Abstract
Public concern for food safety and environmental issues and the increase in fungicide-resistant pathogen have enhanced the interest in developing alternative methods to fungicides to control postharvest fruit decay. In this study, a bacterial strain isolated from stale potato vermicelli was identified as Bacillus pumilus HN-10 based on morphological characteristics and 16S rRNA gene sequence analysis. Furthermore, two novel cationic antifungal peptides named P-1 and P-2 were purified from B. pumilus HN-10 using macroporous adsorbent resin AB-8, Sephadex G-100 chromatography, and reversed-phase high-performance liquid chromatography. The primary structure of P-1 and P-2, which were proved to be novel antifungal peptides by BLAST search in NCBI database, was PLSSPATLNSR and GGSGGGSSGGSIGGR with a molecular weight of 1142.28 and 1149.14 Da, respectively, as indicated by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Both P-1 and P-2 exhibited strong antifungal activity against Trichothecium roseum with minimum inhibitory concentrations starting from 1 μg/mL. The two novel antifungal peptides were stable below 80 °C for 2 h, but lost their activity in 15 min at 121 °C. In addition, they were resistant to the proteolytic action of pepsin, trypsin, and papain, and stable within a wide range of pH (2.0–12.0). These results showed that P-1 and P-2 are novel cationic antifungal peptides with specific activity against T. roseum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichothecium roseum is one of the most important pathogenic fungi causing postharvest diseases in a variety of plants. Besides economic losses, T. roseum also produces mycotoxins, such as trichothecenes, which are harmful to humans and animals (Niu et al. 2016; Tang et al. 2014). In general, fungicides are the primary means to control postharvest diseases. However, fungicide toxicity, fungicide residues, and development of fungicide resistance in pathogens can have potential harmful effects on human health and environment (Ge et al. 2015; Nunes 2012; Li et al. 2012). Therefore, it is imperative to develop new antimicrobial strategies against postharvest diseases.
Antimicrobial peptides (AMPs) can be used in several biotechnological applications with different purposes (Plácido et al. 2017). Also known as host defense peptides, AMPs are biologically active molecules produced by a wide variety of organisms as an essential component of their innate immune response. These molecules have been considered as potential therapeutics because of their broad-spectrum activities and proven ability to evade antimicrobial resistance (Midura-Nowaczek and Markowska 2014; Chan et al. 2006; Li et al. 2016).
However, despite their therapeutic utility, AMPs are also toxic, have low stability and high manufacturing cost, and are susceptible to proteases released by pathogens. Therefore, many studies have been performed to develop novel AMPs with low toxicity and highly improved stability (Regmi et al. 2017; Mookherjee and Hancock 2007).
Certain Bacillus strains are important producers of AMPs with significant potential for biological control. Several Bacillus spp. produce AMPs that are considered safe for industrial use and are commercially available, such as kanosamine or zwittermycin A from Bacillus cereus (Leaes et al. 2016; Marc and Philippe 2007). Recent advances in the synthesis of AMPs and the broad-spectrum activity of these compounds have led to successful isolation and characterization of novel peptides with improved characteristics. (Regmi et al. 2017).
In this study, we described the isolation and characterization of two novel cationic peptides P-1 and P-2 from Bacillus pumilus HN-10, including their antifungal activity against T. roseum, physical and chemical properties, and potential use in the control of several postharvest diseases in plants.
Materials and methods
Microorganisms and media
The bacterial strain B. pumilus HN-10 was isolated from stale potato vermicelli (Wushan Green Source Trading Co., Ltd., Gansu, China) and inoculated onto Luria Bertani medium at 37 °C for 48 h. T. roseum CGMCC 3.4509 was obtained from China Center for Type Culture Collection (Beijing, China) and inoculated onto potato dextrose agar (PDA) at 28 °C for 6 days.
Bacterial strain identification
The bacterial strain HN-10 was identified based on the morphological characteristics and 16S rRNA gene sequence analysis. The 16S rRNA gene sequence was amplified using the eubacteria-specific primers 27F (5ʺ-AGAGTTTGATCCTGGCTCAG-3ʺ) and 1492R (5ʺ-TACGGCTACCTTGTTACGACTT-3ʺ) under the following PCR conditions: initial denaturation at 95 °C for 5 min, followed by 33 cycles of 95 °C, 30 s, 58 °C for 30 s, and 72 °C for 80 s, and a final elongation at 72 °C for 7 min. The sequence of the PCR product was analyzed using the online tool of NCBI BlAST Beta version (https://www.ncbi.nlm.nih.gov/) for taxonomic resolution (Kayalvizhi and Gunasekaran 2010). Neighbor-joining method was used for phylogenetic tree construction and analysis using MEGA 4.0 (Tamura et al. 2007).
Antifungal activity assay
The antifungal activity of the identified bacterium was tested using agar plate diffusion assay. One 5-mm disk of pure T. roseum culture was placed on the side of a PDA plate, inoculated a point with a loop of identified bacterium culture at a distance of 2 cm far away the disk of pure T. roseum culture. The plates were incubated for 5 days at 28 °C. Each experiment was conducted in triplicate.
The antifungal activities of the peptides were tested using agar plate diffusion assay (Huang et al. 2012). One 5-mm disk of pure T. roseum culture was placed on the center of a PDA plate, surrounded by four wells with antifungal peptides at a distance of 2 cm. The control comprised PBS (20 mM, pH 6.8) instead of antifungal peptides. All the plates were incubated for 5 days at 28 °C. Each experiment was conducted in triplicate and repeated at least three times, and a clear zone of inhibition surrounding the well was measured in millimeter (mm).
Peptide production and purification
Bacillus pumilus HN-10 was cultivated in 1000 mL Erlenmeyer flasks containing 500 mL of LB broth at 37 °C for 3 days with agitation (180 rpm). Cell-free supernatant was collected after centrifugation (10,000×g) at 4 °C for 20 min. The harvested culture supernatant was mixed with ammonium sulfate (70% saturation) and stored at 4 °C overnight with constant stirring. The next day, the mixture was centrifuged (10,000×g) at 4 °C for 20 min and precipitate was recovered and dialyzed overnight against phosphate buffered solution (PBS, 20 mM, pH 6.8) using a dialysis membrane (MWCO-12000, Yuanye Biotech, Shanghai, China) (Regmi et al. 2017). Subsequently, the biologically active portion was loaded onto AB-8 resin (Guangfu Biochemical, Tianjin, China), washed with 70% ethanol at a flow rate of 2 BV/h, and monitored at 280 nm. The active fractions were concentrated by lyophilization and then loaded onto Sephadex G-100 gel (Solarbio Life Science, Beijing, China) and eluted with PBS (20 mM, pH 6.8) at a flow rate of 0.5 mL/min, and the elution was monitored at 280 nm.
Furthermore, the concentrated active fractions were loaded onto C18 column (SunFire™ Prep C18, 10 μm, 10 × 150 mm column) and purified by reverse-phased high-performance liquid chromatography (RP-HPLC; Waters 1525) with mobile phase consisting of eluent A [0.1% trifluoroacetic acid (TFA) (v/v) in acetonitrile] and eluent B (0.1% TFA (v/v) in distilled water) at a flow rate of 1 mL/min. A linear gradient of solvent A was applied with the following time schedule: 10–5%, 0–5 min; 5–0%, 5–10 min; 0–10%, 10–11 min; and 10%, 11–30 min. Fractions with antifungal activity were lyophilized for subsequent analysis.
Amino acid sequence analysis of the antifungal peptides
Determination of the molecular ions of the antifungal peptides was performed using the fractions obtained by RP-HPLC by matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI–TOF/MS) with an AB Sciex Voyager Elite MALDI–TOF/MS (Foster City, CA, USA). The ionization matrix used for MALDI–TOF/MS was 3,5-dimethoxy-4-hydroxycinnamic acid (Sigma-Aldrich). The purified peptides were subjected to amino acid sequencing by liquid chromatography tandem-mass spectrometry (LC-MS/MS) on Acclaim PepMap high-performance liquid chromatography (HPLC, Waters, USA) coupled with quadrupole-time of flight (Q-TOF) Premier mass spectrometer (Waters, Milford, MA, USA). A total of 5 µL of the sample were analyzed using a linear gradient of 6–95% acetonitrile with 0.1% (v/v) formic acid at a flow rate of 300 nL/min on a nano-viper column (75 μm × 150 mm, C18 3 μm, 100 Å, Waters). The mass spectra obtained were analyzed and the amino acid sequences of the antifungal peptides were determined according to the fragments observed.
The minimum inhibitory concentration (MIC) of the antifungal peptides
The minimum inhibitory concentration (MIC) of the antifungal peptides from B. pumilus HN-10 was determined by using agar plate diffusion assay. Peptide concentrations varying from 1 to 5000 μg/mL were prepared by two-fold serial dilution. Briefly, one 5-mm disk of pure T. roseum culture was placed on the center of a PDA plate, surrounded by four wells with different concentrations of peptides at a distance of 2 cm, and the blank sample contained 20 mM PBS (pH 6.8). The MIC was defined as the lowest peptide concentration that could inhibit T. roseum growth after incubation for 5 days at 28 °C (Plácido et al. 2017).
Physicochemical properties of the antifungal peptides
The thermal stability of the antifungal peptide samples was determined by heating the samples at 20, 40, 60, 80, and 100 °C for 120 min and 121 °C/105 KPa for 15 min, respectively, before analyzing the residual activity. Similarly, the pH stability of the peptide samples was ascertained over a pH range of 2–12 using 1 M NaOH or HCl (Rahman et al. 2017). To investigate the effect of proteases on the stability of the antifungal peptides, the peptide samples were treated with trypsin (1 mg/mL, pH 8.0), pepsin (1 mg/mL, pH 2.0), and papain (1 mg/mL, pH 7.0), respectively, at 37 °C for 2 h (Miao et al. 2014). Both three proteases purchased by Yuanye Bio-Tech Co., Ltd, Shanghai, China. The reaction was stopped by boiling the mixture at 100 °C for 2 min.
Results
The antifungal activity of the bacterial strain HN-10 and it’s strain identification
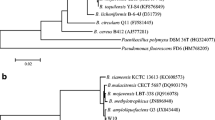
The result of antifungal activity assay showed that the bacterial strain HN-10 exhibited significant antifungal activity against the growth of T. roseum (Fig. 1). The strain identification was based on morphological characteristics and 16S rRNA gene sequence analysis. Gram staining and microscopic analysis revealed that the bacterial strain HN-10 was a Gram-positive, rod-shaped bacterium, with the ability to form endospores during the cultivation, suggesting that the strain could be a Bacillus sp. 16S rRNA sequence analysis revealed that the bacterial strain HN-10 was related to B. pumilus HN-30 (KT003271.1) with 99% identity (Fig. 2). The GenBank accession number for the bacterial strain HN-10 KT003256.1, and the strain was named as B. pumilus HN-10 after further phylogenetic analysis using sequence alignment.
Neighbor-joining tree based on nearly complete 16S rRNA gene sequences showing relationships between the bacterial strain HN-10 and some closely related taxa of the genus Bacillus. The percentages at the nodes are the levels of bootstrap support based on neighbor-joining analyses of 500 resampled data sets
Purification of the antifungal peptides
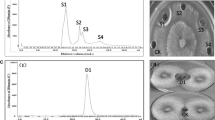
The antifungal peptides were extracted from 2 L B. pumilus HN-10 culture supernatant using ammonium sulfate precipitation, followed by three stages of chromatographic separation on AB-8 resin, Sephadex G-100, and RP-HPLC. The active fractions obtained were pooled on the basis of antifungal activities and absorbance (280 nm) (Fig. 3). Four single peaks (A, B, C, and D) were observed at the final step of the purification process (Fig. 3c), and samples corresponding to peaks A, B, and C showed obvious antifungal activity against T. roseum (Fig. 4d).
Results of antifungal activity assay against T. roseum. Among them, a the crude extract was obtained by ammonium sulfate precipitation, in the plate, except for CK, the other three wells were the same crude extract; b except for CK, the other three wells were the same fraction purified from AB-8 resin; c except for CK, the other three wells were the same fraction purified from Sephadex G-100 gel chromatography; d A, B, C and D was the four fractions purified above from RP-HPLC chromatography and their antifungal activity against T. roseum. All the CK was 20 mmol/L PBS (pH 6.8)
Characterization of the antifungal peptides
The sequences of the antifungal peptides corresponding to the obtained molecular masses were determined by comprehensive proteomic analysis using bioinformatics tools available online, including NCBI protein database, BLAST (https://blast.ncbi.nlm.nih.gov/), APD database (http://aps.unmc.edu/AP/main.php), and ExPASy (http://web.expasy.org/cgi-bin/protparam/protparam/). Three active fractions were analyzed by MALDI–TOF/MS for peptide identification. Fraction A consisted of 11 amino acid residues (P-L-S-S-P-A-T-L-N-S-R) with a molecular mass of 1142.28 Da (Fig. 5a) and high hydrophobic ratio (27%), and was named P-1 (Table 1). Fractions B and C were identified to be the same peptide comprising 15 amino acid residues (G-G-S-G-G-G-S-S-G-G-S-I-G-G-R) with a molecular mass of 1149.14 Da (Fig. 5b, c) and overrepresentation of Gly residues (60.0%), and was named P-2 (Table 2). The obtained sequences were compared with those in the NCBI protein database. The results revealed that no full query sequence was covered, and that the amino acid sequence of P-1 had up to 41.66% identity with Temporin H (AP00859) from Rana temporaria (Table 1), while that of P-2 showed up to 45% identity with KDAMP 19-mer (AP02231) from corneas (Table 2). These findings confirmed that both the peptides obtained were novel.
Determination of the MICs of the antifungal peptides
Antifungal activity with the increasing P-1 and P-2 peptide concentrations graphed in Fig. 6. The results showed that both of the P-1 and P-2 peptides exhibited significant antifungal activity against T. roseum while the peptide concentration greater than or equal to 1 μg/mL. And the maximum inhibition zone was 10 ± 0.5 and 12 ± 0.5 mm while the P-1 and P-2 peptide concentration reached 156 and 78 μg/mL, respectively.
MIC is the lowest concentration of an antifungal agent at which the growth of a microbial strain is inhibited. The results of the present study showed that the MICs of P-1 and P-2 against T. roseum growth were in micromolar concentrations, and that the purified P-1 and P-2 displayed good antifungal activity against T. roseum with an MIC of 1 μg/mL.
Physicochemical properties of the antifungal peptides
The physicochemical properties of the antifungal peptides P-1 and P-2 are shown in Table 3. Although both P-1 and P-2 did not show any activity losses at temperature as high as 60 °C, a complete loss of activity was noted at temperatures exceeding 80 °C. In other words, low temperature (20–60 °C) did not alter the activity of P-1 and P-2, indicating that the antifungal peptides had a certain tolerance to temperature. With regard to the pH stability of P-1 and P-2, the results showed that both the peptides retained 100% of the initial activity within a pH range of 2.0–12.0. In addition, the antifungal peptides P-1 and P-2 were 100% resistant to papain, but retained only about 53 and 76% of their activity after exposed to pepsin and trypsin, respectively (Table 3), revealing that pepsin or trypsin could only destroy a part of the active site of these peptides.
Discussion
Screening and characterization of novel AMPs are attractive owing to the fact that AMPs are very efficient, have the ability to evade antimicrobial resistance, and have potential therapeutic uses (Migliolo et al. 2016; Rahman et al. 2017). To date, more and more AMPs have been found in different species, and it is certain that there are still abundant antimicrobial compounds have yet to be discovered. Thus, from another point of view, bacterial contains a large number of bioactive compounds, are perceived as a potential valuable source for the discovery of new antibacterial peptides.
In the present study, we described the isolation, purification, and characterization of two novel cationic antifungal peptides named P-1 and P-2 from B. pumilus HN-10. The two antifungal peptides with amino acid sequences of P-L-S-S-P-A-T-L-N-S-R and G-G-S-G-G-G-S-S-G-G-S-I-G-G-R, respectively, were confirmed to be novel. Both P-1 and P-2 exhibited significant antifungal activity against T. roseum with MICs of 1 μg/mL, and presented pH stability, thermo-stability, and protease stability. Moreover, the two novel antifungal peptides could be easily synthesized owing to their short sequence, which could significantly increase their application scope.
Accumulating evidences indicate that certain AMPs from B. pumilus could be applied as biological control agents in agricultural fields (Shali et al. 2010; Wang et al. 2017). For example, Rishad et al. (2017) identified antifungal metabolites produced by B. pumilus MCB-7 which showed significant antimycotic activity against agricultural pathogens such as Aspergillus flavus, Aspergillus niger, Aspergillus fumigatus, Ceratorhiza hydrophila and Fusarium oxysporum.
In fact, most of the AMPs are believed to interact with the bacterial membranes and cause cell death by deregulating the properties of the phospholipid bilayer or by causing membrane leakage, although some have been identified to have downstream cytoplasmic targets as well (Brogden 2005; Huang et al. 2010).
In a previous study, observation under scanning electron microscopy and transmission electron microscopy revealed that the fermentation broth of B. pumilus HN-10 destroyed the cell wall and membrane of T. roseum as well as exhibited antifungal activity in melon (Cucumis melo L.) (Huang et al. 2017).
Many AMPs attack microorganisms with their cationic components because microbial membranes are rich in anionic phospholipids and cause pore formation with their amphipathic structure, resulting in leakage of essential metabolites. It is believed that it is very difficult for bacteria to develop resistance to AMPs because most of the AMPs quickly kill the bacterial cells through their actions on the entire bacterial cytoplasmic membrane or through other complex mechanisms (Miyoshi et al. 2016; Lee et al. 2002; Hancock 2001; Onaizi and Leong 2011). In the present study, both P-1and P-2 were cationic AMPs (Tables 1, 2), and their mechanism of action may be similar to that of other AMPs, suggesting that it may be difficult for microorganisms to develop resistance to P-1 and P-2.
It has been reported that peptides with abundant Gly residues, including those from arthropods such as diptericin A, coleoptericin, holotricin 3, tenecin 3, acanthoscurrins, ctenidins and hymenoptaecin, can act against Gram-negative bacteria and fungi including yeasts (Dutta et al. 2017). For example, removal of the Gln and Glu residues at the C-terminus by a carboxypeptidase would expose the Gly residue, which has been proposed to enhance the activity of the peptide while protecting it from enzymatic degradation (Thompson et al. 2007). Thus, it can be concluded that Gly is a key factor involved in the antimicrobial activity of AMPs. In the present study, P-2 was noted to be a Gly-rich (60%) with Gly residues sequentially occurring at one end, which could be responsible for the higher antifungal activity of P-2, when compared with that of P-1 (Fig. 4c).
Higher hydrophobicity has associated with stronger hemolytic activity (Chen et al. 2007). As P-2 exhibited low hydrophobic ratio (6%) (Table 2), it may have low hemolytic activity, and thus may have considerable range of applications. Moreover, fractions B and C (Fig. 3c) identified by MS represented the same molecule, and may possibly be isomers. While the preliminary structure of P-1 and P-2 has been described in the present study, the complex mechanism of action of these antifungal peptides on cell membrane must be further explored.
References
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhbitors in bacteria? Nat Rev Microbiol 3(3):238–250. https://doi.org/10.1038/nrmicro1098
Chan DI, Prenner EJ, Vogel HJ (2006) Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. BBA-Biomembranes 1758:1184–1202. https://doi.org/10.1016/j.bbamem.2006.04.006
Chen YX, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS (2007) Role of peptide hydrophobicity in the mechanism of action of α-Helical antimicrobial peptides. Antimicrob Agents Ch 51(4):1398–1406. https://doi.org/10.1128/AAC.00925-06
Dutta SR, Gauri SS, Ghosh T, Halder SK, DasMohapatra PK, Mondal KC, Ghosh AK (2017) Elucidation of structural and functional integration of a novel antimicrobial peptide from Antheraea mylitta. Bioorg Med Chem Lett 27(8):1686–1692. https://doi.org/10.1016/j.bmcl.2017.03.003
Ge YH, Deng HW, Bi Y, Li CY, Liu YY, Dong BY (2015) Postharvest ASM dipping and DPI pre-treatment regulated reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Postharvest Biol Technol 99:160–167. https://doi.org/10.1016/j.postharvbio.2014.09.001
Hancock RE (2001) Cationic peptides effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1(3):156–164. https://doi.org/10.1016/s1473-3099(01)00092-5
Huang YB, Huang JF, Chen YX (2010) Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell 1(2):143–152. https://doi.org/10.1007/s13238-010-0004-3
Huang XQ, Zhang N, Yong XY, Yang XG, Shen QR (2012) Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol Res 167(3):135–143. https://doi.org/10.1016/j.micres.2011.06.002
Huang YQ, Yun JM, Zhang WW, Ai DY, Qi QY, Yao B (2017) Effect of Bacillus pumilus NH-10 on antagonistic activity and cell structure of Trichothecium roseum. J Food Sci Biotechnol 36(7):689–692 (in Chinese)
Kayalvizhi N, Gunasekaran P (2010) Purification and characterization of a novel broad-spectrum bacteriocin from Bacillus licheniformis MKU3. Biotechnol Bioproc E 15(2):365–370. https://doi.org/10.1007/s12257-009-0164-2
Leaes FL, Velho RV, Gomes Caldas DG, Ritter AC, Tsai SM, Brandelli A (2016) Expression of essential genes for biosynthesis of antimicrobial peptides of Bacillus is modulated by inactivated cells of target microorganisms. Res Microbiol 167:83–89. https://doi.org/10.1016/j.resmic.2015.10.005
Lee DG, Kim HN, Park Y, Kim HK, Choi BH, Choi CH, Hahm KS (2002) Design of novel analogue peptides with potent antibiotic activity based on the antimicrobial peptide, HP(2–20), derived from N-terminus of Helicobacter pylori ribosomal protein L1. BBA 1598:185–194
Li WH, Bi Y, Ge YH, Li YC, Wang JJ, Wang Y (2012) Effects of postharvest sodium silicate treatment on pink rot disease and oxidative stress-antioxidative system in muskmelon fruit. Eur Food Res Technol 234(1):137–145. https://doi.org/10.1007/s00217-011-1611-9
Li SM, Hao LL, Bao WG, Zhang P, Su D, Cheng YY, Nie LY, Wang G, Hou F, Yang Y (2016) A novel short anionic antimicrobial peptide isolated from the skin of Xenopus laevis with broad antimicrobial activity and inhibitory activity against breast cancer cell. Arch Microbiol 198(5):473–482. https://doi.org/10.1007/s00203-016-1206-8
Marc O, Philippe J (2007) Bacillus lipopeptides: versatile weapons for plant disease bio control. