022). Currently 95% of the astaxanthin is industrially produced by chemical synthesis mainly under the brand CAROPHYLL® and its cost reaches 2000 dollars per kilo, which represents up to 10% of the total cost of the food used in the breeding of fish (Johnson and An 1991; Stachowiak and Szulc 2021). Synthetic astaxanthin is composed by 3 different enantiomers (3S-3′S, 3R-3′S, and 3R-3′R) with different spatial orientation of the hydroxyl groups. The 3R-3′S represents 50% of the mixture, does not exist in nature, their antioxidant activity is considerably lower than the observed in natural forms and there are concerns about their safety in human health (Capelli et al. 2013), for these reasons the FDA (Food and Drug Administration, USA) approved their use only for animal feed while is not permitted for humans (Brendler and Williamson 2019; Stachowiak and Szulc 2021). On the other hand there is a growing demand for natural foods that has stimulated the use of natural compounds in replacement of the synthetic, in this context the production of astaxanthin from natural sources has become more relevant, and several countries approved their use as food supplements (Yaqoob et al. 2021).
Natural production of astaxanthin for aquaculture can be achieved with two different microorganisms, the algae Haematococcus pluvialis, that produces 3S-3S′ astaxanthin mainly in monoester forms (Lorenz and Cysewski 2000) and the yeasts species Phaffia rhodozyma that produces 3R-3R′ enantiomer, mainly in free form (Johnson and An 1991; Sanderson and Jolly 1994; Johnson 2003). Each microorganism has their own strengths and weaknesses at the moment of their use for the biotechnological astaxanthin production. Regarding P. rhodozyma, the main limitation is that wild strains produce an average of 300 μg g−1 of astaxanthin which is considered a very low content for industrial production. For this reason a large number of strategies to improve astaxanthin have been carried out; including the obtention of hyperproducing mutants (An et al. 1989; Fang and Cheng 1993; Meyer et al. 1993; Calo et al. 1995; Bon et al. 1997; Rubinstein et al. 1998; Stachowiak 2013a; Luna-Flores et al. 2022), the induction of astaxanthin accumulation have been reported using biotic and abiotic factors, including ethanol, acetic acid, hydrogen peroxide, singlet oxygen, mevalonic acid, citrate, and metals such as copper, phytohormones, changes in temperature and pH, among others (Calo et al. 1995; Gu et al. 1997; Santopietro et al. 1998; Flores-Cotera and Sánchez 2001; Liu and Wu 2006, 2007; Barbachano-Torres et al. 2012; Zhang et al. 2019; Nutakor et al. 2022), also different carbon sources, including raw materials, and nitrogen sources in different C/N relations (Meyer and du Preez 1994a; Florêncio et al. 1998; Flores-Cotera et al. 2001; Ramı́rez et al. 2001; Domínguez-Bocanegra and Torres-Muñoz 2004; Ni et al. 2007; Silva et al. 2012; Jiang et al. 2017; Gervasi et al. 2018; Lai et al. 2022), and finally different strategies of culture were tested, including fed-batch, continuous, and perfusion systems achieving very high cell densities (>50 g dry cell weight per liter) (Meyer and du Preez 1994a; Reynders et al. 1996; Yamane et al. 1997; Ducrey Santopietro et al. 1998; Chan and Ho 1999; Ho et al. 1999; Liu and Wu 2007; Luna-Flores et al. 2022). The combination of this strategies allows an accumulation of total astaxanthin ranging between 1000 and 2500 μg g−1, and companies like ADM or Nextferm report the production of 4000–15,000 μg g−1 for industrial production (Jacobson et al. 2002; Paz et al. 2022).
In addition to the high productivity, obtained with these strategies, P. rhodozyma strains also produce another biotechnological relevant compound called mycosporine-glutaminol-glucoside, MGG (Libkind et al. 2011a, b). Mycosporines (3,5-dihydroxy-5-(hydroxymethyl)-2-methoxycyclohex-2-enone) are water-soluble molecules with an absorption in the UV region of the spectrum present in many yeasts species (Libkind et al. 2005, 2006, 2011a, b; Moliné et al. 2014). To the date, a single mycosporine compound, MGG, has been identified in yeasts, including P, rhodozyma (Sommaruga et al. 2004; Libkind et al. 2005, 2008; Kogej et al. 2006; Volkmann and Gorbushina 2006), this molecule absorb radiation in the UV-B wavelengths with maximum absorbance at 310 nm, and it has a photoprotective and antioxidant role in yeasts (Moliné et al. 2010a; Libkind et al. 2011a, b). In this sense MGG physico-chemical characteristics reveal that they are molecules with great potential to be used in the formulation of cosmetic products (Colabella et al. 2014). Although the biotechnological potential of these family of molecules is under evaluation (Chrapusta et al. 2017), it has been observed that the application of these compounds on human skin prevents the formation of erythema caused by UVR (Torres et al. 2004), and its commercial application in the formulation of protective cosmetic products (Rosic 2019; Singh et al. 2021) as well its use as an additive in the chemical industry in plastics, paints, etc., is promising (Bandaranayake 1998). Moreover the mycosporine like amino-acid porphyra-334 obtained from the red algae Porphyra umbilicalis is the active principle for preparing the commercial product HELIOGUARD® 365 (Schmid et al. 2006; Singh et al. 2021) used to prevent cellular aging of the skin caused by solar radiation. However, the production costs of mycosporines obtained from algae are high due to low productivity and the difficulty involved in obtaining large quantities of this red algae, reaching more than 1500 dollars per gram (estimated from the market price of HELIOGUARD® 365 and the declared concentration). In this context, the MGG produced by yeasts represents a promising alternative for obtaining new active principles for the formulation of photoprotective products of natural origin, and at the same time allows obtaining astaxanthin for aquaculture (Colabella et al. 2014).
The objective of this study was to obtain a P. rhodozyma mutant strains characterized by enhanced production capacities for economically significant metabolites, specifically astaxanthin and mycosporines. Our focus extended to the establishment of optimal cultivation conditions conducive to maximizing the accumulation of both compounds. This entailed a systematic exploration of the influence of distinct carbon/nitrogen ratios in the culture media and an examination of how various abiotic factors affected their accumulation in cultures. In the course of our investigation, we successfully isolated a hyperproducer mutant of P. rhodozyma capable of synthesizing elevated levels of both astaxanthin and mycosporines. Our study revealed a correlation between high carbon/nitrogen ratios and enhanced carotenoid production, whereas low ratios favored mycosporines accumulation. Furthermore, we demonstrated that exposure to light and controlled levels of hydrogen peroxide stimulated the accumulation of both metabolites.
