Abstract
Being around for several decades, there is a vast amount of academic research on biomining, and yet it contributes less to the mining industry compared to other conventional technologies. This critique briefly comments on the current status of biomining research, enumerates a number of primary challenges, and elaborates on some kinetically-oriented strategies and bottom-up policies to sustain biomining with focus on critical material extraction and rare earth elements (REEs). Finally, we present some edge cutting developments which may promote new potentials in biomining.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: current state
Caveat
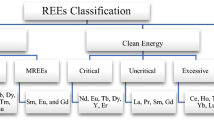
There exists an inconsistency on the definition of “biomining” in the scientific literature but herein it literally refers to extraction of minerals via biological systems. In this definition, phytomining is subcategory of biomining.
Having to work with biological systems in biomining adds extra complications beyond what investors and miners often deal with. This stems from the following factors: (1) biomining, as a whole, is a slow process (Senthil Kumar et al. 2018) (extraction and separation), (2) extreme conditions of traditional mining processes conflict with the sensitive nature of biological systems, and (3) typical mining infrastructures are not designed for bio-processes, and changing it requires high capital investments. Economic pressures for a faster return on invested capital (ROIC) have slowed progress in developing sustainable biomining opportunities. Mining companies mainly invest in pre-examined technologies, some of which have been refined over centuries, such as pyrometallurgical technologies (Johnson 2014). On the other hand, majority of experimental/theoretical studies on bioextraction make simplifications that are not well-suited to biological systems (e.g., linear extrapolation), resulting in misestimations of the kinetic and thermodynamic efficiency of the process (Jin et al. 2017; Thompson et al. 2018). Albeit, those results remain largely unproven, especially at scale. As an example, bioextraction of rare earth elements (REEs) and critical materials are portrayed as a promising method, but still needs validation at a commercial scale (Vahidi and Zhao 2016). Due to this significant gap between research and practice, biomining is still a minor contributor to the entire mining industry; with 15–20% contribution in extraction of copper and 5% of gold at best (Fathollahzadeh et al. 2019; Johnson 2014). With the ROIC being the major concern, slow recovery rate seems to be a bottleneck for the technology readiness level (TRL) of the emerging technologies in biomining. What is the bottleneck of that recovery rate? To answer this question, we shall revisit biomining from the perspective of its constituent elements: (1) bio; the primitive element (bottom), and (2) mining; the derivative element (top).
Biology
From an evolutionary perspective, surviving through gradual and catastrophic terrestrial changes, microorganisms are considered the most successful living organisms on Earth (Francis 2017; Nath et al. 2018). In fact, many microorganisms readily adapt to the slightest environmental changes (Govil et al. 2020; Prado et al. 2019). For instance, bacteria can sense negligible gravitational gradients within small altitudinal variations and adjust their motility (gravitaxis) (Thomas et al. 2018). Owing to this adaptability, microorganisms have been considered for their potential to be used on other planets for the purpose of space mining (Jakhu et al. 2017; Valtonen et al. 2016). Cyanobacteria are one of the most promising microorganisms for self-sustained biomining because of being autotrophs (Mazard et al. 2016). It has been demonstrated that competition between living species requires taking advantage of the fast kinetics of electron-transfer processes, which in turn is promoted by metal ions present in different minerals (Bauer et al. 2007; Sun et al. 2017). Having free electrons and different oxidation states, multivalent metal ions (e.g., transition metal ions) promote diversity in electron-transfer processes in living systems. Electron exchange between extracellular minerals and microorganisms occurs via external receptors as microbial cells are not permeable/conductive to minerals/electrons (Shi et al. 2016). Those external receptors have evolved to selectively attach to specific metals, in a process known as biosorption. For example, S. oneidensis (strain MR-1) is capable of using minerals that contain Fe3+, Mn3+ or Mn4+ as terminal electron acceptors (Shi et al. 2016). Microorganisms exchange electron with metal ions as: (1) energy source, (2) energy sink, (3) electron transfer (electrical signaling), and (4) electron storage to electrically support their own metabolism (Shi et al. 2016). As majority of multivalent metal ions have positive oxidation states, extraction of these ions is preferably made by biooxidation versus bioreduction (e.g., copper and gold). The recent discovery on the role of Ce3+ and La3+ in the enzyme methanol dehydrogenase (MDH) to oxidize methanol to carbon and energy (in some microbes such as Methylacidiphilum fumariolicum SolV) has renewed interests in using microorganisms for bioextraction of REEs (Pol et al. 2014; Skovran and Martinez-Gomez 2015). Bioaccumulation is the intracellular counterpart of biosorption to extract/collect metals. Compared to the relatively well-known mechanisms of outward electron transfer to extracellular minerals, mechanisms of inward electron transfer are yet to be understood, that is in turn very critical to better understand the nature of bioaccumulation (Shi et al. 2016).
