Abstract
Lichenysin, a cyclic lipopeptide biosurfactant produced by Bacillus licheniformis, is composed of aspartate, glutamine, valine, leucine, isoleucine, and branched chain fatty acids. The synthesis of these amino acids and fatty acids requires pyruvate and NADPH as the primary precursor and cofactor. Therefore, a sufficient supply of pyruvate and NADPH is crucial for lichenysin production. This study aimed to increase lichenysin production by constructing a synthetic ED pathway in B. licheniformis WX02 through introducing phosphogluconate dehydratase (encoded by gene edd) and 2-keto-3-deoxygluconate 6-phosphate aldolase (encoded by gene eda) from Escherichia coli. Additionally, the NADP+-dependent glucose-6-phosphate dehydrogenase (encoded by gene zwf) was overexpressed, resulting in an engineered strain WX02/pHY-edda(Ec)-zwf. Analysis of the fermentation process revealed that the concentrations of pyruvate, aspartate, glutamine, valine, leucine, branched-chain fatty acids (iC15:0, aC15:0, iC16:0, iC17:0), and NADPH in WX02/pHY-edda(Ec)-zwf were increased by 77.21%, 80.41%, 85.31%, 141.64%, 44.94%, 35.08%, 38.08%, 19.33%, 21.16%, and 425%, respectively, compared to the control strain WX02/pHY300, which resulted in a 45.43% increase of lichenysin titer. This work took advantage of the ED pathway to increase lichenysin production for the first time, and provides a promising strategy for boosting the productivity of biochemicals that require pyruvate and NADPH as precursor and cofactor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
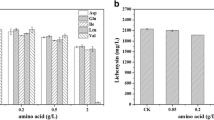
Biosurfactants have attracted extensive attention and studies due to their high surface activity, biodegradability, low toxicity, stability and environmental friendliness compared with chemical surfactants (Das et al. 2008). Lichenysin, one of the important cyclic lipopeptide biosurfactants produced by Bacillus spp., has excellent surface activity and is stable at extremes of pH, temperature, and even in salt (Nerurkar 2010; Simpson et al. 2011), which gives it a broad application prospect in various fields, such as oil exploitation, biological control of agriculture, bioremediation and drug development (Ali et al. 2019). However, the low productivity of lichenysin was not enough to support large-scale production and industrial application. Up to now, several strategies have been performed to improve lichenysin production, which include medium and culture conditions optimization, mutation breeding and promoter substitution. For instance, the yield of lichenysin in B. licheniformis BAS50 was enhanced by 2- and 4-fold, respectively, due to the addition of L-glutamic acid and L-aspartate in the culture (Joshi et al. 2015). Also, random mutagenesis occurred in B. licheniformis KGL11 by nitrosoguanidine causing a 12-fold increase on lichenysin yield (Lin et al. 1998). Besides, the promoter of lichenysin synthase operon was replaced by Psrf, which improved lichenysin titer by 16.8-fold to 2 149 mg/L (Qiu et al. 2019). Yet now, there is still a big challenge for industrial production, which is still limited by the low yield of lichenysin.
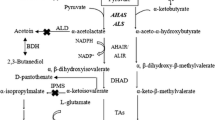
Lichenysin is a lipopeptide composed of a β-hydroxy branched chain fatty acid tail and a circular heptapeptide containing valine, leucine, isoleucine, aspartate and glutamine (Hu et al. 2022). As shown in Fig. 1, pyruvate is the common precursor of fatty acid, aspartate, glutamine, valine, leucine and isoleucine, and their synthesis also needs NADPH as a cofactor. Such as 3-oxoacyl-(acyl-carrier-protein) reductase FabG and enoyl-(acyl-carrier-protein) reductase FabL in fatty acids synthetic pathway (He et al. 2014); glutamate dehydrogenase RocG in aspartate and glutamine synthetic pathway (Yang et al. 2022); ketol-acid reductor-isomerase IlvC and aspartate-semialdehyde dehydrogenase Asd in branched chain amino acid (valine, leucine and isoleucine) synthetic pathway (Westbrook et al. 2018). Therefore, the intracellular concentration of pyruvate and NADPH might be important for lichenysin synthesis.
Pyruvate, as an important organic acid, is a key intermediate in the process of cell metabolism. For increasing the accumulation of pyruvate, Moxley et al. suppressed the carbon flux from pyruvate to acetyl coenzyme A through engineering the pyruvate dehydrogenase complex, and the engineered strain expressing AceEH106V (AceE is the E1 component of pyruvate dehydrogenase complex encoded by aceE) variant increased the accumulation of pyruvate by 11.86% to 0.66 g pyruvate/g glucose (Moxley and Eiteman 2021). Acetoin and acetate are overflow metabolites generated from pyruvate, which is not conducive to bio-chemical production because of wasting a lot of carbon fluxes. Thus, Ma et al. demonstrated that blocking the synthesis of acetoin and acetate by knocking out alsRSD, pta and ackA resulted in at least a 4.5-fold increase in intracellular pyruvate concentration, but this strategy did not successfully improve the production of the target product (N-GlcNAc) because of disturbance in metabolic fluxes (Ma et al. 2018).
