Abstract
Molecular chaperone CbpA from extreme acidophile Acidithiobacillus caldus was applied to improve acid tolerance of Escherichia coli via CRISPR/Cas9. Cell growth and viability of plasmid complementary strain indicated the importance of cbpAAc for bacteria acid tolerance. With in situ gene replacement by CRISPR/Cas9 system, colony formation unit (CFU) of genome recombinant strain BL21-ΔcbpA/AccbpA showed 7.7 times higher cell viability than deficient strain BL21-ΔcbpA and 2.3 times higher than wild type. Cell morphology observation using Field Emission Scanning Electron Microscopy (FESEM) revealed cell breakage of BL21-ΔcbpA and significant recovery of BL21-ΔcbpA/AccbpA. The intracellular ATP level of all strains gradually decreased along with the increased stress time. Particularly, the value of recombinant strain was 56.0% lower than that of deficient strain after 5 h, indicating that the recombinant strain consumed a lot of energy to resist acid stress. The arginine concentration in BL21-ΔcbpA/AccbpA was double that of BL21-ΔcbpA, while the aspartate and glutamate contents were 14.8% and 6.2% higher, respectively, compared to that of wild type. Moreover, RNA-Seq analysis examined 93 genes down-regulated in BL21-ΔcbpA compared to wild type strain, while 123 genes were up-regulated in BL21-ΔcbpA/AccbpA compared to BL21-ΔcbpA, with an emphasis on energy metabolism, transport, and cell components. Finally, the working model in response to acid stress of cbpA from A. caldus was developed. This study constructed a recombinant strain resistant to acid stress and also provided a reference for enhancing microorganisms’ robustness to various conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acidithiobacillus, which plays an important role in the bioleaching process, are a group of acidophilic aerobic bacteria known for their resistance to extreme acid stress (pH 0.5 ~ 2) (Chen et al. 2022). As a perfect extremophile, the acid tolerance of the bacteria to this extreme environment is of increasing concern (Krulwich et al. 2011; Chen et al. 2020). Compared with Acidithiobacillus, the acid resistance of typical industrial microorganism, E. coli, is poor. It has been reported that exposure to low environmental pH leads to a rapid drop in periplasm and cytosol pH, which could cause protein unfolding, denaturation of essential enzymes and DNA damage, inhibiting microbial growth and production (Brameyer et al. 2022; Monteagudo-Cascales et al. 2022).
Chaperones can stabilize misfolded proteins or help refold proteins that have been disrupted by stresses (Wang et al. 2019). To mitigate the toxic effects of isopropanol, an exogenous heat shock protein (Hsp), GroESL, was introduced into the isopropanol producing Cupriavidus necator strain. The resulting strain exhibited increased specific activities of β-ketothiolase and acetyl-CoA transferase, as well as elevated isopropanol titers of 9.8 g L− 1 (Marc et al. 2017; Liu et al. 2021). The overexpression of B. psychrosaccharolyticus Hsp33 in E. coli increased isopropanol tolerance, indicating that psychrophilic proteins function at higher temperatures and bestow a tolerant phenotype. (Kang et al. 2007). Overexpression of the archaeal Pyrococcus horikoshii OT3 chaperone protein and its cofactor prefolding protein in E. coli resulted in higher n-hexane and octane tolerance (Okochi et al. 2008). Weidmann et al. expressed the small molecule Hsp Lo18 of Oenococcus oeni in Lactococcus lactis, which improved its tolerance to acid and heat stress (Weidmann et al. 2017).
CbpA is a stress-responsive protein that binds to nucleic acids to prevent damage (Cosgriff et al. 2010; Molan and Žgur Bertok 2022). CbpA expression is low during the exponential growth phase but increases during the late stationary phase, under phosphate deprivation, and when cultivated in low pH conditions (Chae et al. 2004). The protein belongs to the Hsp40 class of accessory chaperones and can stimulate the folding activity of Hsp70 family chaperones. A number of small heat shock proteins constitute a very varied collection of proteins whose expression is stimulated by stress, and whose primary role is to bind and protect unfolded proteins, keeping them in a non-degradable conformation until they are efficiently refolded by an ATP-driven partner. (Matuszewska et al. 2005; Roncarati and Scarlato 2017).
In this study, we characterized cbpAAc derived from Acidithiobacillus caldus strain conserved in our laboratory and analyzed its sequencing, genetic relationship and structure. Then, the cbpAAc gene was introduced into E. coli using vector and genome editing to evaluate its effect on acid tolerance of the strain. We discovered that the presence of CbpAAc could promote E. coli biomass under acidic conditions and enhance its tolerance to organic acids. Finally, the acid tolerance mechanism of cbpA from A. caldus was developed based on comparative transcriptome analysis.
Materials and methods
Strains, plasmids, and culture conditions
The strain Acidithiobacillus caldus CCTCC M 2,018,727 was stored in our lab and cultured according to Yin et al. (Yin et al. 2022). E. coli strains were cultured in Luria-Bertani (LB) broth at 37 °C. When necessary, antibiotics were added at final concentrations of 100 µg/mL for ampicillin, 50 µg/mL for chloramphenicol, 50 µg/mL spectinomycin, and 50 µg/mL for kanamycin. In addition, the genome of A. caldus ATCC 51,756 was employed as a reference sequence in this study. The strains and plasmids used in this study are listed in Table 1. The primers used for gene cloning and genomic recombination are listed in Table 2.
In silico analysis of the cbpA Ac gene
Several typical DnaJ family proteins were selected according to the report of Bird et al. (Bird et al. 2006), and their amino acid sequences were aligned using MEGA 11 and ESPript 3.0 software. The secondary structure of CbpA was predicted on the PSIPRED web site (http://bioinf.cs.ucl.ac.uk/psipred/). The unrooted phylogenetic tree was created using MEGA 11 software to analyze the genetic relationship between A. caldus CbpA and its homologous proteins. The three-dimensional structure of E. coli CbpA was used as a template to model the ACAty RS00255 encoded protein CbpAAc by AlphaFold 2 software, and the protein structures of A. caldus CbpAAc and E. coli CbpAEc were aligned by PyMol software.
Construction of complementary plasmids and strains
The vectors with different copy numbers, pACYCDuet-1 (about 15 copies) and pETDuet-1 (about 40 copies) were chosen as complementary plasmids. Molecular biology manipulations were carried out using standard techniques (Feng et al. 2022). First, the cbpAAc gene was amplified from the A. caldus genome with primers cbpA-F and cbpA-R, and the pACYCDuet-1 and pETDuet-1 plasmids were reverse amplified to create linearized fragments using the primers plasmid-F and plasmid-R. Then the cbpA with the homologous fragment was respectively ligated with the linearized plasmids by homologous recombination, and transformed into BL21-ΔcbpA to construct plasmid complementary strains. The constructed strains were named BL21-ΔcbpA/pACYC-AccbpA and BL21-ΔcbpA/pETDuet-AccbpA.
Construction of deficient strain and genome recombinant strain
The cbpA gene knockout deficient strain BL21-ΔcbpA was constructed via CRISPR/Cas9 system (Figure S1), described by Jiang et al. (Jiang et al. 2015). A 20-bp guide sequence (CGTCGGTTTCACGCCCATGA) together with the NGGPAM sequence (N20NGG) in the E. coli BL21(DE3) was selected to design sgRNA using CRISPR direct (http://chopchop.cbu.uib.no/). Inverse PCR was used to create the plasmid pTargetF-cbpA from pTargetF using the primers N20-F and N20-R. The PCR fragments were digested with DpnI to remove the template plasmid and subsequently transformed into E. coli DH5α to generate circular plasmids. The sequence was then validated by sequencing using primer pTargetF-S. The editing template fragment, which had two homologous arms corresponding to the upstream and downstream regions, was designed to have 500-bp homology arms on either side, and the sequences were amplified independently before being synthesized together by overlapping PCR. The fusion products should be purified by gel extraction before electroporation.