Materials and methods
Isolation and characterization of P. rhodozyma mutant with increased carotenoids (CAR) and mycosporines (MGG) accumulation
P. rhodozyma CBS 7918T was cultured in modified MMS-bactopeptone (10 g L−1 glucose, 2.5 g L−1 Bactopeptone® Difco, 2.5 g L−1 KH2(PO4), 0.5 g L−1 MgSO4.7H2O, and 1 g L−1 yeast extract). Yeasts were propagated in 250 mL erlenmeyer flasks containing 100 mL of MMS-bactopeptone for 48 hs in a shaker INNOVA4000 (200 rpm at 19 °C). Cells were harvested by centrifugation (3000 rpm, 5 min), rinsed twice with sterile distilled water, and transferred to quartz tubes containing 20 mL of sterile distilled water, at a final cell density of 2 × 105 cells mL−1. Cells were exposed to UV-B radiation provided by a Spectroline XX15-B UV-B lamp during 120 min at a distance of 20 cm. The lamp was covered with a < 280 nm cutoff filter made with a pre-burned (120 min exposure to the UV-B lamp) cellulose acetate film (Moliné et al. 2009). 100 μL aliquots were plated in petri dishes with MMS-agar, spread out with a drigalski spatula, and incubated at 19 °C. After 5 days colonies with changes in pigmentation were picked up and streaked on new plates of the same medium to obtain pure cultures. The parental and the mutant strain were cultured in erlenmeyer flasks containing 100 mL of MMS-bactopeptone for 120 hs. The biomass yield, specific growth rate, and accumulation of CAR and MGG were determined. The assays were conducted in triplicate, and the parameters were determined as described below.
Growth kinetics and metabolites production under different C/N ratio
Twelve unique media broths were prepared, each characterized by different C/N ratios, where the ratio represents the quantity of carbon relative to the amount of nitrogen present in glucose and bactopeptone (Difco), respectively. The nitrogen content was derived from the data sheet provided by the manufacturer, ratios of 14, 7 and 3.5 were tested. Culture media was formulated with 10, 15, 20 and 30 g L−1 of glucose, and bactopeptone concentration was adjusted to obtain the desired C/N ratio (e.g. 1.5; 3 and 6 g L−1 for 10 g of glucose, and 4.5, 9 and 18 for 30 g of glucose). Concentration of the other nutrients was maintained as detailed above. Erlenmeyer flasks containing 100 mL of each culture broth were incubated with the strain 7918Mut under constant agitation at 200 rpm in a Dragon Lab rotary incubator at 250 rpm and 19 °C for 240 hs. Cultures were irradiated with white light using 5 fluorescent tubes (Osram 6500 K, 20W) in a 12:12 hs PAR (photosynthetically active radiation)-darkness cycle. The inoculum consisted of 5 mL of a 48 hs culture under the same conditions described above. Nine samples were taken at different times of the growth curve and CAR and MGG were quantified as described below. Assays were performed in duplicate.
Influence of biotic and abiotic factors on the accumulation of CAR and MGG
The strain 7918Mut was cultivated in MMS-bactopeptone (C/N = 7, glucose 10 g L−1 bactopeptone 3 g L−1) under the conditions described in the previous section. We conducted various test involving both biotic or abiotic factors such as:
-
Sodium Chloride (NaCl, Cicarelli® ASC), at final concentrations of 100 mM, 200 mM and 400 mM;
-
Ethanol (Cicarelli®, 99% ASC) at concentrations of 0.2%, 0.4%, 0.6%, and 0.8%;
-
Hydrogen Peroxide (H2O2 Cicarelli®, 100 vol ASC) at concentrations of 0.01 mM, 0.1 mM, 1 mM, and 2 mM, both independently and in combination with Fe+2 at a concentration of 1 mM. We also included control groups with Fe+2 and Fe+3 (sulfates, Cicarelli® ASC) at 1 mM concentration;
-
Rose bengal at concentrations 1 μM, 2 μM, 4 μM, 6 μM and 8 μM (Anedra® ASC). These tests were conducted under continuous lighting for 120 hs and in total darkness, both from the beginning of the culture and at the beginning of the stationary phase (48 hs);
-
Light exposure during different growth conditions: 120 hs of photosynthetically active radiation exposure (PAR); 70 hs of darkness followed by 50 hs of PAR (DARK-PAR); 50 hs of PAR followed by 70 hs of darkness (PAR-DARK); and 120 hs of continuous darkness (DARK).
Assays for each factor were performed in duplicate.
Extraction and characterization of CAR and MGG
Total carotenoids (CAR) and MGG extractions were performed as described in Moliné et al. (2010a, b). Their respective concentration was expressed as μg of total carotenoids per g of dry biomass (μg g−1) using the astaxanthin extinction coefficient ε = 2.100 at 474 nm (An et al. 1989) and as mg per g of dry biomass (mg g−1), and using the mycosporine-glutaminol molar extinction coefficient, ε = 25,000 at 310 nm (Bouillant et al. 1981). Absorbance was registered using a HEWLETT PACKARD P 8453-E UV–Visible spectrophotometer. CAR of the mutant and parental strain were identified by high performance liquid chromatography (Shimadzu LC-10A-SPD-M10A) with diode array detector (HPLC–DAD), using a Merck RT-18 250 mm column. An isocratic mobile phase composed of HPLC grade acetonitrile 85%, methanol 10% and 2-propanol 5% with a flow rate of 1.0 mL min−1 was used. Astaxanthin and β-carotene standars (Sigma-Aldrich®) were prepared at 10,000 μg L−1 and used to characterize and quantify the carotene content. Other pigments were identified based on their retention times and spectrum, and the absorbance area was related to the astaxanthin content (Britton et al. 2004; Libkind et al. 2018).
Sequential extraction of CAR and MGG
The strain 7918Mut was cultivated in the condition previously described for the characterization of the mutant. Afterwards, 100 mL of the culture were centrifuged at 3000 rpm for 5 min, subsequently resuspended in 25 mL of distilled water and aliquoted into twenty falcon tubes. Five were used for the extraction of MGG using distilled water at 85 °C during 4 hs. After this, centrifugation was performed, and the resulting supernatant was collected for MGG quantification while the pellets were then used for the subsequent extraction of CAR. Concurrently, another set of five tubes was used to determine total MGG content, utilizing 80% ethanol at 85 °C. Five tubes, which were not subjected to MGG extraction, were used to quantify the overall CAR content. Finally, the remaining tubes were used to determine the dry weight. Comparative analysis was conducted to assess the specific MGG extraction yields between water and ethanol extraction methods. Additionally, extraction yields of CAR were compared between the samples from which MGG was extracted using distilled water and those where MGG was left unextracted.
Analytical determinations
Yeast growth was followed by cell counting using a Neubauer chamber by duplicate. Maximum specific growth rate (μ) was calculated by means of the reparameterized Gompertz equation proposed by Zwietering et al. (1990), using the non-linear module of Statistica 6.0 software package (Arroyo Lopez et al. 2009). Dry weight biomass was estimated, in duplicates, using 5 mL of culture, centrifuged and washed twice with distilled water, in a glass tube and dried at 85 °C until constant weight (dry weight, dw). Samples were weighed in a OHAUS AP250D balance.
Statistical analyzes
Differences between treatments were tested be means of an ANOVA test with multiple ad-hoc comparisons applying the method Student-Newman-Keuls (α = 0.05) and with the nonparametric analysis of variance Kruskal-Wallis (Zar 1999), when homoscedasticity and normality test failed. Statistical analyzes were performed using SigmaPlot 12.
