Abstract
Root-knot nematodes (RKN) are sedentary parasites of the roots of plants and are considered some of the most damaging pests in agriculture. Since RKN target the root vascular system, they provoke host nutrient deprivation and defective water transport, causing above-ground symptoms of growth stunting, wilting, chlorosis, and reduced crop yields. In Mexico RKN infestations are primarily dealt with by treating with synthetic chemically based nematicides that are preferred by farmers over available bioproducts. However, due to environmental and human health concerns chemical control is increasingly restricted. Biological control of RKNs can help reduce the use of chemical nematicides as it is achieved with antagonistic organisms, mainly bacteria, fungi, other nematodes, or consortia of diverse microorganisms, which control nematodes directly by predation and parasitism at different stages: eggs, juveniles, or adults; or indirectly by the action of toxic diffusible inhibitory metabolites. The need to increase agricultural production and reduce negative environmental impact creates an opportunity for optimizing biological control agents to suppress nematode populations, but this endeavour remains challenging as researchers around the world try to understand diverse control mechanisms, nematode and microbe life cycles, ecology, metabolite production, predatory behaviours, molecular and biochemical interactions, in order to generate attractive products with the approval of local regulatory bodies. Here, we provide a brief review of the biology of the genus Meloidogyne, biological control strategies, and a comparison between chemical and bioproducts in the Mexican market, and guidelines emitted by national agencies to ensure safety and effectiveness of new developments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematodes are a diverse group of animals that inhabit all ecosystems; it is considered that as many as one million cosmopolitan species exist (Mitreva et al. 2005). Many are free-living, but others have developed parasitic lifestyles (Singh et al. 2021). It is estimated that all vertebrates (including humans) are parasitized at some point in their existence (Brooker 2010). Plants are also parasitized, with over 4100 nematode species described (Decraemer et al. 2006). Phytoparasitic nematodes are found in almost any agricultural crop throughout the world, reducing the production and quality of the crops and thus, causing important economic losses. According to the American Society of Phytopathology, it is estimated that worldwide economic impact due to nematode infestations reaches 14% of all crop yield losses, which is equivalent to almost 125 billion dollars annually (Chitwood 2003). From an economic point of view, root-knot (Meloidogyne spp.) and cyst nematodes (Heterodera spp. and Globodera spp.) are the most important crop-damaging pest nematodes (Jones et al. 2013). They can also be vectors of viruses and affect beneficial plant microbiota (Khan 1993). Nematodes spread by infested seedlings and seedbeds, or by contaminated irrigation water, and the symptoms they produced are non-specific, typically manifesting as yellowing of the leaves, retarded development, and significantly low crop yields. Nematodes are also difficult to detect as they are colourless and microscopic (0.5 mm long by 20 μm wide), confounding producers who might not apply proper measures for their control (Siddique and Grundler 2018).
Meloidogyne is a genus of obligated plant parasites with species distributed worldwide, with an ability to infect almost every vascular plant, both under protected agriculture, greenhouses or in the field. Major Meloidogyne species are M. arenaria, M. incognita, M. javanica, and M. hapla (Wesemael et al. 2011; Jones et al. 2013; Coyne et al. 2018). Although it has a broad host crop selection, the most economically important are: soybean, cereals, tomato, potato and other solanaceous and tubercules (Trudgill and Blok 2001; Charchar et al. 2008; Wesemael et al. 2011; Sikandar et al. 2020).
Biology of the genus Meloidogyne
Meloidogyne is a genus of RKN listed number one on the top ten plant-parasitic nematodes by the scientific community in 2013 (Jones et al. 2013). Approximately, 100 species of RKN belong to Meloidogyne, which given its economic importance is also one of the most studied (Sikandar et al. 2020). Despite the challenges posed by its obligate biotrophic nature, Meloidogyne research encompasses all aspects of its existence: evolution, development, virulence, and plant responses to invasion (Curtis 2007; Ali et al. 2017; Ibrahim et al. 2019). Although transformation techniques have been so far unsuccessful, interference RNA and omics tools have shed light on Meloidogyne molecular mechanisms of its life cycle. Da Rocha et al. (2021), recently reported the parasitism regulatory landscape of Meloidogyne obtained by a thorough transcriptomic analysis at different developmental stages. Meloidogyne research has also benefited from our ample knowledge of Caenorhabditis elegans (a well-studied model organism). Although Meloidogyne and C. elegans diverged more than 500 million years ago, genome microsynteny between the two indicates shared developmental and biochemical pathways (Opperman et al. 2008). WormBase: a comprehensive resource for nematode research originally developed by Harris et al. (2010) for C. elegans, now contains information on parasitic nematodes, including more recent sequences of different Meloidogyne species (https://parasite.wormbase.org/index.html). Since M. hapla (54 Mb) and M. incognita (86 Mb) genomes were sequenced in 2008 (Abad et al. 2008; Opperman et al. 2008), another 19 genome drafts representing six species have been determined, allowing phylogenetic and genomic comparisons (https://www.ebi.ac.uk/ena/browser/view/PRJNA340324) (Mitreva et al. 2005; Lunt et al. 2014).
Reproduction
Most Meloidogyne species reproduce asexually, and despite this being considered an evolutionary dead-end, Meloidogyne is a genus well-adapted to fluctuating environmental conditions, and thus, an attractive model for evolutionary analysis (Castagnone-Sereno et al. 2019). Asexual reproduction occurs either by miotic reduction and subsequent reestablishment of the chromosome number by fusion of the second polar nucleus with the egg pronucleus (for instance in M. chitwoodi, M. exigua, M. fallax and M. hapla), or by parthenogenesis (apomixis), for instance in M. arenaria, M. incognita and M. javanica (Castagnone-Sereno and Danchin 2014). Paradoxically, true asexuality correlates with the most successful evolution of Meloidogyne in terms of its global ubiquity and broad host range, infecting almost all Angiospermae. Asexual reproduction can however epigenetically produce males under stressing environmental conditions, but the sperm nucleus degrades upon female insemination (Baniya et al. 2021). A few minor Meloidogyne species (M. carolinensis, M. megatyla, M. microtyla, M. pini) are sexual with males fertilizing the eggs (Eisenback and Triantaphyllou 2020).
Meloidogyne life cycle
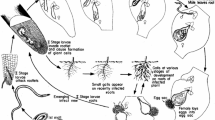
Females deposit ~ 500 eggs into a gelatinous matrix produced by 6 anal glands. The matrix is mainly composed of high and low molecular weight glycoproteins that protect the eggs and function as a temperature and humidity sensor for developmental progress, which is arrested by drought that reduces its volume and hardens the matrix. It also serves as an antimicrobial agent able to agglutinate invading soil microorganisms (Fig. 1). In order to create a canal through which egg masses are pushed outside an enlarged root (gall), it is thought that the plant cell wall is digested. Vieira et al. (2011) identified a carbohydrate binding domain (CBM) in the vulval secretion that might serve this purpose. The transitions from egg to adult throughout consecutive moults takes 25 to 30 days. The first moult occurs before hatching after vermiform stage-1 juveniles (J1) become stage-2 juveniles (J2). This second stage includes a pre-parasitic mobile phase (ppJ2) in the period between hatching, soil migration, root penetration, and establishing a feeding site within the host vasculature, where sedentarism commences, acquiring the parasitic stage (Fig. 1). The next two stages (J3 and J4) are identified by the number of the outer cuticles from previous moults and non-functional stylet; J4 is the stage at which sexual dimorphism distinguishes female and male nematodes. Sedentary mature females resume feeding and swell into a pear-shape and produce eggs. Differential gene expression accompanies these transitions: for instance, upregulated expression of sensory perception and cell wall degradation genes happens from eggs to ppJ2, whereas stress response genes (possibly for contending with plant defences) are upregulated between J2 to J3/J4 and sensory perception genes decrease their expression as the nematode becomes sedentary. Lipid metabolism-related genes are also upregulated at J3/J4 in preparation for the energetically costly adult phase. Mature females without the need for locomotion repress genes for this purpose. Gene regulation in the egg is dominated by developmental processes and includes membrane transport and DNA metabolism. Knowing the gene expression patterns of RNK provides strategies for the rational selection of target genes, and although variable results have been obtained, some genes have been identified through RNA silencing resulting in lower infestation levels (Iqbal et al. 2020).
Life cycle of Meloidogyne. a preparasitic stage-2 juveniles migrate in the soil following plant cues gradients (sugars, amino acids, pH, etcetera); once a susceptible host is found J2 mechanically enter the root moving downwards to avoid the Casparian strip and then up to the vascular cylinder, in which an unknown determinant indicates the establishment site. b Settlement of nematodes J3 to J4 and reprogramming of target cells that will enlarge becoming giant cells that will feed the nematode, one at a time. c As nematodes become adults, enlarged females produce egg masses on the root surface. Figure created with BioRender
Host selection and invasion
Host parasitism by Meloidogyne varies among species, with some infecting a broad range of vegetable crops, ornamental plants, and fruit trees (for instance M. incognita, M. javanica and M. chitwoodi); others grow in restricted geographical regions or are restricted to fewer hosts, such as M. hispanica that infects peach and tomato. In the soil, hatched ppJ2 swim randomly without feeding until they detect a susceptible root by following chemotactic gradients of plant exudates including amino acids, sugars, CO2, and pH. Time of soil residence of ppJ2 juveniles is species-specific, and dependent on their lipid reserves, which when below 65% hinder invasion. When J2s reach the root tip of a suitable host, they access the region of root elongation near the meristematic region by mechanically perforating the least resistant site with their small and delicate stylet. To reach the stele, Meloidogyne first migrates down to the direction of the root tip to avoid the barrier imposed by the Casparian strip (composed of highly lignified and suberized endodermal cells), and moves up to the root differentiation zone where xylem elements are visible, in which they anchor to the central cylinder (von Mende 1997; Holbein et al. 2019). For their journey, Meloidogyne soften the middle lamella by secreting modifying enzymes (such as expansin-like proteins, cellulases, hemicellulases and pectinases) produced in the subventral glands (Vieira et al. 2011; Mitsumasu et al. 2015). This subset of enzymes is encoded by genes acquired laterally (LGT) from bacteria. M. incognita for instance contains more than 60 genes from six different protein families for the digestion of cell wall oligo- and polysaccharides (Abad et al. 2008). Paganini et al. (2012), estimated that up to 3.4% of the RKN genes might have been acquired via LGT, with pathogenic bacteria (Ralstonia solanacearum, Xanthomonas campestris), symbionts (Sinorhizobium meliloti) or rhizobacteria (Burkholderia ambifaria), likely being some of the donor organisms (Danchin et al. 2010; Paganini et al. 2012). Interestingly, intercellular migration causes limited tissue damage.