The construction of cbpAAc gene knockin recombinant strain BL21-ΔcbpA/AccbpA was similar to that of deficient strain, with the exception of the homology arm. There was a cbpAAc gene fragment that needed to be knocked in between the 500-bp upstream and downstream homology arms, which was amplified by primers cbpA-I-F and cbpA-I-R.
Determination of cell growth and cell viability
To assess the growth of plasmid complementary strains, overnight cultures with the same OD600 were transferred as 1% (v/v) inoculum into M9 medium acidified with hydrochloric acid at pH 7.0 and pH 5.0. Appropriate antibiotics were added when necessary, and the cultures were cultured at 37 °C, 200 rpm, until the OD600 reached 0.5. The cultivation was then maintained at 28 °C with the addition of 0.5 mM IPTG. Their OD600 was measured every 2 h for a total of 24 h. For genome recombinant strain, the entire assay was conducted at 37 °C, 200 rpm for a total of 18 h with no antibiotics or IPTG. The specific growth rate (µ) was calculated using Origin 2022 software.
To investigate cell viability, plasmid complementary cells seed solutions with the same OD600 were injected into LB medium with appropriate antibiotics and grown at 37 °C until the OD600 reached 0.5. Afterward, 0.5 mM IPTG was added. Each strain culture was incubated for 2 h at 28 °C before being supplemented with 17.5 mM acetic acid, 35 mM acetic acid, or no organic acid. The strains were stressed for 10 h at 28 °C. Gradient dilutions of the cells were made, and 5 µL of 104, 105, 106, and 107-fold dilutions were deposited on LB solid medium. After an overnight incubation at 37 °C, the colonies were photographed. For genome recombinant strains, seed solutions with the same optical density were inoculated into LB medium and cultivated at 37 °C until OD600 = 0.5, then acetic acid was added to the culture as previously indicated for 10 h stress. The cells were then diluted in the same way and photographed.
Cell morphology observed via Field Emission scanning Electron Microscopy (FESEM)
Strains were cultivated in LB medium to exponential phase with the same optical density and then stressed for 10 h with 35 mM acetic acid. The cells were centrifuged and fixed with 5% glutaraldehyde for 12 h at 4 °C. After that, the samples were washed with 0.1 M phosphate buffer several times to eliminate the glutaraldehyde solution. Gradient dehydration was carried out with 30%, 50%, 70%, 90% and 100% ethanol solution, respectively. The morphological structure of bacteria was observed via FESEM (SU8200, Hitachi, Ltd., Japan) following critical point drying and ion sputtering.
Measurement of intracellular ATP and free amino acids
Strains were grown in LB medium to exponential phase with same optical density and subsequently stressed with 35 mM acetic acid. The cells were sampled at 0 h, 5 and 10 h to measure intracellular ATP concentration using an ATP assay kit (Beyotime, Shanghai, China). Final ATP concentrations were shown in nmol mg− 1 protein.
Cells for the determination of intracellular free amino acids were sampled under 35 mM acetic acid stress. Fifty milliliters of cells were centrifuged (12,000×g, 10 min) and washed twice with ultrapure water after being treated with 35 mM acetic acid for 10 h. Cells were resuspended in 1 mL of 5%(w/v) trichloroacetic acid, which was then mixed and sonicated at room temperature for 20 min. The mixture was left to stand for 2 h. Cell debris was removed by centrifugation at 12,000×g for 15 min, and the supernatant was filtered through a 0.22 μm filter membrane and evaluated using HPLC according to previous report (Fountoulakis and Lahm 1998).
mRNA sequencing and transcriptomic analysis
The exponential phase cells were stressed with 35 mM acetic acid for 10 h. After centrifugation at 5000 rpm min− 1 for 5 min, cells were collected by washing twice with ice-cold deionized water and stored in a -80 ℃ refrigerator until transcriptome analysis. Total RNA was extracted and purified. NanoDrop 2000 was used to detect the pure RNA. The cDNA library was constructed by rRNA removal, mRNA purification and fragmentation, cDNA synthesis, linker ligation, UNG enzyme digestion and polymerase chain reaction (PCR) amplification. Library was sequenced on Illumina Hiseq (Illumina, USA). The RNA-seq data of BL21, BL21-ΔcbpA and BL21-ΔcbpA/AccbpA has been submitted to NCBI (accession ID: PRJNA915998).
Results
Sequence, genetic relationship and structure prediction analysis
The co-chaperone gene cbpA (accession number: OQ126891) in A. caldus genome, which encodes a protein named DnaJ-class molecular chaperone CbpA was identified. As shown in Fig. 1A and Figure S2, A. caldus CbpAAc contains a domain with a highly conserved sequence, which is a characteristic J-domain of DnaJ family proteins, consisting of ~ 75 amino acids (Cosgriff et al. 2010). All J-domains contain a conserved tripeptide (His, Pro, Asp) between the two long helices of the J-domain, which is required for cochaperone activity (Bird et al. 2006; Pepe et al. 2020). According to the results of the phylogenetic tree (Fig. 1B), the three-dimensional structure of E. coli CbpA with the closest genetic relationship was selected as the template to model CbpAAc and aligned with it (Fig. 1D). According to (Fig. 1C), the J-domain of the CbpA protein is composed of four α helices, with helices II and III forming an antiparallel coiled coil fold anchored by helix I. Helices II and III are linked by a solvent-exposed loop. CbpA forms homodimers in solution and dimerizes through the C-terminal domain (Sarraf et al. 2010).
In silico analysis of cbpAAc gene and CbpAAc protein
(A) Amino acid sequence alignment of ACAty_RS00255 product CbpAAc with orthologues (B) unrooted phylogenetic tree of CbpAAc with DnaJ family homologs (C) J-domain structure of the CbpAAc protein (D) structure alignment between CbpAAc and CbpAEc. Blue: CbpAAc from A.caldus; Green: CbpAEc from E. coli
Cell growth and cell viability of BL21-ΔcbpA and plasmid complementary E. coli strains
Cell growth
The deficient strain BL21-ΔcbpA was constructed by deleting cbpA from the E. coli BL21 genome using CRISPR/Cas9. Cell growth of BL21-ΔcbpA and plasmid complementary E. coli strains (BL21-ΔcbpA/pACYC-AccbpA, BL21-ΔcbpA/pETDuet-AccbpA) were analyzed at pH 7.0 and 5.0 (Fig. 2A and B). The final biomass of wild-type BL21 (WT) and deficient strain BL21-ΔcbpA was similar at pH 7.0, but lower than that of the complementary strain. Under this condition, the final OD value of complementary strains BL21-ΔcbpA/pACYC-AccbpA and BL21-ΔcbpA/pETDuet-AccbpA was 15.3% and 10.5% higher than that of BL21-ΔcbpA, respectively. At pH 5.0, The final OD value of the deficient strain was 11.1% lower than that of the wild type BL21. The OD600 of BL21-ΔcbpA/pACYC-AccbpA and BL21-ΔcbpA/pETDuet-AccbpA strains was increased by 22.1% and 22.9%, respectively, when compared to BL21-ΔcbpA. They increased by 8.6% and 9.3%, respectively, when compared to BL21 (WT).
Cell growth and cell viability of plasmid complementary strains under different acid stresses
(A) growth curves at pH 7.0 acidified with hydrochloric acid (B) growth curves at pH 5.0 acidified with hydrochloric acid (C) colonies on plate of complementary bacteria grown with additional acetic acid (diluted 104, 105, 106, and 107-fold)
The strain carrying the cbpAAc overexpression vector showed a growth advantage as the pH of the media declined, suggesting that CbpA may play a role in acid resistance under acidic conditions. The recombinant strain BL21-ΔcbpA/pACYC-AccbpA was chosen for further acetic acid tolerance experiments based on cell growth under different conditions and SDS-PAGE results of different expression vectors (Figure S3).
