Abstract
Chitosan is a versatile biopolymer due to its biocompatibility, biodegradability, antimicrobial, non-toxic, mucoadhesive, and highly adsorptive properties. Chitosan and its derivatives have been used for many biomedical applications. Currently, crustacean shells and other marine organisms are the significant sources of chitin/chitosan production worldwide. However, extraction from marine sources presents several challenges, including an unstable supply of raw materials. Large-scale chitosan extraction from crustacean sources harms the environment by involving harsh processing steps such as alkali deproteinization. Recently many studies have been carried out focusing on alternative sources or eco-friendlier routes for production of chitosan. This paper briefly overviews recent studies on fungi and insect cuticles as alternative chitosan sources. Milder extraction processes for fungal chitosan and the superior quality of the resultant polymer make it highly desirable for biological applications. Biological techniques involving fermentation and enzymatic processing of the raw materials are looked at in detail. In the concluding remarks, the paper highlights the potential of using a combination of “green” technologies and briefly looks at potential biological/biomedical applications of extracted chitinous materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whether from exoskeletons of insects and crustaceans, the radulae of mollusks or fungal species, the sources of chitin and chitosan are abundant and global. Chitin is one of the most abundant biopolymers in nature. It comprises repeating units of N-acetyl glucosamine (GlcNAc) connected by β-(1 → 4) linkages (Vicente et al. 2021). Chitin is a homopolymer of N-acetyl-0-glucosamine (GlcNAc), typically with large molecular weights, and has structural similarity with cellulose. Naturally occurring chitin has O-glucosamine (GlcN) units, randomly interspersed in the polymeric chain. The chitin molecules are abundant in crustacean and insect exoskeletons. They are closely associated with proteins, calcium carbonate, lipids and pigments, all of which are to be separated by chemical or biological methods to obtain pure chitin. Extraction of crustacean or insect chitin is often the forerunner step of chitosan extraction, and thus recent advances in both processes have been discussed in the subsequent sections. In nature, the cell walls of certain fungal species contain chitosan and can be used for direct commercial production of chitosan. Chitosan is the partially or fully deacetylated form of chitin. The copolymer is termed chitosan if the number of deacetylated units (GlcN) are more than the number of N-acetyl-glucosamine (GlcNAc) units, i.e. degree of deacetylation is higher than 50%. Chitooligosachharides (COS) and partially acetylated chitooligosaccharides (paCOS) are the low molecular weight versions of chitin and chitosan (Tabassum et al. 2021). The polymers and oligopolymers are represented in Fig. 1.
Schematic representations of the polymers, a chitin (n > 10), b chitooligosachharide, COS (n < 10), c chitosan (m > n, m + n > 10), d partially acetylated chitooligosachharides, paCOS (m + n < 10). Adapted from (Afroz et al. 2021) with permission for reuse and modification
The source of chitosan, as well as the method of extraction, is a significant research field because these parameters heavily influence the properties and effectiveness of the biopolymers. The molecular weight, degree of deacetylation (DD%), degree of polymerization (DP%), and polydispersity index are the major factors affecting properties such as solubility, crystallinity and hydrophilicity/hydrophobicity. Conventional (or chemical) extraction from crustacean shells involves three primary steps, (1) demineralization of the shells to remove calcium carbonate and calcium phosphate, usually carried out by HCl or other strong acids; (2) deproteination to remove the proteins, adhering to the chitosan, by incubating in strong base like NaOH, at high temperatures (Dhillon et al. 2013); and (3) deacetylation, to convert chitin to chitosan, using 50%w/w NaOH, at high temperatures. An additional decolorization step is sometimes carried out to remove carotenoid pigments using different solvents (acetone, hydrogen peroxide, sodium hypochlorite) or potassium permanganate. Further steps can be used to convert the chitosan to more biologically active COS. Reducing the use or strength of these chemicals and/or reduction of energy consumed for production could produce chitosan and associated chemicals, not only is an eco-friendlier manner, but in some cases can also give products with higher bioactivity (Mathew et al. 2021).
This review focuses on recent methods of chitin and chitosan/COS/paCOS production, which are more sustainable either by being ‘greener’ or more economic or both. The methods are divided into two broad topics. The first topic looks at alternative sources to shrimp shells, to utilize these readily available waste biomasses into viable sources of chitosan. The second topic looks at using biological or biochemical methods, either using the enzyme producing microorganisms directly, (fermentation mediated extraction), or by using enzymes extracted from various organisms like microbes, fishes, shrimps, plants, and animals (enzyme assisted extraction). In a concluding section, recent studies incorporating a combination of any of the methods to make extraction of chitosan greener and/or more economically favourable, are discussed.
Alternative sources of chitosan
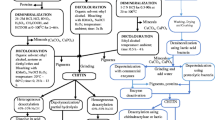
Fungal cell walls and arthropod exoskeletons are rich sources of naturally available chitinous substances and of particular interest is fungal source because there is no requirement to convert chitin to chitosan. The benefits of an alternative to crustacean species as sources of chitin/chitosan include the fact that there is no seasonal dependence for the supply of the biomass, and waste biomass of many pharmaceutical and biotechnological industries are potential sources. In addition to this, the extraction process is usually simpler, and uses lower amounts of or less harsh chemicals. For instance, the demineralization step can be eliminated (as compared to extraction from crustacean sources) since the fungal chitosan is not associated with CaCO3 present in crustacean shells (Jones et al. 2020). The comparison of steps of chitosan extraction from various sources is shown in Fig. 2. Additionally, fungal chitosan has some desirable properties, including lower molecular weights and higher degrees of deacetylation, especially suitable for biomedical applications. The properties are easier to control by controlling the biomass growth conditions, as compared to crustacean chitosan.
Comparison of steps for chitin/chitosan extraction from crustacean shells, fungal and insect biomass. Adapted under Creative Commons Attribution License from (Jones et al. 2020)
Fungal species as alternative sources for chitosan production
Researchers have studied these fungal genera/species for chitosan extraction: Candida spp., Saccharomyces spp. (Afroz et al. 2021), Rhizopus spp. (Chatterjee et al. 2008, 2019; Kleekayai and Suntornsuk 2011; Cardoso et al. 2012), Absidia spp., (Jaworska and Konieczna 2001; Jiang et al. 2011), Mucor spp., Mortierella isabelina, Lentinus edodes, Penicillium spp, (Ebrahimzadeh et al. 2013; Namboodiri and Pakshirajan 2019; Aili et al. 2019), Cunninghamella spp. (de Oliveira et al. 2014; Berger et al. 2014a, b), Gongronella spp. (Nwe and Stevens 2004), Mucor spp. (Tajdini et al. 2010; Karimi and Zamani 2013; Mondala et al. 2015; Safaei et al. 2016; Zininga et al. 2019; Abasian et al. 2020), Rhizomucor spp. (Zamani et al. 2007; Tajdini et al. 2010) and Rhizopus spp. (Chatterjee et al. 2008, 2019; Liao et al. 2008; Cardoso et al. 2012; Tasar et al. 2016) and other species have been studied. The Zygomycetes fungal class has higher chitosan to chitin ratio in their cell walls compared to other classes. The chitinous content in these fungal cell walls typically ranges from 6 to 45%. In a study by Campos-Takaki et al. (2014), the cell wall of fungal species, Cunninghamella blakesleeana, Gongronella butleri, Rhizopus arrhizus, Mucor javanicus, Cunninghamella elegans and Syncephalastrum racemosum were all analyzed and 10–16% of cell walls were found to have chitin, while chitosan was in the range 26–28%. The differences in yield of chitosan arise from difference of species, conditions of fermentation and process of extraction. A detailed review by Crognale et al. (2022) takes a detailed look at the sources available for, and parameters of production of fungal chitosan. Since there are advantages and disadvantages of various genera/species of fungi for chitosan production, and it is to be noted that some high yielding species may be pathogenic, it is important to assess the most suitable organisms for chitosan production according to the desired properties, applications and/or scale up possibilities The species from which chitin and chitosan contents are cultivated are largely affected by the growth medium, fermentation methods and fermentation conditions. Fungal biomass can be produced by solid-state fermentation (SSF) and submerged fermentation (SmF) (Dhillon et al. 2013). Fungal chitosan production requires various ingredients such as, yeast extract, d-glucose and peptone. Also, inexpensive nutrient sources have been studied for culturing fungi for production of chitinous substances (Kannan et al. 2010).
