Abstract
We evaluated the leishmanicidal activity of commercially available 5α-cholest-7-en-3β-ol [5α-chol], (+)-4-cholesten-3-one [(+)-4-chol] and the equimolar mixture of the two of them in promastigotes and amastigotes of two different strains of Leishmania mexicana (LCL) and (DCL). The leishmanicidal effectiveness of these sterols was determined by promastigote growth-kinetic experiments and promastigote viability using the propidium iodide staining procedure. The proliferation test was performed using the CFSE (5-Carboxyfluorescein N-succinimidyl ester) staining of parasites at different time points. To determine the leishmanicidal effectiveness of these sterols in amastigotes, we evaluated parasite killing inside of macrophages at different time points. The trypan blue exclusion test was used to determine cytotoxicity of sterols in uninfected macrophages. We included in all experiments a control group of parasites treated with 2% DMSO (Dimethyl Sulfoxide) and another one treated with the reference drug sodium stibogluconate (Sb). Our results showed that the equimolar mixture at 2000 times lower concentration presented similar leishmanicidal activity as Sb. This mixture was similarly effective at 100 times lower concentration than individual sterols tested separately indicating the existence of a synergistic effect against LCL and DCL parasites. The therapeutic index of the equimolar mixture was 10,000—16,000 times higher than the one recorded by Sb and was not cytotoxic to macrophages. Therefore, the equimolar mixture of 5α-Chol and (+)-4-chol may represent a potential alternative for the treatment of cutaneous leishmaniasis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites of the Leishmania genus transmit leishmaniasis during hematophagy from female mosquitoes of the Lutzomyia genus in the Americas and Phlebotomus genus in the rest of the world. The disease burden from leishmaniasis is 2.35 million disability-adjusted life years lost (DALYs, WHO 2021). Leishmaniasis is the second most prevalent vector-borne parasitic disease globally in terms of morbidity and mortality (Ning et al. 2020). Its distribution is the tropical and subtropical areas of 102 countries or territories of all continents except Oceania and Antarctica. Estimations indicate that 10% of the world's population is at risk (PAHO 2020). This parasitism belongs to the group of neglected diseases because it affects the poorest people with deficient health services. Cutaneous leishmaniasis (CL) is the most frequent clinical condition, yet not deadly. It frequently causes disfigurement, mutilations, social marginalization, loss of work capacity, and psychological trauma (Okwor and Uzonna 2016; Isaac-Márquez et al. 2018). Control of leishmaniasis is focused on chemotherapy as there is no effective vaccine. The intralesional and systemic application of N-methylglucamine or sodium stibogluconate is the first choice of treatment (WHO 2021). Second-line chemotherapy includes amphotericin B (Amphotericin B deoxycholate and liposomal amphotericin B), paromomycin and pentamidine. Additional treatment includes the use of oral miltefosine (Ghorbani and Farhoudi 2017). Some of these drugs are characterized by high toxicity and cost. There is an increase of parasite's drug resistance and patient's suffering during parenteral application of some of these drugs. These inconveniences sometimes cause treatment abandonment (Croft and Olliaro 2011; Ponte-Sucre et al. 2017). Thus, identifying less toxic compounds with more significant leishmanicidal activity is mandatory (DNDi 2021; WHO 2021). Some phytochemicals and synthetic sterols have previously reported have leishmanicidal activity. Other reports have demonstrated that clerosterol isolated from Cassia fistula effectively kills extra- and intra-cellular L. chagasi parasites in vitro (Sartonelli 2007). Silva et al. (2014) showed killing activity towards L. chagasi of a mixture of β-sitosterol and stigmasterol isolated from Musa paradisiaca fruit peel. Also, reports show that sterols isolated from the fungus Trametes Versicolor kill intra- and extra-cellular L. amazonensis (Leliebre-Lara et al. 2016). Ghosh et al. (2016) and Bazin et al. (2006) synthesized oxysterols with leishmanicidal activity against L. donovani. In a previous report, we demonstrated antileishmanial activity of ( +)-4-cholesten-3-one [(+)-4-chol] isolated from hexane extract of P. andrieuxii (Pan et al. 2012). We also showed this extract heals ear´s L. mexicana infections in mice (Lezama-Dávila et al. 2014). In this work, we selected [(+)-4-chol] and 5α-cholest-7-en-3β-ol [5α-chol], which are commercial chemical analogs. There are no literature reports of the use of [5α-chol] as leishmanicidal compound. In this work, we also studied the individual bioactivity of 5α-chol and (+)-4-chol and the possible synergistic activity of the equimolar mixture against promastigotes and amastigotes of two strains L. mexicana.
