Abstract
Dissolved oxygen (DO) is a significant operational parameter in biological systems. In this study, a pilot-scale biofilter was constructed to investigate the removal efficiency of ammonia, iron and manganese, as well as the microbial community structure evolution at different DO concentrations. Results indicated that when DO decreased from 8 to 4 mg/L, iron and manganese were still completely removed, however the concentration of ammonia in the effluent increased, and exceeded the permitted limit of 0.5 mg/L when DO was about 4 mg/L. The main functional microbes for ammonia and manganese removal were Nitrosomonas and Crenothrix, which was mainly distributed at 0.8 and 0.8 m of the filter bed with a corresponding abundance of 8.61% and 16.87% in sufficient DO considition, respectively; while iron was mainly removed by Crenothrix and Gallionella in 0 m with a corresponding abundance of 30.45% and 9.77%. With the decreasing of DO concentration, iron oxidizing bacteria (IOB, Crenothrix and Gallionella) was not affected, while the abundance of manganese oxidizing bacteria (MnOB, Crenothrix) increased to completely oxidize manganese. However, the amount of ammonia oxidizing bacteria (AOB, Nitrosococcus) at 0.4 and 0.8 m of the filter depth obviously decreased with increased ammonia in the effluent. Kinds of other bacteria which may be related to methane, hydrogen sulfide and organic matter removal, were also found. In addition, small part of archaea was also detected, such as Candidatus Nitrososphaera and Ferroplasma, which could oxdize ammonia and ferrous iron, respectively.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ferrous iron and bivalent manganese associated with ammonia are widely exist in groundwater, especially in Northeast China (Qin et al. 2009; Cheng 2016; Zeng et al. 2015). The presence of iron and manganese in drinking water treatment could result in aesthetic and operational problems, such as staining on laundry and sanitary facilities, giving water an unpleasant metallic taste, and reducing pipe diameter when iron and manganese deposits build up in pipelines (Zeng et al. 2015). Meanwhile, ammonia present in drinking water treatment could affect the chlorination process, since ammonia could react with chlorine to form disinfection by-products (Hasan et al. 2013; Richardson and Postigo 2012; Du et al. 2017), which could produce deteriorate taste and odor of water (Cheng et al. 2017a, b), reduce disinfection efficiency (Hasan et al. 2014), and damage human nervous system (Cheng et al. 2017a). Furthermore, ammonia would interfere with the manganese biofiltration process by consuming excessive oxygen during nitrification stage, resulted in mouldy and earthy tasting water (Hasan et al. 2014). Therefore, the presence of iron, manganese and ammonia in drinking water should be limited and the maximum contaminant levels (MCLs) for total iron of 0.3 mg/L, manganese of 0.1 mg/L and ammonia of 0.5 mg/L have been established in China (GB 5749-2006).
Biological removal of ammonia, iron and manganese is preferable compared with chemical methods, since it is not necessary to add extra chemicals which may introduce other pollutants into the produced water and produce potential hazardous by-products (Cai et al. 2015; Tekerlekopoulou et al. 2013). As demonstrated, ammonia, iron and manganese could be simultaneously removed in biological systems (Tekerlekopoulou and Vayenas 2008; Hasan et al. 2012). Biological ammonia oxidation could be achieved by ammonia oxidizing bacteria (AOB) and nitrite oxidizing bacteria (NOB), such as Nitrosomonas halophila, Nitrosomonas europaea, Comamonas, Nitrospira, Nitrosomonas mobilis and Acinetobacter (Hasan et al. 2012; Li et al. 2013). Iron and manganese could be biologically oxidized by iron oxidizing bacteria (IOB) and manganese oxidizing bacteria (MnOB), respectively. So far, several types of bacteria have been confirmed as IOB [Crenothrix, Leptothrix discophora, Leptothrix, Gallionella and Bacillus (Li et al. 2013; Yang et al. 2014)], and MnOB (Leptothix, Gallionella, Hyphomicrobium, Pseudomonas, Crenothix, Siderocapsa and Metalloaenium (Hasan et al. 2012; Tang et al. 2016; Mckee et al. 2016; Granger et al. 2014)).
Dissolved oxygen is a significant operational parameter in biological systems, since it was utilized to oxidize ammonia, iron and manganese. Moreover, energy is needed in general when oxygen dissolves in water, so the higher DO in water, the higher energy consumption and operational cost (Cheng 2016). In recent years, researches had been conducted to investigate the ammonia, iron and manganese removal from different perspectives, such as interactions among ammonia, iron and manganese removal (Tekerlekopoulou and Vayenas 2007), reaction dynamics of biological ammonia, iron and manganese removal (Tekerlekopoulou and Vayenas 2008), and the microbial community profiles of the microorganisms in the biofilter for iron, ammonia and manganese removal (Li et al. 2013). It should be noted that DO in their biological systems was sufficient for ammonia, iron and manganese oxidization, or even 7–8 mg/L. However, few researchers focused on the removal efficiency of ammonia, iron and manganese, as well as the distribution and genetic diversity of the microorganisms in biofilte when DO was insufficient.