Fungal strain improvement and metabolic engineering for chitosan production
Efforts are being made to increase the relative proportion of chitosan in the fungal cell walls. Maw et al. (2002) carried out UV mutation of G. butleri spores for increased chitin deacetylase (CDA) activity. The mutants were screened in addition to high CDA activity, for different hyphal morphologies. Three mutant strains were found to produce twice the extractable chitosan and double CDA activity as compared to the wild strain. However, more efforts are necessary to isolate commercially feasible fungal strain. There are several patents available on metabolic engineering on enhanced production of chitosan in fungi (Deng et al. 2005; Carr and Hammer 2006). Chitin synthase, glutamine-fructose-6-phosphate aminotransferase (GFA), and CDA—these genes were mutated to achieve chitin production within a cell, and to improve the catalytic potential and substrate specificity of the enzymes. For example, R. oryzae or A. niger strains utilized for lactic acid and citric acid production are an attractive source of spent fungal biomass. Similarly, S. cerevisiae, wine yeast can be engineered to produce chitosan. One of the main concerns of mutating these organisms would be that during the yield of the primary fermentation products should not be affected (Ghormade et al. 2017).
Characteristics of fungal chitosan for potential biological applications
The advantages of fungal approaches to chitosan production include uniform physicochemical properties of fungal chitosan, made possible by accurate bioprocess control. A variety of studies shows that MW, polydispersity, and %DD of fungal chitosan can be manipulated by variations in growth media and process conditions. Obtaining reproducible values of these parameters is fundamental to guaranteeing the acceptance of fungal chitosan in sensitive applications. The allergic concern of chitosan that is extracted from crustaceans is very significant. The major allergen present in crustaceans is the muscle protein tropomyosin, which can cause mild to severe allergic reactions. As a result, the demand to produce chitosan from fungal sources is gaining traction. It is easier to make products of different MW as well as viscosity grades from fungal chitosan compared with crustacean chitosan. Chitosan from fungal sources have 3–5 times lower viscosity and MW, but higher %DD, making them appropriate for usage in food, healthcare, and pharmaceutical industries. In parallel with crustacean chitosan and its derivatives, fungal chitosan can also be a topic of interest as wound dressing material, natural preservative in food, cosmetics and pharmaceutical industries. Fungal chitosan is soluble in physiological pH ranges with its poly-cationic characteristics, lower antigen effect and lesser inorganic materials than crustacean chitosan. So, it can be used as a potential drug carrier and non-viral gene delivery system (Moussa et al. 2013; Khan et al. 2015; Duan et al. 2019; Wang et al. 2019; Morin-Crini et al. 2019; Tang et al. 2020; Joseph et al. 2021).
Use of waste biomass as alternative sources or alternative carbon sources
Waste fungal biomass from biotech industries
In different bioprocesses such as fungi originated food, antibiotics and pharmaceuticals, organic acid and enzyme production and in brewing and baking, a very large portion of biotech industries use fungi. Authors have suggested that organic agro-industrial residues such as waste fungal biomass can be used as inexpensive medium to produce chitosan (Leite et al. 2015). Furthermore, many yeasts species are used for wine production, and some are used for isolation of β-glucan. The spent species are all disposed into landfills or sent for incineration; however these wastes have the potential to generate chitinous products (Ghormade et al. 2017). Solely from citric acid production more than 80,000 ton of waste Aspergillus niger biomass per year is generated. Most of the enzymes are produced in the growth medium leaving huge amounts of waste biomass which could be a good source for chitin and chitosan isolation. Production of an edible mushroom Agaricus bisporus generates more than 50,000 ton of waste materials per year consisting of stalks and fruit bodies and it is reported that the extraction of chitin from these waste biomasses could be 1000 ton per year (Wu et al. 2004). Easily available local waste biomass sources were used by Afroz et al. (2021). To produce fungal chitosan from Aspergillus niger obtained from moldy onions, and locally purchased yeast (Saccharomyces cerevisiae).
Value addition to existing mycotech products
Mycotech products are products that are synthesized commercially from fungal sources. Fungal residues, byproducts and other organic wastes are very common in the large scale myco-products’ production processes. It was reported that Melanobatrachus indicus and R. oryzae were used to produce proteins, oil and chitosan on organic wastes (Satari et al. 2016). Metarhizium anisopliae (Entomopathogenic fungus) has been used commercially for the control of insect pests in the agriculture field. The mycelial biomass can be used to isolate cell wall polymers after removing the conidia. It was observed that M. anisopliae was able produce equal % of chitin and chitosan in the cell wall (100 mg chitosan/10 g of dry biomass) (Nahar et al. 2004). Chitosan extraction from the mycelia of M. anisopliae can be one of the approaches for value addition. M. anisopliae also produced CDA enzyme which is useful for the enzymatic deacetylation of chitosan (Kulkarni et al. 2008; Ghormade et al. 2017).
Commercial perspectives of fungal chitosan: present and future
Fungal-based chitosan products are still limited at commercial scale. In the past decades, fungal chitosan applications are focused on biomedical and food technologies, such as carriers for drug/gene delivery, wound dressing, haemo-compatible biomaterials, and preservatives as antimicrobial agents in food and antibacterial food packaging. Fungal-based chitosan products are still limited in the market but are slowly gaining popularity. First fungal based chitosan was patented by an US based company named Cargill in 2005. Cargill’s chitosan was highly deacetylated and obtained from microbials biomass. In Europe, a Belgium based company, KitoZyme first brought fungal based product (KitoZyme) to market. In June 2011, KitoZyme obtained Generally Recognized as Safe (GRAS) status approval from Food and Drug Administration (FDA) for their chitosan product named KiOnutrime-CsG® to be used in beverage applications. The KiOnutrime-CsG® is also registered in the European Union (EU) under the novel food regulations and is approved by the European Food Safety Authority (EFSA). Kitozyme’s non-animal source chitosan products on market are: 1)KiOfine®-B, a new tool for winemakers that helps to prevent and cure contamination by Brettanomyces bruxellensis; 2)KiOfine®-CsG, an antioxidant used in different stages of the winemaking process (clarification and fining) and 3) Slim MED® ADVANCED, a dietary fiber for weight loss. KitoZyme has a spin-off company named KiOmed Pharma (previously Synolyne Pharma) with University of Liège to develop a chitosan-based microbeads hydrogel for the treatment of Osteoarthritis. In Canada, it was Mycodev (in 2013) who brought fungal based chitosan to public attention to commercialize a new fermentation-based product in the Canadian market, with the focus on biomedical and pharmaceutical applications. Another Canadian based company Chinova Bioworks started to commercialize their first mushroom-based chitosan in 2016. Chinova has been developing mushroom chitosan-based formulations to replace synthetic preservatives from food and beverage applications. Chinova Bioworks’ mushroom chitosan ‘Chiber’ has already entered the marketplace. China-based biotech company Chibio has non-animal source chitosan for food and pharmaceutical applications. Chitosan wound dressing commercial products currently available in the market are mostly obtained from crustaceans’ source chitosan. The production cost for the traditional crustacean- based chitosan is cheap compared with fungal based chitosan. Crustacean raw materials are readily available and cheap whereas the cost of raw materials is the main bottleneck for fungal chitosan production. Crustacean chitosan can be found from 10 US dollar per kg to 1000 US dollar per kg depending on product quality and application. On the other hand, about 5 out of 100 persons get affected by crustacean allergy, which means that about 300 million persons need non-crustacean ingredients for food and biotechnological products. This is the reason that people are interested in replacing the animal source products with non-animal source products (Huq et al. 2022).
Switching from conventional to fungi-based processes requires economic and environmental factors to be carefully evaluated. Utilizing agro-industrial residues (rice and wheat straws, hardwood sawdust, cottonseed hulls, soybean residues, wine lees, grape marc, apple pomace, corn steep liquor, sweet potato distillery waste, cassava wastewater, sugarcane syrup and date waste syrup) in the fermentation process and applying milder acid-alkaline treatment conditions in the extraction process—are some of the economic and environmental advantages over the crustacean chitosan production process. Due to the increased environmental regulations fungi-based biotech industries are associated with raised pollution abatement costs and lowering the disposal cost of fungal waste can be achieved by implementing integrated bioprocess focused on fungal chitosan production. In addition to the primary product, yielding chitosan as a co-product from waste mycelia, might boost and increase the diffusion of this technology. For processing crustacean materials, new methods such as hot water, mechanochemical and glycerol treatments have been demonstrated to be greener compared with traditional methods in terms of chemical consumption and wastewater production—these novel greener methods may be used for fungal chitin/chitosan extraction and processing too. Fungal species which are pathogenic to animals and other plants are subjects to further research for safe large scale chitosan production and improvised waste management technology. As green technology, sustainable circular economy and health safety have been getting more priority in recent years, fungal chitosan-based products will gain marketability over the next decades.
