Abstract
Objectives
Bacillus licheniformis WX-02 is used for the production of many valuable chemicals. Here, we have sought to improve l-valine production by blocking the metabolic pathways related to branched-chain amino acids.
Results
The synthesis genes of l-leucine (leuA) and l-isoleucine (ilvA) were deleted to obtain mutant strains. l-Valine yields of WX-02ΔleuA and WX-02ΔilvA reached 33.2 and 21.1 mmol/l, respectively, which are 22 and 14 times higher than the wild-type WX-02 (1.53 mmol/l). After further deletion of l-lactate dehydrogenase gene (ldh) from WX-02ΔleuA, the productivity reached 0.47 mmol/l h, an increase of 19 %.
Conclusion
We provide a possibility to over-produce l-valine using genetically-modified B. licheniformis using remodeling of the biosynthetic pathway to l-valine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Valine (l-Val) is a branched-chain amino acid which is essential for vertebrates and is widely used as a reagent for infusion solutions, cosmetics, foods, and feed industries (Blombach et al. 2008). As an essential amino acid, l-Val is also used for total artificial nutrition and for synthesis of antiviral drugs (Blombach et al. 2007). The fermentative production of l-Val has attracted increasing attentions (Bartek et al. 2008) with a variety of microorganisms being reported as l-Val producers including Corynebacterium (Bartek et al. 2010), Serratia marcescens (Khachatryan 1986), Brevibacterium (Iborra et al. 1992), Escherichia (Park et al. 2007) and Bacillus (Chattopadhyay and Banerjee 1978).
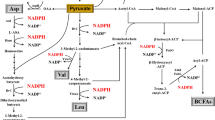
Biochemically, l-Val is synthesized from two molecules of pyruvate, in a pathway comprising four reactions, catalyzed by acetohydroxyacid synthase (AHAS/ALS), acetohydroxy acid isomero reductase (AHAIR/ALIR), dihydroxyacid dehydratase (DHAD), and transaminase B. As shown in Fig. 1, two pyruvates are condensed by AHAS/ALS to acetolactate, which is reduced and isomerised to dihydroxyisovalerate (DHIV) by AHAIR/ALIR, and further dehydrated to ketoisovalerate (KIV) catalyzed by DHAD. KIV is finally transformed to l-Val by transaminase B, with glutamate as an amino donor (Bartek et al. 2010). In most organisms, these enzymes also catalyze the biosynthesis of l-isoleucine from pyruvate and α-ketobutyrate. α-Ketobutyrate is synthesized from l-threonine by threonine dehydratase (TD) (Fig. 1). Ketoisovalerate, the last intermediate of l-Val synthesis, also serves as the precursor for l-leucine and pantothenate biosynthesis (Blombach et al. 2007; Bartek et al. 2008).
Biosynthesis pathway of l-Val in B. licheniformis. AHAS/ALS acetohydroxyacid synthase, AHAIR/ALIR acetohydroxy acid isomero reductase, DHAD dihydroxyacid dehydratase, TAs transaminases, LDH lactate dehydrogenase, ALD acetolactate decarboxylase, IPMS isopropylmalate synthase. The filled forks mean the successfully deleted genes in this study
Bacillus subtilis and B. licheniformis can produce l-Val (Chattopadhyay and Banerjee 1978). B. licheniformis WX-02 is a previously isolated strain in our laboratory for poly-γ-glutamic acid (γ-PGA) production (Wei et al. 2010), and l-Val was also found in its culture broth (unpublished data). B. licheniformis as a l-Val producer has several advantages, such as rapid growth in the media with minimal nutrient requirement, and being classified as “generally recognized as safe”. B. licheniformis is also a thermophile which can grow above 40 °C thereby reducing the risk of bacterial contamination. However, the wild type of B. licheniformis WX-02 only produces a moderate amount of l-Val.
In B. licheniformis, biosynthesis of the other branched-chain amino acids and other metabolic pathways consumes the substrate, energy, coenzymes and NADH/NADPH, which then decreases the production of l-Val. Therefore, remodeling the metabolic pathway is needed for enhanced production of l-Val. The aim of this work is to redirect the metabolic fluxes towards l-Val by blocking the other branched-chain amino acid biosynthesis and overflow metabolic pathways in B. licheniformis WX-02.
Materials and methods
Cell strains, plasmids, primers and growth media
Experiments were performed with the strains and plasmids listed in Tables 1 and 2. The oligonucleotide primers, listed in Supplementary Table 1, were designed based on the B. licheniformis WX-02 genome sequence (GenBank accession no. AHIF00000000) (Yangtse et al. 2012). LB medium was used for culture of Escherichia coli DH5α and B. licheniformis. Medium used for the fermentation of l-Val (Radmacher et al. 2002) was prepared by previously described methods with minor modifications, consisting of (per liter): 100 g glucose, 2 g corn steep liquor, 15 g (NH4)2SO4, 1 g KH2PO4, 0.5 g MgSO4·7H2O, 3 g CaCO3.