Feeding sites (giant cells)
Giant cells (GCs) are reprogramed cells induced by RKN effectors (reviewed by Haegeman et al. (2012)), which serve a nourishing function for the sedentary phase of the parasite. Molecular determinants of the selection of the feeding site remain unknown but must locate to the vascular cylinder at the differentiated region of the root, where five to eight parenchymal cells are selected. Through successive induction of mitosis and endoreplication in the absence of cytokinesis these cells enlarge. Each GC will feed, one at a time, the female in its progression from J2 to adult (Escobar et al. 2015). GCs are metabolically very active, with dense cytoplasm, a large number of organelles and expanded nuclei and nucleoli. Cell fate of GCs is epigenetically regulated by differential expression of host micro RNAs (Jaubert-Possamai et al. 2019), DNA hypomethylation (Atighi et al. 2020), and histone modification (Hassanaly-Goulamhoussen et al. 2021), which together establish a new balance of growth hormones (e.g. auxin/cytokinin), cell cycle progression, and suppression of jasmonic acid-dependent immune responses via gibberellin signalling (Hewezi 2020). Simultaneously, galls form around GCs by increased cell proliferation of the vascular system and hypertrophy of the endodermis and the cortex; galls along the root give the infection its common name of root-knots. Gall organogenesis involves root apical meristem reprogramming for transient pluripotency, quiescent centre identity and procambium-associated increase of genes and regulators in a nematode-dependent manner, which also brings about changes in the cell wall composition that consistent with the dedifferentiated state, they contain more galactose and less xylose (Ishida et al. 2020; Olmo et al. 2020).
Strategies of nematode control
A wide variety of chemical pesticides and other management tools are available for crop growers to control phytoparasitic nematodes, but none is efficient in the sense of efficacy against new nematode biotypes, nematode resistance and adaptability, high cost and expense, and environmental safety (Chitwood 2003). As current options become non-sustainable, new environmentally friendly and sustainable strategies for nematode control must be developed, as this is a key component for sustainable food safety and enhancing the quality of life in a growing world population (Barker et al. 1994). The activity of bioactive agents (microorganisms) contributes to the suppression of populations of phytopathogenic nematodes, whether through: (i) antibiosis or competition for rhizosphere colonization by the production of toxic compounds. Two examples are: hydro-soluble compounds fervenulin, avermectin and nemadectin isolated from different strains and species of Streptomyces with activity against M. incognita [preprint by Hu et al. (2021)], and iturin, surfactin, and fengycin produced by Bacillus strains (Ramyabharathi et al. 2020). (ii) Plant growth promotion and plant induced resistance (IR). Plant resistance to infection by RKN involves treatment with inducing agents (microorganisms or metabolites) that prepare the plant for a prospective RKN infection, during which defence responses would be expected to occur faster or at a higher level compared to non-treated crops (Walters et al. 2005; Hilker and Schmülling 2019). Upon RKN detection plant responses include a burst of reactive oxygen species (ROS), cell signalling through the salicylic acid (SA), ethylene (ET) and jasmonic acid (JA) pathways, upregulation of pathogenesis-related protein genes (PR), and defensin and antimicrobial peptide coding genes (Przybylska and Obrępalska-Stęplowska 2020). (iii) Predation and parasitism, which is carried out by natural enemies of nematodes, including bacteria, fungi, mites, and other predatory nematodes (Khan and Kim 2007; Yang et al. 2020). All mechanisms are illustrated in Fig. 2, and examples of particular cases from the abundant literature on this topic are provided in the following section.
Biocontrol strategies against RKN by different organisms. a Attack by spores and various toxic and lethal metabolites (represented as symbols around the nematode) released by fungi, bacteria, and plants. b Predatory nematodes feeding on parts of the body of RNKs. c Nematodes that become entangled in a sticky web of mycelium and fungal constrictor rings. d Immobilized nematodes by fungal toxins and colonized with ingested or attached fungal spores that germinate and invade the interior and exterior of the nematode. e Fungi that parasitize adult females in the reproductive stage or eggs of nematodes. Figure created with BioRender
Biological control
Control of nematodes by bacteria
Plant growth-promoting bacteria (PGPB) establish close associations with plants and can enhance plant growth and protection from disease and abiotic stress (de Souza et al. 2015; Gupta et al. 2015). The biological control potential of PGPB against phytoparasitic nematodes has been analysed in species belonging to Agrobacterium, Arthrobacter, Azotobacter, Clostridium, Desulfovibrio, Pasteuria, Serratia, Burkholderia, Azospirillum, Bacillus, Chromobacterium, Pseudomonas, and Corynebacterium (Jatala 1986; Migunova and Sasanelli 2021). Bacillus and Pseudomonas are of particular interest, as these widely occur in natural environments, are currently used and commercialized, and in the last two decades they have shown the highest efficacy for biological control (Berlitz et al. 2014; Meena 2014; Dehghanian et al. 2020; Migunova and Sasanelli 2021).
The use of strains belonging to the genus Bacillus as biological control agents is increasing, as this is a widely occurring genus, abundant in the rhizosphere. Beneficial activity of some Bacillus strains has been well studied, and various molecular mechanisms of nematode control have been described (Ongena and Jacques 2008; Lugtenberg and Kamilova 2009; Sivasakthi et al. 2014). The potential of Bacillus as bionematicides is based on the production of nematocidal proteases and chitinases, antibiotics, crystal proteins, secondary metabolites, and the induction of plant systemic resistance (Engelbrecht et al. 2018).
For example Bacillus cereus produces C16 sphingosine and phytosphingosine, two nematocidal compounds that induce reactive oxygen species (ROS) in M. incognita, destroying its genital area, and thus, inhibiting nematode reproduction (Gao et al. 2016). Sphingosine is safe for the environment, humans, and animals, but it is very toxic to nematodes with a nematocidal LC50 of 0.64 μg/ml, making it a safe and effective agent. Gao et al. (2016), also analyzed the plant defense-related enzymes phenylalanine ammonia-lyase (PAL), polyphenol oxidase (PPO), and peroxidase (POD) in tomato treated with B. cereus S2, and found increases of 43.8%, 51.8%, and 86.2%, respectively, when compared to the untreated control. This indicates that B. cereus also activates plant defence systems for controlling M. incognita. Additionally, Geng et al. (2016) identified multiple potential nematocidal factors from the genome of B. firmus DS-1, but focused on a peptidase S8 superfamily protein Sep1 (sep1). Authors demonstrated that B. firmus DS-1 is toxic against C. elegans and M. incognita due to serine protease activity and degradation of the nematode intestinal tissues. Another report detected genes associated with nematocidal activity in B. subtilis OKB105, since it controls M. javanica through the activity of the purL gene (phosphoribosylformylglycinamidine synthase, FGAM synthase). Nematocidal activity was lost by disruption of the purL gene and restored when complemented with either plasmid pMA5-purL or pUC18-purL, demonstrating a role for this gene in mediating nematocidal activity (Xia et al. 2011). Bacillus thuringiensis is a well-known biocontrol agent, characterized by the production of crystal proteins, encoded by cry genes. These toxins have insecticidal and nematocidal activity. Several studies have identified that B. thuringiensis Cry toxins (e.g., Cry6A, Cry5B, Cry1Ea11, Cry1Ab, Cry14Ab) can control a wide spectrum of nematodes, among them Meloidogyne spp., Bursaphelenchus xylophilus, Heterodera glycines, and C. elegans by inhibiting egg hatching and killing stage-2 juveniles (Khan et al. 1995; Höss et al. 2008; Radhakrishnan et al. 2017; Huang et al. 2018; Kahn et al. 2021; Migunova and Sasanelli 2021). Bacillus nematocida has a peculiar nematocidal mechanism, luring nematodes to their death using a “Trojan horse” strategy. The attraction is mediated by potent volatile organic compounds such as benzaldehyde and 2-heptanone, which makes this species more attractive to worms than other dietary bacteria (Niu et al. 2010). Once the bacterium is consumed by nematodes, it secretes extracellular proteases, such as alkaline serine protease Bace16 (bace16 gene), to attack the host intestinal tissues, eventually killing it (Niu et al. 2010). B. megaterium strain YMF3.25 is an efficient biocontrol agent against M. incognita. Huang et al. (2010) identified nematocidal volatile metabolites produced by this bacterium (benzeneacetaldehyde, 2-nonanone, decanal, 2-undecanone, and dimethyl disulphide) that are active against both, juveniles and eggs at 0.5 mmol. Finally, secondary metabolites (such as lipopeptides) produced by Bacillus are of great interest. These are low-molecular-weight amphipathic compounds with antimicrobial properties (Ongena and Jacques 2008). Strains of B. subtilis were tested for their nematocidal activity against M. incognita, and strain Bs5, with biosynthetic capability for surfactin and iturin, was promising in reducing hatching of M. incognita eggs. Crude antibiotics extracted from strain Bs5 exerted lethal effects on eggs and juveniles (Kavitha et al. 2012). Similarly, B. subtilis strain Bbv57 also produced surfactin and iturin, and the presence of the ItuD, srfA, and sfp genes was experimentally confirmed by PCR and chromatography of the products. Application of crude antibiotics obtained from bacterial cultures at concentrations of 25, 50, and 100% in in vitro assays to egg masses of M. incognita or stage-2 juveniles, showed that at the highest concentration egg hatching was 92% reduced and juveniles mortality increased by 87% in comparison with untreated controls (Ramyabharathi et al. 2018). Beneficial microorganisms can trigger resistance in plants, and this is well studied for several Bacillus spp. (Shafi et al. 2017).