In this study, a pilot-scale biofilter system was constructed for ammonia, iron and manganese removal from real groundwater. The biofilter was operated approximately 160 days to evaluate removal efficiency of ammonia, iron and manganese, and the microbial community structures at different DO concentrations were analyzed and compared using 454 HTP (DO in the influent was about 8, 6 and 4 mg/L, respectively, and theoretical oxygen demand to completely oxidize Fe2+, Mn2+ and NH4+–N in raw water is calculated as follows (Li et al. 2013), [O2] = 0.14[Fe2+] + 0.29[Mn2+] + 4.57[NH4+–N] = 7.23 mg/L, where the concentration of Fe2+, Mn2+ and NH4+–N was calculated as average concentration in the formula). The work herein aimed at gain to a deep insight into the relationship between the microbial community structures and the removal efficiency of the pollutants, which would provide significant guidance for optimization of operation parameters for biofilter to reduce energy consumption and operational cost.
Materials and methods
Description of biofilter system
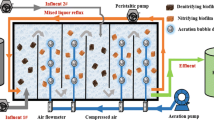
A pilot-scale biofilter system employed to simultaneously remove ammonia, iron and manganese was established in a groundwater treatment plant (GWTP) located in Harbin city, P.R. China (Fig. 1). The biofilter consisted of a plexiglass column with a height of 3000 mm and an inner diameter of 250 mm. A mixing chamber used to mix the incoming waters was set at the top of the biofilter, and a perforated plate used to support the media and collect the treated water was located at the bottom. 3 media sampling ports at 0, 400 and 800 mm and 17 water sampling ports at 100 mm intervals were distributed along the height of the column from media top to bottom. Tanks 1 (volume 1000 L) and 2 (volume 50 L) were used to collect raw groundwater, which was obtained from the GWTP. The raw groundwater was sprayed to tank 1, and then aerated to achieve the concentration of DO at about 8 mg/L; while DO in tank 2 was about 0.2 mg/L.
Two kinds of support materials were packed in the biofilter, and the upper part of the media (300 mm) was columnar anthracite with a height of 5 mm and a mean diameter of 1 mm, while the lower part (1200 mm) was manganese sand with a mean diameter of 0.8–1 mm. Down-flow pattern was adopted, and the amount of flow was controlled at the entry point (Cheng 2016). In addition, the biofilter was operated about 8 months before this experiment. In this experiment, real groundwater extracted from the wells with a depth of 40–50 m, in Harbin city, P.R. China was used. The concentration of ammonia, total iron and manganese in raw groundwater was about 0.9–1.4, 6–14 and 0.9–1.4 mg/L, respectively, and the temperature was 7.8 °C.
Operation of the biofilter system
Water in tank 1 was pumped to the biofilter to investigate the removal efficiency of ammonia, iron and manganese at sufficient DO condition. About a month later, the waters in tanks 1 and 2 were both pumped into the biofilter with a suitable proportion to ensure DO in the influent was about 6 and 4 mg/L, respectively. The flow rate of the water in tank 2 was almost constant, because DO was very low and few ferrous irons were oxidized to iron hydroxides, which could reduce the pipe diameter and flow rate. Therefore, the flow rate of the water in tank 1 was regulated to ensure the proportion of the waters in tanks 1 and 2 was suitable. The biofilter was operated about 2 months when DO in the influent was about 6 or 4 mg/L. The flow rate of the biofilter was fixed at 4 m/h in this experiment because of high iron concentration in the influent.
Sampling and analytical procedure
Analysis of DO and pH were conducted using a DO meter (Oxi 315i-WTW) and a pH meter (pH 315i-WTW), respectively. Ammonia, iron and manganese concentration was measured by photometric method according to Standard Methods for the Examination of Water and Wastewater (Rittmann and Snoeyinck 1984). The filter media (manganese sand or anthracite) were collected at 0, 400 and 800 mm of the filter bed, and stored at − 80 °C for further analysis. Furthermore, the microorganisms in mature media from the biofilter (day 156) was examined by scanning electron microscopy (SEM, JSM-6480LV) according to previous studies (Tang et al. 2016).
