Abstract
The accumulation of acetate in Escherichia coli inhibits cell growth and desired protein synthesis, and cell density and protein expression are increased by reduction of acetate excretion. Dissolved oxygen (DO) is an important parameter for acetate synthesis, and the accumulation of acetate is inversely correlated to DO level. In this study, the effect of DO levels on glutamate dehydrogenase (GDH) expression was investigated, and then different DO control strategies were tested for effects on GDH expression. DO control strategy IV (50% 0–9 h, 30% 9–18 h) provided the highest cell density (15.43 g/L) and GDH concentration (3.42 g/L), values 1.59- and 1.99-times higher than those achieved at 10% DO. The accumulation of acetate was 2.24 g/L with DO control strategy IV, a decrease of 40.74% relative to that achieved for growth at 10% DO. Additionally, under DO control strategy IV, there was lower expression of PoxB, a key enzyme for acetate synthesis, at both the transcriptional and translational level. At the same time, higher transcription and protein expression levels were observed for a glyoxylate shunt gene (aceA), an acetate uptake gene (acs), gluconeogensis and anaplerotic pathways genes (pckA, ppsA, ppc, and sfcA), and a TCA cycle gene (gltA). The flux of acetate with DO strategy IV was 8.4%, a decrease of 62.33% compared with the flux at 10% DO. This decrease represents both lower flux for acetate synthesis and increased flux of reused acetate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus suis (S. suis) is a Gram-positive, facultative anaerobic bacterium that is economically important, because it causes a wide range of diseases in pigs (Lun et al. 2007). The glutamate dehydrogenase (GDH) of S. suis is an effective antigen targeted by a vaccine that protects swine from S. suis infection, and GDH is also used as the target gene in serological assays to diagnose S. suis infection (Kutz and Okwumabua 2008; Wang et al. 2020). In our previous study, we constructed a recombinant strain of E. coli, SSGPA by deletion of pta-ackA from E. coli strain SSG. SSGPA expresses a high level of GDH and a decreased concentration of acetate, but previous work had shown that the accumulation of acetate in E.coli inhibits cell growth and GDH expression (Wang et al. 2020; Parimi et al. 2017; Dong et al. 2017).
Escherichia coli generates acetate as an undesirable byproduct with negative effects on cell growth and expression of recombinant protein (Bemal et al. 2016; Rajaraman et al. 2016), and an effective strategy to optimize protein production is to limit acetate accumulation (Eiteman and Altman 2006). Dissolved oxygen (DO) is a key parameter in fermentation, affecting cytochrome activity and enzymes related to the glycolytic (EMP) pathway, the pentose phosphate (PP) pathway, and the tricarboxylic acid (TCA) cycle (Zheng and Kwon 2013). Higher bacterial cell concentration is achieved by controlling the level of DO (Negrete and Shiloach 2017). The concentration of acetate is accumulated mainly through the phosphotransacetylase (pta)-acetate kinase (ackA) pathway and the pyruvate oxidase B (poxB) pathway; both pathways are also affected by gluconeogenesis (Kumar and Shimizu 2010). Acetate consumption occurs by its conversion back to acetyl-CoA by acetyl-CoA synthetase (acs), which is the reverse action of Pta-AckA and the glyoxylate shunt pathway (aceBAK) (Rajaraman et al. 2016; Wang et al. 2013). Thus, the concentration of accumulated acetate during cultivation of E. coli is the combined result of acetate formation and consumption (Phue and Shiloach 2005).
A well-known disadvantage of oxygen limitation during cultivation is an increased rate of acetate accumulation relative to that under aerobic conditions (Veeravalli et al. 2018). Acetate accumulation is inversely correlated to DO levels, with enhanced acetate production at lower DO levels due to decreased TCA cycle activity and changes in the transcriptional levels of genes associated with glucose and acetate metabolism (Phue and Shiloach 2005; Zhao et al. 2020). Northern blot analysis of proteins that act in the acetate synthesis pathway indicated that only the Pta-AckA pathway genes are transcribed at high oxygen levels, but at low oxygen levels, these Pta-AckA pathway genes and PoxB are transcribed (Phue and Shiloach 2005). Analysis of L-tryptophan production by E. coli at different DO levels revealed high transcription levels of key genes for tryptophan biosynthesis and low transcription levels of key genes involved in acetate synthesis at a relative high DO level, leading to reduced acetate excretion and improved L-tryptophan production (Zhao et al. 2020). However, the concentration of reactive oxygen species (ROS) increases with increasing DO concentration during E. coli growth, and high levels of ROS results in irreversible damage to cellular components (Baez and Shiloach 2013).