Fluorescent pseudomonads (Pseudomonas fluorescens, P. putida, P. aeruginosa, and P. aureofaciens) are effective against certain insects and nematodes. Molecular mechanisms of some Pseudomonas strains to reduce nematode populations include production of metabolites and induction of plant systemic resistance (Meena 2014). Cronin et al. (1997) evaluated strain Pseudomonas fluorescens F113 as a biological control agent against the potato cyst nematode Globodera rostochiensis, and found that it controlled mobile nematode juveniles by producing secondary metabolites such as 2,4-diacetylphloroglucinol (DAPG). Dehghanian et al. (2020) examined the induction of tomato resistance against M. javanica, as well as changes in the expression of plant defense gene PR1 that codes for protein PR1 (involved in the protection towards pathogenic fungi and oomycetes) after applying salicylic acid (SA) and P. fluorescens strain CHA0. The combined treatment slightly improved plant growth, and reduced egg-masses by 92% and the reproduction factor of M. javanica by 11-fold compared to the untreated controls. Meanwhile PR1 gene showed a two-fold increase in relative expression after the combined treatment of P. fluorescens + SA and nematode addition to the pots; however, PR1 increased expression was transitory returning to basal levels 48 h after nematode inoculation.
The Pasteuria genus is of particular interest as these are obligate, mycelial, endospore-forming bacterial parasites of phytoparasitic nematodes (Tian et al. 2007). The spores of Pasteuria spp. attach to the cuticles of stage-2 juveniles of RKNs and germinate after entering the roots and begin feeding. Germ tubes can penetrate the cuticle, and vegetative microcolonies then form and proliferate inside the body of the developing female; due to reproductive system degeneration, the adult female is almost devoid of eggs (Jatala 1986; Gowen et al. 2007; Tian et al. 2007). Bhuiyan et al. (2018) established an experiment where sugarcane was grown in pasteurized sand containing different concentrations of endospores of P. penetrans for the control of M. javanica showing that the severity of root galling and the number of nematode eggs decreased as the endospore concentration increased: at the highest endospore level, egg numbers decreased 96%. Other studied bacteria include Serratia proteamaculans Sneb 851 that has shown high nematocidal potential against M. incognita with 99% and 61% mortalities for stage-2 juveniles and eggs, respectively (Zhao et al. 2018). Commercial products containing Serratia sp. were tested (Nemaless = S. marcescens and Nemafree = Serratia sp.) against RKN M. incognita, and results showed a reduction in the number of stage-2 juveniles, egg masses, egg numbers, and low reproduction factor (Raddy et al. 2013). Additionally, Rhizobium etli G12 suppresses early infection of Globodera pallida and M. incognita (Hallmann et al. 2001). Other bacteria-based products include Nortica (B. firmus), Econem (species of Pasteuria) and Sudozone (P. flourescens Schroeter) (Abd-Elgawad and Vagelas 2015).
Control of RKN by nematophagous fungi
Most research on different biological control agents against phytoparasitic nematodes is focused on nematophagous fungi (Bilgrami et al. 2008). Some species of the genus Trichoderma (Sharon et al. 2001; Al-Hazmi and TariqJaveed 2016; Fan et al. 2020) have been studied for these purposes, but other genera have also shown high nematocidal activity, for example Dactylella, Arthrobotrys, Nematoctonus, Aspergillus, Penicillium, Pochonia, Paecilomyces, Metarhizium and Verticillium (Sánchez Portillo et al. 2016; Thongkaewyuan and Chairin 2018; Peiris et al. 2020; Naz et al. 2021). Nematophagous fungi employ diverse mechanisms to control nematode populations, such as: (i) nematode-trapping (predatory) fungi produce extensive hyphal networks and constricting rings as trapping devices to catch nematodes; (ii) endoparasitic fungi are obligate parasites that infect nematodes by either adhering to their surface or through direct ingestion followed by germination, growth, and nematode killing; (iii) egg-and female-parasitic fungi, which as facultative parasites grow on and parasitise the sedentary stages of nematodes such as eggs and cysts; and (iv) production of toxins that immobilise the nematodes before hyphal penetration through the nematode cuticle (Jatala 1986; Lopez-Llorca et al. 2007; Zhang et al. 2020). Most of these fungi are facultative saprotrophs, meaning that in the absence of nematodes, they feed on decomposing organic matter, and therefore soils rich in organic matter promote their persistence (Lopez-Llorca et al. 2007). Representative species of nematophagous fungi from diverse taxa, are Arthrobotrys oligospora and Drechslerella sp. (nematode-trapping fungi); Metacordyceps chlamydosporia (≡Pochonia chlamydosporia), P. rubescens and Purpureocillium lilacinum (≡Paecilomyces lilacinus; egg- and female-parasitic fungi); Drechmeria coniospora (endoparasitic fungus); and Pleurotus ostreatus (toxin-producing fungus) (Lopez-Llorca et al. 2007; Zhang et al. 2020).
Arthrobotrys oligospora is one of the most extensively studied nematophagous fungi and it is considered as the model of the nematode-trapping group (Peiris et al. 2020; Soliman et al. 2021). This fungus acts as a facultative nematode-trapper for nitrogen, but it also decomposes organic matter as a saprophyte as the source of carbon and energy (Cooke 1963; Jaffee 2004). In vitro experiments have demonstrated high efficacy of the fungus in capturing and suppressing M. incognita stage-2 juveniles, and microscopic observations showed that it traps prey with adhesive loops of hyphae; A. oligospora also significantly suppressed root-knot in tomato plants showing 74% predatory activity towards M. incognita in comparison to 36% of the control (Soliman et al. 2021).
Pochonia chlamydosporia is a facultative parasite that first colonizes the rhizosphere as a saprotroph but which, upon encounter with cyst nematode (CN) and RKN, it infects the nematodes eggs, as these are its main target, although stage-2 juveniles of Meloidogyne within egg masses can be colonized and parasitized, and consequently, P. chlamydosporia has been isolated from the nematodes (Manzanilla-López et al. 2013; Peiris et al. 2020). Some isolates of P. chlamydosporia also induce plant resistance against M. incognita by up-regulation of the salicylic acid pathway in tomatoes inoculated with both fungus and nematode. This was assessed in a co-inoculation experiment to determine the expression of genes related to the salicylic acid and jasmonic acid pathways through the study of pathogenesis-related protein 1, PR1, and lipoxygenase, LoxD, respectively. For the experiment, the soil was inoculated with chlamydospores just before transplanting, and with stage-2 juveniles of M. incognita 1 week later. PR1 expression in roots treated with P. chlamydosporia was upregulated at 0, 7, and 42 days after nematode inoculation compared to control plants, and the LoxD gene was upregulated only 7 days after nematode inoculation (Ghahremani et al. 2019). P. lilacinus is another egg nematode parasite currently used as a biological control agent against various nematodes (Khan et al. 2006). Commercial P. lilacinus strain 251 (PL251) was evaluated for its potential to control M. incognita in tomatoes, showing reduction of root galling by 66%, number of egg masses by 74%, and final nematode population by 71% compared to the non-inoculated control (Kiewnick and Sikora 2006). The main mechanism of action of P. lilacinus is direct infection of sedentary stages, in particular the egg stage, through the production of leucinotoxins, chitinases, proteases, and acetic acid (Djian et al. 1991; Khan et al. 2004).
The endoparasitic fungus D. coniospora is an obligate parasite and mostly exists as conidia in the environment. This species infects nematodes via the adhesion of conidia to the host cuticle, which upon germination produces an appressorium to pierce the cuticle and extend hyphae into the nematode epidermis (Lebrigand et al. 2016). Conidia of D. coniospora adhere to different phytoparasitic nematode species, such as Ditylenchus spp., Pratylenchus penetrans, Cephalenchus sp. and Heterodera schachtii, but conidial attachment to a particular nematode species does not always lead to infection, as specific recognition signals are required (Jansson 1993; Lebrigand et al. 2016; Zhang et al. 2020).
Toxin-producing nematophagous fungi have nematode-immobilizing activity that can also kill their hosts. A particular example of this predatory mechanism is represented by the basidiomycete oyster mushroom P. ostreatus that preys on nematodes to supplement nitrogen intake under nutrient-limiting conditions. Recent studies have shown that P. ostreatus triggers a massive calcium influx and rapid cell necrosis in the neuromuscular system of C. elegans via nematode sensory cilia; it was also effective in paralyzing a diversity of other species of the genera Diploscapter, Oscheius, Rhabditis, Pristionchus, Panagrellus, Acrobeloides, Cephalobus, Mesorhabditis, and Pelodera, which after immobilisation, hyphal growth and penetration, the nematodes were subsequently digested (Satou et al. 2008; Lee et al. 2020). Fewer studies have been conducted with oyster mushrooms as a biological control agent of phytoparasitic nematodes, but they have shown potential for controlling sugar beet nematode Heterodera schachtii and the M. incognita in cowpea (Palizi et al. 2009; Youssef and El-Nagdi 2021).
Control of nematodes by predatory nematodes
As biological control agents against phytoparasitic nematodes, predatory nematodes can be applied, but this is challenging because their biology, behaviour, feeding preferences, prey relationships, and other ecological parameters are important to consider for evaluation of their potential. Predatory nematodes belong to the orders Mononchida, Diplogasterida, Rhabditida, Aphelenchida; superfamilies Dorylaimoidea, Nygolaimoidea, Actinolaimoidea; and families Ironidae, Oncholaimidae, Monohysteridae, and Thalassogeneridae. They have different types of feeding apparatus, modes of searching and catching prey, and feeding mechanisms (Bilgrami 2008; Roy and Borah 2020), which serve for classification in three categories: (i) some feed by cutting the prey body and sucking its contents, as they are unable to engulf intact prey (Diplogasterida); (ii) others feed by a combined action of cutting and sucking and occasionally engulfing a whole prey (Mononchida); and, (iii) those which feed only by puncturing the prey cuticle and sucking out the body contents (suborders Dorylaimina, Aphelenchida, and Nygolaimina) (Roy and Borah 2020).