Materials and methods
Parasites
We used L. mexicana (MNYC/BZ/62/M379) and L. mexicana (MHOM/MX/01//Tab3), strains isolated of a patient from Belize with localized cutaneous leishmaniasis (LCL) and another patient from Tabasco, México with chronic disseminated cutaneous leishmaniasis (DCL). They were maintained by subcutaneous inoculation of amastigotes into shaved rumps of Balb/c mice. Promastigotes were obtained by culturing amastigotes recovered from the lesions in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 IU of penicillin and 100 µg streptomycin (Sigma-Aldrich, Mexico) (Lezama-Dávila et al. 2016; Isaac-Márquez et al. 2018). The use of animals was carried out according to Mexican Official Standard NOM-062-ZOO-1999 (SAGARPA 2001). In this work L. mexicana (MNYC/BZ/62/M379) and L. mexicana (MHOM/MX/01/Tab3) will be called L. mexicana LCL and L. mexicana DCL respectively.
Sterols and reference drug
5α-cholest-7-en-3β-ol, (+)-4-cholesten-3-one and the equimolar mixture of these sterols were studied. These sterols are manufactured by Sigma-Aldrich (México) and in this work they will be abbreviated as 5α-chol and (+)-4-chol respectively. We selected sodium stibogluconate (Sb) as the reference drug because it is used as first-line drug in the treatment of leishmaniasis (de Menezes et al. 2015; Ghorbani and Farhoudi 2017).
Growth´s kinetics of promastigotes
One million LCL and DCL promastigotes/mL of supplemented media were added into individual wells of 24-well plates (Corning Inc.). The parasites were treated with the following concentrations of 0.1, 1, 10, 50, 100 and 200 µM of 5α-chol or (+)-4-chol or their equimolar mixture and were incubated at 280C for 5 days. Reference drug (Sb) was used at concentrations of 200, 300 and 400 µM. Control group consisted of parasites treated with 2% dimethyl sulfoxide (DMSO) from Sigma-Aldrich, Mexico in supplemented media (this concentration of DMSO is not toxic to LCL or DCL promastigotes). Parasites were counted at 12 h and then every 24 h using the Neubauer chamber. These data were used to record the kinetics of parasite growth and calculate the average IC50 of at least three different experiments. After 120 h of incubation, plate containing parasites was spun down and parasites were fed with fresh media to determine parasite survival in all different experimental and control groups. Inhibitory concentration fifty (IC50) was defined as the concentration of sterols or Sb necessary to kill 50% of promastigote population (Lezama-Dávila et al. 2012). We also observed promastigote mobility and its values were assigned according to criteria reported by Isaac-Márquez et al. (2018).
Promastigotes’ killing by propidium iodide (PI) staining
We added one million LCL and DCL promastigotes into individual wells of 24 well plates (Corning Inc.) and treated them with sterols or Sb and then they were incubated at 28 °C as described in the previous section. We recorded parasite viability at different time points using individual plates for every incubation time tested. We also included a control group containing 2% DMSO. After each incubation period, parasites were stained with 10 µg/mL of PI for 30 min. This compound only binds to DNA of dead but not alive parasites (Foglieni et al. 2001; Isaac-Márquez et al. 2018). Stained and unstained parasites were counted in a Newbawer chamber under fluorescence microscopy at 535 nm and under light microscopy respectively. Viable parasites were represented by the difference between the total number of parasites and the number of PI-stained promastigotes. The experiment was carried out in triplicate.