In previous studies, the regeneration of NADPH is mainly achieved through the pentose phosphate pathway (PPP) and the transhydrogenase system. For instance, the NADPH/NADP+ ratio and lysine production in Corynebacterium glutamicum were increased through replacing the NAD+ dependent glyceraldehyde 3-phosphate dehydrogenase encoding gene gapA in the glycolysis pathway with the NADP+ dependent glyceraldehyde 3-phosphate dehydrogenase encoding gene gapN from Streptococcus mutans (Takeno et al. 2016). Xu et al. increased intracellular NADPH concentration by 102.86% by over-expressing NAD kinase PpnK in Corynebacterium crenatum, resulting in an improvement of polyhydroxybutyrate (PHB) yield by 15.7% (Xu et al. 2016). In Escherichia coli, over-expression of NADPH transhydrogenase UdhA was used as an effective way to increase PHB yield from 49 to 66% (g PHB / g CDW) (Sanchez et al. 2006). Therefore, increasing NADPH regeneration rates is a common strategy to enhance bio-chemical production. However, all of the above approaches have their limitations, the release of carbon dioxide within PPP would lower carbon availability and the overexpression of zwf reduced cell growth significantly (Cai et al. 2017; Zhang et al. 2014). Moreover, NAD kinase would consume a lot of NADH and ATP, not suitable for cell metabolism. To solve this contradiction, it is possible to redirect carbon flux through the Entner–Doudoroff (ED) pathway, which regenerates NADPH without a concomitant carbon loss (Ng et al. 2015).
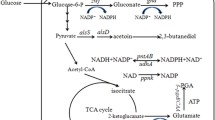
The ED pathway mainly cleaves 6-phosphogluconate into glyceraldehyde 3-phosphate and pyruvate through two steps: dehydration mediated by 6-phosphogluconate dehydratase (encoded by gene edd) and cleavage mediated by 2-keto-3-deoxygluconate-6-phosphate (KDPG) aldolase (encoded by gene eda). And then, glyceraldehyde 3-phosphate will be further converted to pyruvate, while NADH and ATP are regenerated in this process (Okano et al. 2020) (Fig. 2). In comparison, 1 mol of glucose can produce equimolar amounts of ATP, NADH, and NADPH through the ED pathway, while the well-known glycolysis pathway (EMP) can produce two moles each of NADH and ATP. Thus, ED pathway generally produces lower amount of ATP in cells, but can produce more NADPH, and has been shown to require fewer enzymes to synthesize pyruvate than glycolysis pathway (Ng et al. 2015). In addition, previous studies showed that the introduction or overexpression of the ED pathway could also significantly increase intracellular pyruvate accumulation (Liu et al. 2022; Zhang et al. 2014). Therefore, many researchers intend to introduce or overexpress the ED pathway to achieve high production of metabolites. For instance, Ng et al. constructed the synthetic ED pathway with an optimized terpenoid pathway and increased the terpenoid titer by 97% (Ng et al. 2015). In Corynebacterium glutamicum, the introduction of the exogenous ED pathway effectively enhanced glucose consumption and isobutanol productivity (Hasegawa et al. 2020).
The aim of this study was to increase the lichenysin production by improving the accumulation of intracellular pyruvate and NADPH. We attempted to construct a synthetic ED pathway in B. licheniformis WX02 to enhance NADPH generation and reduce the carbon loss, which were beneficial for the synthesis of lichenysin precursors. This work will provide a feasible strategy for improving bio-chemical productivity.
Methods
Strains and plasmids
The strains and plasmids used in this study are listed in Table 1. Escherichia coli DH5α was used for gene cloning. Expression plasmid pHY300PLK with tetracycline resistance was used for gene overexpression.
The gene edd-eda overexpressed strain was constructed as an example, in brief, P43 promoter from B. subtilis 168, gene edd-eda from Z. mobilis ZM4 and amyL terminator from B. licheniformis WX02 were respectively amplified, and fused by SOE-PCR. The fused fragment was inserted into pHY300PLK at the restriction sites EcoR I/Xba I, resulting in the plasmid pHY-edda. Then, pHY-edda was introduced into WX02 by electro-transformation, resulting in the edda overexpression strain, named WX02/pHY-edda. All primers used in this study were listed in Table 2.