From the biological control application perspective Diplogasterid predatory nematodes have some advantages over other predatory nematodes. As reported by Siddiqi et al. (2004) and Bilgrami et al. (2005) diplogasterid predators are easy to culture in vitro, have high rates of reproduction and predation, have short life cycles, and can detect and respond to prey attractants (showing rare cannibalism), while juveniles possess great tolerance to unfavourable environmental conditions. The first field release of a diplogasterid predator Mononchoides gaugleri was performed to determine its effect on existing parasitic nematode populations in turfgrass fields, with a 24.3% reduction of the phytoparasitic nematode population, although unfortunately the rate of predator persistence was low (Bilgrami et al. 2008).
Members of Mononchida have probably received the most attention as possible candidates for control of phytoparasitic nematodes (Jatala 1986), but these are non-specific predators and exert only partial control (Bilgrami 2008). On the other hand, Iotoncus kherai preys on phytoparasitic nematodes M. incognita, Hirschmanniella oryzae, and Rotylenchulus reniformis (Mohandas and Prabhoo 1980). Small, (1979) reported that predatory nematodes Prionchulus punctatus had a significant influence on the M. incognita-induced galling, reducing the number of females. P. punctatus was also effective in the reduction of populations of G. rostochiensis and stage-3 juveniles of Rotylenchus fallorobustus (Small and Grootaert 1983; Bilgrami 2008).
In addition to nematodes, predators Dorylaimina, Aphelenchina and Nygolaimina switch to feeding on bacteria and fungi, which presumably enhances their survival when prey nematodes are scarce (Bilgrami 2008). A comparative study of the predation by Allodarylaimus americanus and Discolaimus silvicolus on different species of phytoparasitic nematodes showed that both species preferred stage-2 juveniles of M. incognita, Anguina tritici, Hetherodera mothi, and Tylenchulus semipenetrans over other nematodes (Khan et al. 1995).
Control of nematodes by microbial consortia
As mentioned before, biological control agents against phytoparasitic nematodes possess a wide range of mechanisms and modes to exert control, combining different strategies by consortia for effective and optimal bioactivity. Synergistic interactions and results at least similar to the most effective control agent used individually, are expected with consortia containing different mechanisms of control (Xu and Jeger 2013). The application of a consortium that includes bacterial and fungal nematode-antagonists is one of the most promising methods. Combined applications of Fusarium oxysporum non-pathogenic strain 162, egg pathogen P. lilacinus 251, and antagonistic bacteria B. firmus were evaluated to assess control of Radopholus similis in banana plants; mixtures of F. oxysporum and P. lilacinus caused a 68.5% reduction in nematode density and combined application of F. oxysporum and B. firmus was the most effective treatment in controlling R. similis on banana (86.2%) compared to the control treatment (Mendoza and Sikora 2009). Similarly, a combination of fungal strains can effectively control a wide range of phytoparasitic nematodes. P. lilacinus and the nematode-trapping fungus Monacrosporium lysipagum were assayed for their ability to reduce the populations of three economically important phytoparasitic nematodes (M. javanica, H. avenae and R. similis), resulting in nematode populations substantially controlled by both individual and combined applications of the fungi (Khan et al. 2006).
Several formulations consisting of two or more bacterial components have been proposed for the control of RKNs (Migunova and Sasanelli 2021). The inoculants Equity (47 strains of bacilli), BioYield (B. subtilis strain GB03 and B. amyloliquefaciens strain GB99) induce significant reductions of M. incognita eggs, juveniles, and galling in tomato (Burkett-Cadena et al. 2008); whereas bioinoculant Micronema (Serratia spp., Pseudomonas spp., Azotobacter spp., B. circulans and B. thuringiensis) caused a significant reduction of stage-2 juveniles, galls and egg masses (97%, 80%, and 88%, respectively) (Youssef et al. 2017).
Biological control strategies for RKNs differ in their efficacy level. A comparison between advantages, disadvantages and compatibility of control strategies is provided in Table 1; while synergistic effects among control agents is presented in Table 2.
Current control strategies of Meloidogyne RKN in Mexico
Being M. incognita a cosmopolitan nematode occurring in various agricultural areas and with the ability to infest more than 2000 plant species including grasses, vegetables, fruit trees and forestry, in Mexico it can also cause damage to commercially important crops such as tomatoes, coffee, eggplant, bananas, beans, papaya, chili, gardenias, cucumbers, and guavas, severely affecting yields and productivity. Infestation with M. incognita is widespread in warm climates, and although the exact distribution of species and races of this pathogen in Mexico is not established, it is known to be the most representative species, accounting for more than 60% prevalence in surveyed states (Cid del Prado Vera et al. 2001); M. incognita is also found in combination with other species occurring in some locations as they occupy the same ecological niche. Although M. incognita is an RKN with a wide distribution and prevalence under different environmental conditions, it is more common in tomato crops in warm climates, whereas M. javanica is more common in temperate regions (Cid del Prado Vera et al. 2001). Field conditions vary widely in different agricultural regions in Mexico, hindering population management with phytosanitary measures.
The Official Mexican Norm (NOM—EM -034-FITO-2000; http://www.dof.gob.mx/nota_detalle_popup.php?codigo=2062881), establishes that the processes of production, harvesting, selection, storage and transportation of fresh fruits and vegetables must be regulated by the Manual of Good Agricultural Practices, considering that the phytosanitary status of these products may be affected or contaminated by biological, chemical and physical elements that may pose a risk to public health if the necessary measures are not implemented. These measures regulate the management of crops from pre-sowing, cleaning of fields and cultivated areas, pest control, farmer hygiene, among others, to the handling, packaging, and transport of final products for national consumption or international export. Before sowing, aeration of the soil and natural disinfection must be promoted. For this purpose, solarization (covering the soil with plastic and exposing it to the sun to reach high temperatures that are lethal to microorganisms) or disinfection with steam is suggested. Similarly, it is recommended to use seeds or propagation materials that are pest-free or resistant to pests, or that have been treated with methods and products effective against plant pests that verify the suitability of the planting material. Irrigation must be conducted with water that is free of microorganisms and avoiding the use of water from ponds or canals, also the washing and decontamination of farm equipment after each use, to name a few measures.
The origin of infections may have multiple causes or arise from different sources, e.g., infected seeds, contaminated vegetative material, contaminated growing soil or substrate, use of contaminated equipment or tools, among others. Damage at the seedling stage is more evident at the time of transplanting at the definitive growing site (FAO, http://www.fao.org/3/v9978e/v9978e05.htm).
To prevent propagation, Mexico follows recommendations from the Food and Agriculture Organization of the United Nations (FAO) to avoid transporting infected seedlings into the field, which consist of inspecting the roots and removing seedlings showing symptoms of nematode infestation at the time of transplanting. Weeds are also recommended to be controlled regularly as many are infested with nematodes and often host RKNs. Fertilization with compost high in organic matter is recommended. To reduce nematode populations, it is advisable to plant trap crops such as marigold (Tagetes spp.) or rattlepods (Crotalaria spp.) before sowing, in rotation or after cultivation.
Genus Tagetes distributes across the American continent and includes more than 50 species, mainly found in Mexico (Kurpis et al. 2019). Mexican culture employs Tagetes for various purposes: antioxidant, medicine, food pigment, flavouring, perfume, resin, ornamental and insecticide; in agriculture it is used as a nematicide, larvicide, attractant or insect repellent, and as fertilizer (Serrato-Cruz et al. 2008). The most used species of Tagetes in pest control are. T. erecta, T. patula, T. minuta and T. filifolia, due to biocidal activity extracted from roots, stems, leaves, inflorescences, or the whole plant. To control nematodes, Tagetes is applied in the form of organic manure to the plants or in the form of aqueous extracts and powders, and it is used in crop rotation. Pyrethrins and thiophenes (such as α-terthienyl) found in marigold roots are responsible for its nematocidal and nematostatic activity (Serrato-Cruz et al. 2008; Hamaguchi et al. 2019).
Rattlepod plants (Crotalaria spp.) for their part are not nematode hosts and are used as a preceding crop or cover crop to suppress nematode populations by disrupting their life cycle (especially at the reproductive stage) (Osei et al. 2010). They can also provide a niche for natural nematode enemies (Wang et al. 2002), or release lethal or toxic metabolites for nematodes.
Currently, the use of chemical products and crop resistant varieties are the most popular strategies to control RKNs in Mexico. However, the risks of chemical nematicides to the environment and human health have led to the discontinuation of several products in some crops (Peiris et al. 2020). Globally, the demand for effective and high-quality natural enemies that can replace chemical pesticides to control phytopathogenic nematodes has increased in the last 20 years (Rodríguez-del-Bosque et al. 2015), but in Mexico, biological control of nematodes is hardly used in farms. As mentioned before, nematode control with microorganisms represents a green solution that in some cases also promotes plant growth, significantly reducing the incidence or severity of various other diseases in different host plants. However, farmers expect products formulated based on bioactive (mainly microbial) agents to have similar properties to chemical pesticides, i.e., high efficacy, long shelf life (2 years on average), ease of application, and preferably without toxicity during application, among others (Perlatti et al. 2013). Therefore, bioactive agents must solve two important problems inherent to their nature: (1) loss of viability/efficacy during transport, storage or after application; and (2) loss of stability within the storage range (− 5 to 30 °C), for both marketers and farmers.
Some disadvantages of bioactive agents (microorganisms or natural extracts) are their low residual activity in the field, mainly due to inactivation by solar radiation, removal by rain, and limited shelf life (Tamez-Guerra et al. 2005), obstacles that in many cases discourage commercialization and use. This is the case of Purpureocillium and Trichoderma species-based products, which are the best-studied and the most used fungal genera for controlling nematodes that can even show improvement in growth and plant performance, but this unique control method cannot eradicate nematodes. Fungi partially control M. incognita and improve plant growth and yield (Peiris et al. 2020); however, on their own, population reduction is only up to 45% compared to untreated conditions, with varying levels of performance. This level of control is lower than chemical products, which can reach efficiencies between 80 and 90% (Jennings 2009). Thus, chemical nematicides remain the first choice for nematode control especially when population thresholds are high. Biological control, on the other hand, is appropriate in soils with low populations, well below the thresholds of nematode population able to affect a crop, which in most cases is quite low [even as low as one RKN/200 g soil for sweet potato (Stirling 2000; Peiris et al. 2020)]. It is a fact that inferior products affect consumer confidence and positive perception towards organic products in general. Many of these commercial products have been labelled "weak products” due to their variable efficacy and questionable quality control (Jenkins and Grzywacz 2003; Montesinos-Matías et al. 2020).