Potential of insect chitosan
Insects are a viable source of chitin and chitosan, although little attention has been given to them so far. Two billion people worldwide eat 1900 various species of insects for nutrition as a reliable food source containing 30–45% protein, 25–40% fat, and 10–15% chitin in total. Southeast Asia, the Pacific, Sub-Saharan Africa and Latin America represent major insect consumers (van Huis 2013; Spranghers et al. 2017). According to Huet et al. (2020), chitin of various purity grades (45, 89.7, and 93.3%) were extracted and physicochemically characterised from Bombyx eri larvae revealing that insect chitins had identical crystallographic structures, thermal stability, and degree of acetylation (> 87%) to commercially accessible and isolated chitin from shrimp shell. The residues from the insect’s breeding for human consumption can be used to successfully obtain chitin/chitosan. Having a low amount of minerals, insects as raw material for chitosan production have made it possible to obtain chitosan only with the deproteinization step. Utilization of enzymes has been proven efficient in removing the proteins contained in the matrix. It has shown a sufficient reduction in harsh treatment to justify the non-use of solvents harmful to the environment (Silva et al., 2021).
Insects have certain advantages over crustaceans in that they are not seasonal and can be readily bred due to their high fertility rate (Luo et al. 2019). As insect breeding centers are springing up all over the world, insects can be used as a viable source of chitin and chitosan for larger ecological and economic sustainability as bioconverters which are reared for organic waste management and animal feed processing. It is noteworthy that insect chitin contains less calcium carbonate (6%) than crustacean chitin (30–50%), allowing for easier extraction and a more eco-sustainable process (Sajomsang and Gonil 2010). In the last few years, numbers of scientific papers have been published on chitin processing and subsequent conversions into bioproducts from various insect species(Badariotti et al. 2007; Sajomsang and Gonil 2010; Liu et al. 2012; Kaya et al. 2014, 2015a, b; Ibitoye et al. 2018; Marei et al. 2019). Insect chitosan has been further formulated into nanoparticles and showed antimicrobial propreties and also showed wound healing characteristics (Al-Saggaf 2021). Insect chitosan from Tenebrio molitor and Brachystola magna were formulated into films for food packaging purposes (Saenz-Mendoza et al. 2020). Insect farm side streams show promise as a source of chtin (Brigode et al. 2020). The various insect species biomass can thus be used by commercial chitosan producers as a profitable alternative source (Iber et al. 2022).
Biological methods of extraction
Using microorganisms or their enzymes has been explored in the last few decades as an environmentally friendly method of chitinous material extraction. The biological methods are broadly categorized into two sections as follows: (1) fermentation mediated extraction, (the microorganisms are incubated along with the raw material) and (2) enzyme mediate extraction, (extracted or commercially available enzymes are added to the raw material). The comparison of biological to chemical methods of chitin extraction is summarized in Fig. 3.
Comparison of chemical and biotechnological extraction. Advantages (green boxes) and disadvantages (red boxes) compared for chemical extraction (bottom left, large red dotted box) and using green extraction methods (bottom right, large green dotted box) Adapted with permission from (Arnold et al. 2020; Mohan et al. 2022)
Fermentation mediated chitin/chitosan extraction
Deproteination and demineralization during fermentation
For processing shrimp and other waste, microbes can be present within the chitosan source (autofermentation) or inoculated into the source for deproteination and/or demineralization. During these fermentation steps, deproteination is by proteolytic enzymes and demineralization is by the organic acids produced by the microorganisms. Ghorbel-Bellaaj showed chitin extraction using six protease producing bacteria (Bacillus pumilus A1, Bacillus mojavencis A21, Bacillus licheniformis RP1, Bacillus cereus SV1, Bacillus amyloliquefaciens An6 and Bacillus subtilis A26), all of which showed DP > 80%. However B. subtilis A26 showed a high degree of demineralization (DM) as well, when supplemented with glucose (Ghorbel-bellaaj et al. 2012). Fermentation of shrimp shells, producing chitin using microorganisms such as Bacillus Licheniformis (Liu et al. 2014a), and Pseudomonas aeruginosa (Sedaghat et al. 2017), all showed high DP.
Halophilic bacteria grow in saline conditions and secrete extracellular proteolytic enzymes. Two such bacterial species Halobacterium salinarum and Halobacterium dombrowskii showed DP as high as 98.99% when producing chitin (Dayakar et al. 2021). The microbes in the species Paenibacillus mucilaginosus TKU032 are interesting because the typical nutrient source of this bacteria is from the marine industry waste including waste shrimp shells (Doan et al. 2020). Microorganisms isolated from the shellfish wastes (and other habitats) can also be used for the breakdown of the shellfish wastes. Protease producing bacteria Alcaligens faecalis were isolated from soil samples of shrimp culture ponds (Rakshit et al. 2021), while B. pumilus A1 was isolated from slaughterhouse polluted water for chitin production (Ghorbel-Bellaaj et al. 2013). In the latter case, the same microbe also carried out demineralization, and further steps to demineralize the product were not carried out.
Demineralization during fermentation
For biochemical demineralization of chitosan sources, fermentation with lactic acid producing bacteria (LAB) is used. These bacteria can also perform deproteination in the correct conditions. Authors have used LAB such as, Lactobacillus plantarum isolated from sausages (Neves et al. 2017), salmon (Castro et al. 2018), or from fermented food (Francisco et al. 2015). Serratia marcescens B742 and Lactobacillus plantarum ATCC 801 were used to produce chitin, and the DP and DM rates were 94.5 and 93.%, respectively (Zhang et al. 2012). Another study used Lactobacillus brevis for demineralization and Rhizopus oligosporus as deproteinizing fungi (Aranday-García et al. 2019). Arbia et al. carried out several studies on demineralization and deproteination of shrimp shell Parapenaeus longirostris using Lactobacillus helveticus (Arbia et al. 2013, 2017, 2019).
Various authors have attempted to get maximum deproteinization and demineralization rates using the same microbial species, however this requires careful optimization of parameters of fermentation: nutrients (C/N ratio, in particular), temperature and duration of fermentation. Alternatively, two (or more) microbial species are simultaneously or separately inoculated into the source, to perform deproteination and demineralization, not necessarily in that order, in a process termed as cofermentation. A detailed study on cofermentation using Bacillus licheniformis 21886 and Gluconobacter oxydans DSM-2003 showed the best DM and DP results were obtained when the waste was first inoculated with Bacillus followed by Gluconobacter, rather than simultaneous inoculation of the two species (Liu et al. 2014a). A study was carried out by Zhang et al. (2021) for successive co-fermentation with B. subtilis and A. pasteurianus for extracting chitin from shrimp shells, showing efficiencies of DP of 94.5% and DM of 92.0%. Bahasan et al. (2017) isolated demineralization microbe Kurthia gibsonii from fermented milk and deproteination microbe, Aspergillus spp. from bread, and inoculated two shrimp species, Fenneropenaeus semisulcatus and Fenneropenaeus indicus to extract chitin. Liu et al. (2020) used successive fermentation with Lactobacillus rhamnoides and Bacillus amyloliquefaciens (BA01) strain. Some recent work has summarized in the Table 1.
Both successive fermentation and cofermentation approaches can lead to > 95% DP and DM degrees, and do not produce environmentally harmful by-products. Previous studies illustrate the importance of optimizing the nutrient source and other parameters and the need for considering the compatibility of the cofermentable species. It is also advisable to carry out the demineralization prior to deproteination since the minerals present in the crustacean shells can affect the enzymatic proteolytic accessibility and thus DP efficiencies (Younes et al. 2015b). Thus if carried out properly cofermentation can reduce expensive double sterilization, replacing the medium, and collecting residues between DP and DM processes (Zhang et al. 2021).
Enzymes and other non-fermentative means of biochemical extraction
Demineralization
For demineralization commercially available organic acids such as lactic acid and citric acid are viable alternatives to conventionally used harsh mineral acids. Lactic acid has proved to be effective as the calcium carbonate of the crustacean shells can react with the lactic acid to form calcium lactate, which is easily removed and lowers environmental pollution (Greene et al. 2016; Marzieh et al. 2019). Formic acid has been used for demineralization in a recent study (Baron et al. 2017). (The fermentation of sources with lactic acid producing bacteria, LAB, has been discussed in “Fermentation Mediated Extraction” section).
Deproteination using enzymes
For deproteination, enzymes are available by extraction from various organisms, and can be used with chitinous sources without fermentation (organisms added to chitinous sources). Crude proteases can be extracted from the natural sources mentioned; alternatively, commercially available proteases can also be used for the deproteination step. It is to be noted however that commercial enzymes are usually more expensive and the crude proteases can sometimes give more effective results owing to the fact that a mixture of proteases is available when extracted from the source. On the other hand, use of commercial enzymes can give a more reliable product and can be easily scaled up for industrial extraction of chitosan (Mathew et al., 2021). Proteases typically used are alcalases, pepsin, papain, pancreatin, delvolase, trypsin, chemotrypsin, proteinase K and pectinase among others (Mathew et al. 2021). A few works have been summarized in Table 2.