In this study, the effect of different DO levels on GDH expression by E. coli SSGPA was investigated, and then different DO control strategies were tested for effects on the expression of GDH. The transcription levels and enzyme activities of key genes for acetate synthesis were analyzed, and the metabolic flux distribution of key nodes included in the acetate synthesis pathway were determined to explain the low excretion of acetate and high expression of GDH at an optimized DO control level.
Materials and methods
Strain and media
The strain E. coli SSGPA used for GDH expression in this study was constructed from E. coli SSG by deletion of pta-ackA as described in our previous work (Wang et al. 2020). The strain was maintained on Luria–Bertani (LB) agar containing 100 mg/L of ampicillin (Am).
The seed medium for GDH expression was Luria Broth (LB), with the following components (in g/L): tryptone 10, yeast extract 5, and NaCl 10. The fermentation medium for GDH expression had the following composition (in g/L): glucose 10, tryptone 5, yeast extract 10, (NH4)2SO4 2, MgSO4·7H2O 2.5, KH2PO4 1.5, citrate 2, FeSO4 0.1, and betaine 1.5. The pH value of the seed and fermentation media was adjusted to 7.0 by addition of 4 mol/L NaOH solution.
Culture condition
The expression of GDH protein by E. coli SSGPA was carried out in a 10-L fermenter (Biotech 2012, Shanghai Bailun Biotech Co., Ltd., Shanghai, China). A sample of 600 mL seed medium in a 3000 mL baffled flask was inoculated with a single colony of E. coli SSGPA and cultivated at 35 °C by shaking at 200 rev/min for 12 h. This culture grown in the baffled flask was then inoculated aseptically (10% v/v) into 6 L of fermentation medium in a 10-L fermenter. The temperature and pH were controlled at 35 °C and 7.0, respectively, while DO levels were maintained to meet experimental requirements by adjusting the agitation and aeration rates. Once the cell density (OD600) increased to about 10.0, 0.5 mmol/L isopropyl thiogalactose (IPTG) was added into the fermenter to induce GDH expression. When the initial glucose was depleted, glucose solution (50%, w/v) was added to the fermenter according to the DO feedback strategy (Zhao et al. 2016).
DO control strategy
The DO levels during GDH expression by E. coli SSGPA were controlled at 10%, 20%, 30%, 50%, or 100%. Four DO control strategy were developed and tested for the expression of GDH protein: strategy I [20% (0–9 h), 30% (9–18 h)], strategy II [30% (0–9 h), 20% (9–18 h)], strategy III [50% (0–9 h), 20% (9–18 h)], and strategy IV [50% (0–9 h), 30% (9–18 h)].
Transcriptome analysis of E. coli
The total RNA of E. coli samples during mid-logarithmic growth of GDH expression was extracted using the RNAiso Plus kit (Takara, China), and transcriptome analysis of E. coli with different DO levels was performed as described previously (Zhao et al. 2020; Mortazavi et al. 2008). The transcription of several genes involved in glucose and acetate metabolism were examined, including an acetate formation gene (poxB), a glyoxylate shunt gene (aceA), an acetate uptake gene (acs), gluconeogensis and anaplerotic pathways genes (pckA, ppsA, ppc, and sfcA), and a TCA cycle gene (gltA) (Phue and Shiloach 2005).