Inhibition of promastigote proliferation
Logarithmic phase of growth of LCL and DCL promastigotes were used and cultured in complete culture media. Parasites were then stained with 1 mL of 10 µM CFSE solution (5-Carboxyfluorescein N-succinimidyl ester, Sigma-Aldrich, México) for 10 min at 280C. When the stained parasites divide, only half of the offspring remain with the fluorescent tag and, consequently, with each cell division the number of fluorescent parasites decreases by half.
At the end of this incubation period fluorescein dye was inactivated with 1 mL of culture media without FBS. Parasites were then washed twice with culture media and finally resuspended in 1 mL of complete culture media. One million stained parasites/mL of culture media were placed into individual wells of 24 well plates (Corning Inc.). Parasites were treated with 1, 10, 50 and 100 µM of individual or mixed sterols (5α-chol and (+)-4-chol) over a period of 120 h at 28 0C. We also included Sb at different concentrations such as 200 y 300 µM and control group was only treated with 2% DMSO. We counted parasite number at 12 h and every 24 h in triplicate using a Neubauer chamber to record growth-kinetics of LCL and DCL promastigotes. Likewise, fluorescent parasites were counted under the fluorescence microscope at a wavelength of 480 nm and results correspond to average number of fluorescent parasites per field counted in three different fields. The experiment was carried out in triplicate (Messaritakis et al. 2010; Isaac-Márquez et al. 2018).
Leishmanicidal effectiveness of sterols against amastigotes inside of macrophages
In this test we used the J774A.1 macrophage cell line derived from Balb/c mice (CLMM). A glass coverslip was placed on the bottom of each well from a 24-well plate (Corning Inc.). Macrophages (3 X 105/mL) were then incubated for 2 h in a CO2 incubator (95% air plus 5% CO2). Immediately after 6 X 106 promastigotes in 1 mL of culture media were added and overnight incubated at 370C in a CO2 incubator. After this incubation period, non-internalized parasites were removed by washing with PBS solution. Infected macrophages were treated with 0.1, 1, 10 and 50 µM of individual or mixed 5α-chol and (+)-4-chol. Macrophages were also treated with 200 and 300 µM of Sb, control groups included treatment with 2% of DMSO. All different groups were incubated at 370C in a CO2 incubator for a period of 120 h. All experiments were performed in triplicate. At the end of each incubation period, macrophages adhered to coverslips were fixed with methanol and stained with Giemsa. Leishmanicidal effectiveness was determined by counting the number of parasites per 100 CLMM. These data were also used to report the concentration of sterols or Sb required to kill 50% of the amastigote population (IC50; Lezama-Dávila et al. 2014; Isaac-Márquez and Lezama-Dávila 2020).
Cytotoxicity of sterols in uninfected macrophages
In this section we used the trypan blue exclusion test to assess the viability of macrophages not infected with parasites. Macrophages at a density of 3 X 105 CLMM/mL were adhered on circular coverslips placed on the bottom of individual wells from 24-well plates (Corning Inc.). Phagocytes were treated with 1, 10, 50, 75 and 100 µM of 5α-chol or ( +)-4-chol or of their equimolar mixture for 72 h at 370C in a CO2 incubator. Reference drug at concentrations of 100–400 µM and controls treated with 2% DMSO were also included. The experiment was carried out in triplicate. Viable and non-viable macrophages were counted to calculate macrophage cytotoxicity (CC50), which is the concentration of sterols or Sb required to destroy 50% of macrophages. The therapeutic index resulted from the division of CC50 by IC50 of amastigotes (Isaac-Márquez et al. 2018).
Statistical analysis
Statistical testing was performed using Mann Whitney U test that was used to compared independent group of data that did not follow a normal distribution. IC50 and CC50 values were calculated with LdP Line® software.