To address this problem, several government agencies are working together to establish programs to control the quality of commercialised products, with the main objective of ensuring that raw materials meet manufacturer's specifications, that production batches and their quality are consistent, and that finished products meet the criteria established for their use. In implementing quality control plans, formulators must follow guidelines without viewing them as a constraint or burden on production processes, but rather as a mechanism to benefit interested parties, i.e., the biological control industry and its customers (Montesinos-Matías et al. 2020).
Mexican regulation guidelines for nematicides
The world market demands that agricultural products are safe for humans (Codex Alimentarius Commission). Therefore, international parameters for maximum levels (ML) of pesticides have been established, and an increasing number of importing countries require certifications of good agricultural practices (GAP) leading to the promotion of the study of biological control alternatives based on microorganisms associated with plants. In Mexico, the Federal Commission for Protection against Sanitary Risks (COFEPRIS in Spanish) is the official body responsible for monitoring the biosafety of agricultural inputs used in the national territory, which can be classified as low-risk due to their pesticide activity and have specificity and safety towards the environment in which they are released. Approval of pesticides for use in agriculture also requires opinions from the Ministry of Health, the Ministry of Agriculture and Rural Development (SADER), and the Ministry of Environment and Natural Resources (SEMARNAT). For its part, the National Service of Agri-Food Health, Safety and Quality (SENASICA) supervises, according to its assignments:
-
1.
That the pesticides for agricultural use to be registered to comply with the characteristics of the application pattern (crop, pest, dose, number, and application intervals), by evaluating their efficacy, and that they can obtain their Biological Effectiveness Opinion.
-
2.
The provision of technical opinions on the biological efficacy of pesticides for agricultural use and on the phytosanitary aspects of the maximum levels (ML) of pesticide residues in the cases specified in the Regulations on Registration, Importation and Export Authorizations (http://www.dof.gob.mx/nota_detalle.php?codigo=5332473&fecha=13/02/2014).
-
3.
That the process of manufacture, formulation, assembly, importation, commercialization, and aerial application of pesticides for agricultural use conforms to phytosanitary and good use specifications, which must be followed in accordance with the technicians' Opinion of Biological Effectiveness (dose, pests, cultivation, expiration).
The following link shows the current list of pesticides approved in Mexico by COFEPRIS: http://siipris03.cofepris.gob.mx/Resoluciones/Consultas/ConWebRegPlaguicida.asp. Supplementary Table 1 lists approved nematicide products currently registered with COFEPRIS. At the time of writing of this paper (October 2021), 43 products with nematocidal activity were registered, 11 of which are synthetic chemical formulations, 13 are products formulated from plant extracts, 17 are bionematicides derived from bacteria and fungi, and two are mixtures of plant extracts and microbial consortia. The proportion of products made from natural compounds including living organisms or extracts, therefore is higher than the proportion of chemical-synthetic products recognised by the national regulatory body (Fig. 3).
Nematocidal products approved by COFEPRIS. a Percentage of product types of different formulations and method of production of commercially available products in the Mexican market. b Type of organisms used in formulations of commercial bionematicides. c Species of organisms present in approved products with nematocidal activity presented as a percentage
Although the most used method of controlling nematodes in Mexico is still chemical-based, and they have not yet being adopted as a complementary solution by Mexican farmers, bionematicides have gained ground in the national market (Supplementary Table 1; Fig. 3); these offer a wider range of products that could help meet the needs of farmers of different crops and whose properties can be adapted to the agricultural conditions of soils in different regions of the country. The availability of several biological formulations indicates a promising future for the use of natural nematode enemies in Mexican major crops, with the hope that they will achieve comparable results to chemical agents when combined with other physical and natural methods for nematode control. To accomplish this goal Mexican government programs (from SENASICA-and the National Reference Centre for Biological Control -CNRCB in Spanish) have been developed to encourage producers and farmers to adopt practices of biocontrol use, based on a recently implemented national development plan that includes integral policies linking environmental sustainability with costs and benefits to society (Williams et al. 2013). Additionally, the Mexican National Society of Biological Control promotes collaboration and training among researchers, farmers, producers, and companies.
Despite numerous studies using a variety of fungal and bacterial species with nematocidal activity, products approved by COFEPRIS are limited to the bacterial species B. subtilis and B. methylotrophicus GF267 and the fungi Trichoderma spp., P. lilacinus and Myrothecium verrucaria. However, other formulations in the market exist without COFEPRIS certification, not because the bioactive ingredients are ineffective, but because production processes do not yet meet quality control guidelines or because the requirements demanded by government regulators are still to be met, or because certification process (which takes several years) is still in process. In these cases, the user must monitor the biological safety of the product and its efficacy based on the concentration of the active ingredient and its viability, as well as other quality control parameters such as correct storage, expiration date, toxicological level, persistence in the environment, waste management (Montesinos-Matías et al. 2020). Bioassays performed under rigorous conditions are expected to select the most virulent strains among isolated candidates. A desirable attribute of these strains is that they remain genetically stable even after scaling processes to maintain infectivity or production of active ingredients with high yields. Strains of genetically improved microorganisms with this characteristic can be obtained as alternative biocontrol agents (Robledo-Monterrubio et al. 2009; Lovett and St. Leger 2018); however, due to current regulation policies in Mexico the use of strains obtained by recombinant DNA techniques is not currently allowed.
Conclusion
The limitations and disadvantages of conventional control provide an important opportunity for the integrated management and biological control of Meloidogyne RKN to deliver effective and sustainable alternatives. Products based on PGPB such as Bacillus, Pseudomonas, and Serratia are promising strategies since they not only suppress phytoparasitic nematodes but also stimulate plant growth and control other plant pathogenic microorganisms. Similarly, control through nematophagous fungi offers multiple beneficial traits, as many of these fungi can also control diverse diseases, insect pests and have the potential to stimulate induced resistance on the plant. Predatory nematodes also offer an ecologically safe alternative to chemical nematicides, but maybe not as an attractive option as previously mentioned, as other beneficial traits besides predation of phytoparasitic nematodes are not widely researched. To improve efficiency beyond current levels of biological control strategies it is desirable to develop a tailored microbial control method that considers environmental factors, crop type (as susceptibility varies among crops), soil parameters (moisture, pH, temperature), and whether trials are conducted in greenhouses or in the field. Future studies should consider all these factors to produce merchandise that maintains viability and improves the predatory efficiency of nematophagous (approximately 45% so far), so that biological control becomes a truly viable alternative to achieve a real impact in the control of Meloidogyne. For this purpose, collaborative academic, scientific and industrial work is needed to formulate new products or to establish practical and real-world parameters for the use of new and existing products under specific growing conditions, in quality control programs, and in accordance with regulatory guidelines to provide a broader range of bionematicidal products with better yields that will eventually replace or reduce the use of synthetic chemical pesticides for nematode control.
