Abstract
Bioremediation of arsenic (As) pollution is an important environmental issue. The present investigation was carried out to isolate As-resistant novel bacteria and characterize their As transformation and tolerance ability. A total of 170 As-resistant bacteria were isolated from As-contaminated soils at the Kangjiawan lead–zinc tailing mine, located in Hunan Province, southern China. Thirteen As-resistant isolates were screened by exposure to 260 mM Na2HAsO4·7H2O, most of which showed a very high level of resistance to As5+ (MIC ≥ 600 mM) and As3+ (MIC ≥ 10 mM). Sequence analysis of 16S rRNA genes indicated that the 13 isolates tested belong to the phyla Firmicutes, Proteobacteria and Actinobacteria, and these isolates were assigned to eight genera, Bacillus, Williamsia, Citricoccus, Rhodococcus, Arthrobacter, Ochrobactrum, Pseudomonas and Sphingomonas. Genes involved in As resistance were present in 11 of the isolates. All 13 strains transformed As (1 mM); the oxidation and reduction rates were 5–30% and 10–51.2% within 72 h, respectively. The rates of oxidation by Bacillus sp. Tw1 and Pseudomonas spp. Tw224 peaked at 42.48 and 34.94% at 120 h, respectively. For Pseudomonas spp. Tw224 and Bacillus sp. Tw133, the highest reduction rates were 52.01% at 48 h and 48.66% at 144 h, respectively. Our findings will facilitate further research into As metabolism and bioremediation of As pollution by genome sequencing and genes modification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is one of the most prevalent and toxic elements and it ranks first on the Agency for Toxic Substances and Disease Registry Priority List of Hazardous Substances. Long-term exposure to As leads to development of not only various forms of cancer but also a range of other illnesses, including cardiovascular and peripheral vascular diseases, neurological disorders, diabetes mellitus and chronic kidney disease (Ralph 2008; Tapio and Grosche 2006). Among human activities, mining practices and wastewater irrigation are the major causes of As contamination (Abedin et al. 2002; Shagol et al. 2014). Crops grown in contaminated soils take up As and transport it to the grains; this represents a potential threat to human health (Jia et al. 2012; Wu et al. 2016). Arsenite and arsenate are the primary As species in nature, and the former is more soluble and over 100-fold more toxic than the latter. As a structural analog of phosphate, arsenate exerts toxicity by inhibiting oxidative phosphorylation. The toxicity of arsenite stems from its affinity for the sulfhydryl groups of cysteine residues (Krumova et al. 2008).
The biogeochemical cycling of As in nature is dependent on microbial transformation, which affects its mobility and species distribution in the environment. Because of its advantages such as good effect, low cost and no secondary pollution, microbial arsenic remediation technology has drawn a widely public attention and has become a research hotspot. Iron oxidizing bacteria could remove arsenic more than 80% under optimal conditions (Katsoyianni et al. 2002). When the initial concentrations of As3+ and As5+ in the water were both 5 mg/L, the arsenic removal efficiency of sulfate-reducing bacteria could reach 60% and 80%, respectively (Teclu et al. 2009). After 21 days of cultivation at 10 mg/L As5+, the strains Trichoderma sp. Sterilemycelial sp., Neoco-smospora sp. Rhizopus sp. and Penicillium sp. could evaporate 29.86%, 27.65%, 26.69%, 25.22% and 22.31% of arsenic into the atmosphere, respectively (Srivastava et al. 2011). Bacteria of the genera Pseudomonas, Bacillus, Actinobacteria, Microbacterium, Rhodococcus, Ochrobactrum, Acinetobacter, Arthrobacter and Rhizobium have been reported to be resistant to As (Anderson and Cook 2004; Cai et al. 2009; Jareonmit et al. 2012; Paul et al. 2014; Sanyal et al. 2016) due to redox or methylation activity. Resistance (ars) genes have been detected in the genomes of the vast majority of bacterial species sequenced to date, suggesting As to be ubiquitous in the environment (Yang and Rosen 2016). As-resistance genes are species-specific and comprise an arsenite-responsive repressor (ArsR), an arsenite efflux permease (ArsB), which functions to extrude trivalent arsenite from cells, and an arsenate reductase (ArsC), which is required for resistance to arsenate via its reduction to arsenite. The arsenite-stimulated ATPase (ArsA) and arsenite-metallochaperone (ArsD), which are always associated within ars operons, appear to be later adaptations that enhance the ability of ArsB to extrude arsenite and thereby increase the level of resistance (Cavalca et al. 2013; Cordi et al. 2015; Yang and Rosen 2016). In addition, the arsenate respiratory reductase (arrA) and oxidase (aio) gene systems have been found in some bacterial taxa (Saltikov and Newman 2003; Inskeep et al. 2007). A global cycle of As methylation that includes ArsM methyltransferases, ArsIC–As bond lyases and ArsH NADPH-flavin mononucleotide-dependent oxidoreductases was identified recently (Yang and Rosen 2016).