Microbial diversity analysis
DNA extraction and polymerase chain reaction (PCR) amplification
Media samples from 0, 400 and 800 mm of the filter layer were explored to deeply understand the microbial community structure evolution at different DO concentrations. These samples are analyzed by 454 HTP at days 35 (DO8-0, DO8-0.4 and DO8-0.8), 95 (DO6-0, DO6-0.4 and DO6-0.8) and 156 (DO4-0, DO4-0.4 and DO4-0.8). DNA was extracted from the media samples by Powersoil DNA Isolation Kit (MoBio Laboratories Inc, USA). The quality of the DNA was examined by 1% (w/v) agarose gel electrophoresis and the concentration was measured with a UV–Vis spectrophotometer (NanoDrop 2000, USA) (Li et al. 2013). The V3–V4 region of the 16S rRNA gene was amplified using bacterial primers 515F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′). PCR amplifications were carried out in a GeneAmp 9700 thermocycler (ABI, USA) according to previous research (Cheng et al. 2017b).
454 high-throughput 16S rRNA gene pyrosequencing
Sequencing of the DNA samples was carried out using the same method as previous research (Cheng et al. 2017b). Raw sequence data of this study was deposited to the NCBI Sequence Read Archive with accession No. SRP095713.
Biodiversity analysis and phylogenetic classification
Low quality reads (ambiguous nucleotides and quality value < 20) were removed from the raw sequence data as described in (Caporaso et al. 2011). The paired-end reads from the samples were overlapped to assembly V3–V4 tags of 16S rRNA gene using SeqPrep (https://github.com/jstjohn/SeqPrep), and chimera sequences from the tags were removed by usearch6. Eventually, the numbers of high quality sequences were 11, 430 (DO8-0), 11, 691 (DO8-0.4), 11, 123 (DO8-0.8), 15, 512 (DO6-0), 14, 181 (DO6-0.4), 22, 134 (DO6-0.8), 12, 793 (DO4-0), 5, 427 (DO4-0.4) and 16, 520 (DO4-0.8) with an average length of 291 bp. The sequences were processed using the same method as previous research (Cheng et al. 2017b).
Results
Overall performance of the biofilter and SEM images
When DO in the influent was sufficient (about 8 mg/L), ammonia, total iron and manganese in the effluent was lower than 0.1, 0.1 and 0.05 mg/L (Table 1), and the corresponding removal efficiencies were 97.2%, 98.7% and 99.6%, respectively. When DO in the influent was reduced to about 6 and 4 mg/L, ammonia in the effluent obviously increased to 0.2 and 0.6 mg/L, respectively, which exceeded the MCL. However, removal efficiency of iron and manganese was not affected by decreased DO concentrations.
Iron and ammonia were removed from the top of the filter depth, and total iron and ammonia decreased to 0.13 and 0.11 mg/L in 0.2 and 0.8 m of the filter depth (Table 2), respectively. While manganese was removed from 0.2 m of the filter depth, and decreased to 0.024 mg/L in 1.2 m. When DO was reduced to about 6 mg/L, iron and manganese were mainly removed in 0–0.2 and 0.2–0.8 m, respectively. Ammonia was quickly oxidized in 0–0.2 m, oxidized in 0.2–0.8 m, and slowly oxidized in 0.8–1.5 m, since DO was only 0.43 mg/L in 0.8 m. When DO was reduced to about 4 mg/L, the pollutants along the filter depth was varied in the same way.
SEM images of the mature media were examined to verify the existence and growth of bacteria in the biofilter. Plenty of microorganisms were observed in the micrograph, and the surfaces of these bacteria were covered by large amounts of iron and manganese oxides (Fig. S1). Two species of microorganisms, which had a very typical structure of twisted stalk and a rod-shaped, were observed.
Richness and diversity of microbial community
The parameters related to the alpha diversity of microbial community for the nine samples at distance cutoff level of 0.03 were shown in Table 3. 1, 021 (DO8-0), 1, 118 (DO8-0.4), 1, 150 (DO8-0.8), 972 (DO6-0), 984 (DO6-0.4), 1, 674 (DO6-0.8), 1, 037 (DO4-0), 613 (DO4-0.4) and 1, 487 (DO4-0.8) OTUs were clustered at a 3% distance. The total number of OTUs estimated by Chao1 estimator were 1, 929 (DO8-0), 2, 554 (DO8-0.4), 2, 079 (DO8-0.8), 1, 715 (DO6-0), 1, 921 (DO6-0.4), 2, 411 (DO6-0.8), 1, 945 (DO4-0), 1, 775 (DO4-0.4) and 2, 351 (DO4-0.8) with infinite sampling, indicating that DO8-0.4 had the greatest richness and DO6-0 had the lowest richness. The pyrosequencing of the nine samples revealed that the bacterial communities were obviously different along the filter depths at different phases. The Shannon diversity index provides not only the simply species richness, but also how the abundance of each species is distributed among all the species in the community. DO6-0.8 had the highest diversity (Shannon = 8.54) among the nine communities, while DO4-0.4 had the lowest diversity (Shannon = 6.04).