Results
Growth´s kinetics of promastigotes
In Fig. 1 we show the chemical structure of individual sterols studied in this work. It can be observed in Figs. 2, 3 that from 24 h and onwards (not at 12 h of incubation) the equimolar mixture of 5α-chol and (+)-4-chol showed a significantly higher (p < 0.05) leishmanicidal activity. This activity was compared to individual sterols or Sb tested in both LCL and DCL L. mexicana promastigotes. After 5 days of incubation, the number of LCL and DCL promastigotes was dramatically reduced by using individual sterols or their mixture ((Figs. 2A, 3A). Sterol mixture at 1 µM proved to be the most efficient leishmanicidal concentration. The reduction of the parasite population at low concentrations of sterols and high concentrations of the reference drug is time dependent (Figs. 2A, 3A). It is important to state that Sb at concentrations below 200 µM and sterols used individually at concentration of 0.1 µM do not have significant (p > 0.05) activity at any incubation time. However, the mixture of two sterols presented significant effect (p < 0.01) even at 0.1 µM from 24 h of incubation onwards. After 120 h of incubation all parasite groups tested were spun down and fed with fresh media and only control group of parasites re-enter into a logarithmic phase of growth. All experimental groups of parasites presented gross morphological alterations suggesting that this represented dead parasites that did not grow after re-feeding with fresh media. Regarding mobility of promastigotes after 5 days of incubation, 75% surviving LCL and DCL promastigotes treated with individual sterols remained mobile and 100% of those treated with the mixture of sterols lost mobility. Whereas with 200 µM of Sb only 25% of the promastigotes were immobile at this incubation time. In the control group, all parasites showed rapid movements. Table 1 presents the IC50 values obtained with sterols or Sb at 24-h interval over a period of 120 h. The leishmanicidal effect observed with the equimolar mixture of sterols was considerably greater compared to the reference drug. At 72 h, IC50 with the equimolar mixture was 1160 and 510 times lower than the one recorded with Sb for LCL and DCL promastigotes, respectively. This effectiveness increased at 120 h, the IC50 of sterol mixture was 1680 (LCL promastigotes) and 1600 (DCL promastigotes) times lower than IC50 of Sb. Also, at this time-period the equimolar mixture was more effective than the sterols used separately.
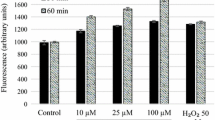
Effect of the equimolar mixture of 5α-chol and (+)-4-chol on promastigotes of L. mexicana (LCL). A Growth kinetics; B Promastigotes stained with propidium iodide (PI). Total number of parasites were counted under light microscopy, while PI-stained ones were counted under fluorescence microscopy at a wavelength of 535 nm. The difference between the total number of parasites and the number of PI-stained ones represents the number of living parasites. Data represent a replica of three independent experiments, *p < 0.01 Statistical analysis was performed using the Mann Whitney U test
Effect of the equimolar mixture of 5α-chol and (+)-4-chol on promastigotes of L. mexicana (DCL). A Growth kinetics; B Promastigotes stained with propidium iodide (PI). Total number of parasites were counted under light microscopy, while PI-stained ones were counted under fluorescence microscopy at a wavelength of 535 nm. The difference between the total number of parasites and the number of PI-stained ones represents the number of living parasites. Data represent a replica of three independent experiments, *p < 0.01 Statistical analysis was performed using Mann Whitney U test
Promastigote killing recorded by propidium iodide (PI) staining
In Figs. 2 and 3we show kinetic experiments of parasite growth (Figs. 2A, 3A) and parasite killing (Figs. 2B, 3B). The killing capacity of sterols for L. mexicana promastigotes was evaluated by PI binding to DNA of dead parasites. The sterol mixture at 1 µM proved to be the most efficient killing preparation from 72 h of incubation and onwards, at this time points we found no viable parasites. Even at 0.1 µM of sterol mixture the proportion of viable parasites was closer to that shown by Sb used at a 2000 higher molar concentration (Figs. 2B, 3B). Moreover, at 1 µM concentration, the mixture of sterols showed more efficient killing compared to individual ones. At this concentration, mixed sterols killed all parasites at 72 h of incubation while individual sterols presented considerable parasite number even at 120 h (Figs. 2B, 3B). This is a clear indication of synergism between these compounds to kill parasites. We also observed that only the sterol mixture was effective to kill parasites with as little as 0.1 µM.
Inhibition of promastigotes proliferation
In these set of experiments, we used the CFSE parasite staining assay to determine the effect of sterols on the reproductive capacity of LCL and DCL promastigotes. In our kinetics of parasite growth´s experiment we observed that control group presented a progressive increase in the number of unstained LCL and DCL promastigotes showing parasites dividing actively (Figs. 4A, B). Therefore, the control group of promastigotes presented significant (p < 0.01) reduction of fluorescent parasites. We also showed that the equimolar mixture of sterols deeply affected parasite proliferation (Figs. 4A, B), that correlated positively well with parasite killing (Figs. 2B, 3B). After 24 h of incubation the group of parasites treated with 1 µM of the equimolar mixture presented more than 90% of fluorescent LCL and DCL parasites/fluorescent microscopic field. This shows that cell division of parasites in this group was considerably reduced. At a concentration of 1 µM the sterol mixture showed more inhibition of proliferation as compared to individual ones. (+)-4 chol presented less inhibition of proliferation than 5α-chol or the mixture of them. Fluorescent LCL and DCL parasites treated with (+)-4 chol for 120 h represented 45% and 59% respectively of the total population per microscopic field tested. This clearly indicates that at this time point of incubation about half population of parasites treated with this sterol retained its reproductive capacity. However, at 0.1 µM concentration of individual or mixed sterols there were not inhibition of proliferation at any time point. Eighty percent of LCL or DCL parasites treated with Sb at concentrations as high as 200 and 300 µM remain fluorescently labeled. However, at lower concentrations such as 100 µM it does not inhibit the proliferation of parasites.
CFSE assay: effect in proliferative activity of L. mexicana (LCL) A and (DCL) B promastigotes. Fluorescent parasites were counted under the fluorescent microscope at a wavelength of 480 nm, while total number of parasites were counted under light microscopy. Data represents a percentage of CFSE-stained parasites. Data represent a replica of three independent experiments, *p < 0.01 Statistical analysis was performed using Mann Whitney U test
Leishmanicidal effectiveness of individual or mixed sterols against amastigotes inside of macrophages
In this section we showed that equimolar mixture of sterols presented greater leishmanicidal effect on LCL and DCL amastigotes internalized by J774A.1 macrophages (CLMM) (Fig. 5). At all different incubation periods, control group registered the highest parasite counts both for LCL and DCL amastigotes/100 CLMM respectively. In contrast both 0.1 and 1 µM of sterol mixture reduced the amastigote population more than three times as compared to the control group. Considerably higher doses of Sb (200 µM) presented a leishmanicidal effect equivalent to that displayed by equimolar mixture. Additionally, at 120 h Sb was highly toxic towards murine macrophages showing 100% of macrophage´s destruction. We also showed that a 100-fold higher concentration (10 µM) of individual sterols was required to obtain a similar effect with the sterol mixture. At 96 h of incubation, the IC50 of the equimolar mixture was 14,000 and 26,000 times lower than the IC50 of the Sb for LCL and DCL amastigotes respectively. At 120 h of incubation the IC50 of the equimolar mixture was 1290 and 660 times lower than 5α-chol and 1500 and 530 times lower than IC50 of (+)-4-chol for LCL and DCL amastigotes respectively. As can be seen in Table 2, the effectiveness of the equimolar mixture of sterols increased significantly over the time.
Effectiveness of the equimolar mixture of 5α-chol and (+)-4-chol in amastigotes of L. mexicana (LCL) A and (DCL) B internalized by murine macrophages J774A.1, Sb effect was recorded up to 96 h only since at 120 h it was cytotoxic and destroyed all murine macrophages. Data is a replica of three independent experiments,* p < 0.01 Statistical analysis was performed using Mann Whitney U test
Cytotoxicity of individual or mixed sterols in uninfected macrophages
Table 3 shows the cytotoxicity values of uninfected macrophages (CC50). The CC50 value of sterol mixture was about 3 times greater than that shown by 5α-chol or (+)-4-chol or Sb. Finally, the therapeutic index of the sterol mixture was 10,000 and 16,000 times greater than that registered by Sb and 150 and 250 times higher than that exhibited by the sterols used individually for LCL and DCL amastigotes respectively.