Cell viability
In the absence of organic acids, there was no significant difference in cell viability between wild type and deficient strain, as shown in Fig. 2C. However, the survival rate of BL21-ΔcbpA/pACYC and BL21-ΔcbpA/pACYC-AccbpA was relatively reduced because they consumed nutrients during growth to over-express proteins. The viability of the deficient strain BL21-ΔcbpA fell by 40.0% compared with that of BL21 (WT) under 17.5 mM acetic acid. The exogenous introduction of cbpAAc did not enhance the acid tolerance of E. coli in this case. The survival rate was lower than that of wild type E. coli BL21. When the four strains were grown in the medium supplemented with 35 mM acetic acid, the survival rates of each strain differed significantly. The cell viability of the deficient strain was reduced by 82.2% when compared to the wild type, while the survival rate of BL21-ΔcbpA/pACYC cells carrying the empty vector was reduced by over 100 times. Moreover, the survival of BL21-ΔcbpA/pACYC-AccbpA cells with cbpAAc overexpression recovered to the wild-type level.
Construction of recombinant E. coli by CRISPR/Cas9 and physiological parameters analysis under acid stress
Cell growth of BL21-ΔcbpA/AccbpA
With the assistance of CRISPR/Cas9 genome editing, the cbpAAc gene was successfully replaced in situ to the corresponding position in E. coli BL21, which was verified by nucleic acid electrophoresis and sequencing. The growth status was assessed at pH 7.0 and pH 5.0, and the results were shown in Fig. 3A and B. At pH 7.0, the growth status of the three E. coli strains was nearly identical, and the specific growth rate also showed a similar trend. When the pH was decreased to 5.0, the three strains revealed some variations. The final OD600 of the deficient strain was 8.7% lower than that of the wild type, and the final biomass of recombinant BL21-ΔcbpA/AccbpA was 4.5% higher than that of the deficient strain.
Cell growth and cell viability of genome recombinant strain under different acid stresses
(A) growth curves at pH 7.0 acidified with hydrochloric acid (B) growth curves at pH 5.0 acidified with hydrochloric acid (C) colonies on plate of genome recombinant bacteria grown with additional acetic acid (diluted 104, 105, 106, and 107-fold)
Cell viability of BL21-ΔcbpA/AccbpA
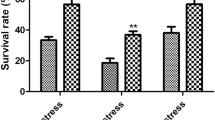
The cell viability was detected under acetic acid stress (Fig. 3C). In the absence of organic acids, the cell viability of the three strains were nearly same. The survival rate of BL21-ΔcbpA was reduced by 69.7% under 17.5 mM acetic acid stress, whereas the survival rate of the recombinant strain BL21-ΔcbpA/AccbpA was 5.7 times higher than that of the deficient strain and slightly higher than that of the wild type. Acid stress significantly affected the cell viability as the concentration of acetic acid increased to 35 mM. When compared to no organic acid, the survival rate of BL21 and BL21-ΔcbpA decreased by about 100 times, and the survival rate of BL21-ΔcbpA/AccbpA decreased by 83.3%. Meanwhile, the survival rate of BL21-ΔcbpA/AccbpA was 7.7 times higher than that of the deficient strain and 2.3 times higher than that of the wild type.
Cell morphology via FESEM observation
The cell morphology was observed via FESEM, as shown in Fig. 4. In the absence of acid, BL21-ΔcbpA cell was somewhat longer and had a minor membrane damage. Conversely, both wild-type BL21 and recombinant strain BL21-ΔcbpA/AccbpA maintained normal cell morphology. Under 35 mM acetic acid stress, the length of BL21 and BL21-ΔcbpA/AccbpA was over 1.5 times that of their acid-free counterparts, while BL21-ΔcbpA showed no significant elongation. As the outmost element, the cell envelope is the first defensive line for environmental stress. The cell envelope of BL21-ΔcbpA showed obvious breakage, the external pressure may affect the components related to the cell envelope, thereby causing changes in cell morphology (Ultee et al. 2019).
Intracellular ATP level
Most metabolism for revisiting stress requires energy, so intracellular ATP level is another crucial parameter to determine the effect of defensive mechanism. As shown in Fig. 5A, the ATP level of BL21-ΔcbpA was higher than that of BL21 (WT) and BL21-ΔcbpA/AccbpA under varied stress time, suggesting that CbpA is involved in an ATP-dependent reaction to prevent aggregation of denatured proteins (Chae et al. 2004). Besides, it gradually decreased in all strains with the extension of stress time, indicating that the strain consumed a lot of energy to resist the damage caused by acid stress. The ATP level of genome recombinant strain BL21-ΔcbpA/AccbpA was 26.2%, 56.0% and 38.0% lower than that of deficient strain at corresponding stress time, respectively. When the ATP level of each strain was compared at 5 and 10 h stress time, it was reduced by 64.5% of BL21 (WT) at 10 h compared to 5 h, and 50.0% of BL21-ΔcbpA, but only 29.5% of BL21-ΔcbpA/AccbpA integrated with acidophile gene.
Intracellular free amino acid composition
Intracellular free amino acid compositions were shown in Fig. 5B. The glutamate, arginine, lysine and histidine contents in the deficient strain showed significant changes, which were reduced by 12.4%, 59.1%, 68.3% and 24.4%, respectively, when compared to the wild type strain. The concentrations of several amino acids in recombinant strain were higher than those in deficient strain, with arginine being roughly twice as high. Furthermore, the aspartate and glutamate contents were 14.8% and 6.2% greater, respectively, than in the wild type strain. The higher intracellular accumulation of aspartate in the recombinant strain may be because it can be converted to alanine and thus consume intracellular H+ (Fernández and Zúñiga 2006). Besides, the levels of lysine and histidine in recombinant strain were 1.0 and 0.7 times greater than in deficient strain, respectively.
Comparative transcriptomic analysis of the recombinant strain
DEGseq software was employed for differentially expressed genes (DEGs) analysis. Parameters were set to padjust < 0.05, |log2FC| ≥ 1.5 to screen DEGs between wild type BL21 (WT) and deficient type BL21-ΔcbpA. A total of 173 genes were up-regulated and 93 genes were down-regulated in deficient strain compared to wild type were screened. Parameter set to padjust < 0.05, |log2FC| ≥ 1.0 to screen DEGs between deficient type and recombinant strain BL21-ΔcbpA/AccbpA. A total of 123 genes were up-regulated and 43 genes were down-regulated in recombinant strain compared to deficient strain. Most of the genes were related to membrane transport, translation and metabolism (Fig. 6 and Table S1), which are the processes most commonly affected by acid stress (Zhu et al. 2019). It also indicated that the function of cbpA in bacteria is relevant to these physiological processes.
Defense and repair mechanisms
The stabilization of macromolecules is a crucial strategy for adaptation to environmental stresses. Heat shock protein (HtpG) functions as a molecular chaperone in a variety of bacterial cellular activities, including protein folding, repair, and signal transduction, especially under environmental stress (Dong et al. 2021). The deletion of cbpA in deficient strain BL21-ΔcbpA allowed the bacteria to enhance the expression of other chaperones (HtpG) in response to stress.
ATP-dependent DEAD-box RNA helicases may play an important role in macromolecular metabolism, particularly in translation and mRNA degradation (Vinnemeier and Hagemann 1999; Prud’homme-Généreux et al. 2004). It is speculated that the knockout of cbpA may slow down the repair of damaged proteins, which in turn slows the function of these helicases. Nth is a DNA glycosylase used to scavenge oxidized pyrimidine base residues in DNA (Luna et al. 2000). The down-regulation (1.5 times) of nth gene in the deficient strain may be due to the weakened or impaired physiological activities, The deficient strain’s down-regulation (1.5 times) of nth gene may be attributed to weakened or impaired physiological activities,, whereas the recombinant strain’s expression was up-regulated. These results implied that CbpA plays a role in protein and DNA repair, and that cbpAAc in recombinant strain can restore this function to a certain extent.