Principal component analysis (PCA) is one of the most commonly used tools in the analysis of ecological data. This method reduces the effective dimensionality of a multivariate data set by producing linear combinations of the original variables (i.e., components) that summarize the predominant patterns in the data. Therefore, PCA was used to analyze the relationship between the DO concentrations and microbial community structure, the results indicated that DO obviously affected the microbial community structure (Fig. 2). The bacterial communities in DO8-0.8 were obviously different from the others, but the bacterial communities in DO4-0.8 and DO6-0.8 were similar.
Taxonomic complexity of the bacterial community
The phylogenetic diversity of bacterial communities from the nine samples at phylum level is shown in Fig. 3a, demonstrating a very high diversity, reflected in the fact that 32 (DO8-0), 31 (DO8-0.4), 29 (DO8-0.8), 31 (DO6-0), 29 (DO6-0.4), 38 (DO6-0.8), 30 (DO4-0), 27 (DO4-0.4) and 39 (DO4-0.8) identified bacterial phyla were detected. 43 identified bacterial phyla and 3 identified archaea phyla in total were observed. Even so, 1.31% (DO8-0), 1.18% (DO8-0.4), 2.91% (DO8-0.8), 1.51% (DO6-0), 2.84% (DO6-0.4), 3.78% (DO6-0.8), 1.79% (DO4-0), 1.45% (DO4-0.4) and 2.92% (DO4-0.8) of the total reads in each sample were not classified, indicating these bacteria are unknown. Proteobacteria which existed widely in biofilters treated groundwater containing iron and manganese (Katsoyiannis and Zouboulis 2004), was the dominant bacterial phyla, and accounted for 76.32% (DO8-0), 78.20% (DO8-0.4), 67.99% (DO8-0.8), 84.59% (DO6-0), 79.64% (DO6-0.4), 49.16% (DO6-0.8), 80.46% (DO4-0), 82.74% (DO4-0.4) and 54.85% (DO4-0.8) of the total reads. Nitrospirae which involved in nitrification (Lu et al. 2012), existed in the nine communities, and was highest in abundance in DO4-0.8 (1.86%).
The class level identification of the bacterial communities in the nine samples was illustrated in Fig. 3b. 108 bacterial classes and 5 archaea classes were detected in the nine communities, and 71, 65, 71, 71, 72, 94, 72, 54 and 90 bacterial classes were detected in DO8-0, DO8-0.4, DO8-0.8, DO6-0, DO6-0.4, DO6-0.8, DO4-0, DO4-0.4 and DO4-0.8, respectively. Betaproteobacteria and Gammaproteobacteria were the dominant bacterial community in all nine communities, and the sum of these two classes accounted for 66.61% (DO8-0), 68.05% (DO8-0.4), 59.37% (DO8-0.8), 69.25% (DO6-0), 65.25% (DO6-0.4), 38.43% (DO6-0.8), 73.05% (DO4-0), 75.91% (DO4-0.4) and 44.07% (DO4-0.8) of the total reads.
At the genus level, 6.19% (DO8-0), 5.82% (DO8-0.4), 9.34% (DO8-0.8), 7.22% (DO6-0), 5.05% (DO6-0.4), 8.15% (DO6-0.8), 5.50% (DO4-0), 4.46% (DO4-0.4) and 9.79% (DO4-0.8) of the total reads in each sample were not classified (Fig. 3c). The dominant population in DO8-0 (30.45%), DO8-0.4 (29.60%), DO6-0 (30.21%), DO6-0.4 (30.76%), DO6-0.8 (18.50%), DO4-0 (31.83%), DO4-0.4 (37.24%) and DO4-0.8 (20.30%) was Crenothrix, which could oxidize not only iron but also manganese; while the dominant population in DO8-0.8 (18.14%) was Achromobacter, which could oxidize organic matter (Lasík et al. 1993). Five kinds of IOB were found in the biofilter, including Crenothrix, Gallionella, Leptospirillum, Bacillus and Pseudomonas, and six kinds of MnOB were found, including Crenothrix, Bacillus, Gallionella, Pseudomonas, Arthrobacter and Hyphomicrobium. The appearance of AOB (Nitrosomonas, Nitrosococcus and Nitrosovibrio) and NOB (Nitrospira) demonstrated that nitritification and nitratification process occurred in the biofilter to remove ammonia and nitrite, respectively (Lu et al. 2012; Li et al. 2017). In addition, several kinds of other genera were present in the biofilter, for example, Methylotenera and Methylomonas may oxidize methane, Paludibacter, Novosphingobium, Flavobacterium and Burkholderia may be organic matter oxidizers, and Sulfuritalea may be sulfur oxidizing bacterium. This is because the chemical composition in the real groundwater was complex.
When DO in the influent was sufficient (day 35), IOB-Crenothrix (30.45%) and Gallionella (9.77%) were found in 0 m of the filter depth with a high abundance, which was agreed with the previous result that iron was mainly removed in 0–0.2 m. In the biofilter, Crenothrix and Gallionella not only oxidized iron but also manganese, and manganese removal was occurred after ferrous iron was completely oxidized (Cheng 2016); therefore, Crenothrix and Gallionella oxidized manganese in 0.4–0.8 m of the filter depth (the concentration of ferrous iron in 0.4 m could not be detected). The abundance of Crenothrix and Gallionella decreased from 29.60% and 8.16% (0.4 m) to 16.87% and 3.28% (0.8 m), which was consistent with the concentration of manganese that decreased from 0.33 mg/L (0.4 m) to 0.16 mg/L (0.8 m). The abundance of Nitrosococcus increased from 1.51% (DO8-0) to 2.46% (DO8-0.4) and 8.61% (DO8-0.8) along the filter depth, while ammonia decreased from 1.18 mg/L (0 m) to 0.15 (0.4 m) and 0.11 mg/L (0.8 m).
When DO in the influent decreased to about 6 mg/L (day 95), DO was insufficient to completely oxidize ammonia, iron and manganese. The abundance of IOB (Crenothrix and Gallionella) in 0 m of the filter depth was hardly changed with decreased DO concentration. The abundance of MnOB-Crenothrix and Gallionella slightly increased in 0.4 m of the filter depth, but the concentration of manganese was hardly varied. And the abundance of Crenothrix in 0.8 m increased from 16.87% (DO8-0.8) to 18.50% (DO6-0.8). AOB-Nitrosococcus was affected significantly by DO, and the abundance of Nitrosococcus along the filter depth obviously decreased from 1.51% (DO8-0), 2.46% (DO8-0.4) and 8.61% (DO8-0.8) to 0.69% (DO6-0), 0.72% (DO6-0.4) and 3.94% (DO6-0.8), respectively; correspondingly the concentration of ammonia in 0.4 and 0.8 m of the filter depth increased from 0.15 to 0.11 mg/L to 0.36 and 0.31 mg/L, respectively.
When DO in the influent decreased to about 4 mg/L (day 156), iron was also completely removed, and the abundance of AOB-Crenothrix and Gallionella in 0 m of the filter depth was hardly varied, too. Manganese in 0.4 and 0.8 m of the filter depth was slightly changed, but the abundance of MnOB-Crenothrix in 0.4 and 0.8 m obviously increased from 30.76% (DO6-0.4) and 18.50% (DO6-0.8) to 37.24% (DO4-0.4) and 20.30% (DO4-0.8), respectively. The abundance of Nitrosococcus in 0 and 0.4 m decreased from 0.69% (DO6-0) and 0.72% (DO6-0.4) to 0.22% (DO4-0) and 0.20% (DO4-0.4), respectively, and ammonia in 0.4 m increased from 0.36 to 0.75 mg/L accordingly. Surprisingly, the abundance of Nitrosococcus in 0.8 m increased from 3.94% (DO6-0.8) to 4.21% (DO4-0.8), although ammonia obviously increased from 0.31 to 0.69 mg/L.
Composition of the archaea community
The numbers of reads of the archaea 16S rRNA gene accounted for 0.33% (DO8-0), 0.34% (DO8-0.4), 0.29% (DO8-0.8), 0.61% (DO6-0), 1.19% (DO6-0.4), 1.18% (DO6-0.8), 0.76% (DO4-0), 0.47% (DO4-0.4) and 0.54% (DO4-0.8) of the total 16S rRNA genes. 3 archaea phyla-[Parvarchaeota], Crenarchaeota and Euryarchaeota, and 5 archaea classess-Thaumarchaeota, MCG, Thermoplasmata, Methanomicrobia and Methanobacteria were detected in all nine communities (Fig. 4). The dominant phylum in the nine communities was Euryarchaeota, while the dominant class was Thermoplasmata. And the dominant genera were Ferroplasma (40.00%, 46.88%, 32.14%, 38.46%, 19.23%, 20.75%, 40.98%, 47.06%, and 27.27%) and Thermogymnomonas (30.00%, 31.25%, 25.00%, 34.62%, 46.15%, 33.96%, 39.34%, 17.65%, and 33.77%) (Fig. 5). In addition, Ferroplasma is iron oxidizing archaea (IOA) (Rawlings 2005), Candidatus Nitrososphaera is ammonia oxidizing archaea (AOA) (Oishi et al. 2012), and Methanosarcina, Methanospirillum, Methanobacterium, Methanosaeta, Methanoculleus and Candidatus Methanoregula, were possible related to methane oxidizing (Bharathi and Chellapandi 2017; Rocheleau et al. 1999).
Discussion
In this study, the removal performance of ammonia, iron and manganese in the biofliter at different DO conditions was investigated. An excellent removal efficiency for ammonia, iron and manganese was achieved in sufficient DO condition. This due to plenty of AOB, NOB, IOB and MnOB attached on the media of biofilter, which could oxidize the pollutants quickly. When DO in the influent was reduced to about 6 and 4 from 8 mg/L, ammonia in the effluent obviously increased, but removal efficiency of total iron and manganese were not affected. The reasons could be explained as followed: (1) Iron was removed at the top of the filter depth, where DO was sufficient. (2) MnOB could utilize DO preferentially to oxidize manganese when DO was insufficient (Cheng 2016). The variation of ammonia, iron and manganese along the filter depth indicated that iron and ammonia were simultaneouly removed from the top of filter media. Since manganese removal only took place after ferrous iron was oxidized completely due to ferrous iron could react with manganese oxides, iron was mainly removed in 0–0.2 m, while ammonia and manganese were simultaneouly removed in 0.2–0.8 m. The concentration of iron and manganese along the filter depth was not affected by DO, while ammonia increased with the decreasing of DO concentrations. The biofilter could be operated at relatively low DO condition to save energy consumption and reduce operating cost, since when DO in the influent was about 6 mg/L, ammonia, iron and manganese were all below the MCLs in this experiment.
Large amounts of iron and manganese oxides, which covered on the surfaces of the bacteria in SEM images, evidenced that microorganisms acted as catalytic role in iron and manganese removal from groundwater (Yang et al. 2014). A type of microorganisms which presented typical structure of twisted stalk may be recognized as the most common IOB and MnOB (Gallionella), and it is very common microorganisms in groundwater treatment plants for iron and manganese removal (Li et al. 2013; Hallbeck and Pedersen 1990; Katsoyiannis and Zouboulis 2004). Another type of microorganisms presented rod-shaped characteristic may be recognized as bacillus, which is an endospore-forming bacterium commonly found in water sources and soil (Kunst et al. 1995). This bacterium has the ability to catalyze manganese oxidation (Yang et al. 2014).
The distribution and genetic diversity of the microorganisms in the biofilter at different DO conditions was also investigated. Under sufficient DO condition, iron was mainly oxidized by IOB-Crenothrix and Gallionella in 0 m of the filter depth, while MnOB-Crenothrix and Gallionella oxidized manganese in 0.4–0.8 m, since manganese removal could be occurred after ferrous iron was completely oxidized (Cheng 2016). The abundance of Nitrosococcus (AOB) increased form 0 to 0.4 and 0.8 m, but removal amount of ammonia decreased. The reason could be explained as the quantity of microbe in DO8-0 was much higher than that in DO8-0.4 and DO8-0.8, and although the quantity of Nitrosococcus obviously decreased from DO8-0 to DO8-0.4 and DO8-0.8, the abundance of Nitrosococcus increased. The main functional microbes for ammonia and manganese removal were Nitrosomonas and Crenothrix, respectively, which was mainly distributed at 0.8 and 0.8 m of the filter bed with a corresponding abundance of 8.61% and 16.87% under sufficient DO condition, respectively; while iron was mainly removed by Crenothrix and Gallionella in 0 m with a corresponding abundance of 30.45% and 9.77%.
Under insufficient DO condition, the abundance of IOB (Crenothrix and Gallionella) in 0 m of the filter depth was hardly changed, since iron was mainly removed at the top of the filter depth where DO was sufficient, and the activity of IOB was not affected. When DO decreased to about 6 and 4 mg/L, the abundance of MnOB-Crenothrix increased in 0.4 and 0.8 m, and MnOB-Gallionella slowly increased in 0.4 m, and silightly varied in 0.8 m; while the concentration of manganese was hardly varied. The reasons may be as follows: (1) kinds of bacteria cannot adapt the low DO condition, resulting in the amount of total bacteria decreased with the decreasing of DO concentration. (2) The activity of Crenothrix and Gallionella decreased with the decreasing of DO concentration, and larger number of Crenothrix and Gallionella was needed to oxidize manganese. (3) Adaptive ability of Crenothrix was stronger than Gallionella at low DO condition. MnOB have an advantage over AOB in utilizing DO at low DO condition (DO < 0.5 mg/L) (Cheng 2016), leading to the increase of ammonia in 0.4 and 0.8 m in insufficient DO condition. With the decreasing of DO concentration, the abundance of AOB-Nitrosococcus decreased in 0 and 0.4 m, agreed with the increase of ammonia in 0.4. But Nitrosococcus first decreased and then increased in 0.8 m, The reason may be that the total amount of microorganism in 0.8 m largely decreased as DO decreased to 0.21 mg/L, while the AOB slightly decreased, so the abundance of Nitrosococcus increased.