Media and culture conditions
LB medium, containing 10 g L−1 tryptone, 5 g L−1 yeast extract, and 10 g L−1 NaCl, was used for pre-cultures. Lichenysin fermentation medium comprised the following: 30 g L−1 glucose, 4 g L−1 NH4SO4, 5 g L−1 NaNO3, 8.2 g L−1 KH2PO4, 28.7 g L−1 Na2HPO4·12H2O, 0.11 g L−1 FeSO4·7H2O, 0.07 g L−1 MnSO4·H2O, 0.2 g L−1 MgSO4·7H2O, 0.78 mg L−1 CaCl2, 1.2 mg L−1 EDTA, pH 7.0. For fermentation of lichenysin, cells were pre-cultured in 50 mL LB medium for 12 h at 37 °C with 230 rpm as seed medium, then 3% (v/v) of seeds were inoculated into 50 mL lichenysin fermentation medium and cultured for 36 h at 37°C with 230 rpm.
Analytical methods
Cell growth was measured by measuring the absorbance at 600 nm (OD600) using a spectrophotometer (UV-160A; Shimadzu, Japan). Residual glucose was measured using a biosensor analyzer (SBA 40C, Shandong Academy of Sciences, China) with an immobilized enzyme membrane.
The concentration of lichenysin was measured using HPLC (Agilent 1260 series; Agilent Technologies) equipped with a C18 column (Hypersil ODS2, 5 μm, 4.6 mm × 150 mm; Elite, China) and an ultraviolet detector as follows. The pH of supernatant separated from 8 mL of culture broth was adjusted to 2.0, and centrifuged at 12 000 rpm for 8 min after quiescence at 4 °C overnight. Then, the precipitation was resuspended with 1.6 mL methanol and stilled for 1 h to dissolve lichenysin. The mobile phase consisted of 100% acetonitrile-trifluoroacetic acid (3.8 mM) as solvent A and 80% acetonitrile-trifluoroacetic acid (3.8 mM) as solvent B. The eluted conditions were as follows: 50% solvent A at 0–3 min, then raised from 50 to 60% in 12 min, then quickly dropped to 50% and kept for 5 min. The flow rate was 0.9 mL/min at 25 °C, and the substances eluted were monitored by UV absorption at 210 nm.
The concentration of intracellular pyruvate was detected with the method reacting with 2, 4-dinitrophenylhydrazine (DNPH). The experimental procedure was described as follows. Dissolved 594 mg DNPH powder into 1000 mL 2 M hydrochloric acid as a DNP reagent for subsequent experiments. Before detecting the intracellular pyruvate concentration, wet cells from 1 mL broth were collected and washed twice with 0.85% NaCl solution. And then, collected cells were broken up by a cell crusher and resuspended with 500 μL deionized water. Subsequently, 100 μL of supernatant liquid was obtained and reacted with 1 mL of DNP reagent at 37 °C for 20 min. After the reaction, 10 mL 0.4 M NaOH solution was added to the mixture to stop the reaction. Finally, the absorbance of the supernatant was detected with spectrophotometer at a wavelength of 520 nm. The different concentration of pyruvate standard was linked with the OD520 nm for calculating pyruvate concentration in samples.
The concentration of amino acids was measured using HPLC (Agilent 1260). Wet cells from 2 mL fermentation broth were collected and washed twice with 0.85% NaCl solution. The cell pellets were suspended with 0.5 mL deionized water and crushed by the cell crusher at 50 Hz for 4 min (the cell crusher was stopped for 3 s every 10 s). Then, 100μL 1 M triethylamine solution (dissolved with acetonitrile) and 100μL 0.2 M phenyl isothiocyanate solution (dissolved with acetonitrile) were added into 200 μL supernatant samples, mixed and reacted at room temperature for 1 h. Subsequently, 400 μL n-hexane was added into the reaction mixture and violently shaken for 5–10 s. After the mixture was stratified, 200 μL of subnatant was mixed with 800 μL of water, and then filtered with 0.22 μm needle filter. Agilent 1260 high performance liquid chromatograph was used to detect the concentration of amino acids. The column was Ultimate Amino Acid Plus (300 × 4.6 mm, 5 μm, SN 60210503021). The flow rate was 1.0 mL/min, the detection wavelength was UV 254 nm, the column temperature was set at 45ºC, and the sample volume was 10 μL. Mobile phase A was 0.05 M sodium acetate solution (pH 6.5), and mobile phase B was a mixture with 20% of methanol, 60% of acetonitrile and 20% of deionized water. Gradient program was set as follows: Mobile phase A was dropped to 52% from 95% and mobile phase B was increased to 48% from 5% in 39 min; then, mobile phase B was set as 100% in the following 6 min; after that, mobile phase A was increased to 95% and mobile phase B was dropped to 5% in 1 min and stayed for 14 min to the end.
The concentration of fatty acids was measured using GC–MS (Gas chromatograph Trace, Thermo; Triple Quadrupole Mass Spectrometer, Thermo; column: TG-5MS, 30 m × 0.25 mm × 0.25 mm, Thermo; USA) when they were methylated by methanol. The detailed methods were according to the previous study. The concentrations of by-products (acetoin, 2,3-butanediol and acetate) were determined according to the method described previously by using GC (7890B; Agilent Technologies, USA) equipped with a TG-WAX column (0.25 μm, 30 m × 0.25 mm). The concentration of NADPH was detected by using the NADP+/NADPH Assay Kit, and the concentration of NADH was detected by using NAD+/NADH Assay Kit. Detailed experimental procedure was referred to the instructions.