References
Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EGJ, Deleury E et al (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26:909–915. https://doi.org/10.1038/nbt.1482
Abd-Elgawad MMM, Vagelas IK (2015) Nematophagous bacteria: field application and commercialization. Biocontrol Agents Phytonematodes. https://doi.org/10.1079/9781780643755.0276
Al-Hazmi AS, TariqJaveed M (2016) Effects of different inoculum densities of Trichoderma harzianum and Trichoderma viride against Meloidogyne javanica on tomato. Saudi J Biol Sci 23:288–292. https://doi.org/10.1016/j.sjbs.2015.04.007
Ali MA, Azeem F, Li H, Bohlmann H (2017) Smart parasitic nematodes use multifaceted strategies to parasitize plants. Front Plant Sci 8:1–21. https://doi.org/10.3389/fpls.2017.01699
Atighi MR, Verstraeten B, Meyer TD, Kyndt T (2020) Genome-wide DNA hypomethylation shapes nematode pattern- triggered immunity in plants. New Phytol 227:545–558. https://doi.org/10.1111/nph.16532
Baniya A, Joseph S, Duncan L, Crow W, Mengistu T (2021) The role of Caenorhabditis elegans sex-determination homologs, Mi-sdc-1 and Mi-tra-1 in Meloidogyne incognita. Eur J Plant Pathol 161:439–452. https://doi.org/10.1007/s10658-021-02335-3
Barker KR, Hussey RS, Krusberg LR, Bird GW, Dunn RA, Ferris H et al (1994) Plant and soil nematodes: Societal impact and focus for the future. J Nematol 26:127–137
Berlitz DL, Knaak N, Cassal MC, Fiuza LM (2014) Bacillus and biopesticides in control of phytonematodes. In: Sahayaraj K (ed) Basic and applied aspects of biopesticides. Springer, New Delhi, pp 3–16. https://doi.org/10.1007/978-81-322-1877-7_1
Bhuiyan SA, Garlick K, Anderson JM, Wickramasinghe P, Stirling GR (2018) Biological control of root-knot nematode on sugarcane in soil naturally or artificially infested with Pasteuria penetrans. Australas Plant Pathol 47:45–52. https://doi.org/10.1007/978-81-322-1877-7_110.1007/s13313-017-0530-z
Bilgrami AL (2008). In: Ciancio A, Mukerji KG (eds) Biological control potentials of predatory nematodes. Springer, Dordrecht. https://doi.org/10.1007/978-81-322-1877-7_110.1007/978-1-4020-6063-2
Bilgrami AL, Gaugler R, Brey C (2005) Prey preference and feeding behaviour of the diplogastrid predator Mononchoides gaugleri (Nematoda: Diplogastrida). Nematology 7:333–342. https://doi.org/10.1007/978-81-322-1877-7_110.1163/156854105774355563
Bilgrami AL, Brey C, Gaugler R (2008) First field release of a predatory nematode, Mononchoides gaugleri (Nematoda: Diplogastrida), to control plant-parasitic nematodes. Nematology 10:143–146. https://doi.org/10.1007/978-81-322-1877-7_110.1163/156854108783360177
Brooker S (2010) Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers—a review. Int J Parasitol 40:1137–1144. https://doi.org/10.1016/j.ijpara.2010.04.004
Burkett-Cadena M, Kokalis-Burelle N, Lawrence KS, van Santen E, Kloepper JW (2008) Suppressiveness of root-knot nematodes mediated by rhizobacteria. Biol Control 47:55–59. https://doi.org/10.1007/978-81-322-1877-7_110.1016/j.biocontrol.2008.07.008
Castagnone-Sereno P, Danchin EGJ (2014) Parasitic success without sex—the nematode experience. J Evol Biol 27:1323–1333. https://doi.org/10.1111/jeb.12337
Castagnone-Sereno P, Mulet K, Danchin EGJ, Koutsovoulos GD, Karaulic M, Da Rocha M et al (2019) Gene copy number variations as signatures of adaptive evolution in the parthenogenetic, plant-parasitic nematode Meloidogyne incognita. Mol Ecol 28:2559–2572. https://doi.org/10.1111/mec.15095
Charchar JM, Eisenback JD, Charchar MJ, Boiteux MENF (2008) Meloidogyne phaseoli n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitising bean in Brazil. Nematology 10:525–538. https://doi.org/10.1007/978-81-322-1877-7_110.1163/156854108784513842
Chitwood DJ (2003) Research on plant-parasitic nematode biology conducted by the United States department of agriculture—agricultural research service. Pest Manage Sci: Former Pestic Sci 753:748–753. https://doi.org/10.1007/978-81-322-1877-7_110.1002/ps.684
Cid del Prado Vera I, Tovar Soto A, Hernández JA (2001) Distribución de especies y razas de meloidogyne en México. Rev Mex Fitopatol 19:32–39
Cooke RC (1963) Succession of nematophagous fungi during the decomposition of organic matter in the soil. Nature 197:205–205. https://doi.org/10.1038/197205a0
Coyne DL, Cortada L, Dalzell JJ, Claudius-Cole AO, Haukeland S, Luambano N et al (2018) Plant-parasitic nematodes and food security in Sub-Saharan Africa. Annu Rev Phytopathol 56:381–403. https://doi.org/10.1146/annurev-phyto-080417-045833
Cronin D, Moënne-Loccoz Y, Fenton A, Dunne C, Dowling DN, O’Gara F (1997) Role of 2,4-diacetylphloroglucinol in the interactions of the biocontrol pseudomonad strain F113 with the potato cyst nematode Globodera rostochiensis. Appl Environ Microbiol 63:1357–1361. https://doi.org/10.1128/aem.63.4.1357-1361.1997
Curtis RHC (2007) Plant parasitic nematode proteins and the host-parasite interaction. Brief Funct Genom Proteom 6:50–58. https://doi.org/10.1093/bfgp/elm006
Da Rocha M, Bournaud C, Dazenière J, Thorpe P, Bailly-Bechet M, Pellegrin C et al (2021) Genome expression dynamics reveal the parasitism regulatory landscape of the root-knot nematode Meloidogyne incognita and a promoter motif associated with effector genes. Genes (basel). https://doi.org/10.3390/genes12050771
Danchin EGJ, Rosso MN, Vieira P, De Almeida-Engler J, Coutinho PM, Henrissat B et al (2010) Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proc Natl Acad Sci USA 107:17651–17656. https://doi.org/10.1073/pnas.1008486107
de Souza R, Ambrosini A, Passaglia LMP (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38:401–419. https://doi.org/10.1590/S1415-475738420150053
Decraemer W, Hunt DJ, Perry RN, Moens M (2006) Structre and classification. In: Perry RN, Moens M (eds) Plant nematology. CABI, Wallingford, pp 3–32. https://doi.org/10.1079/9781845930561.0003
Dehghanian SZ, Abdollahi M, Charehgani H, Niazi A (2020) Combined of salicylic acid and Pseudomonas fluorescens CHA0 on the expression of PR1 gene and control of Meloidogyne javanica in tomato. Biol Control 141:104134. https://doi.org/10.1016/j.biocontrol.2019.104134
Djian C, Pijarowski L, Ponchet M, Arpin N, Favre-Bonvin J (1991) Acetic acid: a selective sematicidal metabolite from culture filtrates of Paecilomyces Lilacinus (Thom) Samson and Trichoderma Longibrachiatum Rifai. Nematologica 37:101–112
Eisenback JD, Triantaphyllou HH (2020) Root-knot nematodes: Meloidogyne species and races. Manual of agricultural nematology. CRC Press, Boca Raton, pp 191–274. https://doi.org/10.1201/9781003066576-6
Engelbrecht G, Horak I, Jansen van Rensburg PJ, Claassens S (2018) Bacillus-based bionematicides: development, modes of action and commercialisation. Biocontrol Sci Technol 28:629–653. https://doi.org/10.1080/09583157.2018.1469000
Escobar C, Barcala M, Cabrera J, Fenoll C (2015) Overview of root-knot nematodes and giant cells. In: Escobar C, Barcala M, Cabrera J, Fenoll C (eds) Plant nematode interactions. Academic Press, Cambridge, pp 1–32. https://doi.org/10.1016/bs.abr.2015.01.001
Fan H, Yao M, Wang H, Zhao D, Zhu X, Wang Y et al (2020) Isolation and effect of Trichoderma citrinoviride Snef 1910 for the biological control of root-knot nematode, Meloidogyne Incognita. BMC Microbiol 20:299. https://doi.org/10.1186/s12866-020-01984-4
Gao H, Qi G, Yin R, Zhang H, Li C, Zhao X (2016) Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Sci Rep 6:1–11. https://doi.org/10.1038/srep28756
Geng C, Nie X, Tang Z, Zhang Y, Lin J, Sun M et al (2016) A novel serine protease, Sep1, from Bacillus firmus DS-1 has nematicidal activity and degrades multiple intestinal-associated nematode proteins. Sci Rep 6:1–12. https://doi.org/10.1038/srep25012
Ghahremani Z, Escudero N, Saus E, Gabaldón T, Sorribas FJ (2019) Pochonia chlamydosporia induces plant-dependent systemic resistance to Meloidogyne incognita. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00945
Gowen S, Davies KG, Pembroke B (2007) Potential use of Pasteuria spp in the management of plant parasitic nematodes. Integrated management and biocontrol of vegetable and grain crops nematodes. Springer, Dordrecht, pp 205–219
Gupta G, Parihar SS, Ahirwar NK, Snehi SK, Singh V (2015) Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microb Biochem Technol 7:96–102. https://doi.org/10.4172/1948-5948.1000188
Haegeman A, Mantelin S, Jones JT, Gheysen G (2012) Functional roles of effectors of plant-parasitic nematodes. Gene 492:19–31. https://doi.org/10.1016/j.gene.2011.10.040
Hallmann J, Quadt-Hallmann A, Miller WG, Sikora RA, Lindow SE (2001) Endophytic colonization of plants by the biocontrol agent Rhizobium etli G12 in relation to Meloidogyne incognita infection. Phytopathology 91:415–422. https://doi.org/10.1094/PHYTO.2001.91.4.415
Hamaguchi T, Sato K, Vicente CSL, Hasegawa K (2019) Nematicidal actions of the marigold exudate α-terthienyl: oxidative stress-inducing compound penetrates nematode hypodermis. Biol Open. https://doi.org/10.1242/bio.038646
Harris TW, Antoshechkin I, Bieri T, Blasiar D, Chan J, Chen WJ et al (2010) WormBase: a comprehensive resource for nematode research. Nucleic Acids Res 38:D463–D467. https://doi.org/10.1093/nar/gkp952
Hassanaly-Goulamhoussen R, Augusto RDC, Marteu-garello N, Favery B, Danchin GJ, Abad P et al (2021) Chromatin landscape dynamics in development of the plant parasitic nematode Meloidogyne incognita. bioRxiv. https://doi.org/10.1101/2021.05.11.443567
Hewezi T (2020) Epigenetic mechanisms in nematode—plant interactions. Annu Rev Phytopathol 58:119–138. https://doi.org/10.1146/annurev-phyto-010820-012805