Removal of As from contaminated sites is hampered by its non-biodegradable nature. Remediation using bacteria has received considerable attention due to their biochemical diversity, stability and ability to survive harsh environments. The bacterial oxidation of arsenite to arsenate represents a potential partial detoxification mechanism that generates the less toxic and less mobile form of As and thus facilitates its bioremediation (Simeonova et al. 2004). Following the transformation of arsenate to arsenite by arsenate-reducing bacteria, the global cycle of As methylation transforms arsenite to less toxic organic forms (dimethylarsenite or trimethylarsine).
The Kangjiawan lead–zinc mine is located in the town of Shuikoushan (latitude/longitude: 26°40′N/112°40′E), Hengyang City, Hunan Province, southern China, at an altitude of 300–500 m, and covers an area of approximately 27.95 km2. It is China’s fourth largest lead–zinc mine and has been in operation for more than 100 years. Soil around the mine is contaminated by various heavy metals, including lead, zinc and As. The objectives of the present study were to isolate highly As-resistant bacteria from an As-contaminated lead–zinc tailing mine and to investigate their potential for bioremediation of As-contaminated soils.
Materials and methods
Sample collection and chemical analysis
Three regions in the Kangjiawan tailing mine were selected for sampling: an abandoned tailings (T), a downwind area planted with Broussonetia papyrifera (B), and a waste slag area (S). The pH of the soil at the three sites was measured using a portable pH meter (Jenway, UK). Three soil samples were collected randomly from each site (10–20 cm depth), and each sample was divided into two subsamples for soil chemical analyses and bacterial isolation. The samples for bacterial isolation were stored at 4 °C. For soil heavy metals analysis, the samples were disposed by air-drying, sieving (< 2 mm) and then digesting with 1:1 concentrated HNO3–H2SO4 (Shagol et al. 2014). The concentrations of As and other heavy metals in the collected samples were determined by inductively coupled plasma mass spectrometry (ICP-MS). In terms of the total organic carbon (TOC) in the soil, 1 mol/L HCl should be added to the soil sample until no more bubbles (carbon dioxide) were produced, and TOC was analyzed using a total organic carbon analyzer (Solid TOC, OI, USA) after being fully dried in the oven. The total nitrate and phosphate were estimated by Fourier transform near-infrared spectrometer (NIRLabN-200, BUCHI, Switzerland) after the fresh soil samples being naturally dried and ground into powder.
Isolation of As-resistant bacteria
Each soil sample (1 g) was added to 99 mL distilled water containing about 10 glass beads and then placed in a shaking incubator at 150 rpm and 28 °C for 30 min. The samples were then serially diluted to 10−3, 10−4 and 10−5, and 0.1 mL of each dilution was spread onto chemically defined solid medium (CDM; Weeger et al. 1999) supplemented with 40 mM Na2HAsO4·7H2O. The CDM comprised three solutions: solution A (0.0812 M MgSO4·7H2O, 0.187 M NH4Cl, 0.07 M Na2SO4, 0.574 mM K2HPO4, 4.57 mM CaCl2·2H2O and 0.446 M Na lactate), solution B (4.8 mM FeSO4·7H2O) and solution C (0.95 M NaHCO3). CDM contained (per liter) 100 mL solution A, 2.5 mL solution B, 10 mL solution C and 887.5 mL water; the final pH was adjusted to 7.2. Solution A was sterilized by autoclaving (121 °C, 20 min) and solutions B and C by filtration through a 0.22 µm pore size filter. After incubation for 3–7 days at 28 °C, colony forming units (CFU) were enumerated (Krumova et al. 2008). For screening and confirmation of As-resistant strains, a single colony was inoculated onto CDM agar supplemented with 65, 130 or 260 mM Na2HAsO4·7H2O. All tests were performed in triplicate.
Determination of the minimum inhibitory concentrations (MICs) of As and other heavy metals
The MIC is defined as the lowest concentration that completely inhibits bacterial growth in minimal medium (Paul et al. 2014). The resistance of the isolates to arsenate and arsenite was determined using CDM agar containing sodium hydrogen arsenate heptahydrate (Na2HAsO4·7H2O) at 200, 400, 600 and 800 mM and sodium arsenite (NaAsO2) at 5, 10 and 20 mM. The cultures were incubated at 28 °C for 3–7 days. Resistance to other heavy metals (Cd, Hg, Cu, Ni and Ba) was also evaluated (1–20 mM in 1 mM increments). The MIC value was determined based on the presence or absence of visible growth.