Deproteination using extracted proteases
Microbial sources which are used for protease extraction include Paenibacillus woosongensis TKB2 (Paul et al. 2015a), Micromonospora chaiyaphumensis s103 (Mhamdi et al. 2017) and Bacillus licheniformis can produce Alcalase, a serine endopeptidase. A surfactant and oxidant resistant protease was isolated from Bacillus cereus SV1 giving DP efficiency of 88.8% ± 0.42 (Manni et al. 2010b). In a related work, the chitin produced in this manner was further processed to give chitosan, which in turn showed remarkable antibacterial activity of against different bacteria (Manni et al. 2010a). A recent study was carried out to isolate proteolytic enzymes from halophilic bacteria, since these enzymes can withstand high salt conditions, and are extracted from marine wastes (Maruthiah et al. 2015). Thus, these halophilic naturally occurring proteases are an economically attractive option for biological extraction of chitinous substances. Marine sources like fish and invertebrate aquatic species can also be a source of crude proteases. Mukhin and Novikov (2001) showed that proteolytic enzymes for digestion of crustacean shell components can be isolated from crustacean wastes; this work showed isolation of a potent protease from digestive system of king crab, which was used for digesting the proteins in king crabs and prawn shells. In a comprehensive study by Younes et al. (2015b) proteinases isolated from 6 species of microbes and 3 species of fish, were used to deproteinize shrimp shell wastes. Proteolytic preparations from Bacillus mojavensis A21 Bacillus subtilis A26, Bacillus licheniformis NH1, B. licheniformisMP1, Vibrio metschnikovii J1, Aspergillus clavatus ES1 and crude alkaline protease extracts from Sardinelle (Sardinellaaurita), Goby (Zosterisessor ophiocephalus) and Grey triggerfish (Balistes capriscus) were all optimized for chitin extraction and the highest DP efficiency of 77% was with proteases from B. mojavensis and 78% with those from B. capriscus. The enzyme to solid (E/S) ratio is major governing factor to increase deproteination. In another similar work, highly stable alkaline proteases extracted from red scorpionfish (Scorpaena scrofa) were used for chitin extraction and a DP efficiency (Younes et al. 2015a).
Deproteination using commercially obtainable proteases
Commercially enzymes have been used by many authors for replacing the harsh chemicals used during chitin and chitosan extraction, as well as further processing of these biopolymers. Commercial proteases like papain, alcalase 2.4L, pepsin, trypsin, pectinase, Proteinase K, and endogenous enzymes have been used traditionally for shrimp shell waste processing (Mathew et al. 2021) but these enzymatic processing steps can be applied for chitin and chitosan extraction. The work by Marzieh et al. (2019) showed an interesting insight into DP efficiency by use of trypsin and ficin with/without sodium metabisulfite. The results showed that sodium metabisulfite has an effect on the enzyme activity, enzymatic chitins (especially chitin produced by trypsin) exhibited higher degrees of acetylation, crystalline index as well as a smooth microfibrillar crystalline. The action of commercial protease from Streptomyces griseus was optimized for different parameters (incubation time, pH, enzyme–substrate ratio and particle size), and showed DP of 91.10% (Hongkulsup et al. 2016) A recent study optimized several commercial proteases tryptase, papain, alkaline protease, proteinase K, neutral protease, and Protemax and obtained a maximum DP of 91% using proteinase K (Dun et al. 2019). An interesting application of protease enzymes is the use of those present in shrimp heads, which are rich in various endogenous enzymes. These enzymes can be activated by incubation or UV irradiation, and autolysis occurs on the substrates present in the shrimp heads/wastes. Use of these enzymes can facilitate in economical shrimp waste treatment for deproteinization and chitin production (Cao et al. 2020). Studies on the optimum conditions for autolysis by endogenous enzymes showed that pH, autolysis time and temperatures have an influence on the DP percentage (Cao et al. 2008, 2009; da Silva et al. 2017). Shrimp head was processed by a combination of autolysis and fermentation, and the endogenous enzymes were responsible for autolysis gave a DP of 88.35%. A pilot study of 20 kg of shrimp head autofermentation was carried out, giving insights into the future methods of green extraction of chitinaceous products (Guo et al. 2019).
Both crude or commercial proteases are useful of deproteinization step, but commercially purified enzymes are expensive and sometimes less efficient compared to crude proteases, which usually get co-extracted with useful enzymes. Some studies add an additional chemical treatment to increase the DP efficiency, however the overall decrease in harsh chemicals still provide a greener alternative to the overall process. Chemical deproteination is more thorough than enzymatic deproteination, (Kaur and Dhillon 2014), which is why it is a worthwhile research to look into a combination of chemical and enzymatic processes, or a combination of different proteases, preferably with one of the enzymes being acid or alkaline stable.
Enzymatic modification of chitin (or chitosan)
Commercially obtainable enzymes are available for mediating the different steps of chitosan extraction including deacetylation and molecular weight degradation. Traditionally, chitin and chitosan were converted to oligosachharides using harsh chemicals such as hydrochlocric acid, nitrous acid, phosphorus acid, lactic acid, formic acid and hydrogen peroxide. The chemical processing not only is harmful to the environment, but also produces a range of products, including secondary compounds, and undesired low molecular weight products. Enzymatic modification of native chitin to chitosan, chitin oligosaccharide, chitosan oligosachharides, are gaining interest to obtain more soluble and bioactive chitin derivatives. Previously nonspecific enzymes like cellulases, hydrolases and even proteases were used to degrade the polymers into their oligopolymeric counterparts. However, the recent trends are to use specific regio-selective enzymes to produce COS and paCOS with desired degrees of polymerizations. Enzymes such as chitinases, chitosanases, chitin deacetylases, and the recently discovered lytic polysaccharide monooxygenases (LPMO) are reviewed in detail (Kaczmarek et al. 2019). Chitinases all hydrolyze glycosidic β-(2.4)-links of acetylated d-glucosamine units and produce chitobioases (disachharides of β-1,4-linked glucosamine units). Some chitinases are also able to create new links between small polysachharide fragments, making these an excellent candidate for producing COS (Arnold et al. 2020). Chitosanases (EC 3.2.1.132) constitute a family of enzymes capable of performing endohydrolysis in partially acetylated chitosan, from the reducing end and exo-β-d-glucosaminidase attack chitosan from its non-reducing end. The sources of these chitin modifying enzymes are various and can be isolated from different microorganisms, including bacteria, cyanobacteria, fungi, and plants (Thadathil and Velappan 2014). LPMOs are enzymes that aid in the hydrolysis of chitin by oxidation, leading to the easy accessibility of chitin by chitinases.
In a study by Deng et al. (2020), chitinase rChit46 was used to recover chitin from shrimp shells, but it was successfully done only after deproteinization, since removal of the proteins exposed the chitin to the chitinase for enzymatic action. The natural chitin oligomer recovery rate was 89.9%. In another study recombinant chitinase LlChi18A from L. lactis ssp. was used for production of chitooligomers (Lv et al. 2016). In a comprehensive study using fourteen chitin deacetylases from bacterial, fungal and viral sources Hembach et al. (2017) produced chitosan oligosaccharides of very specific degree and pattern of polymerization. Bacterial chitosanase from Bacillus licheniformis GA11 was extracted and its activity optimized for shrimp chitosan depolymerization and further tests were carried out to evaluate the COS antioxidant, antibacterial and antifungal activity (Affes et al. 2020). In another study bacterial chitosanase was extracted from Bacillus thuringiensis and used to depolymerize colloidal chitosan (Olicón-Hernández et al. 2017).
Chitin deacetylases (ChDa) are a group of enzymes catalyzing the hydrolysis of acetamido groups of N–acetyl–d–glucosamine residues in chitin and chitosan whereas chitooligosaccharides deacetylases (CODa) perform the same catalysis on chitooligosaccharides. These enzymes and their mode of action can be found in the review by Kaczmarek et al. (2019), and these enzymes can be utilized for future research on COS and paCOS production. One of the factors to consider when using these chitinolytic enzymes is that they are unable to penetrate the crystalline chitin or chitosan structure, and pretreatment is usually carried out chemical or mechanically to produce “colloidal chitin/chitosan” or swollen chitin/chitosan. Another factor is that chitinase/chitosonases producing species by fermentation or extracted enzymes are both viable methods to produce oligopolymers of interest for potential commercial production (Arnold et al. 2020).
Biological extraction on non-crustacean sources
Fermentative extraction could potentially be carried out on non-crustacean, specifically fungal sources of chitosan. When fungal species and shrimp shells are combined in a single reactor, the fungi produce proteases, which helps produced deproteinized shells and hydrolyzed proteins. The proteins were a nutrient source to enhance fungal growth this in turn lowers the pH of the fermentation broth helping in demineralization process (Teng et al. 2001). In a recent study, dual extraction of crustacean and fungal chitosan was carried out, the fungal species Mucor circinelloides acting as a protease secreting source for prawn shells, and subsequently being cofermented with Lactobacillus plantarum and Bacillus subtilis (Yun Nian Tan et al. 2021).