Energy metabolism
The genes encoded well-known tricarboxylic acid cycle enzymes aconitase (AcnB) (Brock et al. 2002), fumarate hydratase (Akram 2014), malate dehydrogenase (Akram 2014), and succinate dehydrogenase complex (Moosavi et al. 2020) were up-regulated by 1.51 ~ 1.68 times in BL21-ΔcbpA, respectively, suggesting that deficient cells synthesized a lot of energy to resist acid stress. Acetyl-CoA synthetase (Ace) catalyzes the binding of acetic acid with CoA to produce acetyl-CoA, which is employed in various metabolic pathways such as fatty acid and cholesterol synthesis as well as the tricarboxylic acid cycle (Fujino et al. 2001; Martínez-Reyes and Chandel 2020). Succinyl-CoA synthase (Scs) synthesizes GTP with ATP as phosphate donor in the presence of GDP, and it synthesizes ATP with GTP as a phosphate donor in the presence of ADP (Kapatral et al. 2000). The α and β subunits of Scs were up-regulated by about 2.7 times. Acetate kinase (AckA) catalyzes the reversible transfer of a phosphate group from acetyl phosphate to ADP to produce acetate and ATP (Chittori et al. 2011). Cytochrome oxidase completes the last step of the electron transport chain during aerobic respiration and promotes the formation of charge gradients across the membrane for ATP synthesis. (VanOrsdel et al. 2013).
Amino acid metabolism
D-amino acid is an important component of bacterial peptidoglycan participating in cell wall remodeling and biofilm decomposition. D-amino acid dehydrogenase (DadA) catalyzes D-amino acid degradation and has a broad substrate specificity (Ohide et al. 2011). It was suggested that Pseudomonas aeruginosa could use various D-amino acids as nutrients (He et al. 2011). Meanwhile, the arginine succinyltransferase (AST) pathway was responsible for the majority of arginine catabolism in E.coli (Itoh 1997; Shirai and Mizuguchi 2003). The genes astA, astB, astC, astE, and astD related to this pathway in BL21-ΔcbpA were all up-regulated to varying degrees. The bacterial aldolase-dehydrogenase complex could produce pyruvate for other metabolic pathways (Baker et al. 2012). The glycine cleavage system is a multienzyme complex that catalyzes reversible glycine oxidation to produce carbon dioxide, ammonia, etc. (Okamura-Ikeda et al. 1993). Multifunctional proline utilization A from E. coli oxidize proline to glutamate in two catalytic steps (Moxley et al. 2011). Tryptophan enzyme can catalyze the degradation of L-tryptophan to indole, pyruvate and ammonia in vivo. The enzyme can reverse the synthesis to L-tryptophan under conditions of excessive pyruvate, ammonia, and moderate indole availability (Isupov et al. 1998). Glutaminase catalyzes glutamine hydrolysis to glutamic acid and ammonia, which is very critical for cellular bioenergetics (Márquez et al. 2006).
Cell envelope
The envelope of Gram-negative bacteria is mainly composed of outer membrane, peptidoglycan cell wall and inner membrane, which protects the cell from adverse environmental damage while enabling the passage of external nutrients and internal wastes (Silhavy et al. 2010). mdoC encodes a transmembrane protein necessary for glucan backbones replacement, which catalyzes the transfer of succinyl residues to the new glucan backbone (Lacroix et al. 1999). Putrescine can be synthesized from ornithine and arginine, and the putrescine synthesis pathway components SpeA and SpeC are required for the formation of normal biofilms (Kurihara et al. 2011). Lecithin plays a main role in the structure and function of cell membrane. Most integral membrane proteins from the large CDP-alcohol phosphatidyltransferase family are involved in phospholipids biosynthesis (Nogly et al. 2014). This family protein was down-regulated by 4.14 times in deficient strain BL21-ΔcbpA.
Entericidin is involved in the synthesis of two small cell envelope lipoproteins that regulate membrane stability reciprocally (Bishop et al. 1998). The GABA pathway consists of glutamate decarboxylase (Gad) and glutamate/GABA antiporter. The pathway’s function is to promote local alkalization of extracellular environment (Le Vo et al. 2013). Antiporter proteins were up-regulated in recombinant strain. The expression of proteins involved in gluconate transport was increased by 1.46 times. In addition, proteins related to membrane transport systems such as CydX, PTS system, ATP-binding cassette system, permease and secretion system were down-regulated in the deficient strain, but most of them were up-regulated in recombinant strain.
Carbohydrate metabolism
Malate dehydrogenase catalyzes the NAD/NADH-dependent interconversion of malate and oxoacetate substrates, which is important for the shuttle of malate/aspartate across the mitochondrial membrane and the tricarboxylic acid cycle within the mitochondrial matrix (Minárik et al. 2002). IdnK catalyzes ATP-dependent phosphorylation of D-gluconate to 6- phosphogluconate, which is further metabolized by the enter-doudoroff pathway (Bausch et al. 1998). The pyruvate dehydrogenase (PDH) complex is a major bridge between bacterial glycolysis and TCA cycle, leading to oxidative decarboxylation of pyruvate to acetyl-CoA (Yang and Zhang 2017). The protein’s E1 and E2 components were both downregulated.
Bacterial L-rhamnose kinase, an enzyme that catalyzes the transfer of γ-phosphate from ATP to the L-hydroxyl group of L-rhamnose, is involved in L-rhamnose degradation (Grueninger and Schulz 2006). The ptsG gene of glucose permease was up-regulated 2.02 times in recombinant strain compared to the deficient strain to supply cell physiological activities. idnK was up-regulated by 1.41 times. Deoxyribosylalase, a key enzyme in the pentose phosphate pathway, catalyzes the reversible reaction of deoxyribose 5-phosphate with glyceraldehyde 3-phosphate and acetaldehyde (Valentin-Hansen et al. 1982).
Gene replication, transcription and translation
Ribosome is a basic cellular complex that is highly conserved in all organisms and is responsible for translating mRNA genetic information into the amino acid sequence of proteins. Most genes associated to the large and small subunits of the ribosome were down-regulated, such as rpsO (RP-S15) and rpsQ (RP-S17), which were down-regulated by 1.51 and 1.64 times, respectively, in the deficient strain. S15 is located at the edge of the 30 S subunit and, together with S17 and S20, is considered to be crucial for initiating subsequent cooperative binding of secondary and late assembly proteins (Bubunenko et al. 2007). The intact r protein L31 of E. coli plays an important role in the formation and stabilization of 70 S ribosomes and is involved in translational activity (Arnold and Reilly 1999; Ueta et al. 2017). The E. coli 50 S subunit lacking L36 contains aberrations extending from the L36 binding site ~ 60 Å to the peptidyl transferase center (Jomaa et al. 2014). Other ribosomal protein subunits were likewise down-regulated to varied degrees, suggesting that the translation process was severely affected. However, ribosomal-related proteins L31, L34, and L36 were up-regulated in recombinant strain, indicating that the genome editing strain enhanced translation processes.
Transport system
Microorganisms could modulate nutrient transport and ion exchange to resist acid stress by altering the activity of transporters (Guan et al. 2017). Members of the ATP-binding cassette (ABC) superfamily are one of the largest classes of systems that utilize ATP to transport a variety of solutes in prokaryotic and eukaryotic cells, including amino acids, ions, proteins, and polysaccharides (Stewart and Hermodson 2003; Locher 2016). The N-acetylgalactosamine transporter components agaE and agaW were related to cell wall components. These transport system related proteins were up-regulated, indicating that cell wall synthesis is enhanced under stress. However, the expression of genes related to fructose, glucose phosphate transfer protein were down-regulated as well as secretion system. It was consistent with the results of carbon metabolism, indicating that the cell activity is mostly used to defend against stress when bacteria suffer from acid environment.
The outer membrane of most Gram-negative bacteria contains lipopolysaccharide (LPS). LPS is a biologically important molecule that restricts the entry of hydrophobic molecules in bacteria by establishing a barrier (Li et al. 2020). The secretion system-related proteins GspG and GspK are also up-regulated. Secretion protein homologues may polymerize into a columnar structure that mediates the flow of released proteins across the cell membrane (Reeves et al. 1993).