Bacterial identification by analysis of the 16S rRNA sequence
Genomic DNA was extracted using the TIANamp Bacteria DNA kit (TIANGEN, China), following the manufacturer’s instructions. 16S rRNA genes were amplified by polymerase chain reaction (PCR) in 50 µl mixtures using the primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-CGGCTACCTTGTTACGACTT-3′) (Yanagi and Yamasato 1993). PCR was performed in the PTC-200 gene amplifier (Bio-Rad, USA) using the following parameters: initial denaturation at 94 °C for 4 min, followed by 30 cycles of 94 °C for 1 min, 55 °C for 1 min and 72 °C for 1.5 min, and a final extension at 72 °C for 10 min. To obtain nearly full-length 16S rRNA genes, the PCR amplicons were purified using the DNA Purification Kit (TaKaRa) and inserted into the pMD18-T vector using a pMD18-T cloning kit (TaKaRa) according to the manufacturer’s instructions. The inserted 16S rRNA genes were sequenced using the M13-47/RV-M primers provided in the cloning kit. The resultant 16S rRNA gene sequences were compared with those of reference strains with validly published names using the EzTaxon-e server (http://www.eztaxon-e.ezbiocloud. net/; Kim et al. 2012). After multiple sequence alignment of the data via CLUSTAL_X (Thompson et al. 1997), a phylogenetic tree was constructed using the neighbor-joining method (Saitou and Nei 1987) in MEGA version 5 (Tamura et al. 2011). The topologies of the phylogenetic tree were evaluated by the bootstrap resampling method of Felsenstein (1985) with 1000 replicates.
Determination of functional genes related to As metabolism
Thirty strains with high As resistance isolated from contaminated soils were analyzed for genes related to As resistance. Eleven pairs of primers (degenerate and specific) were used to amplify functional genes involved in As resistance, including an As oxidase gene (aioA), arsenate reductase genes (arrA and arsC), efflux transporter genes [arsB, ACR3(1) and ACR3(2)] and an arsenite methyltransferase gene (arsM) (Table 1). The PCR reaction mixtures contained 14.75 µL 2 × Taq PCR Mix (Bioeasy, Shanghai, China), 0.2 mM each primer and 25 ng DNA template in a final volume of 25 µL. The PCR cycling conditions were as reported previously (Table 1). The PCR products were purified, ligated into the pMD18-T vector and sequenced, and phylogenetic trees were constructed as described for the 16S rRNA analysis.
Qualitative evaluation of As transformation
The As-transformation capacity of the isolates was evaluated using the silver nitrate (AgNO3) method (Krumova et al. 2008). The isolates were cultured in Luria–Bertani (LB) liquid medium supplemented with 7.7 mM NaAsO2 at 28 °C for 24 h. The cell suspension was centrifuged at 4750×g for 10 min and adjusted to an optical density (OD600nm) of 1.0. A 20 µL aliquot of the cell suspension was transferred to 130 µL CDM and the mixture was plated on CDM agar containing 2 mM NaAsO2 or 20 mM Na2HAsO4·7H2O. The CDM agar plates were incubated in the dark at 28 °C for 48 h and then flooded with 0.1 M AgNO3 to assess the As-transforming ability of the isolates. A brownish precipitate revealed the presence of arsenate around the isolates, while the presence of arsenite was indicated by a yellow precipitate (Branco et al. 2009; Lett et al. 2001).
Quantification of As transformation using the molybdenum blue method
As transformation was also quantified using the molybdenum blue method (Tsang et al. 2007). This method takes advantage of the molybdenum blue color complex produced by the reaction between arsenate ions and the molybdenum blue reagent, and the absorbance at 865 nm is measured. Molybdenum blue reagent comprised (per liter) 6 g ammonium molybdate, 10.8 g ascorbic acid, 0.136 g potassium antimony tartrate and 67.3 mL sulfuric acid. A single colony from each bacterial strain was cultured in LB liquid medium without NaAsO2 and Na2HAsO4·7H2O at 28 °C for 24 h at 150 rpm. Suspensions were centrifuged at 4750×g for 10 min, and then the pellets were washed twice with PIPES buffer (20 mM, pH 7.0). The washed cells were resuspended in PIPES buffer supplemented with 1 mM NaHAsO2 or 1 mM Na2HAsO4·7H2O, and the OD600 was adjusted to 1.0. The cells were incubated at 28 °C with shaking at 150 rpm. Aliquots (1 mL) were taken at different time intervals and centrifuged (4750×g for 10 min) to remove the cell-precipitation. The supernatant was diluted with PIPES buffer (pH 7.0) and subjected to determination of arsenate and arsenite concentrations. Samples (300 µL) were oxidized with 100 µL KIO3 solution (5 mM KIO3 in 50 mM HCl). Unoxidized samples were prepared by acidifying 300 µL of the samples with 100 µL 25 mM HCl. After incubation at 25 °C for 10 min, 600 µL molybdenum blue reagent were added to each sample, followed by incubation at 78 °C for 10 min and for 5 min on ice (Jareonmit et al. 2012; Niggemyer et al. 2001). Arsenate concentrations were determined by measuring the absorbance at 865 nm using the Infinite M200 PRO spectrophotometer (Tecan, Switzerland). An arsenate standard curve was prepared (0—100 µM). Arsenite concentrations were determined by subtracting the absorbance values of oxidized samples from those of unoxidized samples.