Thriving on far-from-equilibrium stability, thermodynamic efficiency is not the only priority in living systems; ~ 10 kBT/cycle is the lower bound of energy dissipation in macromolecular biological machinery (Feng and Crooks 2008; Procacci 2017; Riechers and Crutchfield 2017). However, biomining is restricted less by efficiency (energy) and more by effectivity (time); it is a kinetically controlled rather than a thermodynamically controlled process (Johnson 2014; Senthil Kumar et al. 2018). As an examples, a vast number of microorganisms used in biomining gain (versus consuming) energy by oxidizing metals (Johnson 2014). As the result, biooxidation processes can be more lavish in exploring energy intensive strategies in exchange for enhancement of the process rate, which in turn serves the overall success of biomining. With this in mind, an effective strategy triggers tight competitions among microbial species (single/multiple) over common targets (single/multiple) within natural cell approaches (NCA). Those strategies are based on providing highly competitive environments that select for organisms that carry “selfish genes” that help them outcompete others in a specific mineral rich environment (Silva and Gatenby 2011). Within laboratory-bound experiments (i.e., evolution in action), one not only could devise adaptation strategies to give privilege to survival of desired species (Table 1) but also could drive a population of species to adapt new diets (Blount et al. 2012). We argue that carrier-focused strategies are promising candidates to address the slow recovery rate of biomining (Table 1) as they adapt effective/fast species; eat more in less time (Smułek et al. 2019; Zdarta et al. 2020). A proposed strategy is to start with multispecies assemblages with enough food only for a few generations to adjust, and gradually reducing the food to pass on more selfish genes. As a rule of thumb, diversity improves adaptation in natural biological processes (e.g., multi-species/target) but not necessarily selectivity (Carratalà et al. 2017; Vignuzzi et al. 2006).
While NCA is being gradually explored, sudden breakthroughs rely on engineered cell approaches (ECA). Relatively speaking, the latter (micro-controlled) is complementary to the former (macro-controlled), and faster within certain criteria (Table 2). Nonetheless, successful ECA strategies should eventually be customized within NCA strategies.
Highly selective microbial strategies become more kinetically favorable along with declining ore grades as they simply ignore what is not considered their target. For such cases, an alternative is engineering of a scaffold protein with a specific metal binding domain to enrich for specific targets (i.e., biosorption). This approach includes adding metal binding domains to proteins that form inclusion bodies, and thereby provide a novel strategy to sequester bound elements into an insoluble cellular fraction (Singh and Panda 2005). While inclusion bodies are often considered a problem to be avoided when purifying functional proteins, we suggest that this limitation actually represents a unique opportunity to express insoluble proteins that can sequester bound elements. Inclusion bodies are dense particles comprised of aggregated protein and range in size from 0.15 to 1.3 μm with a density of ~ 1.3 mg/ml (Singh and Panda 2005). They can easily be fractionated from other cellular components by a high-speed centrifugation step. Despite their high density, they are hydrated and have a porous architecture. While proteins trapped in inclusion bodies are typically blocked from folding into complex secondary and tertiary structures, small hydrophilic metal binding motifs are more resilient. Thus, inclusion bodies derived from polypeptides with short metal binding motifs offer a powerful strategy to bind excess metals and sequester those metals inside a cell. The ability of inclusion bodies to sequester elements also creates an expectation that the microorganisms should gain some degree of resistance to otherwise toxic levels of specific elements. Thus, this biosorption strategy provides a flexible engineering platform in which other novel proteins can be designed to specifically bio-enrich different target elements. However, because genetically manipulated strains cannot be simply dumped into the environment, there are additional environmental constraints that need to be dealt with in scaling up this technology. Hence, ECA has drawn less attentions in biomining compared to other biotechnologies (Johnson 2014). However, this problem has environmentally safe solutions. The harvesting and processing of bacteria can be done is contained environments. For extracting metals that are sequestered in microorganisms, the organism will be killed as a part of the harvesting process.