Discussion
Leishmaniasis has become a severe problem for tourism and industrial development in several countries and represents a significant public health problem, mainly in rural or peri-urban areas. There is an urgent need to develop new therapeutic formulations that kill parasites more efficiently and represent less toxicity to humans. There also needs to be an affordable and more straightforward method to administer to patients affected by this medical condition. Therefore, it is imperative to identify new compounds with leishmanicidal potential. Our work represents the first report of leishmanicidal activity of an equimolar mixture of 5α-chol and (+)-4-chol tested in vitro. Our results showed a time-dependent synergistic effect when combining 5α-chol with (+)-4-chol. The equimolar mixture registered higher leishmanicidal activity against LCL and DCL promastigotes and amastigotes of L. mexicana than sterols tested individually or the reference drug. The synergistic effect was more effective in amastigotes compared to promastigotes. This consideration is essential since amastigotes constitute the parasite form found in patients with chronic infections. On the other hand, the high therapeutic index of the equimolar mixture of sterols suggests that it may be safe for its use in in vivo studies. Some reports showed that some phytochemical sterols such as clerosterol effectively kill L. chagasi (Sartonelli et al. 2007). Our previous work (Pan et al. 2012) showed killing activity of cholesta-4,20,24-trien-3-one, 24-methylcholesta-4,24 (28)-dien-3-one, 6,7-dihydroneridienone, and neridienone against L. mexicana. However, the IC50 in these reports is considerably higher than the mixture tested in this work. Also, there are reports of a higher IC50 of 7α, β-aminocholesterol, and 7β-aminomethylcholesterol and the mixture of β-sitosterol and stigmasterol. These compounds were tested against L. donovani (Bazin et al. 2006) and L. chagasi (Silva et al. 2014).
Ergosterol and its derivatives are the main components of the Leishmania plasma membrane. Cholesterol in the parasite membrane needs to be acquired exogenously. These sterols are essential for the permeability and fluidity of Leishmania plasma membrane and can influence its interactions with potential drugs (Torres-Santos et al. 2009; Yao and Wilson 2016). They also serve as precursors and regulators of the life cycle and the parasite development in the host (Bansal et al. 2020). For example, amphotericin B disrupts the osmotic integrity of the cell membrane by interacting primarily with ergosterol (Purkait et al. 2012). In this work, we demonstrated that 5α-chol and (+)-4-chol inhibit the multiplication of L. mexicana, and when mixed, this action potentiates. As for the mechanism of action of these two sterols, we can speculate that they can replace endogenous sterols of parasite cell´s membrane, causing membrane disruption and parasite death. Another field to explore is the mitochondrial damage related to apoptosis or programmed cell death of parasites, possibly through metacaspase activation. It is also possible that different types of steroid receptors in the macrophage membrane could be activated more effectively by the mixture tested in this work. This fact would lead to more efficient parasite killing through different biochemical pathways. These contentions are only speculative, and additional studies are required to focus on the cellular and biochemical mechanism of action of sterols studied in this work. Finally, the possible use of phytochemical sterols such as mycosterols or synthetic sterols for pharmacological treatment of human leishmaniasis presents severe limitations. In the case of phytosterols, large land areas are required to grow plants in sufficient quantities. Costly infrastructure will also be needed to produce enough mycosterols containing biomass to extract the active compound in significant quantities. This situation implies investing a considerable amount of financial capital for a neglected