Concluding remarks on biological extraction and modification of chitinous material
A major hurdle in the usage of the enzymes for hydrolyzing chitinous biomass, is that same enzymes from different species show different activities and the fact that the enzymes are relatively expensive. However, techniques like fermentation, co-fermentation or harvesting a microbe for its enzyme can greatly reduce the cost and nutrient requirement for production of the enzymes. Another major hurdle is that there are not too many for scaling up to commercial or pilot-scale, making this an aspect of further research. Furthermore, much of the literature has focused on valorizing marine waste, and usually looks at chitin as end-product. Future research could be carried out for enzymatic production of chitosan, COS and paCOS for biological and medical research due to increased biocompatibility, biodegradability, low toxicity, antimicrobial, antiviral, antitumor, and antioxidant activities (Kaczmarek et al. 2019).
Concluding remarks
Other than the methods mentioned in this review, there are two major avenues of green extraction: alternative solvents and physicochemical methods of extraction, which have been discussed in other review papers (Mohan et al. 2022). Chitosan from alternative sources such as fungal, mycotech products, or insects have been applied for biological and biomedical applications such as antimicrobial agent (Tayel et al. 2010; de Oliveira et al. 2014; Abdel-Mohsen et al. 2016; Chang et al. 2019), anticancer effects (Mora-Montes et al. 2011), anioxidant effects (Zimoch-Korzycka et al. 2016; Abdel-Gawad et al. 2017; Araújo et al. 2017), wound dressing applications (Tchemtchoua et al. 2011), tissue engineering (Nwe and Tetsuya, 2010) and many others. Futhermore, green solvents can be used for dissolution of chitin/chitosan, opening the avenue for formulating fibers (Shamshina et al. 2014; Zavgorodnya et al. 2017), nanofibers(Li et al. 2018), films (Yang et al. 2019; Haghighi et al. 2020), hydrogels (Wang et al. 2016; Azadi et al. 2018), scaffolds (Wang et al. 2017), sponges (Huang et al. 2018) and other shapes, thus paving the way for biological applications as shown in Fig. 4.
Use of green solvents and natural chitosan for sustainable material formulations. Reprinted with permission from (Kostag and el Seoud 2021)
Green approaches to modifying chitosan to its more soluble and more bioactive derivatives are also now coming into attention. It is evident that only very few studies have been carried out where chitin and chitosan, or its derivatives, are extracted and formulated in fully ecologically friendly method, for biological applications. Thus, further research should focus on combining the alternative routes for production of chitin/chitosan along with green processes for modification for biological applications.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- COS:
-
chitooligosaccharides
- DA:
-
degree of acetylation
- DD:
-
degree of deacetylation
- DM:
-
demineralization, percentage of demineralization
- DP:
-
deproteination/deproteinization, percentage of deproteination
- LPMO:
-
lytic polysaccharide monooxygenases
- paCOS:
-
partially acetylated chitooligosaccharides
References
Abasian L, Shafiei Alavijeh R, Satari B, Karimi K (2020) Sustainable and effective chitosan production by dimorphic fungus Mucor rouxii via replacing yeast extract with fungal extract. Appl Biochem Biotechnol 191:666–678. https://doi.org/10.1007/S12010-019-03220-W/FIGURES/6
Abdel-Mohsen AM, Jancar J, Massoud D et al (2016) Novel chitin/chitosan-glucan wound dressing: isolation, characterization, antibacterial activity and wound healing properties. Int J Pharm 510:86–99. https://doi.org/10.1016/j.ijpharm.2016.06.003
Abdel-Gawad KM, Hifney AF, Fawzy MA, Gomaa M (2017) Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll 63:593–601. https://doi.org/10.1016/J.FOODHYD.2016.10.001
Affes S, Maalej H, Aranaz I et al (2020) Enzymatic production of low-MW chitosan-derivatives: characterization and biological activities evaluation. Int J Biol Macromol 144:279–288. https://doi.org/10.1016/j.ijbiomac.2019.12.062
Afroz MM, Kashem MNH, Piash KMPS, Islam N (2021) Saccharomyces cerevisiae as an untapped source of fungal chitosan for antimicrobial action. Appl Biochem Biotechnol 193:3765–3786. https://doi.org/10.1007/s12010-021-03639-0
Aili D, Adour L, Houali K, Amrane A (2019) Effect of temperature in chitin and chitosan production by solid culture of Penicillium Camembertii on YPG medium. Int J Biol Macromol 133:998–1007. https://doi.org/10.1016/J.IJBIOMAC.2019.04.116
Al-Saggaf MS (2021) Formulation of insect chitosan stabilized silver nanoparticles with propolis extract as potent antimicrobial and wound healing composites. Int J Polym Sci. https://doi.org/10.1155/2021/5578032
Aranday-García R, Saimoto H, Shirai K, Ifuku S (2019) Chitin biological extraction from shrimp wastes and its fibrillation for elastic nanofiber sheets preparation. Carbohydr Polym 213:112–120. https://doi.org/10.1016/j.carbpol.2019.02.083
Araújo D, Freitas F, Sevrin C et al (2017) Co-production of chitin-glucan complex and xylitol by Komagataella pastoris using glucose and xylose mixtures as carbon source. Carbohydr Polym 166:24–30. https://doi.org/10.1016/j.carbpol.2017.02.088
Arbia W, Adour L, Amrane A, Lounici H (2013) Optimization of medium composition for enhanced chitin extraction from Parapenaeus longirostris by Lactobacillus helveticus using response surface methodology. Food Hydrocoll 31:392–403
Arbia W, Arbia L, Adour L et al (2017) Kinetic study of bio-demineralization and bio-deproteinization of shrimp biowaste for chitin recovery | Arbia |. Algerian J Environ Sci Technol 3:1
Arbia W, Arbia L, Adour L et al (2019) Characterization by spectrometric methods of chitin produced from white shrimp shells of Parapenaeus longirostris by Lactobacillus helveticus cultivated on glucose or date waste. Algerian J Environ Sci Technol 5:2
Arnold ND, Brück WM, Garbe D, Brück TB (2020) Enzymatic modification of native chitin and conversion to specialty chemical products. Mar Drugs 18:93. https://doi.org/10.3390/md18020093
Azadi MDA, Hassanjili S, Zarrabi K, Sarkari B (2018) Solidification of hydatid cyst fluid with an injectable chitosan/carboxymethylcellulose/β-glycerophosphate hydrogel for effective control of spillage during aspiration of hydatid cysts. Prog Biomater 7:35–54. https://doi.org/10.1007/S40204-018-0082-5/TABLES/5
Badariotti F, Thuau R, Lelong C et al (2007) Characterization of an atypical family 18 chitinase from the oyster Crassostrea gigas: evidence for a role in early development and immunity. Dev Comp Immunol 31:559–570. https://doi.org/10.1016/j.dci.2006.09.002
Bahasan SHO, Satheesh S, Ba-akdah MA (2017) Extraction of chitin from the shell wastes of two shrimp species <i> Fenneropenaeus semisulcatus <i> and Fenneropenaeus indicus using microorganisms. J Aquat Food Prod Technol 26:390–405. https://doi.org/10.1080/10498850.2016.1188191
Baron R, Socol M, Kaas R et al (2017) Elements for optimizing a one-step enzymatic bio-refinery process of shrimp cuticles: Focus on enzymatic proteolysis screening. Biotechnol Rep 15:70–74. https://doi.org/10.1016/j.btre.2017.01.003
Berger LRR, Stamford TCM, Stamford-Arnaud TM et al (2014a) Effect of corn steep liquor (CSL) and cassava wastewater (CW) on chitin and chitosan production by Cunninghamella elegans and their physicochemical characteristics and cytotoxicity. Molecules 19:2771–2792. https://doi.org/10.3390/MOLECULES19032771
Berger LRR, Stamford TCM, Stamford-Arnaud TM et al (2014b) Green conversion of agroindustrial wastes into chitin and chitosan by Rhizopus arrhizus and Cunninghamella elegans strains. Int J Mol Sci 15:9082–9102. https://doi.org/10.3390/IJMS15059082
Brigode C, Hobbi P, Jafari H et al (2020) Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J Clean Prod 275:122924. https://doi.org/10.1016/J.JCLEPRO.2020.122924
Campos-Takaki GM, Dietrich SMC, Beakes GW (2014) Cytochemistry, ultrastructure and x-ray microanalysis methods applied to cell wall characterization of Mucoralean fungi strains. Microscopy
Cao W, Zhang C, Hong P, Ji H (2008) Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem 109:176–183. https://doi.org/10.1016/J.FOODCHEM.2007.11.080
Cao W, Zhang C, Hong P et al (2009) Autolysis of shrimp head by gradual temperature and nutritional quality of the resulting hydrolysate. LWT Food Sci Technol 42:244–249. https://doi.org/10.1016/J.LWT.2008.05.026
Cao W, Tian S, Wang H et al (2020) Release principle of peptides and amino acids during the autolysis of shrimp head from Litopenaeus vannamei after UV-C irradiation stress. Food Sci Nutr 8:170–178. https://doi.org/10.1002/fsn3.1288
Cardoso A, Lins CIM, dos Santos ER et al (2012) Microbial enhance of chitosan production by Rhizopus arrhizus using agroindustrial substrates. Molecules 17:4904–4914. https://doi.org/10.3390/MOLECULES17054904
Carr B, Hammer PE (2006) Methods for production of chitin and chitosan. 1.