Discussion
It has been reported that microorganisms require the transient and rapid expression of certain proteins, such as heat shock proteins, in order to survive under stress environments (Liu et al. 2021). In silico analysis showed that CbpAAc from A. caldus contained a distinct J-domain which can stimulate Hsp70/DnaK ATPase activity, thereby interacting with DnaK (Wall et al. 1994; Mayer 2021). The J-domain and its chaperone regulatory mechanism are highly conserved from bacteria to humans (Sarraf et al. 2014).
To verify the effect of CbpAAc protein on acid tolerance of E. coli, a recombinant vector carrying the target gene was introduced into the gene knockout strain BL21-ΔcbpA. Different copy number plasmids were selected, and strain BL21-ΔcbpA/pACYC-AccbpA showed a better growth performance under acid stress. Acetic acid is a growth inhibitory substance and a typical byproduct in carbohydrate metabolism. During biomass utilization in industrial production, a substantial amount of acetic acid may be released, leading to increased acid stress (Gao et al. 2018). Cell viability experiment was conducted to evaluate the bacteria acid tolerance, which showed a greater improvement of BL21-ΔcbpA/pACYC-AccbpA to increase in acetic acid concentration rather than wild type. The increased cell viability indicated that cbpAAc plays an important part in acid tolerance process. Under stress circumstances, molecular chaperone CbpA could stimulate the assembly of DnaK and client proteins, and further engaged in cell signaling and stress response. (Genest et al. 2015). However, an unavoidable metabolic burden and higher adaption costs are frequently brought about by the introduction of engineered genetic systems in bacteria (Diaz Ricci and Hernández 2000; Rouches et al. 2022).
With the assistance of CRISPR/Cas9 genome editing, we further constructed a genome recombinant strain BL21-ΔcbpA/AccbpA. Genomic integration of cbpAAc didn’t show a significant growth advantage, which may be because the associated genes varied among hosts in terms of base composition and codon preference (Harnischfeger et al. 2021). In addition, the plasmid-complementary strain stimulated the target gene’s expression, which was higher than that of the genome recombinant strain due to the coexistence of the T7 promoter on the plasmid and the E. coli T7 RNA polymerase. Surprisingly, compared to no acetic acid, the cell viability of recombinant strain was significantly increased when the strain suffered 35 mM acetic acid. On the contrary, cell viability of wild type BL21 was decreased dramatically. The introduction of stress response factor in the genomic context is a promising technique for engineering acid-tolerant phenotypes (Lin et al. 2021). Chen et al. reported that Lactobacillus kefiranofaciens adapts to stress by increasing the production of Hsps and chaperone proteins, which play an important role in correct folding and post-translational modification of newly synthesized proteins (Chen et al. 2017). Besides, the shape and size change of bacteria, known as morphological plasticity or adaptive morphogenesis, is a positive response to combat environmental stress and promote survival (Yang et al. 2016; Ultee et al. 2019).
When microbial cells are exposed to acid stress, they will typically consume ATP for a variety of physiological pathways to maintain intracellular pH homeostasis (Guan and Liu 2020). The presence of molecular chaperone CbpA requires a cycle of ATP binding and hydrolysis to act on unnatural peptides, promoting their folding or unfolding (Saibil 2013). The recombinant strain’s ATP level showed the minimal change after 5 h of stress, indicating that it was more stable to withstand long-term acid stress. Studies have shown that the stress of most weak acids such as sorbate, propionic acid and acetic acid can stimulate the unfolded protein response (Kawazoe et al. 2017). In addition, the accumulation of weakly acidic anions in cytoplasm disrupts electron transport chains in the inner mitochondrial membrane, leading to ATP depletion (Piper et al. 2001; Peetermans et al. 2021).
Amino acids also play an important role in the resistance of microorganisms to acid stress, including the regulation of intracellular pH, energy production, and redox capacity (Lund et al. 2014; Liu et al. 2015). The arginine deaminase (ADI) system, which could produce ATP and NH3, can stabilize intracellular H+ and thus protect cells from acid stress (Diez-Gonzalez and Karaibrahimoglu 2004; Wang et al. 2020). In addition, aspartate can be converted to arginine to enter the ADI pathway, and it can also consume protons to form alanine (Fernández and Zúñiga 2006; Wang et al. 2020). Glutamate decarboxylase (GAD) system is another important acid tolerance mechanism in bacteria. The accumulation of glutamate under acid stress conditions is conducive to the decarboxylation reaction catalyzed by glutamate decarboxylase, which generates CO2 and γ-aminobutyric acid (GABA) while consuming a molecule of H+ to alleviate intracellular acid stress (Krulwich et al. 2011). The proton consumption reaction mediated by lysine decarboxylase, as well as the decarboxylation of histidine to histamine and CO2, also are common acid stress coping methods (Fernández and Zúñiga 2006; Guo et al. 2019).
The DEGs of the wild-type strain BL21, the deficient strain BL21-ΔcbpA, and the recombinant strain BL21-ΔcbpA/AccbpA under 35 mM acetic acid stress were illustrated to further investigate the putative mechanism of acid tolerance enhancement mediated by the molecular chaperone protein CbpAAc. CbpA was shown to be involved in a variety of cellular processes, including repair, cell envelope integrity, carbohydrate metabolism, gene replication, transcription and translation as well as transport system. In the absence of cbpA, the strain initiated other repair mechanisms to resist acid. It also enhanced energy mechanism and amino acid mechanism. The down-regulation of membrane proteins and associated components was consistent with the cell membrane breakage observed via FESEM. Furthermore, the transport system on membrane was damaged. Based on the findings, the model of acid tolerance mechanism of cbpA in A. caldus was preliminarily developed as Fig. 7.
Conclusion
In this study, with the assistance of CRISPR/Cas9, molecular chaperone CbpA from extreme acidophile A. caldus was applied to enhance the acid tolerance of E. coli. Both of the cell growth and cell viability were improved under acid stress. FESEM observation showed a well cell morphology. More ATP was consumed, while the contents of intracellular aspartate and glutamate participated in arginine deaminase system and glutamate decarboxylase system were increased. Taking together, a potential model of the molecular chaperone CbpA’s acid tolerance mechanism was developed. This study constructed an engineered strain which could be utilized to reduce the protein misfolding under acid stress. Furthermore, the work also shines a light on how to enhance microorganisms’ robustness to various environmental stresses.
Data availability
The data supporting the findings of this study are available within this article.
References
Akram M (2014) Citric acid cycle and role of its Intermediates in Metabolism. Cell Biochem Biophys 68(3):475–478. https://doi.org/10.1007/s12013-013-9750-1
Arnold RJ, Reilly JP (1999) Observation of Escherichia coli ribosomal proteins and their posttranslational modifications by mass spectrometry. Anal Biochem 269(1):105–112. https://doi.org/10.1006/abio.1998.3077
Baker P, Hillis C, Carere J, Seah SYK (2012) Protein–protein interactions and substrate channeling in Orthologous and chimeric aldolase–dehydrogenase complexes. Biochemistry 51(9):1942–1952. https://doi.org/10.1021/bi201832a
Bausch C, Peekhaus N, Utz C, Blais T, Murray E, Lowary T, Conway T (1998) Sequence analysis of the GntII (subsidiary) system for gluconate metabolism reveals a novel pathway for L-idonic acid catabolism in Escherichia coli. J Bacteriol 180(14):3704–3710. https://doi.org/10.1128/jb.180.14.3704-3710.1998
Bird JG, Sharma S, Roshwalb SC, Hoskins JR, Wickner S (2006) Functional analysis of CbpA, a DnaJ homolog and nucleoid-associated DNA-binding protein. J Biol Chem 281(45):34349–34356
Bishop RE, Leskiw BK, Hodges RS, Kay CM, Weiner JH (1998) The entericidin locus of Escherichia coli and its implications for programmed bacterial cell death. J Mol Biol 280(4):583–596. https://doi.org/10.1006/jmbi.1998.1894
Brameyer S, Schumacher K, Kuppermann S, Jung K (2022) Division of labor and collective functionality in Escherichia coli under acid stress. Commun Biology 5(1):327. https://doi.org/10.1038/s42003-022-03281-4
Brock M, Maerker C, Schütz A, Völker U, Buckel W (2002) Oxidation of propionate to pyruvate in Escherichia coli. Involvement of methylcitrate dehydratase and aconitase. Eur J Biochem 269(24):6184–6194. https://doi.org/10.1046/j.1432-1033.2002.03336.x
Bubunenko M, Baker T, Court DL (2007) Essentiality of ribosomal and transcription antitermination proteins analyzed by systematic gene replacement in Escherichia coli. J Bacteriol 189(7):2844–2853. https://doi.org/10.1128/jb.01713-06
Chae C, Sharma S, Hoskins JR, Wickner S (2004) CbpA, a DnaJ homolog, is a DnaK co-chaperone, and its activity is modulated by CbpM. J Biol Chem 279(32):33147–33153. https://doi.org/10.1074/jbc.M404862200
Chen MJ, Tang HY, Chiang ML (2017) Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol 66:20–27. https://doi.org/10.1016/j.fm.2017.03.020
Chen XK, Li XY, Ha YF, Lin JQ, Liu XM, Pang X, Lin JQ, Chen LX (2020) Ferric Uptake Regulator provides a new strategy for Acidophile Adaptation to Acidic Ecosystems. Appl Environ Microbiol 86(11). https://doi.org/10.1128/aem.00268-20
Chen J, Liu Y, Diep P, Mahadevan R (2022) Genetic engineering of extremely acidophilic Acidithiobacillus species for biomining: progress and perspectives. J Hazard Mater 438:129456. https://doi.org/10.1016/j.jhazmat.2022.129456
Chittori S, Savithri HS, Murthy MR (2011) Preliminary X-ray crystallographic studies on acetate kinase (AckA) from Salmonella typhimurium in two crystal forms. Acta Crystallogr Sect F Struct Biol Cryst Commun 67(Pt 12):1658–1661. https://doi.org/10.1107/s1744309111043740
Cosgriff S, Chintakayala K, Chim YT, Chen X, Allen S, Lovering AL, Grainger DC (2010) Dimerization and DNA-dependent aggregation of the Escherichia coli nucleoid protein and chaperone CbpA. Mol Microbiol 77(5):1289–1300. https://doi.org/10.1111/j.1365-2958.2010.07292.x
Diaz Ricci JC, Hernández ME (2000) Plasmid effects on Escherichia coli metabolism. Crit Rev Biotechnol 20(2):79–108. https://doi.org/10.1080/07388550008984167
Diez-Gonzalez F, Karaibrahimoglu Y (2004) Comparison of the glutamate-, arginine- and lysine-dependent acid resistance systems in Escherichia coli O157:H7. J Appl Microbiol 96(6):1237–1244. https://doi.org/10.1111/j.1365-2672.2004.02251.x
Dong T, Wang W, Xia M, Liang S, Hu G, Ye H, Cao Q, Dong Z, Zhang C, Feng D, Zuo J (2021) Involvement of the heat shock protein HtpG of Salmonella Typhimurium in infection and proliferation in hosts. Front Cell Infect Microbiol 11:758898. https://doi.org/10.3389/fcimb.2021.758898
Feng J, Li C, He H, Xu S, Wang X, Chen K (2022) Construction of cell factory through combinatorial metabolic engineering for efficient production of itaconic acid. Microb Cell Fact 21(1):275. https://doi.org/10.1186/s12934-022-02001-1
Fernández M, Zúñiga M (2006) Amino acid catabolic pathways of lactic acid bacteria. Crit Rev Microbiol 32(3):155–183. https://doi.org/10.1080/10408410600880643
Fountoulakis M, Lahm H-W (1998) Hydrolysis and amino acid composition analysis of proteins. J Chromatogr A 826(2):109–134. https://doi.org/10.1016/S0021-9673(98)00721-3
Fujino T, Kondo J, Ishikawa M, Morikawa K, Yamamoto TT (2001) Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J Biol Chem 276(14):11420–11426. https://doi.org/10.1074/jbc.M008782200
Gao X, Yang X, Li J, Zhang Y, Chen P, Lin Z (2018) Engineered global regulator H-NS improves the acid tolerance of E. coli. Microb Cell Fact 17(1):118. https://doi.org/10.1186/s12934-018-0966-z
Genest O, Hoskins JR, Kravats AN, Doyle SM, Wickner S (2015) Hsp70 and Hsp90 of E. coli directly interact for collaboration in protein remodeling. J Mol Biol 427(24):3877–3889. https://doi.org/10.1016/j.jmb.2015.10.010
Grueninger D, Schulz GE (2006) Structure and reaction mechanism of l-Rhamnulose kinase from Escherichia coli. J Mol Biol 359(3):787–797. https://doi.org/10.1016/j.jmb.2006.04.013
Guan N, Liu L (2020) Microbial response to acid stress: mechanisms and applications. Appl Microbiol Biotechnol 104(1):51–65. https://doi.org/10.1007/s00253-019-10226-1
Guan N, Li J, Shin HD, Du G, Chen J, Liu L (2017) Microbial response to environmental stresses: from fundamental mechanisms to practical applications. Appl Microbiol Biotechnol 101(10):3991–4008. https://doi.org/10.1007/s00253-017-8264-y
Guo J, Ma Z, Gao J, Zhao J, Wei L, Liu J, Xu N (2019) Recent advances of pH homeostasis mechanisms in Corynebacterium glutamicum. World J Microbiol Biotechnol 35(12):192. https://doi.org/10.1007/s11274-019-2770-2
Harnischfeger J, Beutler M, Salzig D, Rahlfs S, Becker K, Grevelding CG, Czermak P (2021) Biochemical characterization of the recombinant schistosome tegumental protein SmALDH_312 produced in E. coli and baculovirus expression vector system. Electron J Biotechnol 54:26–36. https://doi.org/10.1016/j.ejbt.2021.08.002
He W, Li C, Lu CD (2011) Regulation and characterization of the dadRAX locus for D-amino acid catabolism in Pseudomonas aeruginosa PAO1. J Bacteriol 193(9):2107–2115. https://doi.org/10.1128/jb.00036-11
Isupov MN, Antson AA, Dodson EJ, Dodson GG, Dementieva IS, Zakomirdina LN, Wilson KS, Dauter Z, Lebedev AA, Harutyunyan EH (1998) Crystal structure of tryptophanase. J Mol Biol 276(3):603–623. https://doi.org/10.1006/jmbi.1997.1561
Itoh Y (1997) Cloning and characterization of the aru genes encoding enzymes of the catabolic arginine succinyltransferase pathway in Pseudomonas aeruginosa. J Bacteriol 179(23):7280–7290. https://doi.org/10.1128/jb.179.23.7280-7290.1997
Jiang Y, Chen B, Duan C, Sun B, Yang J, Yang S (2015) Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl Environ Microbiol 81(7):2506–2514. https://doi.org/10.1128/aem.04023-14