Chemicals and materials for cloning
All other chemicals were of analytical grade supplied by Sinopharm Chemical Reagent (China). T4 DNA ligase, DNA marker and TransStart Fast Pfu DNA Polymerase were purchased from Takara Bio (China) or TransGen Biotech (China). Nucleotide sequences were determined by Beijing Genomics institution (China).
Construction of plasmids
Bacillus licheniformis WX-02 was cultured in LB medium overnight, then collected for extraction of genomic DNA (Sohail 1998). The gene, ilvA, encoding TD was deleted by the double-crossover homologous recombination method (Qi et al. 2014). First, two approximately 500 bp homologous arms of the 5′- and 3′-coding regions of ilvA gene were amplified by PCR from the genomic DNA of B. licheniformis WX-02 with the primers ilvAKF1 and ilvAKR1, ilvAKF2 and ilvAKR2, respectively (Supplementary Table 1). These two homologous arms were ligated by overlapping extension PCR (SOE-PCR) with the primers ilvAKF1 and ilvAKR2 (Supplementary Table 1) (Wang et al. 2012; Qi et al. 2014). The DNA fragment was subcloned into vector T2(2)-ori (Qi et al. 2014) joined by BamHI and XbaI restriction sites. The resulting plasmid was further verified by sequencing. A recombinant vector for ilvA knock-out was designated as T2(2)-oriΔilvA. The other two T2(2)-ori-based plasmids including T2(2)-oriΔleuA and T2(2)-oriΔldh were also constructed with the appropriate primers (Supplementary Table 1) by the above methods for deletion of leuA encoding for α-isopropylmalate synthase and ldh encoding for lactate dehydrogenase in B. licheniformis WX-02.
Construction of ilvA and leuA knock-out strains of WX-02
Competent cells of E. coli DH5α and B. licheniformis WX-02 were prepared for transformation of constructed plasmids using standard protocols (see also Xue et al. 1999). E. coli DH5α was transferred with T2(2)-oriΔilvA or T2(2)-oriΔleuA plasmid and then cultured in LB medium with kanamycin (20 μg/ml). The plasmid T2(2)-oriΔilvA or T2(2)-oriΔleuA isolated from the recombinant E. coli was used for transforming B. licheniformis WX-02.
The knock-out of leuA or ilvA gene in B. licheniformis WX-02 was done by our previously established methods (Qi et al. 2014). Briefly, WX-02 was electrotransformed with T2(2)-oriΔilvA or T2(2)-oriΔleuA, the transformants were then selected by kanamycin resistance (20 μg/ml) followed by verification with PCR using the primers ilvAKF1 and ilvAKR2 for WX-02ΔilvA, and leuAKF1 and leuAKR2 for WX-02ΔleuA (Supplementary Table 1), respectively. The selected positive transformants were cultured in LB medium containing kanamycin (20 μg/ml) at 45 °C for 8 h to promote the first crossover in the cells.
The successful first crossover was analyzed by PCR. Briefly, the cells with kanamycin resistance were further selected by PCR with primers of ΔilvA A single crossover-F and ΔilvA A single crossover-R for crossover at upstream of ilvA, and with primers of ΔilvA B single crossover-F and ΔilvA B single crossover-R for crossover at downstream of ilvA, respectively (Supplementary Table 1). The single crossover of leuA in WX-02 was also verified by PCR with the appropriate primers (Supplementary Table 1).
The selected colonies with single crossover were repeatedly cultured in LB medium at 37 °C for 8 h. After serial transfer without antibiotics, cells were plated on LB agar plates for selection of kanamycin-sensitive colonies. The ilvA or leuA knock-out strain was selected by the second crossover, and confirmed by PCR with primers of ilvAYF and ilvAYR for WX-02ΔilvA, and with leuAYF and leuAYR for WX-02ΔleuA, respectively (Supplementary Table 1).
Construction of WX-02ΔleuAΔldh
The mutant strain of WX-02ΔleuAΔldh was constructed based on the parental strain of WX-02ΔleuA. T2(2)-oriΔldh was used for transforming WX-02ΔleuA, then the transformants were selected and verified for the successful single crossover also the double crossover using PCR with the appropriate primers (Supplementary Table 1) by the methods described above, followed with verification by nucleotide sequencing.