In recent years researches were used OMICs (genomics, proteomics, transcriptomics, metabolomics) to identify genes and proteins involved in bioleaching (Buetti-Dinh et al. 2020). Understanding the genetic basis for cell attachment on metal and what gene expression patterns are enriched in a microbial community will help in the design of strategies to stimulate bioleaching rates, speed up the initiation of bioleaching operations, and improve the persistence of active cells in heap bioleaching operations (Christel et al. 2017).
Mining
Traditional (bio)mining has a top-down structure in the following order: (1) decision makers issue a list of so-called “critical materials” based on provisional policies, (2) investors outline future directions for organizations, and (3) organizations adjust their own policies accordingly. In this picture, it is the supreme order, i.e., the list, which guides policies from top to bottom. On the other hand, nature functions in a bottom-up fashion. As far as bioextraction is concerned, minerals have been naturally concentrated based on the needs at the bottom level; i.e., what is critical for microorganisms, versus that of human decision makers. Consequently, we argue that sustainable biomining should emphasize (1) finding pre-existing ecosystems that already concentrate minerals of interest (bottom up approach) or (2) developing new artificial ecosystems (for example, with organisms engineered to accumulate a specific metal). In a bottom-up perspective, bioextraction processes are inherently more selective than those based on a general dissolution of the surrounding matrix, particularly in extraction of low-grade minerals, such as REEs and gold. From the operational perspective, current strategies are based on proper selection and adaptation of microorganisms to meet the harsh mine conditions. However, we suggest consideration should be given to alternative mining strategies that better accommodate growth requirements for different bioextracting microorganisms.
Sustainable biomining and its outlook
Taken together, as a natural way of extracting minerals, the use of bioextraction has the potential to be a major and sustainable addition to traditional mining. Here, we enumerate some reasons for such claim: (1) compared to other extraction techniques, bioextraction can be more energy conservative and environmentally friendly (Johnson 2014), (2) rich primary resources for many minerals are increasingly being exhausted or becoming cost prohibitive, especially for critical and REEs, and (3) inspired by the fact that many minerals are already bioextracted by living organisms, biomining only needs to expedite this process within kinetically-oriented strategies that combine top-down policies with bottom-up processes.