Physiological and biochemical characterization
Physiological and biochemical characterization of isolates (Tw1, Tw133 and Tw224) was performed using the API system (bioMérieux). Urease, arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase and tryptophan deaminase activities, citric acid utilization, indole production, H2S production, Voges–Prokauer test, hydrolysis of o-nitrophenyl b-d-galactopyranoside (ONPG) and gelatin were determined using the API 20E system (bioMérieux). Other enzymatic activities were assessed using API ZYM test strips (bioMérieux) according to the manufacturer’s instructions. Utilization of sugars and acid production from carbohydrates as carbon sources were determined using API 50CH kits (bioMérieux) according to the manufacturer’s instructions, using API 50CHB as the inoculation medium.
Results
Physicochemical analysis of soils and isolation of As-resistant bacteria
The physicochemical characteristics of the soils used in this study are shown in Table 2. The soil samples had abundant organic carbon and potassium, little nitrogen and phosphorus, high concentrations of heavy metals and pH values of 5.0–8.0. The concentrations of As and lead (Pb) were markedly higher than those of other heavy metals. Indeed, the As concentrations at sites T2 (3875.1 mg/kg) and B3 (4534.8 mg/kg) were over 100-fold higher than the national standard (30 mg/kg), which alerted us to focus on As-resistant bacteria.
A total of 170 bacterial strains were isolated from the soils based on their ability to grow in the presence of 40 mM arsenate. CDM containing high concentrations of arsenate was used to further determine the tolerance of the 170 isolates to arsenate. Among them, 46, 31 and 13 strains exhibited growth in the presence of 65, 135 and 260 mM As, respectively (Fig. 1). The 13 As-resistant isolates capable of growing in the presence of 260 mM arsenate were selected for assessment of As-resistance properties.
MICs for As and other heavy metals
Arsenate- and arsenite-resistance properties were determined by monitoring bacterial growth in CDM supplemented with various concentrations of arsenate or arsenite (Table 3). All of the bacterial strains evaluated showed very high resistance to both arsenate (MIC ≥ 600 mM) and arsenite (MIC ≥ 10 mM), with the exception of strain Bw218 (arsenate, MIC = 400 mM) and strain Tw31 (arsenite, MIC = 5 mM). Several strains (Tw1, Tw49, Tw196, Bw206, Tw222, Tw224 and Tw251) exhibited resistance to arsenite (MIC ≥ 20 mM); indeed, strain Tw196 was tolerant to greater than 800 mM arsenate and 20 mM arsenite.
Resistance to other heavy metals (Ni, Cu, Cd, Ba and Hg) was also assessed (Table 3). It was observed that most of the isolates showed high resistance to Ni and Cu, moderate resistance to Hg and Ba, and sensitivity to Cd. Furthermore, strains Tw196, Bw206, Tw222 and Tw224 exhibited a high level of resistance to multiple heavy metals.
Identification of the isolates by 16S rRNA gene sequencing
Nearly complete 16S rRNA gene sequences of the 13 isolates were determined and have been deposited in the GenBank database (accession numbers are shown in Fig. 2). Comparison of these sequences with those in public databases demonstrated that these strains belong to three phyla: Firmicutes, Proteobacteria and Actinobacteria (Fig. 2); Actinobacteria accounted for the majority of the isolates. Phylogenetic analysis of the 16S rRNA gene sequences suggested that the isolates belong to eight different genera Bacillus (strains Tw1 and Tw133), Williamsia (strain Tw49), Citricoccus (strain Tw196), Rhodococcus (strains Sw125 and Tw151), Arthrobacter (strains Sw149, Bw206 and Bw218), Ochrobactrum (strain Tw31), Pseudomonas (strains Tw222 and Tw224) and Sphingomonas (strain Tw251).