disease. Pharmaceutical companies do not consider this investment a sound investment. In the case of industrial chemical synthesis, the cost of this process will depend on the complexity of the sterol structure and would be costly. One possibility to abate this cost will be identifying affordable and commercially available chemical compounds with leishmanicidal potential. This case happens with the equimolar mixture 5α-chol and (+)-4-chol. Our in vitro studies showed that the equimolar mixture at low concentrations turned out to be an excellent leishmanicidal agent. It merits serious consideration as a future promise for treating cutaneous leishmaniasis. However, in vivo studies in animals are required to complete the pre-clinical phase of drug development prior to a clinical trial in humans.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Bansal R, Sankar Sen S, Muthuswammi R, Madhubala R (2020) Stigmasterol as a potential biomarker for amphotericin B resistance in Leishmania donovani. J Antimicrob Chemother 75:942–950. https://doi.org/10.1093/jac/dkz515
Bazin MA, Loiseaub PM, Boriesb C, Letourneuxc Y, Raulta S, El Kihel L (2006) Synthesis of oxysterols and nitrogenous sterols with antileishmanial and trypanocidal activities. Eur J Med Chem 41:1109–1116. https://doi.org/10.1016/j.ejmech.2006.03.033
Croft SL, Olliaro R (2011) Leishmaniasis chemotherapy—challenges and opportunities. Clin Microbiol Infect 17:1478–1483. https://doi.org/10.1111/j.1469-0691.2011.03630.x
de Menezes JP, Guedes CE, Petersen AL, Fraga DB, Veras PS (2015) Advances in development of new treatment for Leishmaniasis. Biomed Res Int 2015:815023. https://doi.org/10.1155/2015/815023
Drugs for Neglected Diseases Initiative (DNDi) (2021) Cutaneous leishmaniasis. https://dndi.org/diseases/cutaneous-leishmaniasis/. Accessed 2 January 2021
Foglieni C, Meoni C, Davall AM (2001) Fluorescent dyes for cell viability: an application on prefixed conditions. Histochem Cell Biol 115(3):223–229. https://doi.org/10.1007/s004180100249
Ghorbani M, Farhoudi R (2017) Leishmaniasis in humans: drug or vaccine therapy? Drug Des Devel Ther 12:25–40. https://doi.org/10.2147/dddt.s146521
Ghosh P, Ghosh A, Mandal A, Sultana SS, Dey S, Pal C (2016) Oxysterols: synthesis and anti-leishmanial activities. Steroids 107:65–73. https://doi.org/10.1016/j.steroids.2015.12.020
Isaac-Márquez AP, Lezama-Dávila CM (2020) Cytokine regulation of female-macrophage resistance to Leishmania mexicana parasites. Role of IL-12p70. Parasite Immunol. https://doi.org/10.1111/pim.12685
Isaac-Márquez AP, Talamás-Rohana P, Galindo-Sevilla N, Gaitan-Puch SE, Díaz-Díaz NA, Hernández-Ballina GA, Lezama-Dávila CM (2018) Decanethiol functionalized silver nanoparticles are new powerful leishmanicidals in vitro. World J Microbiol Biotechnol 34:38. https://doi.org/10.1007/s11274-018-2420-0
Leliebre-Lara V, Monzote Fidalgo L, Pferschy-Wenzig EM, Kunert O, Nogueiras Lima C, Bauer R (2016) In vitro antileishmanial activity of sterol from Trametes versicolor (Bres. Rivardens). Molecules 21:1045. https://doi.org/10.3390/molecules21081045
Lezama-Dávila CM, Isaac-Márquez AP, Kapadia G, Owens K, Oghumu S, Beverley S, Satoskar AR (2012) Leishmanicidal activity of two naphthoquinones against Leishmania donovani. Biol Pharm Bull 35:1761–1764. https://doi.org/10.1248/bpb.b12-00419
Lezama-Dávila CM, Pan L, Isaac-Márquez AP, Terrazas C, Oghumu S, Isaac-Márquez R, Pech-Dzib MY, Barbi J, Calomeni E, Parinandi N, Kinghorn DA, Satoskar AR (2014) Pentalinon andrieuxii root extract is effective in the topical treatment of cutaneous leishmaniasis caused by Leishmania mexicana. Phytother Res 28:909–916. https://doi.org/10.1002/ptr.5079
Lezama-Dávila CM, McChesney JD, Bastos JK, Miranda MA, Tiossi RF, da Costa JC, Bentley MV, Gaitan-Puch SE, Isaac-Márquez AP (2016) A new antileishmanial preparation of combined solamargine and solasonine heals cutaneous leishmaniasis through different immunochemical pathways. Antimicrob Agents Chemother 60:2732–2738. https://doi.org/10.1128/aac.02804-15