Jomaa A, Jain N, Davis JH, Williamson JR, Britton RA, Ortega J (2014) Functional domains of the 50S subunit mature late in the assembly process. Nucleic Acids Res 42(5):3419–3435. https://doi.org/10.1093/nar/gkt1295
Kang H-J, Heo D-H, Choi S-W, Kim K-N, Shim J, Kim C-W, Sung H-C, Yun C-W (2007) Functional characterization of Hsp33 protein from Bacillus psychrosaccharolyticus; additional function of HSP33 on resistance to solvent stress. Biochem Biophys Res Commun 358(3):743–750. https://doi.org/10.1016/j.bbrc.2007.04.184
Kapatral V, Bina X, Chakrabarty AM (2000) Succinyl coenzyme A synthetase of Pseudomonas aeruginosa with a broad specificity for nucleoside triphosphate (NTP) synthesis modulates specificity for NTP synthesis by the 12-kilodalton form of nucleoside diphosphate kinase. J Bacteriol 182(5):1333–1339. https://doi.org/10.1128/jb.182.5.1333-1339.2000
Kawazoe N, Kimata Y, Izawa S (2017) Acetic acid causes endoplasmic reticulum stress and induces the unfolded protein response in Saccharomyces cerevisiae. Front Microbiol 8:1192. https://doi.org/10.3389/fmicb.2017.01192
Krulwich TA, Sachs G, Padan E (2011) Molecular aspects of bacterial pH sensing and homeostasis. Nat Reviews: Microbiol 9(5):330–343. https://doi.org/10.1038/nrmicro2549
Kurihara S, Suzuki H, Oshida M, Benno Y (2011) A Novel Putrescine Importer required for type 1 Pili-driven Surface Motility Induced by Extracellular Putrescine in Escherichia coli K-12*. J Biol Chem 286(12):10185–10192. https://doi.org/10.1074/jbc.M110.176032
Lacroix JM, Lanfroy E, Cogez V, Lequette Y, Bohin A, Bohin JP (1999) The mdoC gene of Escherichia coli encodes a membrane protein that is required for succinylation of osmoregulated periplasmic glucans. J Bacteriol 181(12):3626–3631. https://doi.org/10.1128/jb.181.12.3626-3631.1999
Le Vo TD, Ko J-s, Park SJ, Lee SH, Hong SH (2013) Efficient gamma-aminobutyric acid bioconversion by employing synthetic complex between glutamate decarboxylase and glutamate/GABA antiporter in engineered Escherichia coli. J Ind Microbiol Biotechnol 40(8):927–933. https://doi.org/10.1007/s10295-013-1289-z
Li Z, Jiang B, Zhang X, Yang Y, Hardwidge PR, Ren W, Zhu G (2020) The role of bacterial cell envelope structures in acid stress resistance in E. coli. Appl Microbiol Biotechnol 104(7):2911–2921. https://doi.org/10.1007/s00253-020-10453-x
Lin Z, Li J, Yan X, Yang J, Li X, Chen P, Yang X (2021) Engineering of the small noncoding RNA (sRNA) DsrA together with the sRNA chaperone hfq enhances the Acid Tolerance of Escherichia coli. Appl Environ Microbiol 87(10). https://doi.org/10.1128/aem.02923-20
Liu Y, Tang H, Lin Z, Xu P (2015) Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation. Biotechnol Adv 33(7):1484–1492. https://doi.org/10.1016/j.biotechadv.2015.06.001
Liu H, Qi Y, Zhou P, Ye C, Gao C, Chen X, Liu L (2021) Microbial physiological engineering increases the efficiency of microbial cell factories. Crit Rev Biotechnol 41(3):339–354. https://doi.org/10.1080/07388551.2020.1856770
Locher KP (2016) Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol 23(6):487–493. https://doi.org/10.1038/nsmb.3216
Luna L, Bjørås M, Hoff E, Rognes T, Seeberg E (2000) Cell-cycle regulation, intracellular sorting and induced overexpression of the human NTH1 DNA glycosylase involved in removal of formamidopyrimidine residues from DNA. Mutat Res 460(2):95–104. https://doi.org/10.1016/s0921-8777(00)00015-x
Lund P, Tramonti A, De Biase D (2014) Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev 38(6):1091–1125. https://doi.org/10.1111/1574-6976.12076
Marc J, Grousseau E, Lombard E, Sinskey AJ, Gorret N, Guillouet SE (2017) Over expression of GroESL in Cupriavidus necator for heterotrophic and autotrophic isopropanol production. Metab Eng 42:74–84. https://doi.org/10.1016/j.ymben.2017.05.007
Márquez J, de la Oliva AR, Matés JM, Segura JA, Alonso FJ (2006) Glutaminase: a multifaceted protein not only involved in generating glutamate. Neurochem Int 48(6–7):465–471. https://doi.org/10.1016/j.neuint.2005.10.015
Martínez-Reyes I, Chandel NS (2020) Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 11(1):102. https://doi.org/10.1038/s41467-019-13668-3
Matuszewska M, Kuczyńska-Wiśnik D, Laskowska E, Liberek K (2005) The small heat shock protein IbpA of Escherichia coli cooperates with IbpB in stabilization of thermally aggregated proteins in a disaggregation competent state. J Biol Chem 280(13):12292–12298. https://doi.org/10.1074/jbc.M412706200
Mayer MP (2021) The Hsp70-Chaperone machines in Bacteria. Front Mol Biosci 8:694012. https://doi.org/10.3389/fmolb.2021.694012
Minárik P, Tomásková N, Kollárová M, Antalík M (2002) Malate dehydrogenases–structure and function. Gen Physiol Biophys 21(3):257–265
Molan K, Žgur Bertok D (2022) Small prokaryotic DNA-Binding proteins protect Genome Integrity throughout the life cycle. Int J Mol Sci 23(7). https://doi.org/10.3390/ijms23074008
Monteagudo-Cascales E, Martín-Mora D, Xu W, Sourjik V, Matilla MA, Ortega Á, Krell T (2022) The pH robustness of bacterial sensing. mBio 13(5):e0165022. https://doi.org/10.1128/mbio.01650-22
Moosavi B, Zhu XL, Yang WC, Yang GF (2020) Genetic, epigenetic and biochemical regulation of succinate dehydrogenase function. Biol Chem 401(3):319–330. https://doi.org/10.1515/hsz-2019-0264
Moxley MA, Tanner JJ, Becker DF (2011) Steady-state kinetic mechanism of the proline:ubiquinone oxidoreductase activity of proline utilization A (PutA) from Escherichia coli. Arch Biochem Biophys 516(2):113–120. https://doi.org/10.1016/j.abb.2011.10.011
Nogly P, Gushchin I, Remeeva A, Esteves AM, Borges N, Ma P, Ishchenko A, Grudinin S, Round E, Moraes I, Borshchevskiy V, Santos H, Gordeliy V, Archer M (2014) X-ray structure of a CDP-alcohol phosphatidyltransferase membrane enzyme and insights into its catalytic mechanism. Nat Commun 5:4169. https://doi.org/10.1038/ncomms5169
Ohide H, Miyoshi Y, Maruyama R, Hamase K, Konno R (2011) D-Amino acid metabolism in mammals: biosynthesis, degradation and analytical aspects of the metabolic study. J Chromatogr B Analyt Technol Biomed Life Sci 879(29):3162–3168. https://doi.org/10.1016/j.jchromb.2011.06.028
Okamura-Ikeda K, Ohmura Y, Fujiwara K, Motokawa Y (1993) Cloning and nucleotide sequence of the gcv operon encoding the Escherichia coli glycine-cleavage system. Eur J Biochem 216(2):539–548. https://doi.org/10.1111/j.1432-1033.1993.tb18172.x
Okochi M, Kanie K, Kurimoto M, Yohda M, Honda H (2008) Overexpression of prefoldin from the hyperthermophilic archaeum Pyrococcus horikoshii OT3 endowed Escherichia coli with organic solvent tolerance. Appl Microbiol Biotechnol 79(3):443–449. https://doi.org/10.1007/s00253-008-1450-1
Peetermans A, Foulquié-Moreno MR, Thevelein JM (2021) Mechanisms underlying lactic acid tolerance and its influence on lactic acid production in Saccharomyces cerevisiae. Microb Cell 8(6):111–130. https://doi.org/10.15698/mic2021.06.751