Statistical analysis
All samples were analyzed in triplicate, and the data were presented as the mean ± standard deviation for each sample point. Significant differences were determined by T-test. Statistical significance was defined as p < 0.05, p < 0.01 or p < 0.005, and p < 0.05 means a statistical difference, p < 0.01 means a significant statistical difference, and p < 0.005 means an extremely significant statistical difference.
Results
Effects of genes edd and eda overexpression on lichenysin production
Phosphogluconate dehydratase and 2-dehydro-3-deoxy-phosphogluconate aldolase are key enzymes in the ED pathway, which are encoded by gene edd and eda respectively. However, the edd gene is not present in B. licheniformis. Thus, edd and eda genes from E. coli or Z. mobilis were introduced to the B. licheniformis WX02 through co-expression plasmid pHY-edda and the engineered strains WX02/pHY-edda(Ec) and WX02/pHY-edda(ZMO) containing complete ED pathway were obtained (Fig. 3a). Then, the strain WX02/pHY300 was used as the control to evaluate the effect of ED pathway on lichenysin production. As shown in Fig. 3b, WX02/pHY-edda(Ec) could produce 179.58 mg/L lichenysin, which increased by 27.65% compared with that of the control strain (140.68 mg/L). While the lichenysin titer of WX02/pHY-edda(ZMO) was 100.90 mg/L, which was decreased by 28.28% compared with that of the control strain. By detecting the concentration of by-products, it was found that the acetate concentration in WX02/pHY-edda(ZMO) was 6.02 g/L, increased by 54.36% compared with the control strain (3.91 g/L), which might be the reason for the decrease of lichenysin in this strain.
Effects of genes edd and eda overexpression on lichenysin production. (a Design and construction of WX02/pHY-edda; b Effects of genes edd and eda overexpression on lichenysin titer and cell growth; c Effects of genes edd and eda overexpression on pyruvate accumulation; d Effects of genes edd and eda overexpression on precursor amino acids synthesis; e Effects of genes edd and eda overexpression on branched chain fatty acids synthesis. Data in (c–e) were detected at the mid-exponential growth phase. The CK refers to the control strain WX02/pHY300. The edda(Ec) means overexpression of edd and eda genes from E. coli; The edda(ZMO) means overexpression of edd and eda genes from Z. mobilis. iC14:0, 12-methyltetradecanoate; iC15:0, 13-methyl-tetradecanoate; aC15:0, 12-methyltetradecanoate; iC16:0, 14-methylpentadecanoate; iC17:0, 15-methyl-pentadecanoate. *p < 0.05, **p < 0.01, ***p < 0.005)
Then, we detected the intracellular concentration of pyruvate, amino acids (Asp, Gln, Val, Ile, Leu) and fatty acids. As shown in Fig. 3c, the concentrations of intracellular pyruvate in WX02/pHY-edda(Ec) and WX02/pHY-edda(ZMO) were 33.33 mg/L and 41.87 mg/L respectively, which were increased by 60.94% and 100.22% compared with WX02/pHY300 (20.71 mg/L). However, the concentrations of essential amino acids for lichenysin synthesis were decreased significantly (Fig. 3d). The concentrations of branch chain fatty acids, such as iC15:0, aC15:0 and iC16:0 increased slightly, while the concentration of iC14:0 had no significant difference between WX02/pHY300, WX02/pHY-edda(Ec) and WX02/pHY-edda(ZMO) (Fig. 3e). The introduction of ED pathway can affect the NAD(P)H regeneration and the intracellular concentration of NADPH is very important for the synthesis of precursor amino acids and fatty acids. Therefore, the concentrations of intracellular NADPH and NADH were detected at the mid-log growth phase. As shown in Table 3, WX02/pHY-edda(Ec) and WX02/pHY-edda(ZMO) could respectively generate 0.24 μM/gDCW and 0.31 μM/gDCW NADPH, which were increased by 1.00- and 1.58-fold than WX02/pHY300 (0.12 μM/gDCW), which was consistent with the previous report (Jojima et al. 2021). Moreover, the overexpression of edda(Ec) and edda(ZMO) increased the NADPH/NADH by 66.67% and 120.00% compared with the control, which might contribute to the improvement of lichenysin yield. Even so, the concentrations of intracellular amino acids were decreased in edda overexpression strains, which suggested that the NADPH might not be enough for the synthesis of lichenysin precursors yet (Fig. 3d).