The concentration of ammonia and manganese in raw groundwater was about 0.9–1.4 and 0.9–1.4 mg/L, respectively, but the abundance of AOB-Nitrosococcus was obviously lower than MnOB in the nine samples, which was agreed with our previous research (Cheng et al. 2017a, b). The reason may be that AOB-Nitrosococcus could oxidize ammonia with much higher efficiency compared with MnOB.
Although the proportion of archaea was very low compared with bacteria in the biofilter, the archaea played an significant role in ammonia and iron removal, such as IOA-Ferroplasma and AOA-Nitrososphaera were found in the biofilter. Leininger et al. (2006) demonstrated that archaea are far more important than bacteria for nitrification in different soils and suggested that they are the major ammonia oxidizing microorganisms in soil ecosystems around the world (Leininger et al. 2006). It is necessary to investigate the distribution and the role of archaea in the biofilters in the near future.
When DO in the influent decreased from about 8 to 4 mg/L, iron and manganese were still completely removed, while ammonia exceeded the MCL when DO was about 4 mg/L, indicating that the biofliter could be operated at relatively low DO condition (6 mg/L) to save energy consumption and reduce operating cost. AOB, NOB, AOA, IOB, IOA, MnOB which were related to ammonia, iron and manganese removal were found in the biofilter treating real groundwater, respectively. IOB-Crenothrix and Gallionella oxidized ferrous iron at the top of the filter depth, therefore, IOB were not affected by DO. The abundance of MnOB-Crenothrix increased in 0.4 and 0.8 m since the activity of the MnOB decreased with the decrease of DO concentration. The amount of AOB-Nitrosococcus in 0.4 and 0.8 m obviously decreased with the increase of ammonia. The microbial community structure was obviously changed as DO decreasing from about 8 to 4 mg/L, proving that DO was an important operational parameter.
References
Bharathi M, Chellapandi P (2017) Intergenomic evolution and metabolic cross-talk between rumen and thermophilic autotrophic methanogenic archaea. Mol Phylogenet Evol 107:293–304
Cai YA, Li D, Liang YW, Luo YH, Zeng HP, Zhang J (2015) Effective start-up biofiltration method for fe, mn, and ammonia removal and bacterial community analysis. Bioresource Technol 176:149–155
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108:4516–4522
Cheng QF (2016) Competitive mechanism of ammonia, iron and manganese for dissolved oxygen using pilot-scale biofilter at different dissolved oxygen concentrations. Water Sci Technol 16(3):766–774
Cheng QF, Nengzi LC, Xu DY, Guo JY, Yu J (2017a) Influence of nitrite on the removal of Mn(II) using pilot-scale biofilters. J Water Reuse Desa 7:264–271
Cheng QF, Nengzi LC, Bao LL, Huang Y, Liu SY, Cheng XW, Li B, Zhang J (2017b) Distribution and genetic diversity of microbial populations in the pilot-scale biofilter for simultaneous removal of ammonia, iron and manganese from real groundwater. Chemosphere 182:450–457
Du X, Liu GY, Qu FS, Li K, Shao SL, Li GB, Liang H (2017) Removal of iron, manganese and ammonia from groundwater using a PAC-MBR system: the anti-pollution ability, microbial population and membrane fouling. Desalination 403:97–106
Granger HC, Stoddart AK, Gagnon GA (2014) Direct biofiltration for manganese removal from surface water. J Environ Eng 140:223–224
Hallbeck L, Pedersen K (1990) Culture parameters regulating stalk formation and growth-rate of Gallionella–Ferruginea. J Gen Appl Microbiol 136:1675–1680
Hasan HA, Abdullah SRS, Kofli NT, Kamarudin SK (2012) Effective microbes for simultaneous bio-oxidation of ammonia and manganese in biological aerated filter system. Bioresource Technol 124:355–363
Hasan HA, Abdullah SRS, Kamarudin SK, Kofli NT (2013) On-off control of aeration time in the simultaneous removal of ammonia and manganese using a biological aerated filter system. Process Saf Environ 91:415–422
Hasan HA, Abdullah SRS, Kamarudin SK, Kofli NT, Anuar N (2014) Kinetic evaluation of simultaneous cod, ammonia and manganese removal from drinking water using a biological aerated filter system. Sep Purif Technol 130:56–64