Identification of genes involved in As resistance
The presence of seven As metabolism genes [aioA, arrA, arsC, arsB, ACR3(1), ACR3(2) and arsM] in As-resistant isolates was determined by PCR amplification using 11 previously reported primer pairs. The As reductase gene arsC was detected in eight isolates (Ochrobactum sp. Tw31, Rhodococcus sp. Sw125 and Tw151, Bacillus sp. Tw133, Williamsia sp. Sw149, Arthrobacter sp. Bw206 and Bw218 and Sphingomonas sp. Tw251). Genes encoding arsenite efflux pumps were present in several isolates, e.g., arsB in Pseudomonas spp. Tw222, Tw224 and Sphingomonas sp. Tw251, ACR3(2) in Arthrobacter sp. Bw218 and Pseudomonas spp. Tw222 and ACR3(1) in Citricoccus sp. Tw196 (Table 4). No aioA, arrA or arsM gene was amplified. Strains Bw218, Tw222 and Tw251 harbored two As-resistance genes: arsC/ACR3(2), arsB/ACR3(2) and arsC/arsB, respectively. The two remaining isolates (Bacillus sp. Tw1 and Williamisia sp. Tw49) were able to grow in the presence of 600 mM arsenate and 20 mM arsenite, but no As-tolerant/resistant genes were detected in these strains. This was likely due to the selectivity of PCR primers or the presence of other resistance systems.
Phylogenetic trees were constructed based on partial ArsB, ACR3 and ArsC amino acid sequences, and the corresponding genes have been deposited in the GenBank database under the accession numbers shown in Fig. S1. The deduced amino acid sequence of arsB from Pseudomonas spp. Tw222 and Tw224 and Sphingomonas sp. Tw251 showed 99% identity to ArsB from Pseudomonas sp. NBRC 111140, Pseudomonas fluorescens and Sphingomonas hankookensis, respectively. Comparison of the 16S rRNA and deduced ArsB phylogenetic trees indicated that their evolutionary relationships were similar. The ACR3 cluster was divided into two phylogenetic groups: ACR3(1) and ACR3(2). The ACR3(1) sequence of Citricoccus sp. Tw196 showed 99% identity to that from Citricoccus sp. CH26A. The ACR3(2) group included two sequences from Pseudomonas spp. Tw222 and Arthrobacter sp. Bw218, which displayed 98% and 96% identities to those from Pseudomonas geniculate and Cupriavidus gilardii, respectively.
The arsenate reductase arsC genes were amplified by PCR using the primer pairs arsC4r/arsC4f and amlt42f/amlt376r (Table 1). The ArsC proteins of Bacillus sp. Tw133 and Bacillus megaterium showed 100% sequence identity. The deduced amino acid sequences of ArsC (amlt42f/amlt376r) from Ochrobactrum sp. Tw31, Rhodococcus sp. Sw125 and Sphingomonas sp. Tw251 displayed 100%, 99% and 100% identities to those of Enterobacteriaceae, Escherichia coli and Ochrobactrum sp. KAs3-20, respectively.
As-transformation capacity
The As-transformation ability of 13 isolates was evaluated using the AgNO3 method. The interaction of silver nitrate with arsenite generates a bright yellow precipitate, while a brownish precipitate forms in the presence of arsenate. Only Pseudomonas spp. Tw222 and Tw224 produced a bright yellow precipitate (Fig. 3). However, none of the strains revealed As-oxidizing activity.
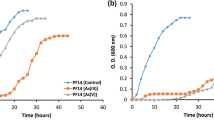
The As-oxidizing or -reducing abilities of the 13 As-resistant isolates were further assessed by the molybdenum blue method using 1 mM arsenite or arsenate. The isolates exhibited various degrees of redox activity. The oxidation and reduction rates of the As-resistant strains at 24–72 h were 5–30% and 10–51.2%, respectively. The oxidation and reduction rates of most isolates were < 20% and 30%, respectively. In contrast, Bacillus sp. Tw1 and Pseudomonas spp. Tw224 oxidized approximately 30% of the total arsenite (Fig. 4a), while Bacillus sp. Tw133, Pseudomonas spp. Tw222 and Tw224 revealed reduction rates of 35.9, 30.1 and 51.2%, respectively, after 72 h (Fig. 4b).
As oxidation and reduction in PIPES buffer supplemented with 1 mM As3+ or As5+. As oxidation (a) and reduction (b) were measured at 24, 48 and 72 h. The As oxidation and reduction rates of Bacillus sp. Tw1, Tw133 and Pseudomonas spp. Tw224 (c) were further determined at different time intervals (0–144 h). Bars show means ± SE of three replicates
The oxidation and reduction rates of Bacillus sp. Tw1, Tw133 and Pseudomonas spp. Tw224 were evaluated over time (Fig. 4c). The oxidation rates of Bacillus sp. Tw1 and Pseudomonas spp. Tw224 peaked at 42.48 and 34.94%, respectively, at 120 h. For Pseudomonas spp. Tw224 and Bacillus sp. Tw133, the highest reduction rates were 52.01% at 48 h and 48.66% at 144 h, respectively; therefore, Pseudomonas spp. Tw224 exhibited considerable oxidation and reduction activities.