Biomining has gazed at the most recent explorations in the forefront of science and technology. For example, scholars are in continuous search to find microorganisms which can survive at high temperatures, where biomining might be faster and more selective (e.g., selective oxidation of iron over sulfur) (Johnson 2014; Mbye et al. 2020). Within resources containing an abundance of sulfide minerals, an active area of research is on how to hinder the oxidation of sulfide all the way to sulfate as it releases a lot of sulfuric acid in the environment (Engelbrektson et al. 2018). In the field of microbiology, there exists an ongoing search on how electron transfers occur from extracellular minerals to internal electron acceptors of microorganisms, while energy remains conserved within such processes (Shi et al. 2016). Inspired by the fact that the active sites of proteins are very specific and selective, one can invest on refining this selectivity within ECA, as already discussed. Albeit, biosafety is a major concern for ECA alternatives, as there exist very strict limitations when engineered cells are potentially released into terrestrial environments. These biosafety-related concerns are less of a constraint in the context of engineering biosystems for space biomining projects, such as BioRock (Loudon et al. 2018; Raafat et al. 2013). Biomining is considered as one of the better economical viable options for mining celestial bodies (e.g., asteroids). As an example, the long-term objective of BioRock (by NASA) is to enable in-situ 3D printing of machinery directly from the minerals extracted by pre-cultivated microorganisms, rather than launching that machinery from earth to space (Loudon et al. 2018; Raafat et al. 2013). A parallel line of investigation has emerged based on cutting edge developments in the field of nonequilibrium chemistry. Promising research in this area has progressively thinned the border between chemistry and biology, i.e., living and nonliving systems (Dhiman et al. 2017; Intoy and Halley 2017; Nakouzi and Steinbock 2016). New (bio)materials are being developed to make proper adjustments in response to environmental changes (Derkus et al. 2020; Dhiman et al. 2020; Huang et al. 2020; Li et al. 2019). Such (bio)materials could ideally be tailored to selectively extract minerals, eliminating biosafety concerns. As an example, macrocyclic/bio-inorganic receptors have been recently designed for highly selective extraction of precious metals, such as gold, platinum, or palladium (Liu et al. 2018). Although, application of life-like materials that mimic biological structures have been extensively investigated, their capability for bioextraction or hyperaccumulation of critical materials have not been reported.
References
Bauer M, Heitmann T, Macalady DL, Blodau C (2007) Electron transfer capacities and reaction kinetics of peat dissolved organic matter. Environ Sci Technol 41:139–145. https://doi.org/10.1021/es061323j
Blount ZD, Barrick JE, Davidson CJ, Lenski RE (2012) Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature 489:513–518. https://doi.org/10.1038/nature11514
Buetti-Dinh A, Herold M, Christel S, Hajjami ME, Bellenberg S, Ilie O, Wilmes P, Poetsch A, Sand W, Vera M, Pivkin IV, Dopson M (2020) Systems biology of acidophile biofilms for efficient metal extraction. Sci Data 7:215. https://doi.org/10.1038/s41597-020-0519-2
Carratalà A, Shim H, Zhong Q, Bachmann V, Jensen JD, Kohn T (2017) Experimental adaptation of human echovirus 11 to ultraviolet radiation leads to resistance to disinfection and ribavirin. Virus Evol. https://doi.org/10.1093/ve/vex035
Christel S, Herold M, Bellenberg S, El Hajjami M, Buetti-Dinh A, Pivkin IV, Sand W, Wilmes P, Poetsch A, Dopson M (2017) Multi-omics reveals the lifestyle of the acidophilic, mineral-oxidizing model species Leptospirillum ferriphilum T. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02091-17
Derkus B, Okesola BO, Barrett DW, D’Este M, Chowdhury TT, Eglin D, Mata A (2020) Multicomponent hydrogels for the formation of vascularized bone-like constructs in vitro. Acta Biomater 109:82–94. https://doi.org/10.1016/j.actbio.2020.03.025