Activity analysis of key enzymes for acetate synthesis
Crude extracts were prepared from cells harvested during the mid-logphase (10 h) of fermentation (Tan et al. 2016). E. coli cells were collected by centrifugation at 8000×g for 10 min at 4 °C, washed twice with 50 mM Tris buffer (pH 7.0), and then suspended in the same buffer with 1 × protease inhibitor (Roche, Switzerland) to an OD550 of 10 for sonication treatment. After centrifugation at 12,000×g and 4 °C for 20 min, the supernatant (crude extract) was transferred to a new tube (Piao et al. 2019). The activities of key enzymes for acetate synthesis were then measured as described previously, including fructose bisphosphatase (Fbn), phosphoenolpyruvate carboxykinase (Pck), phosphoenolpyruvate synthase (Pps), Phosphoenolpyruvate carboxylase (Ppc), citrate synthase (Glt), pyruvate oxidase B (PoxB), and acetyl-COA synthase (Acs) (Lin et al. 2018; Zhang et al. 2018; Zhao et al. 2018).
Metabolic flux distribution analysis of key nodes for acetate synthesis
Based on the law of conservation and the pseudo-steady-state hypothesis (PSSH) of the intracellular intermediate metabolites, a mass metabolic matrix was constructed (Chen and Zeng 2017). The metabolic network and the reaction rate equations of metabolic nodes were established as previously described (Bemal et al. 2016; Castaño-Cerezo et al. 2009). According to the analysis of metabolic flux balance and the stoichiometry model, and based on the strain being in stationary phase near the end of the fermentation period, the metabolic flux was determined for key nodes in the acetate synthesis pathway with different DO levels during the later period of GDH expression using MATLAB (MathWorks, Natick, MA, USA) (Enjalbert et al. 2017; Wang et al. 2019).
Analysis of fermentation products
The cell density of E. coli SSGPA during the GDH expression process was measured by spectrophotometry (OD600), and the dry cell weight (DCW) was assessed using the following relationship: 1 OD600 = 0.42 g/L DCW (Zhao et al. 2020). The concentration of glucose was determined using an SBA-40E biosensor analyzer (Biology Institute of Shandong Academy of Sciences, Jinan, China). The acetate concentration was determined by high-performance liquid chromatography using an Agilent 1200 system (Agilent Technologies, Santa Clara, CA, USA) equipped with an Aminex HPX-87H (Bio-Rad Laboratories, Inc., USA) (Lin et al. 2016). The concentration of GDH protein was determined as described previously (Wang et al. 2020).
Statistical analysis
All experiments were conducted in triplicate, and the data were averaged and are presented as mean values ± standard deviation. One-way analysis of variance was followed by Tukey test for multiple comparison to determine significant differences. Statistical significance was defined as p < 0.05.
Results
GDH expression with different DO levels
Cell density and GDH expression levels
The cell density and GDH expression levels were determined for E. coli SSGPA cultivated at different DO levels (10%, 20%, 30%, 50%, and 100%), as presented in Fig. 1. For DO levels maintained below 50%, the cell density and GDH concentration increased with higher DO level. The highest cell density (13.63 g/L) and GDH concentration (3.05 g/L) were obtained at a DO level of 50%, increased values by 40.08% and 40.55%, respectively, compared with those at 10% DO (9.73 g/L and 2.17 g/L). The cell density values at 20% and 30% DO were 12.25 g/L and 13.29 g/L, respectively, with GDH concentrations of 2.78 g/L and 2.98 g/L, respectively. The cell density and GDH concentration at 50% DO were significantly different from those at 10% and 20% (p < 0.05), and those at 30% and 50% were not significantly different. For cultivation at 100% DO, cell density and GDH concentration were lowest, with values of 9.41 g/L and 2.38 g/L, respectively.
Accumulation and synthesis rate of acetate
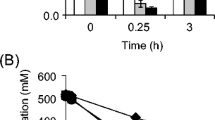
Figure 2 shows the accumulation of acetate by E. coli SSGPA and its synthesis rate at different DO levels. For GDH expression, acetate accumulated during the early fermentation period (0–12 h), and then the accumulated acetate was partially consumed during the later fermentation phase (12–18 h). The accumulation of acetate and acetate synthesis rate both decreased during the first ten hours of culture time with increasing DO levels. The accumulation of acetate at the end of fermentation with 10% DO (3.78 g/L) was highest, while that with 100% DO (1.96 g/L) was lowest. The concentration of acetate accumulated at 50% DO (2.20 g/L) at the end of cultivation was decreased by 14.06% and 6.78% compared with that at 20% and 30% DO. The accumulation of acetate at 100% DO level was significantly different from that at other DO levels (p < 0.05).