Results
P. rhodozyma mutant with increased CAR and MGG accumulation
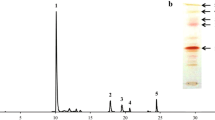
A total of 11,467 colonies distributed in 72 Petri dishes were obtained, after exposure of P. rhodozyma CBS 7918 to UV-B radiation, and visually examined. Tree colonies presented detectable color changes, one was albino, one had an orange pigmentation, and the last one presented an intense red color. HPLC analysis on CAR extracts of the three mutants revealed that only the last red strain, designated as 7918Mut, produced astaxanthin and presented similar proportions of each carotenoid as the parental strain (Fig. 1). The comparison between the parental CBS 7918 and the mutant 7918Mut (Table 1) showed not only a significant increase in CAR content (142%, p = 0.002), but also an increase in MGG content (220%, p = 0.005). No differences in specific growth rates (p = 0.567) or biomass yield (p = 0.201) were observed between both strains.
Growth curves and specific yields of CAR and MGG under different glucose concentrations and C/N ratios
CAR and MGG yields were affected by glucose concentration and C/N ratio (carbon of glucose relative to the amount of nitrogen present in bactopeptone). Batch with an initial concentration of 10 g L−1 of glucose and 6 g L−1 of Bactopeptone presented the best specific productivity yields for MGG (93.4 ± 7.2 mg g−1); these values dropped quickly as the concentration of the carbon source increased although for each concentration the highest productivity was always obtained in the lower C/N ratio. On the other hand, carotenoids maximum accumulation 738 μg g−1 was obtained when the glucose was 10 g L−1 and bactopeptone 1.5 g L−1, and a minimum accumulation of 480 μg g−1 for the batch with glucose 30 g L−1 and bactopeptone 18 g L−1 after 240 hs of culture. The accumulation of carotenoids and MGG occurred at different stages of the growth curve. Carotenoids were detectable after 24 hs of culture (exponential growth) but the maximum specific productivity was reached at the stationary phase (120 hs of culture) with negligible changes after that. On the contrary, MGG accumulation during exponential growth was less than 4 mg g−1 and only began to accumulate after 60 hs of culture, when the stationary phase was reached, reaching maximum specific productivity at 80 hs. Glucose concentration also affected the biomass yield (p < 0.001), while the concentration of bactopeptone did not produce changes (p < 0.001). For glucose concentrations greater than 15 g L−1 a decrease in the biomass, as well as CAR and MGG yields, was observed. The growth and accumulation kinetics of each metabolite for two different culture conditions is presented in Fig. 2 (all the other conditions are provided in Supplementary figures). The yields of biomass and each metabolite is summarized in Table 2.
Influence of biotic and abiotic factors on the accumulation of CAR and MGG
Regarding the different factors studied, their effects in the accumulation of CAR and MGG were diverse (Table 3). Light had a significant positive effect on the accumulation of both metabolites when compared with those cultured under dark. Higher MGG accumulation (~62 mg g−1) was observed in the treatment under continuous light (PAR), and the effect of light during the stationary phase (treatment DARK-PAR, ~55 mg g−1) was greater than during the exponential phase (PAR-DARK, ~22 mg g−1). For factors such as Sodium Chloride, Ethanol, Hydrogen Peroxide, Hydroxyl Radical (via Fenton reaction), the controls with Fe+2 and Fe+3 and singlet oxygen (via Photosensitization with rose bengal under PAR light), MGG accumulation was negatively affected or held constant with the exception of the treatment with Hydrogen Peroxide (H2O2) at a concentration of 0.01 mM, where a slightly positive effect was observed on the accumulation MGG. On the contrary, the accumulation of CAR was stimulated by a larger number of factors, mainly ethanol and singlet oxygen. Results are detailed in Table 3.
Sequential extraction of CAR and MGG
The extraction with water for 4 hs at 85 °C allowed to obtain 44.4 ± 4.2 mg g−1 which represent the 82% of MGG yields obtained in the extraction with ethanol, 54.1 ± 5.8 mg g−1 (p = 0.017). Subsequent extraction of CAR in those samples showed a slightly lower content, 599.4 ± 64.6 μg g−1, than those obtained for the samples without previous MGG extraction, 647.4 ± 45.2 μg g−1 (p = 0.206).
Discussion
In this study we analyzed for the first time the simultaneous production of two biotechnological relevant metabolites in a single batch to establish the best conditions for their production using a hyperproducer mutant obtained by random mutagenesis. Mutagenesis by chemical, mainly using N-Methyl-N′-nitro-N-nitrosoguanidine (NTG), or physical treatments, mainly UV radiation, are common strategies to obtain astaxanthin-hyperproducing mutants, and changes in colonies coloration is used as a screening method for their isolation (An et al. 1989; Meyer et al. 1993; Bon et al. 1997; Rubinstein et al. 1998; Schmidt et al. 2011). Using UV radiation, we successfully generated a carotenoid hyperproducer mutant (selected for its more intense color), but surprisingly the isolate 7918Mut exhibited also a twofold increase in MGG content (colorless). To the best of our knowledge, this represents the first MGG hyperproducer mutant reported, not only in yeasts, but for all mycosporinogenic microorganisms. Given that the metabolic pathways for each of these metabolites are unrelated, carotenoids are synthesized via the mevalonic acid pathway and MGG is synthetized by a cluster of at least three genes using sedoheptulose 7-phosphate as precursor (Sepúlveda et al. 2023), and considering that generation of mutants in the carotenoids content in the applied conditions are unusual (only 3 over 11,467 colonies examinated = 0.26% of the total), the simultaneous mutation of both pathways appears highly improbable. Future genome sequencing of the mutant will help to resolve whether there are common regulatory genes for the expression of both pathways. However, it’s worth considering that both metabolites play roles in yeast photoprotection and antioxidant responses (Moliné et al. 2009, 2010a, b) suggesting the possibility of a shared triggering mechanism for their synthesis, likely through genes that regulate the response to radiation or oxidative stress, as discussed later on. On the other hand, random mutagenesis frequently exerts a detrimental impact on the duplication rate (Szafraniec et al. 2003). However, growth rate of the mutant strain (μ = 0.101) closely resembled the values reported by various authors for different strains of P. rhodozyma under comparable culture conditions (Chan and Ho 1999; Ho et al. 1999; Meyer and du Preez 1994b), moreover it did not exhibit any significant deviation from the growth rate observed in the parental strain (μ = 0.103).
When we examined the kinetics of the biomass growth and the production of CAR and MGG during our experiments, we found that each of these metabolites is produced during different stages of growth. CAR are produced from the beginning of the exponential growth phase and continue until glucose is depleted, with minimal changes in their content during the stationary phase. While carotenoids are typically classified as secondary metabolites in most organisms, it’s important to note that in P. rhodozyma, this kinetics has been previously well-documented (Johnson and Lewis 1979), revealing that CAR are considered primary metabolites in this particular species. In contrast, the accumulation of MGG only begins after entering the stationary phase, and approximately 52 hs later (totaling 100 hs of culture), it reaches its maximum concentration, nearly 100 mg g−1 (under conditions of 10 g L−1 glucose and C/N ratio 3). MGG yields obtained in this study with P. rhodozyma surpass those observed in other microorganisms. For instance, mycosporines produced by cyanobacterias or algaes reaches less than 10 mg g−1 within seven to nine days (Portwich and Garcia-Pichel 1999; Korbee et al. 2005), and the fungus Thichothecium roseum achieves a maximum of 25 mg g−1 (Favre-Bonvin et al. 1987). Our findings suggest that MGG is indeed a secondary metabolite of P. rhodozyma, and it´s accumulation only occurs after reaching the stationary phase. However, it is important to highlight that due to the relatively short cultivation times for P. rhodozyma and the substantial accumulation of this metabolite in our mutant, we achieve significant quantities of MGG within a short culture period compared to other microbial sources.