Dhiman S, Jain A, Kumar M, George SJ (2017) Adenosine-phosphate-fueled, temporally programmed supramolecular polymers with multiple transient states. J Am Chem Soc 139:16568–16575. https://doi.org/10.1021/jacs.7b07469
Dhiman S, Ghosh R, George SJ (2020) Redox-mediated transient reconfiguration of a supramolecular assembly. ChemSystemsChem. https://doi.org/10.1002/syst.201900042
Engelbrektson AL, Cheng Y, Hubbard CG, Jin YT, Arora B, Tom LM, Hu P, Grauel AL, Conrad ME, Andersen GL, Ajo-Franklin JB, Coates JD (2018) Attenuating sulfidogenesis in a soured continuous flow column system with perchlorate treatment. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01575
Fathollahzadeh H, Eksteen JJ, Kaksonen AH, Watkin ELJ (2019) Role of microorganisms in bioleaching of rare earth elements from primary and secondary resources. Appl Microbiol Biotechnol 103:1043–1057. https://doi.org/10.1007/s00253-018-9526-z
Feng EH, Crooks GE (2008) Length of time’s arrow. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.101.090602
Francis D (2017) Antimicrobials from microbes. Bioresources and bioprocess in biotechnology. Springer Singapore, Singapore, pp 291–326. https://doi.org/10.1007/978-981-10-4284-3_12
Govil T, Saxena P, Samanta D, Singh SS, Kumar S, Salem DR, Sani RK (2020) Adaptive enrichment of a thermophilic bacterial isolate for enhanced enzymatic activity. Microorganisms 8:1–16. https://doi.org/10.3390/microorganisms8060871
Huang C, Chen X, Xue Z, Wang T (2020) Effect of structure: a new insight into nanoparticle assemblies from inanimate to animate. Sci Adv 6:eaba1321. https://doi.org/10.1126/sciadv.aba1321
Intoy BF, Halley JW (2017) Energetics in a model of prebiotic evolution. Phys Rev E 96:062402. https://doi.org/10.1103/PhysRevE.96.062402
Jakhu RS, Pelton JN, Nyampong YOM (2017) Space mining and its regulation, space mining and its regulation. Springer International Publishing, Cham. https://doi.org/10.1007/978-3-319-39246-2
Jin H, Park DM, Gupta M, Brewer AW, Ho L, Singer SL, Bourcier WL, Woods S, Reed DW, Lammers LN, Sutherland JW, Jiao Y (2017) Techno-economic assessment for integrating biosorption into rare earth recovery process. ACS Sustain Chem Eng 5:10148–10155. https://doi.org/10.1021/acssuschemeng.7b02147
Johnson DB (2014) Biomining—biotechnologies for extracting and recovering metals from ores and waste materials. Curr Opin Biotechnol 30:24–31. https://doi.org/10.1016/j.copbio.2014.04.008
Li S, Bai H, Shepherd RF, Zhao H (2019) Bio-inspired design and additive manufacturing of soft materials, machines, robots, and haptic interfaces. Angew Chem Int Ed 58:11182–11204. https://doi.org/10.1002/anie.201813402
Liu W, Oliver AG, Smith BD (2018) Macrocyclic receptor for precious gold, platinum, or palladium coordination complexes. J Am Chem Soc 140:6810–6813. https://doi.org/10.1021/jacs.8b04155
Loudon CM, Nicholson N, Finster K, Leys N, Byloos B, Van Houdt R, Rettberg P, Moeller R, Fuchs FM, Demets R, Krause J, Vukich M, Mariani A, Cockell C (2018) BioRock: new experiments and hardware to investigate microbe-mineral interactions in space. Int J Astrobiol 17:303–313. https://doi.org/10.1017/S1473550417000234
Mazard S, Penesyan A, Ostrowski M, Paulsen IT, Egan S (2016) Tiny microbes with a big impact: the role of cyanobacteria and their metabolites in shaping our future. Mar Drugs 14:97. https://doi.org/10.3390/md14050097
Mbye M, Baig MA, AbuQamar SF, El-Tarabily KA, Obaid RS, Osaili TM, Al-Nabulsi AA, Turner MS, Shah NP, Ayyash MM (2020) Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr Rev Food Sci Food Saf 19:1110–1124. https://doi.org/10.1111/1541-4337.12554
Nakouzi E, Steinbock O (2016) Self-organization in precipitation reactions far from the equilibrium. Sci Adv 2:e1601144. https://doi.org/10.1126/sciadv.1601144
Nath K, Bhattacharya MK, Kar S (2018) Antimicrobial potential of ethnomedicinal ferns of southern Assam, India. Indian J Pharm Sci 80:556–560. https://doi.org/10.4172/pharmaceutical-sciences.1000392