GDH expression with different DO control strategies
Cell density and GDH expression level
Based on the above results, the four DO control strategies were applied for GDH expression by E. coli SSGPA, and the resulting cell density and GDH expression levels are displayed in Fig. 3. The cell density (15.43 g/L) and GDH concentration (3.42 g/L) obtained with DO control strategy IV were highest, with increases of 5.98% and 7.89%, respectively, compared with those using DO control strategy III (14.56 g/L and 3.17 g/L). The cell density and GDH concentration with DO control strategy I (12.55 g/L and 2.83 g/L, respectively) was not significantly different from those obtained with DO control strategy II (12.92 g/L and 2.89 g/L, respectively). However, the cell density and GDH concentration with DO control strategy III and IV were significantly different from those obtained with DO control strategies I and II (p < 0.05).
Accumulation and production rate of acetate
The accumulation of acetate for different DO control strategies during GDH expression by E. coli SSGPA is shown in Fig. 4, along with the acetate synthesis rate. Lower concentrations of acetate accumulated at high DO levels, and the acetate synthesis rate increased with lower DO levels during early fermentation. The average values for the accumulation of acetate with DO control strategies I, II, III, and IV at the end of fermentation were 2.49 g/L, 2.41 g/L, 2.30 g/L, and 2.24 g/L, respectively. The accumulated acetate concentrations at the end of fermentation with DO control strategies III and IV were significantly different from those obtained using DO control strategies I and II (p < 0.05).
Mechanism analysis of acetate synthesis at different DO levels
Transcription levels of key genes for acetate synthesis
Based on the above results, due to the oxygen limitation with 10% DO level and the more appropriate DO level with DO control strategy IV, higher and lower concentration of acetate were accumulated, leading to lower and higher expression of GDH, respectively. Therefore, the acetate synthesis mechanism with 10% DO level and DO strategy IV were selected to investigate the effect of DO level on acetate synthesis. The transcription levels of key genes for acetate synthesis were measured at 10 h cultivation time for cultivation using DO strategy IV or a 10% DO level, as shown in Table 1. There were significant changes in the transcription levels of key genes for acetate synthesis at different DO levels. When the DO level was maintained at 30% using DO control strategy IV at 10 h, the transcription level of an acetate-producing gene (poxB) was lower, while levels of acetate-utilizing genes (acs and aceA) were higher. Additionally, higher transcription levels were observed for a TCA cycle-associated gene (gltA) and genes associated with gluconeogenesis and the anaplerotic pathway (pckA, ppsA, ppc, and sfcA).
Activity of key enzymes for acetate synthesis
Figure 5 shows the activities of key enzymes for acetate synthesis in cultures grown for GDH expression with DO control strategy IV or 10% DO. The activity of a key enzyme of the EMP pathway, Fbn, was decreased 6.48% with DO strategy IV compared to its activity at 10% DO. The activities of PoxB were 154 U/g and 196 U/g with DO strategy IV and 10% DO, respectively. Using DO strategy IV, the activities of Acs, Pck, Pps, Ppc, and Glt were increased by 13.71%, 14.97%, 18.60, and 10.71%, respectively, compared to activities at 10% DO.