Effects of gene zwf overexpression on lichenysin production
Glucose-6-phosphate dehydrogenase encoded by gene zwf catalyzes the synthesis of 6-phospho-D-gluconate, the precursor of the ED pathway and this reaction could provide most of NADPH for cells, which is beneficial to the synthesis of amino acids and fatty acids. For increasing the synthesis of 6-phospho-D-gluconate and the NADPH regeneration, gene zwf was overexpressed in WX02 (Fig. 4a). As shown in Fig. 4b, lichenysin titer of WX02/pHY-zwf was 153.63 mg/L lichenysin, which was increased by 9.21% compared with that of control strain WX02/pHY300 (140.68 mg/L). Moreover, WX02/pHY-zwf just produced 14.90 mg/L pyruvate, which was lower than WX02/pHY300 (Fig. 4c). However, it was found that the concentrations of precursor amino acids and branch chain fatty acids in WX02/pHY-zwf were all increased significantly compared with the control strain. As shown in Fig. 4d, the concentrations of aspartate, valine and leucine in WX02/pHY-zwf were increased by 32.31%, 161.56% and 39.46%, respectively. Also, the concentrations of branch-chain fatty acids iC14:0, iC15:0, aC15:0 and iC16:0 in WX02/pHY-zwf were also increased by 60.84%, 20.56%, 36.54% and 81.15%, respectively (Fig. 4e). For figuring out the effects of zwf overexpression on lichenysin precursor synthesis, the intracellular NAD(P)H concentrations were detected (Table 4). It was present that the zwf overexpression strain could produce 1.57 μM/gDCW NADPH, which increased by 12.08-fold compared with the control (0.12 μM/gDCW) and the NADPH/NADH in WX02/pHY-zwf was increased by 14.20-fold compared with WX02/pHY300. The enhancement of NADPH supply might be one of the reasons for the increase of lichenysin precursor synthesis.
Effects of gene zwf overexpression on lichenysin production. (a Design and construction of WX02/pHY-zwf; b Effects of gene zwf overexpression on lichenysin titer and cell growth; c Effects of gene zwf overexpression on pyruvate accumulation; d Effects of gene zwf overexpression on amino acids synthesis; e Effects of gene zwf overexpression on fatty acids synthesis. Data in (c–e) were detected at the mid-exponential growth phase. The CK refers to the control strain WX02/pHY300. The zwf refers to the control strain WX02/pHY-zwf. *p < 0.05, **p < 0.01, ***p < 0.005)
Effects of genes edda and zwf co-overexpression on lichenysin production
According to the above results, the overexpression of edda gene improved the accumulation of pyruvate and the overexpression of zwf gene significantly increase the generation of NADPH, which were beneficial to the synthesis of lichenysin precursors. Therefore, it was intended to co-overexpression of edda and zwf for further enhancing the lichenysin yield. Gene edda was inserted into the multiple cloning site (MCS) of pHY300PLK and the ampicillin resistance gene was replaced by gene zwf to construct the co-overexpression plasmid pHY-edda(Ec)-zwf (Fig. 5a). As shown in Fig. 5b that the lichenysin titer of WX02/pHY-edda(Ec)-zwf was 204.59 mg/L, which was increased by 45.43% compared with that of WX02/pHY300 (140.68 mg/L). As expected, the intracellular concentrations of pyruvate, branch-chain amino acids and branch-chain fatty acids were all increased significantly, which secured the adequate supply of precursors to further improve lichenysin production. The concentrations of pyruvate, aspartate, glutamine, valine, leucine, iC15:0, aC15:0, iC16:0 and iC17:0 in pHY-edda(Ec)-zwf were increased by 77.21%, 80.41%, 85.31%, 141.64%, 44.94%, 35.08%, 38.08%, 19.33% and 21.16%, respectively compared with the control strain (Fig. 5c–e). Moreover, as shown in Table 5, the synthesis of NADPH in co-overexpressed strain was increased by 4.25-fold compared with the control and NADPH/NADH was increased by 3.67-fold. Overall, the co-overexpression of edda and zwf successfully improved the intracellular accumulation of pyruvate and NADPH, which enhanced the synthesis of amino acids and branched chain fatty acid causing the further increase on lichenysin production.
Effects of genes edda and zwf overexpression on lichenysin production. (a Design and construction of WX02/pHY-edda(Ec)-zwf; b Effects of genes edda and zwf overexpression on lichenysin titer and cell growth; c Effects of genes edda and zwf overexpression on pyruvate accumulation; d Effects of genes edda and zwf overexpression on amino acids synthesis; e Effects of genes edda and zwf overexpression on fatty acids synthesis. Data in (c–e) were detected at the mid-exponential growth phase. The CK refers to the control strain WX02/pHY300. The edda-zwf refers to the control strain WX02/pHY-edda(Ec)-zwf. *p < 0.05, **p < 0.01, ***p < 0.005)
Evaluation of fermentation properties of the synthetic strain
The fermentation properties of strain B. licheniformis WX02/pHY-edda-zwf were investigated using the starting strain B. licheniformis WX02/pHY300 as the control with the initial glucose concentration of 30 g/L (Fig. 6). As shown in Fig. 6a, the synthetic strain WX02/pHY-edda-zwf produced more lichenysin than the control strain during the whole fermentation process and both of them reached the highest lichenysin titer at 21 h. Through growth curve analysis, it was found that the maximum biomass of WX02/pHY-edda-zwf was lower than WX02/pHY300, which might be due to the enhancement of ED pathway decreasing the carbon flux of PP pathway (Fig. 6b). Moreover, there was little difference in the amount of residual glucose between those two strains (Fig. 6c), but the glucose uptake rate of WX02/pHY-edda-zwf during logarithmic phase was 11.18 mmol·g−1 DCW·h−1, increased by 21.52% compared with WX02/pHY300 (9.20 mmol·g−1 DCW·h−1). The extracellular concentration of acetoin and 2,3-butanediol was detected, and the results showed no significant difference between these two strains (Fig. 6d).
Fermentation curves of WX02/pHY300 and WX02/pHY-edda(Ec)-zwf. (a The curve of lichenysin titer; b The growth curve; c Glucose uptake curve; d The curve of acetoin and 2,3-butanediol synthesis. The CK refers to the control strain WX02/pHY300. The edda-zwf refers to the control strain WX02/pHY-edda(Ec)-zwf.)
Discussion
In this study, we constructed a synthetic ED pathway in B. licheniformis WX02 by introducing phosphogluconate dehydratase (encoded by gene edd), 2-keto-3-deoxygluconate 6-phosphate aldolase (encoded by gene eda) from E. coli or Z. mobilis (Fig. 2). The results showed that the lichenysin titer and the pyruvate accumulation were successfully increased by introducing the ED pathway (Fig. 3b, c). However, the intracellular concentrations of the precursor amino acids and branched chain fatty acids for lichenysin synthesis were all decreased (Fig. 3d, e), which might be caused by the inadequate supply of NADPH. In addition, the overexpression of edda (Ec) and edda (ZMO) also increased the synthesis of NADH significantly (Table 3), which was not consistent with previous report (Jojima et al. 2021), and the mechanism needs to be further investigated.
By enhancing the expression of gene zwf separately, the accumulation of aspartate, valine, leucine, the synthesis of branch-chain fatty acids and the generation of NADPH were all increased significantly (Fig. 4; Table 4), which were beneficial for lichenysin production. While, the synthesis of NADH was reduced compared to the control, which might affect the synthesis of ATP. ATP is not only important for cell growth and metabolism but also involved in adenylation, the first step to active lichenysin synthesis (Fan et al. 2020). Combined with the above work, co-overexpression edda (Ec) and zwf might be an effective approach.
In edda and zwf co-overexpressed strain, the intracellular concentrations of pyruvate, aspartate, glutamine, valine, leucine, branched chain fatty acids, NADPH and NADH were all increased significantly, resulting in a 45.43% enhancement on lichenysin production (Fig. 5; Table 5). However, the NADPH concentration of WX02/pHY-edda(Ec)-zwf was lower than that of WX02/pHY-zwf (Tables 4, 5). For understanding those results, the transcriptional level of edd gene in WX02/pHY300, WX02/pHY-edda(Ec) and WX02/pHY-edda(Ec)-zwf was detected and the results showed that the transcription level of edd in WX02/pHY-edda(Ec)-zwf was lower than WX02/pHY-edda(Ec) (Fig. 7a). Then, the transcriptional level of zwf gene in WX02/pHY300, WX02/pHY-edda(Ec), WX02/pHY-edda(ZMO), WX02/pHY-zwf and WX02/pHY-edda(Ec)-zwf were detected and it showed no significant difference between WX02/pHY300, WX02/pHY-edda(Ec), WX02/pHY-edda(ZMO), while the transcription level of zwf gene in WX02/pHY-zwf and WX02/pHY-edda(Ec)-zwf were respectively increased by 187.77- and 52.44-fold than that of the control strain (Fig. 7b). The transcription level of zwf gene in WX02/pHY-edda(Ec)-zwf was reduced by 89.70% compared with WX02/pHY-zwf, which might be the main reason for the lower synthesis of NADPH in WX02/pHY-edda(Ec)-zwf (Tables 4, 5).
The relative transcription levels of genes edd (A) and zwf (B) in engineered strains. (The transcriptional levels of edd gene in WX02/pHY-edda(Ec) and zwf gene in WX02/pHY300 were regarded as 1. Transcriptional levels were detected at the mid-exponential growth phase. *p < 0.05, **p < 0.01, ***p < 0.005)
Pyruvate and NADPH are required for the synthesis of many other bio-chemicals. Therefore, pulcherrimin and poly-γ-glutamic acids (γ-PGA) were selected to explore the effect of the synthetic ED pathway on other bio-chemicals. The results showed that WX02/pHY-edda(Ec), WX02/pHY-zwf and WX02/pHY-edda(Ec)-zwf produce 151.24 mg/L, 153.20 mg/L and 173.91 mg/L pulcherrimin, which respectively increased by 16.33%, 17.84% and 33.77% compared with the control strain WX02/pHY300 (130.01 mg/L) (Fig. 8a). As shown in Fig. 8b, the overexpression of edda(Ec), edda(ZMO), zwf and edda(Ec)-zwf were all increased the γ-PGA production significantly. Among them, the synthetic strain WX02/pHY-edda(Ec)-zwf produced 12.88 g/L γ-PGA, which increased by 58.23% compared with the control strain (8.14 g/L). In general, the synthetic ED pathway could work efficiently in the production of bio-chemicals which need pyruvate and NADPH as their precursor or cofactor.
Taken together, this study successfully increased the lichenysin production by introducing the key genes edd and eda of ED pathway to B. licheniformis WX02 and further enhanced NADPH supply by overexpressing zwf gene. The synthetic ED pathway constructed in this study improved the synthesis of lichenysin precursor amino acids and branched chain fatty acids through promoting pyruvate and NADPH accumulation, which also contributed to increasing the synthesis of other products. This work not only increased lichenysin production by introducing ED pathway, but provided a promising strategy for enhancing the production of biochemicals requiring pyruvate and NADPH as precursor and cofactor. In addition, the results showed that the expression of edda gene and zwf gene were negatively affected when they were expressed on one plasmid. Thus, we will try to insert the edda gene into the chromosome DNA and then replace the promoter of zwf gene by stronger promoter, which is hoped to further increase the expression of these genes and also decrease the stress of foreign plasmid on lichenysin production.
Data availability
The data generated or analyzed during this study are included in this published article.
Abbreviations
- Asp:
-
Aspartate
- Gln:
-
Glutamine
- Val:
-
Valine
- Leu:
-
Leucine
- Ile:
-
Isoleucine
- iC14:0:
-
12-Methyl-tetradecanoate
- iC15:0:
-
13-Methyl-tetradecanoate
- aC15:0:
-
12-Methyl-tetradecanoate
- iC16:0:
-
14-Methyl-pentadecanoate
- iC17:0:
-
15-Methyl-pentadecanoate
- C16:0:
-
Hexadecanoate
References
Ali N, Wang F, Xu B, Safdar B, Ullah A, Naveed M, Wang C, Rashid MT (2019) Production and application of biosurfactant produced by Bacillus Licheniformis Ali5 in enhanced oil recovery and motor oil removal from contaminated sand. Molecules. https://doi.org/10.3390/molecules24244448
Cai D, He P, Lu X, Zhu C, Zhu J, Zhan Y, Wang Q, Wen Z, Chen S (2017) A novel approach to improve poly-gamma-glutamic acid production by NADPH regeneration in Bacillus licheniformis WX-02. Sci Rep 7:43404. https://doi.org/10.1038/srep43404
Das P, Mukherjee S, Sen R (2008) Genetic regulations of the biosynthesis of microbial surfactants: an overview. Biotechnol Genet Eng Rev 25:165–185. https://doi.org/10.5661/bger-25-165
Fan W, Liu H, Liu P, Deng X, Chen H, Liu Q, Feng Y (2020) Characterization of protein interaction surface on fatty acyl selectivity of starter condensation domain in lipopeptide biosynthesis. Appl Microbiol Biotechnol 104(2):653–660. https://doi.org/10.1007/s00253-019-10251-0
Hasegawa S, Jojima T, Suda M, Inui M (2020) Isobutanol production in Corynebacterium glutamicum: suppressed succinate by-production by pckA inactivation and enhanced productivity via the Entner-Doudoroff pathway. Metab Eng 59:24–35. https://doi.org/10.1016/j.ymben.2020.01.004
He L, Xiao Y, Gebreselassie N, Zhang F, Antoniewiez MR, Tang YJ, Peng L (2014) Central metabolic responses to the overproduction of fatty acids in Escherichia coli based on 13C-metabolic flux analysis. Biotechnol Bioeng 111(3):575–585. https://doi.org/10.1002/bit.25124
Hu S, He P, Zhang Y, Jiang M, Wang Q, Yang S, Chen S (2022) Transcription factor DegU-mediated multi-pathway regulation on lichenysin biosynthesis in Bacillus licheniformis. Metab Eng 74:108–120. https://doi.org/10.1016/j.ymben.2022.10.003
Jojima T, Igari T, Noburyu R, Watanabe A, Suda M, Inui M (2021) Coexistence of the Entner-Doudoroff and Embden-Meyerhof-Parnas pathways enhances glucose consumption of ethanol-producing Corynebacterium glutamicum. Biotechnol Biofuels 14(1):45. https://doi.org/10.1186/s13068-021-01876-3
Joshi S, Yadav S, Desai AJ (2015) Application of response-surface methodology to evaluate the optimum medium components for the enhanced production of lichenysin by Bacillus licheniformis R2. Biochem Eng J 41(2):122–127. https://doi.org/10.1016/j.bej.2008.04.005
Lin SC, Lin KG, Lo CC, Lin YM (1998) Enhanced biosurfactant production by a Bacillus licheniformis mutant. Enzyme Microb Technol 23(3–4):267–273. https://doi.org/10.1016/S0141-0229(98)00049-0
Liu B, Sun X, Liu Y, Yang M, Wang L, Li Y, Wang J (2022) Increased NADPH supply enhances glycolysis metabolic flux and L-methionine production in Corynebacterium glutamicum. Foods. https://doi.org/10.3390/foods11071031
Ma W, Liu Y, Shin HD, Li J, Chen J, Du G, Liu L (2018) Metabolic engineering of carbon overflow metabolism of Bacillus subtilis for improved N-acetyl-glucosamine production. Bioresour Technol 250:642–649. https://doi.org/10.1016/j.biortech.2017.10.007
Moxley WC, Eiteman MA (2021) Pyruvate production by Escherichia coli by use of pyruvate dehydrogenase variants. Appl Environ Microbiol 87(13):e0048721. https://doi.org/10.1128/AEM.00487-21
Nerurkar AS (2010) Structural and molecular characteristics of lichenysin and its relationship with surface activity. Adv Exp Med Biol 672:304–315. https://doi.org/10.1007/978-1-4419-5979-9_23
Ng CY, Farasat I, Maranas CD, Salis HM (2015) Rational design of a synthetic Entner-Doudoroff pathway for improved and controllable NADPH regeneration. Metab Eng 29:86–96. https://doi.org/10.1016/j.ymben.2015.03.001
Okano K, Zhu Q, Honda K (2020) In vitro reconstitution of non-phosphorylative Entner-Doudoroff pathway for lactate production. J Biosci Bioeng 129(3):269–275. https://doi.org/10.1016/j.jbiosc.2019.09.010
Qiu Y, Wang Q, Zhu C, Yang Q, Zhou S, Xiang Z, Chen S (2019) Deciphering metabolic responses of biosurfactant lichenysin on biosynthesis of poly-gamma-glutamic acid. Appl Microbiol Biotechnol 103(10):4003–4015. https://doi.org/10.1007/s00253-019-09750-x
Sanchez AM, Andrews J, Hussein I, Bennett GN, San KY (2006) Effect of overexpression of a soluble pyridine nucleotide transhydrogenase (UdhA) on the production of poly(3-hydroxybutyrate) in Escherichia coli. Biotechnol Prog 22(2):420–425. https://doi.org/10.1021/bp050375u
Simpson DR, Natraj NR, McInerney MJ, Duncan KE (2011) Biosurfactant-producing Bacillus are present in produced brines from Oklahoma oil reservoirs with a wide range of salinities. Appl Microbiol Biotechnol 91(4):1083–1093. https://doi.org/10.1007/s00253-011-3326-z
Takeno S, Hori K, Ohtani S, Mimura A, Mitsuhashi S, Ikeda M (2016) L-Lysine production independent of the oxidative pentose phosphate pathway by Corynebacterium glutamicum with the Streptococcus mutans gapN gene. Metab Eng 37:1–10. https://doi.org/10.1016/j.ymben.2016.03.007
Westbrook AW, Ren X, Moo-Young M, Chou CP (2018) Metabolic engineering of Bacillus subtilis for L-valine overproduction. Biotechnol Bioeng 115(11):2778–2792. https://doi.org/10.1002/bit.26789
Xu M, Qin J, Rao Z, You H, Zhang X, Yang T, Wang X, Xu Z (2016) Effect of Polyhydroxybutyrate (PHB) storage on L-arginine production in recombinant Corynebacterium crenatum using coenzyme regulation. Microb Cell Fact 15:15. https://doi.org/10.1186/s12934-016-0414-x
Yang F, Liu N, Chen Y, Wang S, Liu J, Zhao L, Ma X, Cai D, Chen S (2022) Rational engineering of cofactor specificity of glutamate dehydrogenase for poly-gamma-glutamic acid synthesis in Bacillus licheniformis. Enzyme Microb Technol 155:109979. https://doi.org/10.1016/j.enzmictec.2021.109979
Zhang Y, Lin Z, Liu Q, Li Y, Wang Z, Ma H, Chen T, Zhao X (2014) Engineering of Serine-Deamination pathway, Entner-Doudoroff pathway and pyruvate dehydrogenase complex to improve poly(3-hydroxybutyrate) production in Escherichia coli. Microb Cell Fact 13:172. https://doi.org/10.1186/s12934-014-0172-6
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFC2100202).
Author information
Authors and Affiliations
Contributions
SW Chen contributed reagents and materials. SW Chen and SY Hu designed experiments. SY Hu, C Zhao, YJ Zhang, XT Wang and PH He performed the experiments. SY Hu, PH He and SW Chen drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, S., Zhao, C., Zhang, Y. et al. Construct a synthetic Entner–Doudoroff pathway in Bacillus licheniformis for enhancing lichenysin production. World J Microbiol Biotechnol 39, 168 (2023). https://doi.org/10.1007/s11274-023-03619-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03619-y