Physiological and biochemical characterization of As-resistant bacteria
Bacillus sp. Tw1, Tw133 and Pseudomonas spp. Tw224 have strong arsenic oxidation or reduction capacity, so the three strains were selected and the Physiological and biochemical characterization was performed using the API 20E, API 50CH and API ZYM systems (bioMérieux). All three strains exhibited acid production from glucose, glycerol, l-arabinose, d-xylose, d-galactose, d-glucose, d-fructose, d-maltose, d-melibiose, d-saccharose (sucrose), d-trehalose, inulin, d-raffinose and glycogen. The strains were positive for esterase lipase (C8), esculin ferric citrate and the Voges–Prokauer test but negative (−) for lysine decarboxylase, ornithine decarboxylase, lipase (C14), cystine arylamidase, β-glucuronidase, N-acetyl-β-glucosaminidase, α-mannosidase and β-fucosidase activities, H2S production, urea hydrolysis and acid production from mannitol, inositol, sorbitol, rhamnose and potassium 2-ketogluconate. Other physiological and biochemical characteristics are shown in Table 5. Therefore, Bacillus sp. Tw1 has characteristics similar to those of Bacillus sp. Tw133, such as ONPG hydrolysis, esterase (C4) and acid production from d-mannitol, N-acetylglucosamine, amidon and d-turanose, but it was negative for arginine dihydrolase, leucine arylamidase, valine arylamidase, trypsin and α-glucosidase. However, Bacillus sp. Tw133 was positive for gelatin hydrolysis and alkaline phosphatase and α-chymotrypsin activities but negative for naphthol-AS-BI-phosphohydrolase, α-galactosidase and β-glucosidase activities. In contrast, Pseudomonas spp. Tw224 was positive for arginine dihydrolase, leucine arylamidase, trypsin and naphthol-AS-BI-phosphohydrolase activities but negative for ONPG hydrolysis and α-galactosidase, β-galactosidase, α-glucosidase and β-glucosidase activities.
Discussion
As pollution is a serious issue due to its toxicity to the environment and human health globally (Fendorf et al. 2010). A low-cost, high-efficiency and eco-friendly bioremediation method is required for contaminated soil or water (Cavalca et al. 2013). In recent years, indigenous and non-indigenous bacteria have received much attention for removal of pollutants from contaminated sites (Luo et al. 2015; Kavamura and Esposito 2010). The isolation and characterization of the diverse As-resistant bacteria will facilitate research into As metabolism and bioremediation of As pollution.
In this study, a total of 170 As-resistant strains were isolated from As-contaminated soils collected from the Kangjiawan lead–zinc mine, located in Hunan Province, southern China. Phylogenetic analysis based on 16S rRNA genes revealed that the 13 strains with marked As resistance belonged to the genera Bacillus, Pseudomonas, Arthrobacter, Citricoccus, Williamsia, Rhodococcus, Sphingomonas and Ochrobactrum (Fig. 2). As-resistant Pseudomonas, Bacillus, Rhodococcus, Arthrobacter, Ochrobactrum, Citricoccus and Sphingomona strains have been reported previously (Cai et al. 2009). To our knowledge, Williamsia sp. Tw49 is the first arsenite-resistant bacterium of this genus to be reported.
The bacteria were tolerant to 10 arsenite and 100 mM arsenate (Jackson and Dugas 2003). Particularly, a few Acinetobacter, Microbacterium, Pseudomonas and Rhizobium strains could withstand up to 600 mM arsenate or 10 mM arsenite (Paul et al. 2014). The majority of the 13 isolates could tolerate 600 mM arsenate, even 800 mM, and more than 50% could tolerate 20 mM arsenite. In addition, the isolates also showed tolerance to several other heavy metals (Cd, Ba, Cu, Ni and Hg).
Bacteria use several mechanisms to survive and grow in As-polluted environments, such as As oxidation, reduction and methylation. Eleven of the 13 As-resistant isolates harbored at least one gene related to As resistance, the most common being the arsenate reductase gene arsC. All 13 isolates were considered arsenite-oxidizing strains, but none harbored the arsenite oxidase gene aioA. This might have been due to the presence of novel arsenite oxidase genes (Sultana et al. 2012) or mutations in primer-binding sites (divergence in the conserved catalytic domain) (Sanyal et al. 2016). No As-resistance gene or methyltransferase gene (arsM) was amplified from Bacillus sp. Tw1 or Williamsia sp. Tw49, suggesting that their As metabolism is mediated by unknown mechanisms.