Pol A, Barends TRM, Dietl A, Khadem AF, Eygensteyn J, Jetten MSM, Op den Camp HJM (2014) Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol 16:255–264. https://doi.org/10.1111/1462-2920.12249
Prado N, Sampayo M, González P, Lombó F, Díaz J (2019) Physicochemical, sensory and microbiological characterization of Asturian Chorizo, a traditional fermented sausage manufactured in Northern Spain. Meat Sci 156:118–124. https://doi.org/10.1016/j.meatsci.2019.05.023
Procacci P (2017) Alchemical determination of drug-receptor binding free energy: where we stand and where we could move to. J Mol Graph Model 71:233–241. https://doi.org/10.1016/j.jmgm.2016.11.018
Raafat K, Burnett JA, Chapman T, Cockell CS (2013) The physics of mining in space. Astron Geophys 54:5.10-5.12. https://doi.org/10.1093/astrogeo/att160
Riechers PM, Crutchfield JP (2017) Fluctuations when driving between nonequilibrium steady states. J Stat Phys 168:873–918. https://doi.org/10.1007/s10955-017-1822-y
Senthil Kumar P, Yaashikaa PR, Baskar G (2018) Biomining of natural resources. Springer, Singapore, pp 313–342. https://doi.org/10.1007/978-981-10-7413-4_17
Shi L, Dong H, Reguera G, Beyenal H, Lu A, Liu J, Yu HQ, Fredrickson JK (2016) Extracellular electron transfer mechanisms between microorganisms and minerals. Nat Rev Microbiol 14:651–662. https://doi.org/10.1038/nrmicro.2016.93
Silva AS, Gatenby RA (2011) Adaptation to survival in germinal center is the initial step in onset of indolent stage of multiple myeloma. Mol Pharm 8:2012–2020. https://doi.org/10.1021/mp200279p
Singh SM, Panda AK (2005) Solubihzation and refolding of bacterial inclusion body proteins. J Biosci Bioeng 99:303–310. https://doi.org/10.1263/jbb.99.303
Skovran E, Martinez-Gomez NC (2015) Just add lanthanides. Science 348:862–863. https://doi.org/10.1126/science.aaa9091
Smułek W, Zdarta A, Pacholak A, Runka T, Kaczorek E (2019) Increased biological removal of 1-chloronaphthalene as a result of exposure: a study of bacterial adaptation strategies. Ecotoxicol Environ Saf 185:109707. https://doi.org/10.1016/j.ecoenv.2019.109707
Sun T, Levin BDA, Guzman JJL, Enders A, Muller DA, Angenent LT, Lehmann J (2017) Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat Commun 8:14873. https://doi.org/10.1038/ncomms14873
Thomas MA, Kleist AB, Volkman BF (2018) Decoding the chemotactic signal. J Leukoc Biol 104:359–374. https://doi.org/10.1002/JLB.1MR0218-044
Thompson VS, Gupta M, Jin H, Vahidi E, Yim M, Jindra MA, Nguyen V, Fujita Y, Sutherland JW, Jiao Y, Reed DW (2018) Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain Chem Eng 6:1602–1609. https://doi.org/10.1021/acssuschemeng.7b02771
Vahidi E, Zhao F (2016) Life cycle analysis for solvent extraction of rare earth elements from aqueous solutions. REWAS 2016: towards materials resource sustainability. Springer International Publishing, Cham, pp 113–120. https://doi.org/10.1007/978-3-319-48768-7_17
Valtonen M, Anosova J, Kholshevnikov K, Mylläri A, Orlov V, Tanikawa K (2016) The solar system. The three-body problem from pythagoras to hawking. Springer International Publishing, Cham, pp 99–116. https://doi.org/10.1007/978-3-319-22726-9_5
Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R (2006) Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439:344–348. https://doi.org/10.1038/nature04388
Zdarta A, Smułek W, Kaczorek E (2020) Multilevel changes in bacterial properties on long-term exposure to hydrocarbons and impact of these cells on fresh-water communities. Sci Total Environ 729:138956. https://doi.org/10.1016/j.scitotenv.2020.138956
Acknowledgements
The authors are inspired by the works of Ilya Prigogine and Gavin Crooks.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors designed the study, and wrote, read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbasi, B., Harper, J. & Ahmadvand, S. A short critique on biomining technology for critical materials. World J Microbiol Biotechnol 37, 87 (2021). https://doi.org/10.1007/s11274-021-03048-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03048-9