Katsoyiannis IA, Zouboulis AI (2004) Biological treatment of Mn(II) and Fe(II) containing groundwater: kinetic considerations and product characterization. Water Res 38:1922–1932
Kunst F, Vassarotti A, Danchin A (1995) Organization of the European Bacillus subtilis genome sequencing project. Microbiology 389:84–87
Lasík J, Koníček J, Roušalová O (1993) Production of an exocellular polymer in a mutant strain of Achromobacter delicatulus, on a new type of stirrer. Folia Microbiol 38:71–73
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammoniaoxidizing prokaryotes in soils. Nature 442(7104):806–809
Li XK, Chu ZR, Liu YJ, Zhu MT, Yang L, Zhang J (2013) Molecular characterization of microbial populations in full–scale biofilters treating iron, manganese and ammonia containing groundwater in Harbin, China. Bioresource Technol 147:234–239
Li D, Stanford B, Dickenson E, Khunjar WO, Homme CL, Rosenfeldt EJ, Sharp JO (2017) Effect of advanced oxidation on N-nitrosodimethylamine (NDMA) formation and microbial ecology during pilot-scale biological activated carbon filtration. Water Res 113:160–170
Lu L, Xing DF, Ren NQ (2012) Pyrosequencing reveals highly diverse microbial communities in microbial electrolysis cells involved in enhanced H2 production from waste activated sludge. Water Res 46:2425–2434
Mckee KP, Vance CC, Karthikeyan R (2016) Biological manganese oxidation by pseudomonas putida in trickling filters. J Environ Sci Heal Part A 51:523–535
Oishi R, Tada C, Asano R, Yamamoto N, Suyama Y, Nakai Y (2012) Growth of ammonia-oxidizing archaea and bacteria in cattle manure compost under various temperatures and ammonia concentrations. Microbial Ecol 63:787–793
Qin SY, Ma F, Huang P, Yang JX (2009) Fe (II) and Mn (II) removal from drilled well water: a case study from a biological treatment unit in Harbin. Desalination 245:183–193
Rawlings DE (2005) Characteristics and daptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microbial Cell Factories 4(1):13
Richardson SD, Postigo C (2012) Drinking water disinfection by-products. The handbook of environmental chemistry emerging organic contaminants and human health, vol 20. Springer, New York, Chap. 4, pp. 93–137
Rocheleau S, Greer CW, Lawrence JR, Cantin C, Larame´e L, Guiot SR (1999) Differentiation of Methanosaeta concilii and Methanosarcina barkeri in anaerobic mesophilic granular sludge by fluorescent in situ hybridization and confocal scanning laser microscopy. Appl Environ Microbiol 65:2222–2229
Tang WW, Gong JM, Wu LJ, Li YF, Zhang MT, Zeng XP (2016) DGGE diversity of manganese mine samples and isolation of a lysinibacillus sp. efficient in removal of high Mn (II) concentrations. Chemosphere 165:277–283
Tekerlekopoulou AG, Vayenas DV (2007) Ammonia, iron and manganese removal from potable water using trickling filters. Desalination 210:225–235
Tekerlekopoulou AG, Vayenas DV (2008) Simultaneous biological removal of ammonia, iron and manganese from potable water using a trickling filter. Biochem Eng J 39:215–220
Tekerlekopoulou AG, Pavlou S, Vayenas DV (2013) Removal of ammonium, iron and manganese from potable water in biofiltration units: a review. J Chem Techno Bio 88:751–773
Yang L, Li XK, Chu ZR, Ren YH, Zhang J (2014) Distribution and genetic diversity of the microorganisms in the biofilter for the simultaneous removal of arsenic, iron and manganese from simulated groundwater. Bioresource Technol 156:384–388
Zeng XP, Xia J, Wang ZZ, Li WH (2015) Removal of iron and manganese in steel industry drainage by biological activated carbon. Desalination Water Trea 56:2543–2550
Acknowledgements
This work was kindly supported by National Natural Science Foundation of China (51808062 and 51608061), and Department of Science and Technology of Sichuan Province (19ZDYF0150).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Research involving human and animal rights
This research did not involve Human Participants and Animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, Q., Huang, Y., Nengzi, L. et al. Performance and microbial community profiles in pilot-scale biofilter for ammonia, iron and manganese removal at different dissolved oxygen concentrations. World J Microbiol Biotechnol 35, 43 (2019). https://doi.org/10.1007/s11274-019-2617-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2617-x