Hilker M, Schmülling T (2019) Stress priming, memory, and signalling in plants. Plant Cell Environ 42:753–761. https://doi.org/10.1111/pce.13526
Holbein J, Franke RB, Marhavý P, Fujita S, Górecka M, Sobczak M et al (2019) Root endodermal barrier system contributes to defence against plant-parasitic cyst and root-knot nematodes. Plant J 100:221–236. https://doi.org/10.1111/tpj.14459
Höss S, Arndt M, Baumgarte S, Tebbe CC, Nguyen HT, Jehle JA (2008) Effects of transgenic corn and Cry1Ab protein on the nematode, Caenorhabditis elegans. Ecotoxicol Environ Saf 70:334–340. https://doi.org/10.1016/j.ecoenv.2007.10.017
Hu Q, Yang M, Bo T, Li Y, Wu C, Mo M et al (2021) Nematicidal Streptomyces against Meloidogyne incognita by hydrosoluble macromolecules. Res Sq. https://doi.org/10.21203/rs.3.rs-199348/v1
Huang Y, Xu CK, Ma L, Zhang KQ, Duan CQ, Mo MH (2010) Characterisation of volatiles produced from Bacillus megaterium YFM3.25 and their nematicidal activity against Meloidogyne incognita. Eur J Plant Pathol 126:417–422. https://doi.org/10.1007/s10658-009-9550-z
Huang T, Lin Q, Qian X, Zheng Y, Yao J, Wu H et al (2018) Nematicidal activity of cry1ea11 from Bacillus thuringiensis BRC-XQ12 against the pine wood nematode (Bursaphelenchus xylophilus). Phytopathology 108:44–51. https://doi.org/10.1094/PHYTO-05-17-0179-R
Ibrahim HMM, Ahmad EM, Martínez-Medina A, Aly MAM (2019) Effective approaches to study the plant-root knot nematode interaction. Plant Physiol Biochem 141:332–342. https://doi.org/10.1016/j.plaphy.2019.06.009
Iqbal S, Fosu-Nyarko J, Jones MGK (2020) Attempt to silence genes of the RNAi pathways of the root-knot nematode, Meloidogyne incognita results in diverse responses including increase and no change in expression of some genes. Front Plant Sci 11:1–13. https://doi.org/10.3389/fpls.2020.00328
Ishida T, Suzuki R, Nakagami S, Kuroha T, Sakamoto S, Nakata MT (2020) Root-knot nematodes modulate cell walls during root - knot formation in Arabidopsis roots. J Plant Res 133:419–428. https://doi.org/10.1007/s10265-020-01186-z
Jaffee BA (2004) Wood, nematodes, and the nematode-trapping fungus Arthrobotrys oligospora. Soil Biol Biochem 36:1171–1178. https://doi.org/10.1016/j.soilbio.2004.03.003
Jansson H-B (1993) Adhesion to nematodes of conidia from the nematophagous fungus Drechmeria coniospora. Microbiology 139:1899–1906. https://doi.org/10.1099/00221287-139-8-1899
Jatala P (1986) Biological control of plant-parasitic nematodes. Annu Rev Microbiol 24:453–489
Jaubert-Possamai S, Noureddine Y, Favery B (2019) MicroRNAs, new players in the plant—nematode interaction. Front Plant Sci 10:1–8. https://doi.org/10.3389/fpls.2019.01180
Jenkins NE, Grzywacz D (2003) Towards the standardization of quality control of fungal and viral biological control agents. In: van Lenteren JC (ed) Quality control and production of biological control agents: theory and testing procedures. CABI, Wallingford. https://doi.org/10.1079/9780851996882.0247
Jennings D (2009) Tropical root and tuber crops. Cassava, sweet potato, yams and aroids. Exp Agric 45:382–382. https://doi.org/10.1017/S0014479709007832
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK et al (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. https://doi.org/10.1111/mpp.12057
Kahn TW, Duck NB, McCarville MT, Schouten LC, Schweri K, Zaitseva J et al (2021) A Bacillus thuringiensis Cry protein controls soybean cyst nematode in transgenic soybean plants. Nat Commun. https://doi.org/10.1038/s41467-021-23743-3
Kavitha PG, Jonathan EI, Nakkeeran S (2012) Effects of crude antibiotic of Bacillus subtilis on hatching of eggs and mortality of juveniles of Meloidogyne incognita. Nematol Mediterr 40:203–206
Khan MW (1993) Mechanisms of interactions between nematodes and other plant pathogens BT. In: Khan MW (ed) Nematode interactions. Springer, Dordrecht, pp 55–78. https://doi.org/10.1007/978-94-011-1488-2_4
Khan Z, Kim YH (2007) A review on the role of predatory soil nematodes in the biological control of plant parasitic nematodes. Appl Soil Ecol 35:370–379. https://doi.org/10.1016/j.apsoil.2006.07.007
Khan Z, Bilgrami AL, Jairajpuri S (1995) A comparative study on the predation by Allodorylaimus americanus and Discolaimus silvicolus (Nematoda : Dorylaimida) on different species of plant parasitic nematodes. Fundam Appl Nematol 18:99–108
Khan A, Williams KL, Nevalainen HKM (2004) Effects of Paecilomyces lilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biol Control 31:346–352. https://doi.org/10.1016/j.biocontrol.2004.07.011
Khan A, Williams KL, Nevalainen HKM (2006) Control of plant-parasitic nematodes by Paecilomyces lilacinus and Monacrosporium lysipagum in pot trials. Biocontrol 51:643–658. https://doi.org/10.1007/s10526-005-4241-y
Kiewnick S, Sikora RA (2006) Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol Control 38:179–187. https://doi.org/10.1016/j.biocontrol.2005.12.006
Kurpis J, Serrato-Cruz MA, Feria Arroyo TP (2019) Modeling the effects of climate change on the distribution of Tagetes lucida Cav. (Asteraceae). Glob Ecol Conserv 20:e00747. https://doi.org/10.1016/j.gecco.2019.e00747
Lebrigand K, He LD, Thakur N, Arguel MJ, Polanowska J, Henrissat B et al (2016) Comparative genomic analysis of Drechmeria coniospora reveals core and specific genetic requirements for fungal endoparasitism of nematodes. PLoS Genet 12:1–41. https://doi.org/10.1371/journal.pgen.1006017
Lee CH, Chang HW, Yang CT, Wali N, Shie JJ, Hsueh YP (2020) Sensory cilia as the Achilles heel of nematodes when attacked by carnivorous mushrooms. Proc Natl Acad Sci USA 117:6014–6022. https://doi.org/10.1073/pnas.1918473117
Lopez-Llorca LV, Maciá-Vicente JG, Jansson HB (2007) Mode of action and interactions of nematophagous fungi. In: Ciancio A, Mukerji KG (eds) Integrated management and biocontrol of vegetable and grain crops nematodes. Integrated management of plant pests and diseases, vol 2. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-6063-2_3
Lovett B, St. Leger RJ (2018) Genetically engineering better fungal biopesticides. Pest Manage Sci 74:781–789. https://doi.org/10.1002/ps.4734
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Lunt DH, Kumar S, Koutsovoulos G, Blaxter ML (2014) The complex hybrid origins of the root knot nematodes revealed through comparative genomics. Peer J 2014:1–25. https://doi.org/10.7717/peerj.356
Manzanilla-López RH, Esteves I, Finetti-Sialer MM, Hirsch PR, Ward E, Devonshire J et al (2013) Pochonia chlamydosporia: advances and challenges to improve its performance as a biological control agent of sedentary endo-parasitic nematodes. J Nematol 45:1–7
Meena B (2014) Biological control of pest and diseases using fluorescent Pseudomonads. In: Sahayaraj K (ed) Basic and applied aspects of biopesticides. Springer, New Delhi, pp 17–29. https://doi.org/10.1007/978-81-322-1877-7_2
Mendoza AR, Sikora RA (2009) Biological control of Radopholus similis in banana by combined application of the mutualistic endophyte Fusarium oxysporum strain 162, the egg pathogen Paecilomyces lilacinus strain 251 and the antagonistic bacteria Bacillus firmus. Biocontrol 54:263–272. https://doi.org/10.1007/s10526-008-9181-x
Migunova VD, Sasanelli N (2021) Bacteria as biocontrol tool against phytoparasitic nematodes. Plants 10:1–15. https://doi.org/10.3390/plants10020389
Mitreva M, Blaxter ML, Bird DM, McCarter JP (2005) Comparative genomics of nematodes. Trends Genet 21:573–581. https://doi.org/10.1016/j.tig.2005.08.003
Mitsumasu K, Seto Y, Yoshida S (2015) Apoplastic interactions between plants and plant root intruders. Front Plant Sci 6:1–17. https://doi.org/10.3389/fpls.2015.00617
Mohandas C, Prabhoo NR (1980) The feeding behaviour and food preferences of predatory nematodes (Mononchida) from the soils of Kerala (India). Rev D’ecologie Biol Du Sol 17:53–60
Montesinos-Matías R, García-Cancino D, Castillo-Minjaréz JM, Ordáz-Hernández A, Sanchez-González JA (2020) La calidad de los enemigos naturales en programas de control biológico. In: Arredondo-Bernal HC, Tamayo-Mejía F, Rodríguez del Bosque LA (eds) Fundamento y práctica del control biológico de plagas y enfermedades. BBA, Biblioteca Básica de Agricultura, Colegio de Posgraduados, Chapingo, pp 451–483
Naz S, Dabral S, Nagarajan SN, Arora D, Singh LV, Kumar P et al (2021) Compromised base excision repair pathway in Mycobacterium tuberculosis imparts superior adaptability in the host. PLoS Pathog 17:e1009452. https://doi.org/10.1371/journal.ppat.1009452
Niu Q, Huang X, Zhang L, Xu J, Yang D, Wei K et al (2010) A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc Natl Acad Sci USA 107:16631–16636. https://doi.org/10.1371/journal.ppat.100945210.1073/pnas.1007276107
Olmo R, Cabrera J, Díaz-Manzano FE, Ruiz-Ferrer V, Barcala M, Ishida T et al (2020) Root-knot nematodes induce gall formation by recruiting developmental pathways of post-embryonic organogenesis and regeneration to promote transient pluripotency. New Phytol 227:200–215. https://doi.org/10.1111/nph.16521
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. https://doi.org/10.1016/j.tim.2007.12.009
Opperman CH, Bird DM, Williamson VM, Rokhsar DS, Burke M, Cohn J et al (2008) Sequence and genetic map of Meloidogyne hapla: a compact nematode genome for plant parasitism. Proc Natl Acad Sci USA 105:14802–14807. https://doi.org/10.1073/pnas.0805946105
Osei K, Gowen SR, Pembroke B, Brandenburg RL, Jordan DL (2010) Potential of leguminous cover crops in management of a mixed population of root-knot nematodes (Meloidogyne spp.). J Nematol 42:173–178
Paganini J, Campan-Fournier A, Da Rocha M, Gouret P, Pontarotti P, Wajnberg E et al (2012) Contribution of lateral gene transfers to the genome composition and parasitic ability of root-knot nematodes. PLoS ONE. https://doi.org/10.1371/journal.pone.0050875