On the other hand, the impact of C/N ratio reveals the existence of a trade-off between maximizing each metabolite. Generally, an increase in the nitrogen source (lower C/N ratio) leads to greater accumulation of MGG and a lower accumulation of pigments and vice versa. While the effect on carotenoids is in line with previous observations in other P. rhodozyma strains using different nitrogen sources (Yamane et al. 1997; Flores-Cotera et al. 2001; Vustin et al. 2004), it is important to note that this response is not universal among yeasts. For instance, in Rhodotorula glutinis, carotenogenesis is favored by a low C/N ratio (Sakaki et al. 1999), while in Rhodotorula mucilaginosa production remains unaffected by the C/N ratio (Libkind et al. 2004). Hence, the relationship between the C/N ratio and the accumulation of carotenoids is species-dependent. In contrast, a lower C/N ratio significantly impacts the accumulation of MGG, reaching nearly 100 mg g−1 (which represents 10% of the dry biomass weight). The positive influence between high nitrogen content and mycosporine production in cyanobacteria and dinoflagellates is well-established (Peinado et al. 2004; Figueroa et al. 2008; Korbee et al. 2010). However, this is the first report documenting the effect of nitrogen on the accumulation of MGG produced by yeast. Our findings underscore the fundamental role of nitrogen in the accumulation of these metabolites in P. rhodozyma and should be considered a crucial factor in designing culture media for industrial metabolite production.
Beyond the C/N ratio, we observed that a high initial glucose concentration has a negative impact on the accumulation of both compounds. This observation aligns with previous findings by other authors, such as Johnson and Lewis (1979) and Meyer and du Preez (1994a), who determined that glucose concentrations exceeding 1.5% w/v hindered the accumulation of astaxanthin, leading to a yield dropping below 300 μg g−1 at glucose concentrations exceeding 3%. Although our strain exhibited a higher yield (480–620 μg g−1), we observed a similar effect caused by the high initial glucose concentration. In a similar trend, we found that MGG accumulation was strongly inhibited by glucose concentrations, resulting in nearly 90% reduction in yield with a concentration of 30 g L−1. The repression of carotenogenesis in Phaffia rhodozyma under these conditions might be linked to the Crabtree effect, which involves the alcoholic fermentation of sugars in the presence of oxygen (Reynders et al. 1997) and by the repression of three genes involved in mycosporinogenesis (Miao et al. 2019). Future research will explore the impact of non-fermentable carbon sources on the accumulation of these metabolites.
Regardless of the culture conditions, numerous factors have been extensively studied in recent decades to determine their impact on P. rhodozyma carotenogenesis. In this study, we explored, for the first time, how those factors affect MGG accumulation as well. In this section, we demonstrate that only light exposure and low concentrations of H2O2 (during stationary growth phase) have a positive effect on the accumulation of both metabolites. Carotenoids and MGG are important compounds for UV-B resistance in different yeasts species (Moliné et al. 2009, 2010a, b, 2014; Villarreal et al. 2016) and their production is photoinduced (Johnson and Lewis 1979; An and Johnson 1990; Meyer and Du Preez 1994a, b; Libkind et al. 2006, 2009; Libkind et al. 2011a, b; Stachowiak 2013b). Furthermore both molecules exhibit antioxidant activity, shielding yeasts from reactive oxygen species (Schroeder and Johnson 1995; Moliné et al. 2010a), and in the particular case of H2O2 it has been established that low concentrations induces astaxanthin content in P. rhodozyma (An et al. 1996; Santopietro et al. 1998; Frengova and Beshkova 2009; Zhang et al. 2019). Here, we established that these two factors are also important for MGG production in P. rhodozyma and could be harnessed for the simultaneous biotechnological production of both metabolites. This suggests a general response in this species to radiation and oxidative stress. In this context, recent findings described that the expression of gene GST1, encoding for a glutathione S-transferase, enhances the production of astaxanthin (Shi et al. 2022). This family of enzymes is known to be involved in the oxidative stress response, in particular with peroxidase activities of yeasts (Veal et al. 2002), and these findings serve as evidence that general systems for regulating responses to oxidative stress exist.
As we discussed above the obtention of a mutant hyperproducer for both compounds suggest that exist a common genetic system that triggers their synthesis but at the same time the production of both compounds appears to be decoupled, not only in terms of their biosynthetic pathways but also in the growth phase in which they are produced. While the genes responsible in carotenogenesis and mycosporinogenesis have been previously characterized in Phaffia rhodozyma CBS 7918 (Bellora et al. 2016), those related with the regulation of each pathway remain unidentified. Despite demonstrating that both compounds are produced in different moments and conditions, the results of our study strongly suggest the existence of a common network that triggers the production of both metabolites. This phenomenon is likely a response to direct and indirect stress induced by radiation exposure.
All the other factors we investigated had either detrimental or contrasting effects on the accumulation of CAR and MGG. Notably, NaCl and Fe+2 + H2O2 (Fenton reaction) resulted in lower concentrations of both compounds compared to the control. In some yeast species like Rhodotorula glutinis, R. mucilaginosa, R. aurantiaca, Sporidiobolus salmonicolor, or Sporidiobolus pararoseus, high concentrations of NaCl have been found to have positive effects on CAR production (Bhosale and Gadre 2001; Li et al. 2017; Marova et al. 2004). Similarly, NaCl positive effect has been reported for mycosporine accumulation in black fungi (Kogej et al. 2006, 2007), which is in line with the hypothesis that these molecules could also act as compatible osmolytes (Oren and Gunde-Cimerman 2007). However, our findings indicate that saline stress, even at low levels, negatively impacts the growth rate, growth yield, and specific productivity of both metabolites in P. rhodozyma suggesting that MGG and carotenoids do not play a role in protecting against osmotic stress in this species. In the same trend, no significant differences were observed in the treatments with Fe+2 + H2O2, either from the beginning of the culture or in the stationary phase, over the accumulation of carotenoids or MGG. The hydroxyl radical generated by the Fenton reaction (Fe+2 + H2O2 → OH− + OH. + Fe+3) was previously shown to have positive effects on astaxanthin accumulation (up to 30%) under conditions similar to those applied here (Santopietro et al. 1998). However, in our mutant strain only a small change in yield was observed regardless of the concentration of the reactant H2O2, suggesting that this response may not be universal in this species. On the contrary, ethanol and singlet oxygen (generated by photosensitization with rose bengal) boost carotenoids accumulation but have a notably adverse effect on MGG. The impact of ethanol on astaxanthin production in P rhodozyma has been documented previously (Gu et al. 1997; Yamane et al. 1997). Ethanol disrupts the balance of NADH/NAD, generating superoxide radicals, and also serves as a substrate for the synthesis of isoprenoids, and upregulates expression of genes involved in carotenogenesis (Marcoleta et al. 2011; Martínez-Cárdenas et al. 2018). Similarly, photogenerators of singlet oxygen (1O2) like rose bengal also are known for their influence on the antioxidant responses of yeasts (Schroeder and Johnson 1995; Brombacher et al. 2006). The effect of singlet oxygen on P. rhodozyma carotenogenesis was previously observed by Schroeder and Johnson (1995), who suggested that this effect results from the activation of specific genes that respond to singlet oxygen, although these genes have not yet been found. The findings of our work support this hypothesis and confirm that the increase in pigment accumulation is independent of the culture stage in which this oxidant is generated. The control treatment with rose bengal in the dark (where singlet oxygen is not formed), shows an accumulation of CAR and MGG similar to the treatment in total darkness without this stain. This observation allowed us to rule out a potential influence of rose bengal on the accumulation of these metabolites and attribute their higher levels of accumulation to the formation of singlet oxygen. Unexpectedly, a substantial reduction in MGG accumulation was observed (23% of the control, in the treatment with rose bengal 8 μM applied from the beginning of the culture). While MGG possesses antioxidant properties against singlet oxygen (Moliné et al. 2010a), the results of this section suggest that this reactive oxygen species does not stimulate MGG accumulation. Considering this, the lower accumulation of MGG is likely due to their oxidation and degradation with no compensatory effect to stimulate its synthesis.
Finally, the sequential extraction of MGG and carotenoids allowed us to demonstrate the feasibility of obtaining both biotechnologically significant products from a single culture.
It’s worth noting that the extraction of yeast´s MGG using hot water was first observed within our research group, resulting in an invention patent (van Broock et al. 2009), In this work, we showed that the water extraction method results in a slightly lower yield compared to the extraction with ethanol. However, it represents an extremely promising method as it not only utilizes the most cost-effective and green solvent available but also because it allows to obtain high concentrations of the metabolite without extracting other less polar compounds (for example, ethanol extraction also results in the extraction of carotenoids) (Tognetti et al. 2013). On the other hand, we have now further illustrated that the residual cells left after this extraction process can be effectively utilized for the subsequent extraction of astaxanthin, with minimal impact on the overall process. Undoubtedly, this procedure is ideal for obtaining both metabolites from a single culture and adds high value to the biotechnological production of this yeast species.
In conclusion, in this study we utilized the biotechnologically relevant yeast P. rhodozyma and demonstrated the feasibility of obtaining two commercially significant metabolites in a single batch for the first time, thereby adding new value to their production. The mutant obtained in this study exhibits unique characteristics due to its high specific yield of both metabolites, being the most relevant strain for industrial exploitation.
Although for most of the conditions studied here, CAR and MGG seem to compete in their production, and therefore, when we optimize one product, it has a negative effect on the accumulation of the other, in this work, we demonstrate that it is possible to achieve a compromise for both with an intermediate C/N ratio. Additionally, we show that the production of both can be stimulated by radiation or small concentrations of hydrogen peroxide. All these conditions are a first step towards optimizing the production of each metabolite, or even achieving intermediate values according to the industrial requirements. This study serves as an initial stepping stone for future work where the production system can be further enhanced and scaled up, or new conditions can be explored to further increase the productivity of these biotechnologically relevant compounds.
Data availability
The data underlying this article are available in the article and in its online supplementary material. The raw datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
An G-H, Johnson EA (1990) Influence of light on growth and pigmentation of the yeast Phaffia rhodozyma. Antonie Van Leeuwenhoek 57:191–203. https://doi.org/10.1007/BF00400151
An G-H, Schuman DB, Johnson EA (1989) Isolation of Phaffia rhodozyma mutants with increased astaxanthin content. Appl Environ Microbiol 55:116–124. https://doi.org/10.1128/aem.55.1.116-124.1989
An G-H, Chang K-W, Johnson E-A (1996) Effect of oxygen radicals and aeration on carotenogenesis and growth of Phaffia rhodozyma (Xanthophyllomyces dendrorhous). J Microbiol Biotechnol 6:103–109
Andrewes AG, Phaff HJ, Starr MP (1976) Carotenoids of Phaffia rhodozyma, a red-pigmented fermenting yeast. Phytochemistry 15:1003–1007. https://doi.org/10.1016/S0031-9422(00)84390-3
Bandaranayake WM (1998) Mycosporines: are they nature’s sunscreens? Nat Prod Rep 15:159–172. https://doi.org/10.1039/A815159Y
Barbachano-Torres A, Ramos-Valdivia AC, Cerda-García-Rojas CM et al (2012) Carotenogenesis induction with hydrogen peroxide in Xanthophyllomyces dendrorhous colored mutants. Microb Appl Res:598–602. https://doi.org/10.1142/9789814405041_0122
Bellora N, Moliné M, David-Palma M et al (2016) Comparative genomics provides new insights into the diversity. physiology. and sexuality of the only industrially exploited tremellomycete: Phaffia rhodozyma. BMC Genomics 17:1–16. https://doi.org/10.1186/s12864-016-3244-7
Bhosale P, Gadre RV (2001) Production of β-carotene by a Rhodotorula glutinis mutant in sea water medium. Bioresour Technol 76:53–55. https://doi.org/10.1016/S0960-8524(00)00075-4
Bjørklund G, Gasmi A, Lenchyk L et al (2022) The role of Astaxanthin as a nutraceutical in health and age-related conditions. Molecules 27:7167. https://doi.org/10.3390/molecules27217167
Bon JA, Leathers TD, Jayaswal RK (1997) Isolation of astaxanthin-overproducing mutants of Phaffia rhodozyma. Biotechnol Lett 19:109–112. https://doi.org/10.1023/A:1018391726206
Bouillant M-L, Pittet J-L, Bernillon J et al (1981) Mycosporins from Ascochyta pisi Cladosporium Herbarum and Septoria Nodorum. Phytochemistry 20:2705–2707. https://doi.org/10.1016/0031-9422(81)85272-7
Brendler T, Williamson EM (2019) Astaxanthin: how much is too much? A Safety Review. Phytother Res 33:3090–3111. https://doi.org/10.1002/ptr.6514
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids: handbook. Springer Science & Business Media
Brombacher K, Fischer BB, Rüfenacht K et al (2006) The role of Yap1p and Skn7p-mediated oxidative stress response in the defence of Saccharomyces cerevisiae against singlet oxygen. Yeast 23:741–750. https://doi.org/10.1002/yea.1392
Calo P, Velazquez JB, Sieiro C et al (1995) Analysis of astaxanthin and other carotenoids from several Phaffia rhodozyma mutants. J Agric Food Chem 43:1396–1399. https://doi.org/10.1021/jf00053a049
Capelli B, Bagchi D, Cysewski GR (2013) Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 12:145–152. https://doi.org/10.1007/s13749-013-0051-5
Chan HY, Ho KP (1999) Growth and carotenoid production by pH-stat cultures of Phaffia rhodozyma. Biotechnol Lett 21:953–958. https://doi.org/10.1023/A:1005638610564
Chrapusta E, Kaminski A, Duchnik K et al (2017) Mycosporine-Like amino acids: potential health and beauty ingredients. Mar Drugs 15:326. https://doi.org/10.3390/md15100326
Colabella F, Moline M, Libkind D (2014) UV sunscreens of microbial origin: mycosporines and mycosporine- like aminoacids. Recent Pat Biotechnol 8:179–193. https://doi.org/10.2174/1872208309666150102104520
Domínguez-Bocanegra AR, Torres-Muñoz JA (2004) Astaxanthin hyperproduction by Phaffia rhodozyma (now Xanthophyllomyces dendrorhous) with raw coconut milk as sole source of energy. Appl Microbiol Biotechnol 66:249–252. https://doi.org/10.1007/s00253-004-1686-3
Ducrey Santopietro LM, Spencer JFT, Siñeriz F (1998) Fed-batch and continuous culture of Phaffia rhodozyma (Xanthophyllomyces dendrorhous). Folia Microbiol 43:169–172. https://doi.org/10.1007/BF02816504
Fang TJ, Cheng Y-S (1993) Improvement of astaxanthin production by Phaffia rhodozyma through mutation and optimization of culture conditions. J Ferment Bioeng 75:466–469. https://doi.org/10.1016/0922-338X(93)90099-T
Favre-Bonvin J, Bernillon J, Salin N et al (1987) Biosynthesis of mycosporines: mycosporine glutaminol in Trichothecium roseum. Phytochemistry 26:2509–2514. https://doi.org/10.1016/S0031-9422(00)83866-2
Figueroa FL, Bueno A, Korbee N et al (2008) Accumulation of mycosporine-like amino acids in Asparagopsis armata grown in tanks with fishpond effluents of Gilthead Sea Bream. Sparus Aurata J World Aquac Soc 39:692–699. https://doi.org/10.1111/j.1749-7345.2008.00199.x
Florêncio JA, Soccol CR, Furlanetto LF et al (1998) A factorial approach for a sugarcane juice-based low cost culture medium: increasing the astaxanthin production by the red yeast Phaffia rhodozyma. Bioprocess Eng 19:161–164. https://doi.org/10.1007/PL00009008
Flores-Cotera LB, Sánchez S (2001) Copper but not iron limitation increases astaxanthin production by Phaffia rhodozyma in a chemically defined medium. Biotechnol Lett 23:793–797. https://doi.org/10.1023/A:1010358517806
Flores-Cotera L, Martín R, Sánchez S (2001) Citrate a possible precursor of astaxanthin in Phaffia rhodozyma: influence of varying levels of ammonium, phosphate and citrate in a chemically defined medium. Appl Microbiol Biotechnol 55:341–347. https://doi.org/10.1007/s002530000498
Frengova GI, Beshkova DM (2009) Carotenoids from Rhodotorula and Phaffia: yeasts of biotechnological importance. J Ind Microbiol Biotechnol 36:163. https://doi.org/10.1007/s10295-008-0492-9
Gervasi T, Pellizzeri V, Benameur Q et al (2018) Valorization of raw materials from agricultural industry for astaxanthin and β-carotene production by Xanthophyllomyces dendrorhous. Nat Prod Res 32:1554–1561. https://doi.org/10.1080/14786419.2017.1385024
Gu W-L, An G-H, Johnson EA (1997) Ethanol increases carotenoid production in Phaffia rhodozyma. J Ind Microbiol Biotechnol 19:114–117. https://doi.org/10.1038/sj.jim.2900425
Ho KP, Tam CY, Zhou B (1999) Growth and carotenoid production of Phaffia rhodozyma in fed-batch cultures with different feeding methods. Biotechnol Lett 21:175–178. https://doi.org/10.1023/A:1005487709974
Hussein G, Sankawa U, Goto H, Matusumoto KH, Watanabe (2006) Astaxanthin a carotenoid with potential in human health and nutrition. J Nat Prod 69:443–449. https://doi.org/10.1021/np050354+
Jacobson GK, Jolly SO, Sedmak JJ et al (2002) Astaxanthin over-producing strains of Phaffia rhodozyma. methods for their cultivation. and their use in animal feeds. Patent US6015684A
Jiang G-L, Zhou L-Y, Wang Y-T et al (2017) Astaxanthin from Jerusalem artichoke: production by fed-batch fermentation using Phaffia rhodozyma and application in cosmetics. Process Biochem 63:16–25. https://doi.org/10.1016/j.procbio.2017.08.013
Johnson EA (2003) Phaffia rhodozyma: colorful odyssey. Int Microbiol 6:169–174. https://doi.org/10.1016/10.1007/s10123-003-0130-3
Johnson EA, An G-H (1991) Astaxanthin from microbial sources. Crit Rev Biotechnol 11:297–326. https://doi.org/10.3109/07388559109040622
Johnson EA, Lewis MJY (1979) Astaxanthin formation by the yeast Phaffia rhodozyma. Microbiology 115:173–183. https://doi.org/10.1099/00221287-115-1-173
Johnson EA, Villa TG, Lewis MJ (1980) Phaffia rhodozyma as an astaxanthin source in salmonid diets. Aquaculture 20:123–134. https://doi.org/10.1016/0044-8486(80)90041-1
Kogej T, Gostinčar C, Volkmann M et al (2006) Mycosporines in extremophilic fungi—novel complementary osmolytes? Environ Chem 3:105–110. https://doi.org/10.1071/EN06012
Kogej T, Stein M, Volkmann M, Gorbushina AA et al (2007) Osmotic adaptation of the halophilic fungus Hortaea werneckii, role of osmolytes and melanization. Microbiol 153:4261–4273. https://doi.org/10.1099/mic.0.2007/010751-0
Korbee N, Figueroa FL, Aguilera J (2005) Effect of light quality on the accumulation of photosynthetic pigments. proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta). J Photochem Photobiol B 80:71–78. https://doi.org/10.1016/j.jphotobiol.2005.03.002
Korbee N, Teresa Mata M, Figueroa FL (2010) Photoprotection mechanisms against ultraviolet radiation in Heterocapsa sp. (Dinophyceae) are influenced by nitrogen availability: mycosporine-like amino acids vs. xanthophyll cycle. Limnol Oceanogr 55:899–908. https://doi.org/10.4319/lo.2010.55.2.0899
Lai J-X, Chen X, Bu J et al (2022) Direct production of astaxanthin from food waste by Phaffia rhodozyma. Process Biochem 113:224–233. https://doi.org/10.1016/j.procbio.2022.01.003
Li C, Zhang N, Li B et al (2017) Increased torulene accumulation in red yeast Sporidiobolus pararoseus NGR as stress response to high salt conditions. Food Chem 237:1041–1047. https://doi.org/10.1016/j.foodchem.2017.06.033
Libkind D, Brizzio S, van Broock M (2004) Rhodotorula mucilaginosa a carotenoid producing yeast strain from a patagonian high-altitude lake. Folia Microbiol 49:19–25. https://doi.org/10.1007/BF02931640
Libkind D, Sommaruga R, Zagarese H et al (2005) Mycosporines in carotenogenic yeasts. Syst Appl Microbiol 28:749–754. https://doi.org/10.1016/j.syapm.2005.05.005
Libkind D, Diéguez MC, Moliné M et al (2006) Occurrence of photoprotective compounds in yeasts from freshwater ecosystems of northwestern Patagonia (Argentina). Photochem Photobiol 82:972–980. https://doi.org/10.1562/2005-09-09-RA-679
Libkind D, Moliné M, de García V et al (2008) Characterization of a novel South American population of the astaxanthin producing yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma). J Ind Microbiol Biotechnol 35:151–158. https://doi.org/10.1007/s10295-007-0275-8
Libkind D, Moliné M, Sampaio JP et al (2009) Yeasts from high-altitude lakes: influence of UV radiation. FEMS Microbiol Ecol 69:353–362. https://doi.org/10.1111/j.1574-6941.2009.00728.x