Pepe S, Scarlato V, Roncarati D (2020) The Helicobacter pylori HspR-Modulator CbpA is a multifunctional heat-shock protein. Microorganisms 8(2). https://doi.org/10.3390/microorganisms8020251
Piper P, Calderon CO, Hatzixanthis K, Mollapour M (2001) Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147(Pt 10):2635–2642. https://doi.org/10.1099/00221287-147-10-2635
Prud’homme-Généreux A, Beran RK, Iost I, Ramey CS, Mackie GA, Simons RW (2004) Physical and functional interactions among RNase E, polynucleotide phosphorylase and the cold-shock protein, CsdA: evidence for a ‘cold shock degradosome’. Mol Microbiol 54(5):1409–1421. https://doi.org/10.1111/j.1365-2958.2004.04360.x
Reeves PJ, Whitcombe D, Wharam S, Gibson M, Allison G, Bunce N, Barallon R, Douglas P, Mulholland V, Stevens S (1993) Molecular cloning and characterization of 13 out genes from Erwinia carotovora subspecies carotovora: genes encoding members of a general secretion pathway (GSP) widespread in gram-negative bacteria. Mol Microbiol 8(3):443–456. https://doi.org/10.1111/j.1365-2958.1993.tb01589.x
Roncarati D, Scarlato V (2017) Regulation of heat-shock genes in bacteria: from signal sensing to gene expression output. FEMS Microbiol Rev 41(4):549–574. https://doi.org/10.1093/femsre/fux015
Rouches MV, Xu Y, Cortes LBG, Lambert G (2022) A plasmid system with tunable copy number. Nat Commun 13(1):3908. https://doi.org/10.1038/s41467-022-31422-0
Saibil H (2013) Chaperone machines for protein folding, unfolding and disaggregation. Nat Reviews: Mol Cell Biology 14(10):630–642. https://doi.org/10.1038/nrm3658
Sarraf NS, Baardsnes J, Cheng J, O’Connor-McCourt M, Cygler M, Ekiel I (2010) Structural basis of the regulation of the CbpA co-chaperone by its specific modulator CbpM. J Mol Biol 398(1):111–121. https://doi.org/10.1016/j.jmb.2010.03.006
Sarraf NS, Shi R, McDonald L, Baardsnes J, Zhang L, Cygler M, Ekiel I (2014) Structure of CbpA J-domain bound to the regulatory protein Cbpm explains its specificity and suggests evolutionary link between Cbpm and transcriptional regulators. PLoS ONE 9(6):e100441. https://doi.org/10.1371/journal.pone.0100441
Shirai H, Mizuguchi K (2003) Prediction of the structure and function of AstA and AstB, the first two enzymes of the arginine succinyltransferase pathway of arginine catabolism. FEBS Lett 555(3):505–510. https://doi.org/10.1016/s0014-5793(03)01314-0
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2(5):a000414. https://doi.org/10.1101/cshperspect.a000414
Stewart JB, Hermodson MA (2003) Topology of RbsC, the membrane component of the Escherichia coli ribose transporter. J Bacteriol 185(17):5234–5239. https://doi.org/10.1128/jb.185.17.5234-5239.2003
Ueta M, Wada C, Bessho Y, Maeda M, Wada A (2017) Ribosomal protein L31 in Escherichia coli contributes to ribosome subunit association and translation, whereas short L31 cleaved by protease 7 reduces both activities. Genes Cells 22(5):452–471. https://doi.org/10.1111/gtc.12488
Ultee E, Ramijan K, Dame RT, Briegel A, Claessen D (2019) Stress-induced adaptive morphogenesis in bacteria. Adv Microb Physiol 74:97–141. https://doi.org/10.1016/bs.ampbs.2019.02.001
Valentin-Hansen P, Boëtius F, Hammer-Jespersen K, Svendsen I (1982) The primary structure of Escherichia coli K12 2-deoxyribose 5-phosphate aldolase. Nucleotide sequence of the deoC gene and the amino acid sequence of the enzyme. Eur J Biochem 125(3):561–566. https://doi.org/10.1111/j.1432-1033.1982.tb06719.x
VanOrsdel CE, Bhatt S, Allen RJ, Brenner EP, Hobson JJ, Jamil A, Haynes BM, Genson AM, Hemm MR (2013) The Escherichia coli CydX protein is a member of the CydAB cytochrome bd oxidase complex and is required for cytochrome bd oxidase activity. J Bacteriol 195(16):3640–3650. https://doi.org/10.1128/jb.00324-13
Vinnemeier J, Hagemann M (1999) Identification of salt-regulated genes in the genome of the cyanobacterium Synechocystis sp. strain PCC 6803 by subtractive RNA hybridization. Arch Microbiol 172(6):377–386. https://doi.org/10.1007/s002030050774
Wall D, Zylicz M, Georgopoulos C (1994) The NH2-terminal 108 amino acids of the Escherichia coli DnaJ protein stimulate the ATPase activity of DnaK and are sufficient for lambda replication. J Biol Chem 269(7):5446–5451. https://doi.org/10.1016/S0021-9258(17)37706-2
Wang J, Wang W, Wang H, Yuan F, Xu Z, Yang K, Li Z, Chen Y, Fan K (2019) Improvement of stress tolerance and riboflavin production of Bacillus subtilis by introduction of heat shock proteins from thermophilic bacillus strains. Appl Microbiol Biotechnol 103(11):4455–4465. https://doi.org/10.1007/s00253-019-09788-x
Wang C, Ren X, Yu C, Wang J, Wang L, Zhuge X, Liu X (2020) Physiological and transcriptional responses of Streptomyces albulus to acid stress in the biosynthesis of ε-Poly-L-lysine. Front Microbiol 11:1379. https://doi.org/10.3389/fmicb.2020.01379
Weidmann S, Maitre M, Laurent J, Coucheney F, Rieu A, Guzzo J (2017) Production of the small heat shock protein Lo18 from Oenococcus oeni in Lactococcus lactis improves its stress tolerance. Int J Food Microbiol 247:18–23. https://doi.org/10.1016/j.ijfoodmicro.2016.06.005
Yang M, Zhang X (2017) Construction of pyruvate producing strain with intact pyruvate dehydrogenase and genome-wide transcription analysis. World J Microbiol Biotechnol 33(3):59. https://doi.org/10.1007/s11274-016-2202-5
Yang DC, Blair KM, Salama NR (2016) Staying in shape: the impact of cell shape on bacterial survival in diverse environments. Microbiol Mol Biol Rev 80(1):187–203. https://doi.org/10.1128/mmbr.00031-15
Yin Y, Tong Y, Yang H, Feng S (2022) EpsR(ac) is a copper-sensing MarR family transcriptional repressor from Acidithiobacillus caldus. Appl Microbiol Biotechnol 106(9–10):3679–3689. https://doi.org/10.1007/s00253-022-11971-6
Zhu Z, Yang J, Yang P, Wu Z, Zhang J, Du G (2019) Enhanced acid-stress tolerance in Lactococcus lactis NZ9000 by overexpression of ABC transporters. Microb Cell Fact 18(1):136. https://doi.org/10.1186/s12934-019-1188-8
Acknowledgements
This study was supported by grants from the National Key Research and Development Program of China (2022YFC3401300), the National Natural Science Foundation of China (No. 21878128; 21776113; 21606110; 31701582), the funding of Key Laboratory of Industrial Biotechnology, Ministry of Education (KLIBKF202005), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Program of Introducing Talents of Discipline to Universities (No. 111-2-06).
Author information
Authors and Affiliations
Contributions
Zhenming Jiang and Shoushuai Feng designed the research and analyzed the data. Zhenming Jiang and Jie Lu conducted experiments. Zhenming Jiang wrote the first draft of the manuscript. Shoushuai Feng, Hailin Yang, and Yanjun Tong contributed to manuscript revision, read, and approved the submitted version. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Z., Lu, J., Tong, Y. et al. Enhancement of acid tolerance of Escherichia coli by introduction of molecule chaperone CbpA from extremophile. World J Microbiol Biotechnol 39, 158 (2023). https://doi.org/10.1007/s11274-023-03613-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03613-4