Production of l-Val by mutant strains
Single colonies of the wild strain WX-02, and the mutant strains of WX-02ΔleuA, WX-02ΔilvA and WX-02ΔleuAΔldh, were transferred into 250 ml flasks containing 50 ml LB medium. The flasks were incubated at 37 °C and 180 rpm for 8 h until the OD600 of culture reached 4.2. The cells were then subcultured for 84 or 96 h at the same conditions for producing l-Val. The samples were collected periodically to determine the time course of cell density, residual glucose and l-Val concentrations.
Analysis
Cell densities were determined from the OD600 values. The concentration of residual glucose was measured by a biosensor equipped with a glucose oxidase electrode. l-Val was determined by GC after derivatization with ethyl chlorocarbonate (Mudiam and Ratnasekhar 2013). The production of acetoin, 2,3-butanediol and lactate in the broth were analyzed, respectively, by GC (Qi et al. 2014) and HPLC (Santos et al. 2014).
Results
Establishment of WX-02ΔilvA and WX-02ΔleuA knock-out strains
The T2(2)-ori plasmid was used for deletion of ilvA and leuA genes. The PCR product amplified from the genomic DNA of ilvA knock-out strain was about 1,498 bp, while a DNA fragment about 2,767 bp containing the ilvA gene was amplified from the genomic DNA of WX-02 using primers of ilvAYF and ilvAYR (Supplementary Table 1). The PCR product from ilvA knock-out strain was purified and sequenced. No other mutation than the deletion was found (data not shown), suggesting the successful construction of the ilvA deficient strain WX-02 ΔilvA.
Similarly, the PCR product amplified from the genomic DNA of leuA knock-out strain was about 1,224 bp, while a DNA fragment about 2,781 bp containing the leuA gene was amplified from the genomic DNA of WX-02 by using primers leuAYF and leuAYR (Supplementary Table 1), suggesting the successful construction of the leuA deficient strain WX-02 ΔleuA.
l-Val production by WX-02ΔilvA and WX-02ΔleuA
l-Val production by the mutant strains is shown in Fig. 2. As expected, the l-Val production of both WX-02ΔilvA and WX-02ΔleuA were higher than that of WX-02. WX-02 produced the maximum 1.53 mmol/l l-Val production, while the l-Val produced by the mutant strains of WX-02ΔilvA and WX-02ΔleuA reached a maximum 21 mmol/l (13.8 times of WX-02) at 72 h and 33.2 mmol/l (21.7 times of WX-02) at 84 h, respectively. These data confirm the deletion both of leuA and ilvA gene significantly increases l-Val production, and the deletion of leuA showed a higher l-Val production than the deletion of ilvA.
Establishment of WX-02ΔleuAΔldh based on WX-02ΔleuA
The T2(2)-ori plasmid was used for deletion of ldh gene. The PCR product amplified from WX-02ΔleuAΔldh was about 1,291 bp, while a DNA fragment about 2,251 bp containing the ldh gene was amplified from the genomic DNA of WX-02ΔleuA using primers of Δldh-F and Δldh-R (Supplementary Table 1) as negative control. The PCR products were purified and sequenced, and no other mutation than the deletion was found, suggesting the successful construction of the deficient strains of WX-02ΔleuAΔldh.
l-Val production by WX-02ΔleuAΔldh
The production of lactate was also detected during fermentation. The results showed no l-lactate was detectable after the ldh gene was deleted from WX-02ΔleuA. Figure 3a shows the biomass of WX-02ΔleuAΔldh is similar to that of WX-02ΔleuA. The trend of glucose consumption of WX-02ΔleuAΔldh is similar to that of WX-02ΔleuA.
Figure 3b shows l-Val production by the mutant strains. l-Val production was similar in WX-02ΔleuAΔldh and WX-02ΔleuA in the first stage of fermentation (12–24 h). After 24 h, WX-02ΔleuAΔldh produced more l-Val than the parental strain WX-02ΔleuA. At 36, 48 and 72 h, the l-Val production of WX-02ΔleuAΔldh increased by 23.4, 20.2 and 18.9 % respectively, compared to that of WX-02ΔleuA. After 72 h, the l-Val production of WX-02ΔleuAΔldh reached the maximum 33.6 mmol/l, higher than that of WX-02ΔleuA (27.2 mmol/l). The fermentation time for the maximum l-Val production was 72 h for WX-02ΔleuAΔldh, shorter than that for WX-02ΔleuA (84 h). The productivity of WX-02ΔleuAΔldh reached 0.47 mmol/l h, 19 % higher than that of WX-02ΔleuA. These data indicated the further deletion of ldh gene improved l-Val productivity.