Libkind D, Moline M, van Broock M (2011a) Production of the UVB-absorbing compound mycosporine–glutaminol–glucoside by Xanthophyllomyces dendrorhous (Phaffia rhodozyma). FEMS Yeast Res 11:52–59. https://doi.org/10.1111/j.1567-1364.2010.00688.x
Libkind D, Moliné M, Sommaruga R et al (2011b) Phylogenetic distribution of fungal mycosporines within the Pucciniomycotina (Basidiomycota). Yeast 28:619–627. https://doi.org/10.1002/yea.1891
Libkind D, Moliné M, Colabella F (2018) Isolation and selection of new astaxanthin-producing strains of Phaffia rhodozyma. In: Barreiro C, Barredo J-L (eds) Microbial carotenoids: methods and protocols. Springer, New York, pp 297–310. https://doi.org/10.1007/978-1-4939-8742-9_18
Lim KC, Yusoff FM, Shariff M et al (2018) Astaxanthin as feed supplement in aquatic animals. Rev Aquac 10:738–773. https://doi.org/10.1111/raq.12200
Liu YS, Wu JY (2006) Hydrogen peroxide-induced astaxanthin biosynthesis and catalase activity in Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol 73:663–668. https://doi.org/10.1007/s00253-006-0501-8
Liu YS, Wu JY (2007) Perfusion culture process plus H2O2 stimulation for efficient astaxanthin production by Xanthophyllomyces dendrorhous. Biotechnol Bioeng 97:568–573. https://doi.org/10.1002/bit.21256
Lorenz RT, Cysewski GR (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18:160–167. https://doi.org/10.1016/S0167-7799(00)01433-5
Luna-Flores CH, Wang A, von Hellens J, Speight R (2022) Towards commercial levels of astaxanthin production in Phaffia rhodozyma. J Biotechnol 350:42–54. https://doi.org/10.1016/j.jbiotec.2022.04.001
Marcoleta A, Niklitschek M, Wozniak A et al (2011) Glucose and ethanol-dependent transcriptional regulation of the astaxanthin biosynthesis pathway in Xanthophyllomyces dendrorhous. BMC Microbiol 11:190. https://doi.org/10.1186/1471-2180-11-190
Marova I, Breierova E, Koci R et al (2004) Influence of exogenous stress factors on production of carotenoids by some strains of carotenogenic yeasts. Ann Microbiol 54:73–86
Martínez-Cárdenas A, Chávez-Cabrera C, Vasquez-Bahena JM et al (2018) A common mechanism explains the induction of aerobic fermentation and adaptive antioxidant response in Phaffia rhodozyma. Microb Cell Factories 17:53. https://doi.org/10.1186/s12934-018-0898-7
Meyer PS, du Preez JC (1994a) Astaxanthin production by a Phaffia rhodozyma mutant on grape juice. World J Microbiol Biotechnol 10:178–183. https://doi.org/10.1007/BF00360882
Meyer PS, Du Preez JC (1994b) Photo-regulated astaxanthin production by Phaffia rhodozyma mutants. Syst Appl Microbiol 17:24–31. https://doi.org/10.1016/S0723-2020(11)80027-5
Meyer PS, du Preez JC, Kilian SG (1993) Selection and evaluation of astaxanthin-overproducing mutants of Phaffia rhodozyma. World J Microbiol Biotechnol 9:514–520. https://doi.org/10.1007/BF00386286
Miao L, Chi S, Wu M et al (2019) Deregulation of phytoene-β-carotene synthase results in derepression of astaxanthin synthesis at high glucose concentration in Phaffia rhodozyma astaxanthin-overproducing strain MK19. BMC Microbiol 19:133. https://doi.org/10.1186/s12866-019-1507-6
Moliné M, Libkind D, del Carmen DM et al (2009) Photoprotective role of carotenoids in yeasts: response to UV-B of pigmented and naturally-occurring albino strains. J Photochem Photobiol B 95:156–161. https://doi.org/10.1016/j.jphotobiol.2009.02.006
Moliné M, Arbeloa EM, Flores MR et al (2010a) UVB photoprotective role of mycosporines in yeast: photostability and antioxidant activity of mycosporine-glutaminol-glucoside. Radiat Res 175:44–50. https://doi.org/10.1667/RR2245.1
Moliné M, Flores MR, Libkind D et al (2010b) Photoprotection by carotenoid pigments in the yeast Rhodotorula mucilaginosa: the role of torularhodin. Photochem Photobiol Sci 9:1145–1151. https://doi.org/10.1039/c0pp00009d
Moliné M, Libkind D, de Garcia V et al (2014) Production of Pigments and photo-protective compounds by cold-adapted yeasts. In: Buzzini P, Margesin R (eds) Cold-adapted yeasts: biodiversity. adaptation strategies and biotechnological significance. Springer, Berlin/Heidelberg, pp 193–224. https://doi.org/10.1007/978-3-642-39681-6_9
Ni H, Chen Q, Ruan H et al (2007) Studies on optimization of nitrogen sources for astaxanthin production by Phaffia rhodozyma. J Zhejiang Univ Sci B 8:365–370. https://doi.org/10.1631/jzus.2007.B0365
Nutakor C, Kanwugu ON, Kovaleva EG et al (2022) Enhancing astaxanthin yield in Phaffia rhodozyma: current trends and potential of phytohormones. Appl Microbiol Biotechnol 106:3531–3538. https://doi.org/10.1007/s00253-022-11972-5
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269:1–10. https://doi.org/10.1111/j.1574-6968.2007.00650.x
Paz S, Tzafra C, Yael L, Khutorian M et al (2022) Astaxanthin over-producing strains of Phaffia rhodozyma. Patent WO2021019409A1
Peinado NK, Abdala Díaz RT, Figueroa FL et al (2004) Ammonium and UV Radiation stimulate the accumulation of Mycosporine-like amino acids in Porphyra columbina (rhodophyta) from Patagonia, Argentina. J Phycol 40:248–259. https://doi.org/10.1046/j.1529-8817.2004.03013.x
Portwich A, Garcia-Pichel F (1999) Ultraviolet and osmotic stresses induce and regulate the synthesis of mycosporines in the cyanobacterium Chlorogloeopsis PCC 6912. Arch Microbiol 172:187–192. https://doi.org/10.1007/s002030050759
Ramı́rezGutierrezGschaedler JHA (2001) Optimization of astaxanthin production by Phaffia rhodozyma through factorial design and response surface methodology. J Biotechnol 88:259–268. https://doi.org/10.1016/S0168-1656(01)00279-6
Reynders MB, Rawlings DE, Harrison STL (1996) Studies on the growth. modelling and pigment production by the yeast Phaffia rhodozyma during fed-batch cultivation. Biotechnol Lett 18:649–654. https://doi.org/10.1007/BF00130759
Reynders MB, Rawlings DE, Harrison STL (1997) Demonstration of the Crabtree effect in Phaffia rhodozyma during continuous and fed-batch cultivation. Biotechnol Lett 19:549–552. https://doi.org/10.1023/A:1018341421122
Rosic NN (2019) Mycosporine-like amino acids: making the foundation for organic personalised sunscreens. Mar Drugs 17:638. https://doi.org/10.3390/md17110638
Rubinstein L, Altamirano A, Santopietro LD et al (1998) Isolation and characterization of Phaffia rhodozyma mutants. Folia Microbiol 43:626–630. https://doi.org/10.1007/BF02816380
Sakaki H, Nochide H, Nakanishi T et al (1999) Effect of culture condition on the biosynthesis of carotenoids in Rhodotorula glutinis No. 21. J Biosci Bioeng 3:400