Castro R, Guerrero-Legarreta I, Bórquez R (2018) Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol Rep. https://doi.org/10.1016/j.btre.2018.e00287
Chang AKT, Frias RR, Alvarez L et al (2019) Comparative antibacterial activity of commercial chitosan and chitosan extracted from Auricularia sp. Biocatal Agric Biotechnol 17:189–195. https://doi.org/10.1016/j.bcab.2018.11.016
Chatterjee S, Chatterjee S, Chatterjee BP, Guha AK (2008) Enhancement of growth and chitosan production by Rhizopus oryzae in whey medium by plant growth hormones. Int J Biol Macromol 42:120–126. https://doi.org/10.1016/J.IJBIOMAC.2007.10.006
Chatterjee S, Guha AK, Chatterjee BP (2019) Evaluation of quantity and quality of chitosan produce from Rhizopus oryzae by utilizing food product processing waste whey and molasses. J Environ Manag 251:109565. https://doi.org/10.1016/J.JENVMAN.2019.109565
Crognale S, Russo C, Petruccioli M, D’annibale A (2022) Chitosan poduction by fungi: current state of knowledge, future opportunities and constraints. Fermentation 8:76
da Silva CP, Bezerra RS, dos Santos ACO et al (2017) Biological value of shrimp protein hydrolysate by-product produced by autolysis. LWT Food Sci Technol 80:456–461. https://doi.org/10.1016/J.LWT.2017.03.008
da Silva J, Lucas A, Quadro Oreste E, Leão Gouveia Costa H et al (2021) Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. https://doi.org/10.1016/j.foodchem.2020.128550
de Oliveira CEV, Magnani M, de Sales CV et al (2014) Effects of chitosan from Cunninghamella elegans on virulence of post-harvest pathogenic fungi in table grapes (Vitis labrusca L.). Int J Food Microbiol 171:54–61. https://doi.org/10.1016/J.IJFOODMICRO.2013.11.006
Dayakar B, Xavier KAM, Das O et al (2021) Application of extreme halophilic archaea as biocatalyst for chitin isolation from shrimp shell waste. Carbohydr Polym Technol Appl 2:100093. https://doi.org/10.1016/j.carpta.2021.100093
Deng M-D, McMullin TW, Grund AD (2005) Metabolic engineering for enhanced production of chitin and chitosan in microorganisms. 1
Deng J-J, Mao H-H, Fang W et al (2020) Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. J Clean Prod 271:122655. https://doi.org/10.1016/j.jclepro.2020.122655
Dhillon GS, Kaur S, Brar SK, Verma M (2013) Green synthesis approach: extraction of chitosan from fungus mycelia. Crit Rev Biotechnol 33:379–403. https://doi.org/10.3109/07388551.2012.717217
Doan CT, Tran TN, Wang CL, Wang SL (2020) Microbial conversion of shrimp heads to proteases and chitin as an effective dye adsorbent. Polymers 12:1–16. https://doi.org/10.3390/polym12102228
Duan C, Meng X, Meng J et al (2019) Chitosan as a preservative for fruits and vegetables: a review on chemistry and antimicrobial properties. J Bioresour Bioprod 4:11–21
Dun Y, Li Y, Xu J et al (2019) Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. Int J Biol Macromol 123:420–426. https://doi.org/10.1016/j.ijbiomac.2018.11.088
Ebrahimzadeh MA, Chabra A, Gharaei-Fathabad E, Pourmorad F (2013) Preparation of chitosan from Penicillium spp. and determination of their degree of deacetylation. Indian J Biotechnol 12:231–235
Francisco FC, Mae R, Simora C, Nuñal SN (2015) Deproteination and demineralization of shrimp waste using lactic acid bacteria for the production of crude chitin and chitosan. Aquac Aquar Conserv Legis 8:107–115
Ghorbel-bellaaj O, Younes I, Maâlej H et al (2012) Chitin extraction from shrimp shell waste using Bacillus bacteria. Int J Biol Macromol 51:1196–1201. https://doi.org/10.1016/j.ijbiomac.2012.08.034
Ghorbel-Bellaaj O, Hajji S, Younes I et al (2013) Optimization of chitin extraction from shrimp waste with Bacillus pumilus A1 using response surface methodology. Int J Biol Macromol 61:243–250. https://doi.org/10.1016/j.ijbiomac.2013.07.001
Ghormade V, Pathan EK, Deshpandev M (2017) Can fungi compete with marine sources for chitosan production? Int J Biol Macromol 104:1415–1421
Greene BHC, Robertson KN, Young JCOC, Clyburne JAC (2016) Lactic acid demineralization of green crab (Carcinus maenas) shells: effect of reaction conditions and isolation of an unusual calcium complex. Green Chem Lett Rev 9:1–11. https://doi.org/10.1080/17518253.2015.1119891
Guo N, Jianan S, Zhang Z, Xiangzhao M (2019) Recovery of chitin and protein from shrimp head waste by endogenous enzyme autolysis and fermentation. J Ocean Univ China. https://doi.org/10.1007/s11802-019-3867-9
Haghighi H, Licciardello F, Fava P et al (2020) Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag Shelf Life. https://doi.org/10.1016/j.fpsl.2020.100551
Hembach L, Cord-Landwehr S, Moerschbacher BM (2017) Enzymatic production of all fourteen partially acetylated chitosan tetramers using different chitin deacetylases acting in forward or reverse mode. Sci Rep. https://doi.org/10.1038/s41598-017-17950-6
Hongkulsup C, Khutoryanskiy V, Niranjan K (2016) Enzyme assisted extraction of chitin from shrimp shells (Litopenaeus vannamei). J Chem Technol Biotechnol 91:1250–1256. https://doi.org/10.1002/JCTB.4714
Huang R, Tan L, Cai B et al (2018) Preparation, characterization and in vitro release study of drug-loaded sodium carboxy-methylcellulose/chitosan composite sponge. PLoS ONE 13:e0206275. https://doi.org/10.1371/JOURNAL.PONE.0206275
Huet G, Hadad C, Husson E et al (2020) Straightforward extraction and selective bioconversion of high purity chitin from Bombyx eri larva: toward an integrated insect biorefinery. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115382
van Huis A (2013) Potential of insects as food and feed in assuring food security. Annu Rev Entomol 58:563–583
Huq T, Khan A, Brown D et al (2022) Sources, production and commercial applications of fungal chitosan: a review. J Bioresour Bioprod 7:85–98
Iber BT, Kasan NA, Torsabo D, Omuwa JW (2022) A review of various sources of chitin and chitosan in nature. J Renew Mater 10:1097–1123
Ibitoye EB, Lokman IH, Hezmee MNM et al (2018) Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed Mater. https://doi.org/10.1088/1748-605X/aa9dde
Jaworska MM, Konieczna E (2001) The influence of supplemental components in nutrient medium on chitosan formation by the fungus Absidia orchidis. Appl Microbiol Biotechnol 56:220–224. https://doi.org/10.1007/s002530000591
Jiang L, Pan S, Kim JM (2011) Influence of nitrogen source on chitosan production carried out by Absidia coerulea CTCC AF 93105. Carbohydr Polym 86:359–361. https://doi.org/10.1016/j.carbpol.2011.04.045
Jones M, Kujundzic M, John S, Bismarck A (2020) Crab vs. Mushroom: a review of crustacean and fungal chitin in wound treatment. Mar Drugs. https://doi.org/10.3390/md18010064
Joseph SM, Krishnamoorthy S, Paranthaman R et al (2021) A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydr Polym Technol Appl. https://doi.org/10.1016/j.carpta.2021.100036
Kaczmarek MB, Struszczyk-Swita K, Li X et al (2019) Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front Bioeng Biotechnol 7:243
Kannan M, Nesakumari M, Rajarathinam K, Singh AJAR (2010) Production and characterization of mushroom chitosan under solid-state fermentation conditions. Adv Biol Res 4:10–13
Karimi K, Zamani A (2013) Mucor indicus: Biology and industrial application perspectives: a review. Biotechnol Adv 31:466–481. https://doi.org/10.1016/J.BIOTECHADV.2013.01.009
Kaur S, Dhillon GS (2014) The versatile biopolymer chitosan: potential sources, evaluation of extraction methods and applications. Crit Rev Microbiol 40:155–175. https://doi.org/10.3109/1040841X.2013.770385
Kaya M, Baublys V, Can E et al (2014) Comparison of physicochemical properties of chitins isolated from an insect (Melolontha melolontha) and a crustacean species (Oniscus asellus). Zoomorphology 133:285–293. https://doi.org/10.1007/s00435-014-0227-6