Metabolic flux distribution of key nodes for acetate synthesis
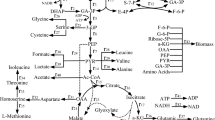
The metabolic flux distribution of key nodes for acetate synthesis was determined using DO control strategy IV and 10% DO during the later fermentation period (12–18 h) of GDH expression by E. coli SSGPA, and the results are presented in Fig. 6. The DO level affected the distribution of carbon metabolic flux. The carbon flux entering the EMP pathway was 86.7% at 10% DO and decreased to 74.4% for DO strategy IV. The flux for pyruvate synthesis from PEP with DO strategy IV was decreased by 17.88% compared with that at 10% DO, and the flux of pyruvate for acetyl-CoA and acetate formation with DO strategy IV also decreased by 7.69% and 27.43%, respectively, compared to those at 10% DO. Using DO strategy IV, more flux of acetyl-CoA went towards citrate formation, with lower flux of acetyl-CoA to formation of acetyl-AMP and acetate. With DO strategy IV, there was 5.9% carbon flux from acetate to acetyl-AMP synthesis, an increase of 12.29% compared with that at 10% DO. With lower flux for acetate synthesis and greater flux of acetate reused, the overall flux of acetate with DO strategy IV reduced to 8.4%, a decrease of 62.33% compared with that at 10% DO.
Discussion
Acetate overflow can reduce cell density and product yields (Bemal et al. 2016), and the variation of DO level could potentially alter bacterial metabolism and acetate formation, affecting both cell growth and recombinant proteins production capability (Phue and Shiloach 2005). Performing GDH expression at different DO levels, the excretion of acetate decreased with higher DO levels, indicating that the accumulation of acetate was inversely correlated to DO level (Zhao et al. 2020). The inhibition of cell growth and protein formation caused by acetate decreased due to reduced acetate excretion, and cell density and GDH concentration increased with DO level below 50%. However, the lowest cell density and GDH expression level were obtained when the lowest concentration of acetate was accumulated at a DO level of 100%, because the formation of ROS with high oxygen concentration caused cellular damage by altering enzymes, proteins, and DNA (Negrete and Shiloach 2017; Rui et al. 2010). For example, the expression level of porcine circovirus type 2 capsid protein by E. coli was lowest when the DO level was maintained at 100% (Cheng et al. 2015). Due to the oxygen limitation with DO level of 10%, the highest concentration of acetate was accumulated, leading to lower cell density and GDH expression. High cell density and GDH expression level were obtained with relatively high DO levels, and both cell density and GDH expression were higher with DO control strategies III and IV than those at 50% DO, which revealed that a DO level of 20% or 30% was better for GDH formation than 50%. Overall, the highest cell density and GDH concentration were obtained with DO control strategy IV because of the appropriate DO level, with values 1.59- and 1.99-times higher than those obtained when cultured at 10% DO, which showed that DO level was the key factor for GDH expression. In addition, cell density and GDH expression with DO control strategy IV were increased by 11.32% and 11.21% compared with those of 50% DO level, which were significantly different (p < 0.05).
Compared to the early fermentation period, there was decreased acetate accumulation with GDH expression during the later culture phase. Acetate can be consumed as a carbon and energy source to support cell growth after consumption of glucose, and the concentration of glucose reached zero when a DO feedback feeding strategy was used (Zhao et al. 2016). A previous study found 10 g/L of acetate accumulated in a strain deleted for ackA, but this amount decreased to 5.0 g/L at the end of fermentation, which was consistent with the change of acetate concentration during GDH expression (Parimi et al. 2017). Acs is a high-affinity enzyme that scavenges low acetate concentrations upon depletion of the main carbon source (Bemal et al. 2016; Pinhal et al. 2019), and the overexpression of Acs has been proposed as a potential strategy to reduce acetate accumulation (Parimi et al. 2017). During continuous culture, the expression of acs can lead to a futile cycle of simultaneous acetate production and scavenging, thus limiting acetate overflow (Bemal et al. 2016). The higher transcription level of acs and activity of Acs with DO control strategy IV increased the flux from acetate to acetyl-AMP synthesis, with decreased concentration of acetate compared to that at 10% DO level.
The PoxB pathway is the major pathway for acetate formation during the stationary phase, and deletion of poxB decreased the accumulation of acetate (Bemal et al. 2016). The lower transcription level of poxB and activity of PoxB resulted in a lower excretion of acetate (Phue and Shiloach 2005). The increasing transcription levels of ppsA, pckA, ppc, and sfcA and increasing enzyme activities of Pck, Pps, and Ppc could decrease the concentrations of pyruvate and Acetyl-CoA, and the synthesis flux of pyruvate and Acetyl-CoA at higher DO level (Phue and Shiloach 2005; Yang et al. 2018). Acetyl-CoA is a key substrate for acetate synthesis, with most acetyl-CoA entering the TCA cycle, leading to its complete oxidation into CO2 (Bemal et al. 2016). The unbalance between carbon source catabolism and cellular respiration forms a bottleneck at the pyruvate node and the accumulation of acetyl-CoA, leading to improved acetate accumulation (De Mets et al. 2019). As a result, higher transcription levels of aceA and gltA and increased enzyme activity of Glt increased the TCA cycle activity with DO control strategy IV, resulting in the accumulation of less acetate compared with that of 10% DO level.
Acetate is considered a typical “overflow” metabolite when the carbon fux into central metabolic pathways exceeds the cellular biosynthetic demands and capacity for energy generation (Parimi et al. 2017). The excretion of acetate can be affected by the flux ratio between the EMP and PP pathways, and acetate yield increased with increased EMP flux (Kamata et al. 2019). Due to the decreased flux for the EMP pathway caused by the transcription level of fbn and activity of Fbn with DO control strategy IV, there was less accumulation of acetate compared with cultivation at DO level of 10%. Additionally, the increased transcription of genes related to gluconeogensis and anaplerotic pathways and increased TCA cycle capacity at higher DO level resulted in less flux for pyruvate and acetyl-CoA synthesis and greater consumption of acetyl-CoA by the TCA cycle (Song et al. 2018). With lower flux for acetate synthesis and increased flux of acetate reused with DO control strategy IV, the overall flux of acetate was lower, and the accumulation of acetate was decreased by 40.74% compared with that at 10% DO level. According to the analysis of acetate synthesis with different DO levels, the accumulation of acetate was decreased obviously with an appropriate DO level, increasing both cell density and GDH expression.
Conclusion
DO level was identified as a key parameter for acetate synthesis and GDH expression. The transcriptional level of key genes, activity of a key enzyme, and metabolic flux of key nodes for acetate synthesis were all affected by DO level. Using DO control strategy IV (50% 0–9 h, 30% 9–18 h), the transcriptional level of an acetate-producing gene was lower, while levels of acetate-utilizing genes, a TCA cycle-associated gene, genes associated with gluconeogenesis and the anaplerotic pathway were higher, compared with the levels obtained at DO level of 10%. Additionally, the activities of key enzymes for acetate synthesis with DO control strategy IV or 10% DO level were consistent with the transcription level variation of these genes. The flux of acetate synthesis with DO strategy IV was 8.4%, a 62.33% decrease compared with that obtained using 10% DO level. Using the appropriate DO level with DO control strategy IV, the accumulation of acetate decreased to 2.24 g/L, which resulted in 15.43 g/L cell density and 3.42 g/L GDH concentration, amounts that were 1.59- and 1.99-times higher than those achieved at 10% DO level.
References
Baez A, Shiloach J (2013) Escherichia coli avoids high dissolved oxygen stress by activation of SoxRS and manganese superoxide dismutase. Microb Cell Fact 12:23
Bemal V, Castano-Cerezo S, Canovas M (2016) Acetate metabolism regulation in Escherichia coli: carbon overflow, pathogenicity, and beyond. Appl Microbiol Biotechnol 100(21):8985–9001
Castaño-Cerezo S, Pastor JM, Renilla S, Bemal V, Iborra JL, Canovas M (2009) An insight into the role of phosphotransacetylase (pta) and the acetate/acetyl-CoA node in Escherichia coli. Microb Cell Fact 8:54
Chen L, Zeng AP (2017) Rational design and metabolic analysis of Escherichia coli for effective production of l-tryptophan at high concentration. Appl Microbiol Biotechnol 101(2):559–568
Cheng LK, Tian FR, Fu Q, Li SG, Fu SJ, Yang XY, Qu GG, Miao LZ, Li F, Shen ZQ (2015) Application of dissolved oxygen stage control and dissolved oxygen feedback feeding to improve production of porcine circovirus type 2 capsid protein by Escherichia coli. J Chem Pharm Res 7(11):447–453
De Mets F, Van Melderen L, Gotlesman S (2019) Regulation of acetate netabolism and coordination with the TCA cycle via a processed small RNA. Proc Natl Aced Sci USA 116(3):1043–1052
Dong X, Chen X, Qian Y, Wang Y, Wang L, Qiao W, Liu L (2017) Metabolic engineering of Escherichia coli W3110 to produce L-malate. Biotechnol Bioeng 114(3):656–664
Eiteman MA, Altman E (2006) Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol 24(11):530–536
Enjalbert B, Milland P, Dinclaux M, Portais JC, Letisse F (2017) Acetate fluxes in Escherichia coli are determined by the thermodynamic control of the Pta-AckA pathway. Sci Rep 7:42135
Kamata K, Toya Y, Shimizu H (2019) Effect of precise control flux ratio between the glycolytic pathways on mevalonate production in Escherichia coli. Biotechnol Bioeng 116(5):1180–1188
Kumar R, Shimizu K (2010) Metabolic regulation of Escherichia coli and its gdhA, glnL, gltB, D mutants under different carbon and nitrogen limitations in the continuous culture. Microb Cell Fact 9:8
Kutz R, Okwumabua O (2008) Differentiation of highly virulent strains of Streptococcus suis serotype 2 according to glutamate dehydrogenase electrophoretic and sequence type. J Clin Microbiol 46(10):3201–3207
Lin CW, Cheng LK, Wang J, Zhang SL, Fu Q, Li SG, Li F, Han WY, Shen ZQ (2016) Optimization of culture conditions to improve the expression level of beta1-epstion toxin of Clostridium perfringens type B in Escherichia coli. Biotechnol Buotechnoll Equip 30(2):324–331
Lin PP, Jaeger AJ, Wu TY, Xu SC, Lee AS, Gao P, Chen PW, Liao JC (2018) Construction and evolution of an Escherichia coli strain relying on nonoxidative glycolysis for sugar catabolism. PNAS 115(14):3538–3546
Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ (2007) Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis 7(3):201–209
Mortazavi A, Williams BA, Mccue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628
Negrete N, Shiloach J (2017) Improving E. coli growth performance by manipulating small RNA expression. Microb Cell Fact 16(1):198
Parimi NS, Durie IA, Wu X, Niyas AM, Eiterman MA (2017) Eliminating acetate formation improves citramalate production by metabolically engineered Escherichia coli. Microb Cell Fact 16(1):114
Phue JN, Shiloach J (2005) Impact of dissolved oxygen concentration on acetate accumulation and physiology of E. coli BL21, evaluating transcription levels of key genes at different dissolved oxygen conditions. Metab Eng 7:353–363
Piao XY, Wang L, Lin BX, Chen H, Liu WF, Tao Y (2019) Metabolic engineering of Escherichia coli for production of L-aspartate ana its derivative β-alanine with high stoichiometric yield. Metab Eng 54:244–254
Pinhal S, Ropers D, Geiselmann J, de Jong H (2019) Acetate metabolism and the inhibition of bacterial growth by acetate. J Bacteriol 201(13):e00147-e219
Rajaraman E, Agrawal A, Crigler J, Seipelt-Thiemann R, Altman E, Eiteman MA (2016) Transcriptional analysis and adaptive evolution of Escherichia coli strains growing on acetate. Appl Microbiol Biotechnol 100(17):7777–7785