Palizi P, Goltapeh E, Pourjam E, Safaie N (2009) Potential of oyster mushrooms for the biocontrol of sugar beet nematode (Heterodera schachtii). J Plant Prot Res 49:27–34. https://doi.org/10.2478/v10045-009-0004-6
Peiris PUS, Li Y, Brown P, Xu C (2020) Fungal biocontrol against Meloidogyne spp. in agricultural crops: a systematic review and meta-analysis. Biol Control 144:104235. https://doi.org/10.1016/j.biocontrol.2020.104235
Perlatti B, de Souza Bergo PL, Fernandes da Silva MF, Fernandes JB, Forim MR (2013) Polymeric nanoparticle-based insecticides: a controlled release purpose for agrochemicals. Insecticides—development of safer and more effective technologies. InTech, London, pp 523–550. https://doi.org/10.5772/53355
Przybylska A, Obrępalska-Stęplowska A (2020) Plant defense responses in monocotyledonous and dicotyledonous host plants during root-knot nematode infection. Plant Soil 451:239–260. https://doi.org/10.1007/s11104-020-04533-0
Raddy HM, Fouad AFA, Montasser SA, Abdel-Lateedf MF, EL-Samadisy AM (2013) Efficacy of six nematicides and six commercial bioproducts against root-knot nematode, Meloidogyne incognita on tomato. J Appl Sci Res 9:4410–4417
Radhakrishnan R, Hashem A, Abd Allah EF (2017) Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol 8:1–14. https://doi.org/10.3389/fphys.2017.00667
Ramyabharathi SA, Meena KS, Rajendran L, Karthikeyan G, Jonathan EI, Raguchander T (2018) Biocontrol of wilt-nematode complex infecting gerbera by Bacillus subtilis under protected cultivation. Egypt J Biol Pest Control 28:1–9. https://doi.org/10.1186/s41938-018-0027-2
Ramyabharathi SA, Meena KS, Rajendran L, Raguchander T, Jonathan EI (2020) Potential of a rhizobacterium Bacillus subtilis (Bbv 57) on Fusarium oxysporum f. sp. gerberae and Meloidogyne incognita infecting Gerbera grown in protected cultivation. Eur J Plant Pathol 158:615–632. https://doi.org/10.1007/s10658-020-02087-6
Robledo-Monterrubio M, Alatorre-Rosas R, Viniegra-González G, Loera O (2009) Selection of improved Beauveria bassiana (Bals.) Vuill. strains based on 2-deoxy-d-glucose resistance and physiological analysis. J Invertebr Pathol 101:222–227. https://doi.org/10.1016/j.jip.2009.05.007
Rodríguez-del-Bosque LA, Arredondo-Bernal HC, Williams JF (2015) Pasado, presente y perspectivas del control Biológico en México. In: Arredondo-Bernal HC, Rodríguez del Bosque LA (eds) Casos de control biológico en México, vol 2. BBA Biblioteca Básica de Agricultura, Colegio de Posgraduados, Montecillo, pp 17–28
Roy P, Borah A (2020) Role of Predeceous nematodes in plant disease management. Pharma Innov J 9:463–467
Sánchez Portillo JF, Lugo García GA, Mundo Ocampo M, Reyes Olivas A, Ley Tandingan ID, Ole Becker J (2016) Búsqueda y aislamiento de hongos nematófagos vs Meloidogyne spp. en el norte de Sinaloa, México. Rev Mex Cienc Agric 7:1829–1839
Satou T, Kaneko K, Li W, Koike K (2008) The toxin produced by Pleurotus ostreatus reduces the head size of nematodes. Biol Pharm Bull 31:574–576. https://doi.org/10.1248/bpb.31.574
Serrato-Cruz MA, Díaz-Cedillo F, Barajas-Pérez JS (2008) Composición del aceite esencial en germoplasma de Tagetes filifolia Lag. de la región centro-sur de México. Agrociencia 42:277–285
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31:446–459. https://doi.org/10.1080/13102818.2017.1286950
Sharon E, Bar-Eyal M, Chet I, Herrera-Estrella A, Kleifeld O, Spiegel Y (2001) Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Biol Control 91:687–693
Siddiqi MR, Bilgrami AL, Tabassum K (2004) Description and biology of Mononchoides gaugleri sp. n. (Nematoda: Diplogasterida). Int J Nematol 14:124–129
Siddique S, Grundler FMW (2018) Parasitic nematodes manipulate plant development to establish feeding sites. Curr Opin Microbiol 46:102–108. https://doi.org/10.1016/j.mib.2018.09.004
Sikandar A, Zhang MY, Wang YY, Zhu XF, Liu XY, Fan HY et al (2020) Review article: Meloidogyne incognita (root-knot nematode) a risk to agriculture. Appl Ecol Environ Res 18:1679–1690. https://doi.org/10.15666/aeer/1801_16791690
Singh PR, Karssen G, Couvreur M, Subbotin SA, Bert W (2021) Integrative taxonomy and molecular phylogeny of the plant-parasitic nematode genus Paratylenchus (Nematoda: Paratylenchinae): linking species with molecular barcodes. Plants. https://doi.org/10.3390/plants10020408
Sivasakthi S, Usharani G, Saranraj P (2014) Biocontrol potentiality of plant growth promoting bacteria (PGPR)—Pseudomonas fluorescens and Bacillus subtilis: a review. Afr J Agric Res 9:1265–1277. https://doi.org/10.5897/AJAR2013.7914
Small RW (1979) The effects of predatory nematodes on populations of plant parasitic nematodes in pots. Nematologica 25:94–130
Small RW, Grootaert P (1983) Observations on the predation abilities of some soil dwelling predatory nematodes. Nematologica 29:109–118
Soliman MS, El-Deriny MM, Ibrahim DSS, Zakaria H, Ahmed Y (2021) Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J Appl Microbiol. https://doi.org/10.1111/jam.15101
Stirling GR (2000) Nematode monitoring strategy for vegetable crops : a report for the rural industries research and development corporation. Rural Industries Research & Development Corporation Barton, A.C.T. https://nla.gov.au/nla.cat-vn1971878
Tamez-Guerra P, Rodriguez-Padilla C, Galán-Wong LJ (2005) Solar radiation (UV) protectants for microbial insecticides. Semiochemicals in pest and weed control ACS symposium series. American Chemical Society, Washington, CD, pp 10–127. https://doi.org/10.1021/bk-2005-0906.ch010
Thongkaewyuan A, Chairin T (2018) Biocontrol of Meloidogyne incognita by Metarhizium guizhouense and its protease. Biol Control 126:142–146. https://doi.org/10.1016/j.biocontrol.2018.08.005
Tian B, Yang J, Zhang KQ (2007) Bacteria used in the biological control of plant-parasitic nematodes: populations, mechanisms of action, and future prospects. FEMS Microbiol Ecol 61:197–213. https://doi.org/10.1111/j.1574-6941.2007.00349.x
Trudgill DL, Blok VC (2001) APOMICTIC, POLYPHAGOUS ROOT-KNOT NEMATODES: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol 39:53–77. https://doi.org/10.1146/annurev.phyto.39.1.53
Vieira P, Danchin EGJ, Neveu C, Crozat C, Jaubert S, Hussey RS et al (2011) The plant apoplasm is an important recipient compartment for nematode secreted proteins. J Exp Bot 62:1241–1253. https://doi.org/10.1093/jxb/erq352
von Mende N (1997) Invasion and migration behaviour of sedentary nematodes. In: Fenoll C, Grundler FMW, Ohl SA (eds) Cellular and molecular aspects of plant-nematode interactions. Springer, Dordrecht, pp 51–64. https://doi.org/10.1007/978-94-011-5596-0_5
Walters D, Walsh D, Newton A, Lyon G (2005) Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology 95:1368–1373. https://doi.org/10.1094/PHYTO-95-1368
Wang K-H, Sipes BS, Schmitt DP (2002) Crotalaria as a cover crop for nematode management: a review. Nematropica 32:35–57
Wesemael WML, Viaene N, Moens M (2011) Root-knot nematodes (Meloidogyne spp.) in Europe. Nematology 13:3–16. https://doi.org/10.1163/138855410X526831
Williams T, Arredondo-Bernal HC, Rodríguez-del-Bosque LA (2013) Biological pest control in Mexico. Annu Rev Entomol 58:119–140. https://doi.org/10.1146/annurev-ento-120811-153552
Xia Y, Xie S, Ma X, Wu H, Wang X, Gao X (2011) The purL gene of Bacillus subtilis is associated with nematicidal activity. FEMS Microbiol Lett 322:99–107. https://doi.org/10.1111/j.1574-6968.2011.02336.x
Xu XM, Jeger MJ (2013) Theoretical modeling suggests that synergy may result from combined use of two biocontrol agents for controlling foliar pathogens under spatial heterogeneous conditions. Phytopathology 103:768–775. https://doi.org/10.1094/PHYTO-10-12-0266-R
Yang S-H, Wang D, Chen C, Xu C-L, Xie H (2020) Evaluation of Stratiolaelaps scimitus (Acari: Laelapidae) for controlling the root-knot nematode, Meloidogyne incognita (Tylenchida: Heteroderidae). Sci Rep 10:5645. https://doi.org/10.1038/s41598-020-62643-2
Youssef MM, El-Nagdi WM (2021) New approach for biocontrolling root-knot nematode, Meloidogyne incognita on cowpea by commercial fresh oyster mushroom (Pleurotus ostreatus). Jordan J Biol Sci 14:173–177
Youssef MMA, Abd-El-Khair H, El-Nagdi WMAEH (2017) Management of root knot nematode, Meloidogyne incognita infecting sugar beet as affected by certain bacterial and fungal suspensions. Agric Eng Int CIGR J 2017:293–301
Zhang Y, Li S, Li H, Wang R, Zhang KQ, Xu J (2020) Fungi–nematode interactions: Diversity, ecology, and biocontrol prospects in agriculture. J Fungi 6:1–24. https://doi.org/10.3390/jof6040206
Zhao D, Zhao H, Zhao D, Zhu X, Wang Y, Duan Y et al (2018) Isolation and identification of bacteria from rhizosphere soil and their effect on plant growth promotion and root-knot nematode disease. Biol Control 119:12–19. https://doi.org/10.1016/j.biocontrol.2018.01.004
Acknowledgements
Authors acknowledge financial support by DGAPA-PAPIIT IN211019 to C. M.-A., and to Instituto Tecnológico de Sonora (ITSON) Projects PROFAPI_2021_0006 and NPTC PRODEP Project 511-6/2020-8594. I. T.-V. received postdoctoral fellowship from the Consejo Nacional de Ciencia y Tecnología (CONACYT). We are grateful to Dr Christopher D. Wood for critical reading of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tapia-Vázquez, I., Montoya-Martínez, A.C., De los Santos-Villalobos, S. et al. Root-knot nematodes (Meloidogyne spp.) a threat to agriculture in Mexico: biology, current control strategies, and perspectives. World J Microbiol Biotechnol 38, 26 (2022). https://doi.org/10.1007/s11274-021-03211-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03211-2