Kaya M, Baublys V, Šatkauskiene I et al (2015a) First chitin extraction from Plumatella repens (Bryozoa) with comparison to chitins of insect and fungal origin. Int J Biol Macromol 79:126–132. https://doi.org/10.1016/j.ijbiomac.2015.04.066
Kaya M, Lelešius E, Nagrockaite R et al (2015b) Differentiations of chitin content and surface morphologies of chitins extracted from male and female grasshopper species. PLoS ONE. https://doi.org/10.1371/journal.pone.0115531
Khan A, Vu KD, Riedl B, Lacroix M (2015) Optimization of the antimicrobial activity of nisin, Na-EDTA and pH against gram-negative and gram-positive bacteria. LWT Food Sci Technol 61:124–129. https://doi.org/10.1016/j.lwt.2014.11.035
Kleekayai T, Suntornsuk W (2011) Production and characterization of chitosan obtained from Rhizopus oryzae grown on potato chip processing waste. World J Microbiol Biotechnol 27:1145–1154. https://doi.org/10.1007/S11274-010-0561-X
Kostag M, el Seoud OA (2021) Sustainable biomaterials based on cellulose, chitin and chitosan composites: a review. Carbohydr Polym Technol Appl 2:100079
Kulkarni SA, Ghormade V, Kulkarni G et al (2008) Comparison of Metarhizium isolates for biocontrol of Helicoverpa armigera (Lepidoptera: Noctuidae) in chickpea. Biocontrol Sci Technol 18:809–828. https://doi.org/10.1080/09583150802366475
Leite MV, Stamford TCM, Stamford-Arnaud TM et al (2015) Conversion of agro-industrial wastes to chitosan production by Syncephalastrum racemosum UCP 1302. Int J Appl Res Nat Prod 8:5–11
Li Z, Ma J, Li R et al (2018) Fabrication of a blood compatible composite membrane from chitosan nanoparticles, ethyl cellulose and bacterial cellulose sulfate. RSC Adv 8:31322–31330. https://doi.org/10.1039/C8RA05536J
Liao W, Liu Y, Frear C, Chen S (2008) Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material—dairy manure—using a pelletized filamentous fungus Rhizopus oryzae ATCC 20344. Bioresour Technol 99:5859–5866. https://doi.org/10.1016/J.BIORTECH.2007.10.006
Liu S, Sun J, Yu L et al (2012) Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 17:4604–4611. https://doi.org/10.3390/molecules17044604
Liu P, Liu S, Guo N et al (2014a) Cofermentation of <i>Bacillus licheniformis</I> and Gluconobacter oxydans for chitin extraction from shrimp waste. Biochem Eng J 91:10–15. https://doi.org/10.1016/j.bej.2014.07.004
Liu P, Liu S, Guo N et al (2014b) Cofermentation of Bacillus licheniformis and Gluconobacter oxydans for chitin extraction from shrimp waste. Biochem Eng J 91:10–15. https://doi.org/10.1016/j.bej.2014.07.004
Liu Y, Xing R, Yang H et al (2020) Chitin extraction from shrimp (Litopenaeus vannamei) shells by successive two-step fermentation with Lactobacillus rhamnoides and Bacillus amyloliquefaciens. Int J Biol Macromol 148:424–433. https://doi.org/10.1016/j.ijbiomac.2020.01.124
Luo Q, Wang Y, Han Q et al (2019) Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr Polym 209:266–275. https://doi.org/10.1016/j.carbpol.2019.01.030
Lv M, Hu Y, Gänzle MG et al (2016) Preparation of chitooligosaccharides from fungal waste mycelium by recombinant chitinase. Carbohydr Res 430:1–7. https://doi.org/10.1016/j.carres.2016.04.019
Manni L, Ghorbel-Bellaaj O, Jellouli K et al (2010a) Extraction and characterization of chitin, chitosan, and protein hydrolysates prepared from shrimp waste by treatment with crude protease from bacillus cereus SV1. Appl Biochem Biotechnol 162:345–357. https://doi.org/10.1007/s12010-009-8846-y
Manni L, Jellouli K, Ghorbel-Bellaaj O et al (2010b) An oxidant- and solvent-stable protease produced by bacillus cereus SV1: application in the deproteinization of shrimp wastes and as a laundry detergent additive. Appl Biochem Biotechnol 160:2308–2321. https://doi.org/10.1007/S12010-009-8703-Z
Marei N, Elwahy AHM, Salah TA et al (2019) Enhanced antibacterial activity of Egyptian local insects’ chitosan-based nanoparticles loaded with ciprofloxacin-HCl. Int J Biol Macromol 126:262–272. https://doi.org/10.1016/j.ijbiomac.2018.12.204
Maruthiah T, Somanath B, Immanuel G, Palavesam A (2015) Deproteinization potential and antioxidant property of haloalkalophilic organic solvent tolerant protease from marine Bacillus sp. APCMST-RS3 using marine shell wastes. Biotechnol Rep 8:124–132. https://doi.org/10.1016/j.btre.2015.10.009
Marzieh MN, Zahra F, Tahereh E, Sara KN (2019) Comparison of the physicochemical and structural characteristics of enzymatic produced chitin and commercial chitin. Int J Biol Macromol 139:270–276. https://doi.org/10.1016/j.ijbiomac.2019.07.217
Mathew GM, Huang CC, Sindhu R et al (2021) Enzymatic approaches in the bioprocessing of shellfish wastes. 3 Biotech 11:1–13
Maw T, Tan TK, Khor E, Wong SM (2002) Selection of Gongronella butleri strains for enhanced chitosan yield with UV mutagenesis. J Biotechnol 95:189–193. https://doi.org/10.1016/S0168-1656(02)00004-4
Mhamdi S, Ktari N, Hajji S et al (2017) Alkaline proteases from a newly isolated Micromonospora chaiyaphumensis S103: characterization and application as a detergent additive and for chitin extraction from shrimp shell waste. Int J Biol Macromol 94:415–422. https://doi.org/10.1016/j.ijbiomac.2016.10.036
Mohan K, Ganesan AR, Ezhilarasi PN et al (2022) Green and eco-friendly approaches for the extraction of chitin and chitosan: a review. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2022.119349
Mondala A, Al-Mubarak R, Atkinson J et al (2015) Direct solid-state fermentation of soybean processing residues for the production of fungal chitosan by Mucor rouxii. J Mater Sci Chem Eng 3:11–21. https://doi.org/10.4236/MSCE.2015.32003
Mora-Montes HM, Netea MG, Ferwerda G et al (2011) Recognition and blocking of innate immunity cells by Candida albicans Chitin. Infect Immun 79:1961–1970. https://doi.org/10.1128/IAI.01282-10
Morin-Crini N, Lichtfouse E, Torri G, Crini G (2019) Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ Chem Lett 17:1667–1692
Moussa SH, Tayel AA, Al-Hassan AA, Farouk A (2013) Tetrazolium/formazan test as an efficient method to determine fungal chitosan antimicrobial activity. J Mycol 2013:1–7. https://doi.org/10.1155/2013/753692
Mukhin VA, Novikov VY (2001) Enzymatic hydrolysis of proteins from crustaceans of the Barents Sea. Appl Biochem Microbiol 37:633–638
Nahar P, Ghormade V, Deshpande MV (2004) The extracellular constitutive production of chitin deacetylase in Metarhizium anisopliae: possible edge to entomopathogenic fungi in the biological control of insect pests. J Invertebr Pathol 85:80–88. https://doi.org/10.1016/j.jip.2003.11.006
Namboodiri MMT, Pakshirajan K (2019) Sustainable and green approach of chitosan production from Penicillium citrinum biomass using industrial wastewater as a cheap substrate. J Environ Manage 240:431–440. https://doi.org/10.1016/J.JENVMAN.2019.03.085
Neves AC, Zanette C, Grade ST et al (2017) Optimization of lactic fermentation for extraction of chitin from freshwater shrimp waste. Acta Scientiarum Technol 39:125–133. https://doi.org/10.4025/actascitechnol.v39i2.29370
Nwe N, Stevens WF (2004) Effect of urea on fungal chitosan production in solid substrate fermentation. Process Biochem 39:1639–1642. https://doi.org/10.1016/S0032-9592(03)00301-7
Nwe N, Tetsuya Tamur H (2010) Production of fungal chitosan by enzymatic method and applications in plant tissue culture and tissue engineering: 11 years of our progress, present situation and future prospects. In: Elnashar M (ed) Biopolymers. Sciyo, Rijeka
Olicón-Hernández DR, Vázquez-Landaverde PA, Cruz-Camarillo R, Rojas-Avelizapa LI (2017) Comparison of chito-oligosaccharide production from three different colloidal chitosans using the endochitonsanolytic system of Bacillus thuringiensis. Prep Biochem Biotechnol 47:116–122. https://doi.org/10.1080/10826068.2016.1181086