Sanderson GW, Jolly SO (1994) The value of Phaffia yeast as a feed ingredient for salmonid fish. Aquaculture 124:193–200. https://doi.org/10.1016/0044-8486(94)90377-8
Santopietro LMD, Spencer JFT, Spencer DM et al (1998) Effects of oxidative stress on the production of carotenoid pigments by Phaffia rhodozyma (Xanthophyllomyces dendrorhous). Folia Microbiol 43:173–176. https://doi.org/10.1007/BF02816505
Schmid D, Schürch C, Zülli F (2006) Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmet 9
Schmidt I, Schewe H, Gassel S et al (2011) Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol 89:555–571. https://doi.org/10.1007/s00253-010-2976-6
Schroeder WA, Johnson EA (1995) Singlet oxygen and peroxyl radicals regulate carotenoid biosynthesis in Phaffia rhodozyma. J Biol Chem 270:18374–18379. https://doi.org/10.1074/jbc.270.31.18374
Sepúlveda D, Campusano S, Moliné M et al (2023) Unraveling the molecular basis of mycosporine biosynthesis in fungi. Int J Mol Sci 24:5930. https://doi.org/10.3390/ijms24065930
Shi Z, He X, Zhang H et al (2022) Whole genome sequencing and RNA-seq-driven discovery of new targets that affect carotenoid synthesis in Phaffia rhodozyma. Front Microbiol 13:837894. https://doi.org/10.3389/fmicb.2022.837894
Silva CM, Borba TM, Burkert CAV, Burkert JFM (2012) Carotenoid production by Phaffia rhodozyma using raw glycerol as an additional carbon source. Int J Food Eng 8. https://doi.org/10.1515/1556-3758.2843
Singh A, Čížková M, Bišová K et al (2021) Exploring mycosporine-like amino acids (MAAs) as safe and natural protective agents against UV-induced skin damage. Antioxidants 10:683. https://doi.org/10.3390/antiox10050683
Sommaruga R, Libkind D, van Broock M et al (2004) Mycosporine-glutaminol-glucoside. A UV-absorbing compound of two Rhodotorula yeast species. Yeast 21:1077–1081. https://doi.org/10.1002/yea.1148
Stachowiak B (2013a) Efficiency of selected mutagens in generating Xanthophyllomyces dendrorhous strains hyperproducing astaxanthin. Pol J Microbiol 62:67–72
Stachowiak B (2013b) Effect of illumination intensities on astaxanthin synthesis by Xanthophyllomyces dendrorhous and its mutants. Food Sci Biotechnol 22:1033–1038. https://doi.org/10.1007/s10068-013-0180-z
Stachowiak B, Szulc P (2021) Astaxanthin for the food industry. Molecules 26:2666. https://doi.org/10.3390/molecules26092666
Szafraniec K, Wloch DM, Sliwa P et al (2003) Small fitness effects and weak genetic interactions between deleterious mutations in heterozygous loci of the yeast Saccharomyces cerevisiae. Genet Res 82:19–31. https://doi.org/10.1017/S001667230300630X
Tognetti C, Moliné M, van Broock M et al (2013) Favored isolation and rapid identification of the astaxanthin-producing yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma) from environmental samples. J Basic Microbiol 53:766–772. https://doi.org/10.1002/jobm.201200274
Torres A, Hochberg M, Pergament I et al (2004) A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. Eur J Biochem 271:780–784. https://doi.org/10.1111/j.1432-1033.2004.03981.x
van Broock M, Libkind D, Moliné M (2009) Composiciones que absorben UVB y antioxidantes. Procedimientos y usos. Patente CONICET-UNComahue. Patent P090103845
Veal EA, Toone WM, Jones N et al (2002) Distinct roles for glutathione S-transferases in the oxidative stress response in Schizosaccharomyces pombe. J Biol Chem 277:35523–35531. https://doi.org/10.1074/jbc.M111548200
Villarreal P, Carrasco M, Barahona S et al (2016) Tolerance to ultraviolet radiation of psychrotolerant yeasts and analysis of their carotenoid, mycosporine, and ergosterol content. Curr Microbiol 72:94–101. https://doi.org/10.1007/s00284-015-0928-1
Volkmann M, Gorbushina AA (2006) A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol Lett 255:286–295. https://doi.org/10.1111/j.1574-6968.2006.00088.x
Vustin MM, Belykh EN, Kishilova SA (2004) Relationship between astaxanthin production and the intensity of anabolic processes in the yeast Phaffia rhodozyma. Microbiology 73:643–649. https://doi.org/10.1007/s11021-005-0004-0
Yamane Y, Higashida K, Nakashimada Y et al (1997) Astaxanthin production by Phaffia rhodozyma enhanced in fed-batch culture with glucose and ethanol feeding. Biotechnol Lett 19:1109–1111. https://doi.org/10.1023/A:1018492611011
Yaqoob S, Riaz M, Shabbir A et al (2021) Commercialization and marketing potential of carotenoids. In: Zia-Ul-Haq M, Dewanjee S, Riaz M (eds) Carotenoids: structure and function in the human body. Springer, Cham, pp 799–826. https://doi.org/10.1007/978-3-030-46459-2_27
Zhang J, Li Q-R, Zhang M-H et al (2019) Enhancement of carotenoid biosynthesis in Phaffia rhodozyma PR106 under stress conditions. Biosci Biotechnol Biochem 83:2375–2385. https://doi.org/10.1080/09168451.2019.1650633
Zwietering MH, Jongenburger I, Rombouts FM, Van't Riet, KJAEM (1990) Modeling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881. https://doi.org/10.1128/aem.56.6.1875-1881.1990
Acknowledgements
This work was supported by Universidad Nacional del Comahue Project (project B247), CONICET (project PIP 11220200102948CO), and FONCYT (project PICT-2017-2083). We thank Julieta Burini and Mailen Latorre for providing a for critical reading and improving the writing style of the manuscript.
Funding
This work was supported by Universidad Nacional del Comahue Project (project B247), CONICET (project PIP 11220200102948CO), and FONCYT (project PICT-2017–2083).
Author information
Authors and Affiliations
Contributions
Martín Moliné (Conceptualization, Investigation, Formal analysis, Writing – original draft), Diego Libkind (Conceptualization, Writing – review & editing, Funding acquisition), and María van Broock (Conceptualization, Supervision).
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose. No conflict of interest exits in the submission of this manuscript.
Ethics approval
Not required.
Consent to participate/consent to publish
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moliné, M., Libkind, D. & van Broock, M.R. Two at once: simultaneous increased production of astaxanthin and mycosporines in a single batch culture using a Phaffia rhodozyma mutant strain. World J Microbiol Biotechnol 40, 87 (2024). https://doi.org/10.1007/s11274-024-03901-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-03901-7