Discussion
Bacillus licheniformis contains a strong metabolic flux from pyruvate to branched-chain amino acids such as l-Val (data not shown), leading us to explore the potential of producing l-Val using this strain. Based on the strong ability of B. licheniformis to convert glucose to various branched-chain amino acids, we modified the metabolic flux towards l-Val synthesis. The syntheses of l-leucine and l-isoleucine complete the precursors, energy, coenzymes and NADH/NADPH with that of l-Val (Blombach et al. 2007; Bartek et al. 2010). Therefore, the inhibition of l-leucine and l-isoleucine syntheses is beneficial for l-Val synthesis by redirecting the metabolic fluxes towards l-Val (Blombach et al. 2007, 2008). As expected, the l-Val synthesis significantly enhanced after the deletion of leuA or ilvA gene in B. licheniformis WX-02.
In addition to branched-chain amino acids, a strong overflow metabolic pathway from pyruvate to lactate was also found in B. licheniformis. We further deleted the key enzyme gene, ldh, for lactate formation, which further increased l-Val productivity. Pyruvate is converted to acetoin and 2,3-butanediol by acetolactate decarboxylase encoded by gene alsD, but the deletion of alsD did not improve l-Val production (data not shown here). Collectively, our studies provided a possibility to highly produce l-Val by remodeling metabolic pathway for l-Val biosynthesis.
References
Bartek T, Makus P, Klein B, Lang S, Oldiges M (2008) Influence of l-isoleucine and pantothenate auxotrophy for l-valine formation in Corynebacterium glutamicum revisited by metabolome analyses. Bioprocess Biosyst Eng 31:217–225
Bartek T, Blombach B, Zönnchen E, Makus P, Lang S, Eikmanns BJ, Oldiges M (2010) Importance of NADPH supply for improved l-valine formation in Corynebacterium glutamicum. Biotechnol Prog 26:361–371
Blombach B, Schreiner ME, Holatko J (2007) l-Valine production with pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum. Appl Environ Microbiol 73:2079–2084
Blombach B, Schreiner ME, Bartek T, Oldiges M, Eikmanns BJ (2008) Corynebacterium glutamicum tailored for high-yield l-valine production. Appl Microbiol Biotechnol 79:471–479
Chattopadhyay SP, Banerjee AK (1978) Production of valine by a Bacillus sp. Z Allg Mikrobiol 18:243–254
Iborra JL, Obon JM, Manjon A, Canovas M (1992) Analysis of a laminated enzyme membrane reactor for continuous resolution of amino acids. Biotechnol Appl Biochem 15:22–30
Khachatryan AG (1986) Dependence of valine production by serratia marcescens on the ion composition of the fermentation. Microbiology 22:554–556
Mudiam MK, Ratnasekhar Ch (2013) Ultra sound assisted one step rapid derivatization and dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometric determination of amino acids in complex matrices. J Chromatogr A 1291:10–18
Park JH, Lee KH, Kim TY, Lee SY (2007) Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci 104:7797–7802
Qi G, Kang Y, Li L, Xiao A, Zhang S, Wen Z, Xu D, Chen S (2014) Deletion of meso-2,3-butanediol dehydrogenase gene budC for enhanced d-2,3-butanediol production in Bacillus licheniformis. Biotechnol Biofuels 7(1):16
Radmacher E, Vaitsikova A, Burger U, Krumbach K, Sahm H, Eggeling L (2002) Linking central metabolism with increased pathway flux: l-valine accumulation by Corynebacterium glutamicum. Appl Environ Microbiol 68(5):2246–2250
Santos CC, Libeck BD, Schwan RF (2014) Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int J Food Microbiol 186(1):32–41
Sohail M (1998) A simple and rapid method for preparing genomic DNA from gram-positive bacteria. Mol Biotechnol 10(2):191–193
Wang Q, Chen T, Zhao X, Chamu J (2012) Metabolic engineering of thermophilic Bacillus licheniformis for chiral pure d-2,3-butanediol production. Biotechnol Bioeng 109:1610–1621
Wei X, Ji Z, Chen S (2010) Isolation of halotolerant Bacillus licheniformis WX-02 and regulatory effects of sodium chloride on yield and molecular sizes of poly-γ-glutamic acid. Appl Biochem Biotechnol 160(5):1332–1340
Xue G, Johnson JS, Dalrymple BP (1999) High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J Microbiol Methods 34(3):183–191
Yangtse W, Zhou Y, Lei Y, Qiu Y, Wei X, Ji Z, Qi G, Yong Y, Chen L, Chen S (2012) Genome sequence of Bacillus licheniformis WX-02. J Bacteriol 194(13):3561–3562
Acknowledgments
This work was supported by National Program on Key Basic Research Project (973 Program, No. 2015CB150505).
Supporting Information
Supplementary Table 1 – Primers used for PCR in this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, C., Huo, Y., Qi, G. et al. Enhancement of l-valine production in Bacillus licheniformis by blocking three branched pathways. Biotechnol Lett 37, 1243–1248 (2015). https://doi.org/10.1007/s10529-015-1783-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1783-7