Rui B, Shen T, Zhou H, Liu JP, Chen JS, Pan XS, Liu HY, Wu JH, Zheng HR, Shi YY (2010) A systematic investigation of Escherichia coli central carbon metabolism in response to superoxide stress. BMC Syst Biol 4:122
Song HS, Seo HM, Jeon JM, Moon YM, Hong JG, Bhatia SK, Ahn J, Lee H, Kim W, Park YC, Choi KY, Kim YG, Yang YH (2018) Enhancd isobutanol production from acetate by combinatorial overexpression of acetyl-CoA synthetase and anaplerotic enzymes in engineered Escherichia coli. Biotechnol Bioeng 115(8):1971–1978
Tan ZG, Chen J, Zhang XL (2016) Systematic engineering of pentose phosphate pathway improves Escherichia coli succinate production. Biotechnol Biofuels 9:262
Veeravalli K, Schindler T, Dong E, Yamada M, Hamilton R, Laird MW (2018) Strain engineering to reduce acetate accumulation during microaerobic growth in Escheriichia coli. Biotechnol Prog 34(2):303–314
Wang J, Cheng LK, Wang J, Liu Q, Shen T, Chen N (2013) Genetic engineering of Escherichia coli to enhance production of L-tryptophan. Appl Microbiol Biotechnol 97:7587–7596
Wang Q, Xu JH, Sun ZJ, Luan LQ, Li Y, Wang JS, Liang QF, Qi QS (2019) Engineering an in vivo EP-bifido pathway in Escherichia coli for high-yield acetyl-CoA generation with low CO2 emission. Metab Eng 51:79–87
Wang J, Shang QM, Zhao CG, Zhang SS, Lin CW, Shen ZQ, Cheng LK (2020) Improvement of Streptococcus suis glutamate dehydrogenase expression in Escherichia coli through genetic modification of acetate synthesis pathway. Lett Appl Microbiol 70(2):64–70
Yang F, Yang L, Chang Z, Chang L, Yang B (2018) Regulation of virulence and motility cy acetate in enteropathogenic Escherichia coli. Int J Med Microbiol 308(7):840–847
Zhang CL, Li YJ, Ma J, Liu Y, He JL, Li YZ, Zhu FZ, Meng J, Zhan JJ, Li ZX, Zhao L, Ma Q, Fan XG, Xu QY, Xie XX, Chen N (2018) High production of 4-hydroxyisoleucine in Corynebacterium glutamicum by multistep metabolic engineering. Metab Eng 49:287–298
Zhao CG, Cheng LK, Xu QY, Wang J, Shen ZQ, Chen N (2016) Improvement of the production of L-tryptophan in Escherichia coli by application of a dissolved oxygen stage control strategy. Ann Microbiol 66(2):843–854
Zhao M, Huang DX, Zhang XJ, Koffas MAG, Zhou JW, Deng Y (2018) Metabolic engineering of Escherichia coli for producing adipic acid through the reverse adipate degradation pathway. Metab Eng 47:254–262
Zhao CG, Fang HT, Wang J, Zhang SS, Zhao XB, Li ZL, Lin CW, Shen ZQ, Cheng LK (2020) Application of fermentation process control to increase L-tryptophan production in Escherichia coli. Biotechnol Prog 36(2):e2944
Zheng S, Kwon I (2013) Controlling enzyme inhibition using an expression set of genetically encoded amino acids. Biotechnol Bioeng 110(9):2361–2370
Acknowledgements
This study was funded by the Major Science and Technology Innovation Project of Shandong (2019JZZY020606), the Development of Science and Technology Plan Program of Binzhou (2015ZC0107), the Technological Innovation Project of Shandong (201741916018), the Key Specialized Research and Development Breakthrough program in Henan Province (202102110101, 182102110347), Young Talent Lifting Project in Henan Province (2020HYTP041,2021HYTP038) , the National Natural Science Foundation of China (31702263) and the Youth Backbone Teacher Project of Colleges and Universities of Henan Province (2020GGJS163).
Author information
Authors and Affiliations
Contributions
LC, XX and ZS designed the research. LC and XX performed the experiments. LC, CZ, ZS, and CL acquired and analyzed the data. XZ, JW, JW, and LW contributed to writing of the manuscript. Finally, all authors have reviewed and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, L., Zhao, C., Yang, X. et al. Application of a dissolved oxygen control strategy to increase the expression of Streptococcus suis glutamate dehydrogenase in Escherichia coli. World J Microbiol Biotechnol 37, 60 (2021). https://doi.org/10.1007/s11274-021-03025-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03025-2