Paul T, Halder SK, Das A et al (2015a) Production of chitin and bioactive materials from Black tiger shrimp (Penaeus monodon) shell waste by the treatment of bacterial protease cocktail. 3 Biotech 5:483–493. https://doi.org/10.1007/s13205-014-0245-6
Rakshit S, Mondal S, Pal K et al (2021) Extraction of chitin from Litopenaeus vannamei shell and its subsequent characterization: an approach of waste valorization through microbial bioprocessing. Bioprocess Biosyst Eng 44:1943–1956. https://doi.org/10.1007/s00449-021-02574-y
Saenz-Mendoza AI, Zamudio-Flores PB, García-Anaya MC et al (2020) Characterization of insect chitosan films from Tenebrio molitor and Brachystola magna and its comparison with commercial chitosan of different molecular weights. Int J Biol Macromol 160:953–963. https://doi.org/10.1016/J.IJBIOMAC.2020.05.255
Safaei Z, Karimi K, Zamani A (2016) Impact of phosphate, potassium, yeast extract, and trace metals on chitosan and metabolite production by Mucor indicus. Int J Mol Sci. https://doi.org/10.3390/IJMS17091429
Sajomsang W, Gonil P (2010) Preparation and characterization of α-chitin from cicada sloughs. Mater Sci Eng C 30:357–363. https://doi.org/10.1016/j.msec.2009.11.014
Satari B, Karimi K, Taherzadeh MJ, Zamani A (2016) Co-production of fungal biomass derived constituents and ethanol from citruswastes free sugars without auxiliary nutrients in airlift bioreactor. Int J Mol Sci. https://doi.org/10.3390/ijms17030302
Sedaghat F, Yousefzadi M, Toiserkani H, Najafipour S (2017) Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: an alternative procedure for chemical extraction of chitin and chitosan. Int J Biol Macromol 104:883–888. https://doi.org/10.1016/j.ijbiomac.2017.06.099
Shamshina JL, Gurau G, Block LE et al (2014) Chitin–calcium alginate composite fibers for wound care dressings spun from ionic liquid solution. J Mater Chem B 2:3924–3936. https://doi.org/10.1039/C4TB00329B
Spranghers T, Ottoboni M, Klootwijk C et al (2017) Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J Sci Food Agric 97:2594–2600. https://doi.org/10.1002/jsfa.8081
Tabassum N, Ahmed S, Ali MA (2021) Chitooligosaccharides and their structural-functional effect on hydrogels: a review. Carbohydr Polym 261:117882
Tajdini F, Amini MA, Nafissi-Varcheh N, Faramarzi MA (2010) Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int J Biol Macromol 47:180–183. https://doi.org/10.1016/J.IJBIOMAC.2010.05.002
Tan JS, Abbasiliasi S, Lee CK, Phapugrangkul P (2020) Chitin extraction from shrimp wastes by single step fermentation with Lactobacillus acidophilus FTDC3871 using response surface methodology. J Food Process Preserv. https://doi.org/10.1111/jfpp.14895
Tan YN, Lee PP, Chen WN (2021) Dual extraction of crustacean and fungal chitosan from a single Mucor circinelloides fermentation. Fermentation 6:40
Tang RH, Li M, Liu LN et al (2020) Chitosan-modified nitrocellulose membrane for paper-based point-of-care testing. Cellulose 27:3835–3846. https://doi.org/10.1007/s10570-020-03031-x
Tasar OC, Erdal S, Taskin M (2016) Chitosan production by psychrotolerant Rhizopus oryzae in non-sterile open fermentation conditions. Int J Biol Macromol 89:428–433. https://doi.org/10.1016/J.IJBIOMAC.2016.05.007
Tayel AA, Moussa S, Opwis K et al (2010) Inhibition of microbial pathogens by fungal chitosan. Int J Biol Macromol 47:10–14. https://doi.org/10.1016/j.ijbiomac.2010.04.005
Tchemtchoua VT, Ganka A, Aqil A et al (2011) Development of a chitosan nanofibrillar scaffold for skin repair and regeneration. Biomacromol 12:3194–3204. https://doi.org/10.1021/bm200680q
Teng WL, Khor E, Tan TK et al (2001) Concurrent production of chitin from shrimp shells and fungi. Carbohydr Res 332:305–316. https://doi.org/10.1016/S0008-6215(01)00084-2
Thadathil N, Velappan SP (2014) Recent developments in chitosanase research and its biotechnological applications: a review. Food Chem 150:392–399. https://doi.org/10.1016/j.foodchem.2013.10.083
Vicente FA, Huš M, Likozar B, Novak U (2021) Chitin deacetylation using deep eutectic solvents: ab initio-supported process optimization. ACS Sustain Chem Eng 9:3874–3886. https://doi.org/10.1021/ACSSUSCHEMENG.0C08976
Wang SL, Kao TY, Wang CL et al (2006) A solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in the deproteinization of squid pen for β-chitin preparation. Enzyme Microb Technol 39:724–731
Wang T, Chen L, Shen T, Wu D (2016) Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int J Biol Macromol 93:775–782. https://doi.org/10.1016/j.ijbiomac.2016.09.038
Wang Y, Qian J, Zhao N et al (2017) Novel hydroxyethyl chitosan/cellulose scaffolds with bubble-like porous structure for bone tissue engineering. Carbohydr Polym 167:44–51. https://doi.org/10.1016/j.carbpol.2017.03.030
Wang Y, Khan A, Liu Y et al (2019) Chitosan oligosaccharide-based dual pH responsive nano-micelles for targeted delivery of hydrophobic drugs. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115061
Wu T, Zivanovic S, Draughon FA, Sams CE (2004) Chitin and chitosan-value-added products from mushroom waste. J Agric Food Chem 52:7905–7910. https://doi.org/10.1021/jf0492565
Yang J, Dahlström C, Edlund H et al (2019) pH-responsive cellulose–chitosan nanocomposite films with slow release of chitosan. Cellulose 26:3763–3776. https://doi.org/10.1007/S10570-019-02357-5/TABLES/3
Younes I, Nasri R, Bkhairia I et al (2015a) New proteases extracted from red scorpionfish (Scorpaena scrofa) viscera: characterization and application as a detergent additive and for shrimp waste deproteinization. Food Bioprod Process 94:453–462. https://doi.org/10.1016/J.FBP.2014.06.003
Younes I, Rinaudo M, Harding D, Sashiwa H (2015b) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13:1133–1174. https://doi.org/10.3390/md13031133
Yu Y, Liu X, Miao J, Leng K (2020) Chitin from Antarctic krill shell: eco-preparation, detection, and characterization. Int J Biol Macromol 164:4125–4137. https://doi.org/10.1016/j.ijbiomac.2020.08.244
Zamani A, Edebo L, Sjöström B, Taherzadeh MJ (2007) Extraction and precipitation of chitosan from cell wall of zygomycetes fungi by dilute sulfuric acid. Biomacromol 8:3786–3790. https://doi.org/10.1021/BM700701W
Zavgorodnya O, Shamshina JL, Bonner JR, Rogers RD (2017) Electrospinning biopolymers from ionic liquids requires control of different solution properties than volatile organic solvents. ACS Sustain Chem Eng 5:5512–5519. https://doi.org/10.1021/ACSSUSCHEMENG.7B00863
Zhang H, Jin Y, Deng Y et al (2012) Production of chitin from shrimp shell powders using Serratia marcescens B742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydr Res 362:13–20. https://doi.org/10.1016/j.carres.2012.09.011
Zhang Q, Wang L, Liu S, Li Y (2021) Establishment of successive co-fermentation by Bacillus subtilis and Acetobacter pasteurianus for extracting chitin from shrimp shells. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2021.117720
Zimoch-Korzycka A, Gardrat C, alKharboutly M et al (2016) Chemical characterization, antioxidant and anti-listerial activity of non-animal chitosan-glucan complexes. Food Hydrocoll 61:338–343. https://doi.org/10.1016/j.foodhyd.2016.05.019
Zininga JT, Puri AK, Govender A et al (2019) Concomitant production of chitosan and lipids from a newly isolated Mucor circinelloides ZSKP for biodiesel production. Bioresour Technol 272:545–551. https://doi.org/10.1016/J.BIORTECH.2018.10.035
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by all the authors. The sections ‘Introduction’, ‘Biological methods of Extraction’, and ‘Concluding Remarks’ were drafted by NI; section; ‘Alternative sources of Chitosan’ by MH and SFT. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Islam, N., Hoque, M. & Taharat, S.F. Recent advances in extraction of chitin and chitosan. World J Microbiol Biotechnol 39, 28 (2023). https://doi.org/10.1007/s11274-022-03468-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03468-1