Messaritakis I, Mazeris A, Koutala E, Antoniou M (2010) Leishmania donovani s.l. evaluation of the proliferation potential of promastigotes using CFSE staining and flow cytometry. Exp Parasitol 125:384–388. https://doi.org/10.1016/j.exppara.2010.03.006
Ning Y, Frankfater C, Hsu F-F, Soares RP, Cardoso CA, Nogueira PM, Lander NM, Docampo R, Zhang K (2020) Lathosterol oxidase sterol C-5 desaturase deletion confers resistance to amphotericin B and sensitivity to acidic stress in Leishmania major. MSphere 5:e00380-20
Oficial Mexicana NOM-062-ZOO-1999 Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. DOF: 12/12/2001
Okwor I, Uzonna J (2016) Social and Economic Burden of Human Leishmaniasis. Am J Trop Med Hyg 94:489–493. https://doi.org/10.4269/ajtmh.15-0408
PAHO Pan American Health Organization (2020) Leishmaniasis: Epidemiological Report in the Americas. https://iris.paho.org/handle/10665.2/51742. Accessed 30 December 2021
Pan L, Lezama-Dávila CM, Isaac-Márquez AP, Calomeni EP, Fuchs JR, Satoskar AR, Kinghorn AD (2012) Sterols with antileishmanial activity isolated from the roots of Pentalinon andrieuxii. Phytochemistry 82:128–135. https://doi.org/10.1016/j.phytochem.2012.06.012
Ponte-Sucre A, Gamarro F, Dujardin J-C, Barrett MP, López Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B (2017) Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11:e0006052. https://doi.org/10.1371/journal.pntd.0006052
Purkait B, Kumar A, Nandi N, Hasan Sardar A, Das S, Kumar S, Pandey K, Ravidas V, Kumar M, De T, Singh D, Das P (2012) Mechanism of amphotericin resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother 56:1031–1041. https://doi.org/10.1128/AAC.00030-11
Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA) (2001) Norma Oficial Mexicana NOM-062-ZOO-1999 Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. DOF: 12/12/2001. https://dof.gob.mx/nota_detalle.php?codigo=5405210&fecha=26/08/2015
Sartonelli P, Andrade SP, Melhem MSC, Prado FO, Tempone AG (2007) Isolation of antileishmanial sterol from the fruits of Cassia fistula using bioguided fractionation. Phytother Res 21:644–647. https://doi.org/10.1002/ptr.2131
Silva AAS, Morais SM, Falcao MJC, Vieira IGP, Ribeiro LM, Viana SM, Teixeira MJ, Barreto FS, Carvalho CA, Cardoso RPA, Andrade-Junior HF (2014) Activity of cycloartane-type triterpenes and sterols isolated from Musa paradisiaca fruit peel against Leishmania infantum chagasi. Phytomedicine 21:1419–1423. https://doi.org/10.1016/j.phymed.2014.05.005
Torres-Santos EC, Sampaio-Santos MI, Buckner FS, Yokoyama K, Gelb M, Urbina JA, Rossi-Bergmann B (2009) Altered sterol profile induced in Leishmania amazonensis by a natural dihydroxymethoxylated chalcone. J Antimicrob Chemother 63:469–472. https://doi.org/10.1093/jac/dkn546
WHO (2021) Leishmaniasis. https://www.who.int/es/news-room/fact-sheets/detail/leishmaniasis Accessed 1 January 2021
Yao C, Wilson ME (2016) Dynamics of sterol synthesis during development of Leishmania spp. Parasites to their virulent form. Parasit Vectors. https://doi.org/10.1186/s13071-016-1470-0
Acknowledgements
This study was financially supported by Universidad Autónoma de Campeche (003/UAC/2016). Authors appreciate Mr Rene Gonzales and Eleazar Velasco´s assistance. We are also in debt to Prof Norma Galindo and Prof Patricia Talamás who provided DCL parasites and J774A.1 cells, respectively.
Funding
This research was supported by Universidad Autónoma de Campeche (003/UAC/2016).
Author information
Authors and Affiliations
Contributions
APIM: designed, performed experiments, and wrote manuscript. CMLD: designed, performed experiments, and revised manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The manuscript has been read and approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Isaac-Márquez, A.P., Lezama-Dávila, C.M. In vitro leishmanicidal activity of two cholesterol derivatives. World J Microbiol Biotechnol 38, 66 (2022). https://doi.org/10.1007/s11274-022-03248-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03248-x