Despite the finding that only two As-resistant isolates (Pseudomonas spp. Tw222 and Tw224) exhibited weak reduction activity by the AgNO3 assay, almost all of the strains were positive for oxidation and reduction activities by the quantitative molybdenum blue assay. Bacillus sp. Tw1 and Tw133 oxidized arsenite and reduced arsenate at rates of 42.48% and 48.66%, compared with 34.94 and 52.01% for Pseudomonas spp. Tw224, respectively. The oxidation activities of Bacillus sp. Tw1 and Pseudomonas spp. Tw224 were greater than those of Pseudomonas koreensis JS123 and Bacillus sp. MC196 (33% and 26.5% of 1 mM arsenite within 72 h, respectively; Jareonmit et al. 2012). However, the reduction activities of Bacillus sp. Tw133 and Pseudomonase spp. Tw224 were lower than that of Pseudomonas sp. DRBS1 (97% of 100 mM arsenate; Srivastava et al. 2010). The possible reasons include inhibition of arsenate reductase activity under the unaccommodated growing conditions or dynamic balance with oxidation and reduction simultaneity.
Isolation and characterization of As-resistant bacteria will enhance our understanding of As biogeochemical pathways and facilitate bioremediation of As pollution. In the present work a large number of phylogenetically different As-resistant bacteria were isolated from tailing mine soils. Bacteria capable of arsenate reduction or arsenite oxidation might be useful for bioremediation of As-contaminated sites. Future work will address the bioremediation potential of the isolates as well as the underlying mechanisms.
References
Abedin MJ, Cotter-Howells J, Meharg AA (2002) Arsenic uptake and accumulation in rice (Oryza sativa L.) irrigated with contaminated water. Plant Soil 240(2):311–319
Achour AR, Bauda P, Billard P (2007) Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol 158(2):128–137
Anderson CR, Cook GM (2004) Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated sites in New Zealand. Curr Microbiol 48(5):341–347
Branco R, Francisco R, Chung AP, Morais PV (2009) Identification of an aox system that requires cytochrome c in the highly arsenic-resistant bacterium Ochrobactrum tritici SCII24. Appl Environ Microbiol 75(15):5141–5147
Cai L, Liu GH, Rensing C, Wang GJ (2009) Genes involved in arsenic transformation and resistance associated with different levels of arsenic-contaminated soils. BMC Microbiol 9(4):1–11
Cavalca L, Corsini A, Zaccheo P, Andreoni V, Muyzer G (2013) Microbial transformations of arsenic: perspectives for biological removal of arsenic from water. Future Microbiol 8(6):753–768
Cordi A, Pagnout C, Devin S, Poirel J, Billard P, Dollard MA, Bauda P (2015) Determination of physiological, taxonomic, and molecular characteristics of a cultivable arsenic-resistant bacterial community. Environ Sci Poll Res 22(18):13753–13763
Escudero LV, Casamayor EO, Chong G, Pedrós-Alió C (2013) Distribution of microbial arsenic reduction, oxidation and extrusion genes along a wide range of environmental arsenic concentrations. PLoS ONE 8(10):e78890
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328(5982):1123–1127
Fisher E, Dawson AM, Polshyna G, Lisak J, Crable B, Perera E, Ranganathan M, Thangavelu M, Basu P, Stolz JF (2008) Transformation of inorganic and organic arsenic by Alkaliphils oremlandiisp. nov. strain OhILAs. Ann N Y Acad Sci 1125(1):230–241
Inskeep WP, Macur RE, Hamamura N, Warelow TP, Ward SA, Santini JM (2007) Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ Microbiol 9(4):934–943
Jackson CR, Dugas SL (2003) Phylogenetic analysis of bacterial and archaeal arsC gene sequences suggests an ancient, common origin for arsenate reductase. BMC Evol Biol 3(1):1–10
Jareonmit P, Mehta M, Sadowsky MJ, Sajjaphan K (2012) Phylogenetic and phenotypic analyses of arsenic-reducing bacteria isolated from an old tin-mine area in Thailand. World J Microbiol Biotechnol 28(5):2287–2292
Jia Y, Huang H, Sun GX, Zhao FJ, Zhu YG (2012) Pathways and relative contributions to arsenic volatilization from rice plants and paddy soil. Environ Sci Technol 46(15):8090–8096
Jia Y, Huang H, Zhong M, Wang FH, Zhang LM, Zhu YG (2013) Microbial arsenic methylation in soil and rice rhizosphere. Environ Sci Technol 47(7):3141–3148
Katsoyianni I, Zouboulis A, Althoff H, Bartel H (2002) As(III)removal from groundwaters using fixed-bed upflow bioreactors. Chemosphere 47(3):325–332
Kavamura VN, Esposito E (2010) Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol Adv 28(1):61–69
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Sys Evol Microbiol 62(3):716–721
Krumova K, Nikolovska M, Groudeva V (2008) Isolation and identification of arsenic-transforming bacteria from arsenic-contaminated sites in Bulgaria. Biotechnol Biotechnol Equip 22(2):721–728
Lear G, Song B, Gault AG, Polya DA, Lloyd JR (2007) Molecular analysis of arsenate-reducing bacteria within Cambodian sediments following amendment with acetate. Appl Environ Microbiol 73(4):1041–1048
Lett MC, Paknikar KM, Lievremont D (2001) A simple and rapid method for arsenite and arsenate speciation. Process Met 11:541–546
Luo X, Liu H, Huang G, Li Y, Zhao Y, Li X (2015) Remediation of arsenic-contaminated groundwater using media-injected permeable reactive barriers with a modified montmorillonite: sand tank studies. Environ Sci Poll Res 23(1):870–877
Niggemyer A, Spring S, Stackebrandt E, Rosenzweig RF (2001) Isolation and characterization of a novel As(V)-reducing bacterium: implications for arsenic mobilization and the genus Desulfitobacterium. Appl Environ Microbiol 67(12):5568–5580
Paul D, Poddar S, Sar P (2014) Characterization of arsenite-oxidizing bacteria isolated from arsenic-contaminated groundwater of West Bengal. J Environ Sci Health Part A 49(13):1481–1492
Ralph SJ (2008) Arsenic-based antineoplastic drugs and their mechanisms of action. Met-Based Drugs 2008(1):260146
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Saltikov CW, Newman DK (2003) Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci USA 100(19):10983–10988
Sanyal SK, Mou TJ, Chakrabarty RP, Hoque S, Hossain MA, Sultanal M (2016) Diversity of arsenite oxidase gene and arsenotrophic bacteria in arsenic-affected Bangladesh soils. AMB Express 6(1):1–11
Shagol CC, Krishnamoorthy R, Kim K, Sundaram S, Sa T (2014) Arsenic-tolerant plant-growth-promoting bacteria isolated from arsenic-polluted soils in South Korea. Environ Sci Poll Res 21(15):9356–9365
Simeonova DD, Lièvremont D, Lagarde F, Muller DAE, Groudeva VI, Lett MC (2004) Microplate screening assay for the detection of arsenite-oxidizing and arsenate-reducing bacteria. FEMS Microbiol Lett 237:249–253
Srivastava D, Madamwar D, Subramanian RB (2010) Pentavalent arsenate reductase activity in cytosolic fractions of a Pseudomonas sp. isolated from arsenic-contaminated sites of Tezpur. Assam Appl Biochem Biotechnol 162(3):766–779
Srivastava PK, Vaish A, Dwivadi S (2011) Biological removal of arsenic pollution by soil fungi. Sci Total Environ 409(12):2430–2442
Sultana M, Vogler S, Zargar K, Schmidt AC, Saltikov C, Seifert J, Schlömann M (2012) New clusters of arsenite oxidase and unusual bacterial groups in enrichments from arsenic-contaminated soil. Arch Microbiol 194(7):623–635
Sun YM, Polishchuk EA, Radoja U, Cullen WR (2004) Identification and quantification of arsC genes in environmental samples by using real-time PCR. J Microbial Methods 58(3):335–349
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Tapio S, Grosche B (2006) Arsenic in the aetiology of cancer. Mutat Res 612(3):215–246
Teclu D, Tivchev G, Laing M, Wallis M (2009) Bioremoval of arsenic species from contaminated waters by sulphate–reducing bacteria. Water Res 42:4885–4893
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Tsang S, Phu F, Baum MM, Poskrebyshev GA (2007) Determination of phosphate/arsenate by a modified molybdenum blue method and reduction of arsenate by S2O4 2–. Talanta 71(4):1560–1568
Weeger W, Lievremont D, Perret M, Lagarde F, Hubert JC, Leroy M, Lett MC (1999) Oxidation of arsenite to arsenate by a bacterium isolated from an aquatic environment. Biometals 12(2):141–149
Wu F, Wang J, Yang J, Li J, Zheng Y (2016) Does arsenic play an important role in the soil microbial community around a typical arsenic mining area. Environ Poll 213:949–956
Yanagi M, Yamasato K (1993) Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett 107(1):115–120
Yang HC, Rosen BP (2016) New mechanisms of bacterial arsenic resistance. Biomed J 39(1):5–13
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (Grant Nos. 2016JX03, YX2014-15), the National Science and Technology Ministry (Grant No. 2012BAC09B03), the National Natural Science Foundation of China (Grant No. J1310005) and the Beijing Nova Program (Grant No. 2011033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, D., Zhang, Z., Gao, Q. et al. Isolation and characterization of aerobic, culturable, arsenic-tolerant bacteria from lead–zinc mine tailing in southern China. World J Microbiol Biotechnol 34, 177 (2018). https://doi.org/10.1007/s11274-018-2557-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2557-x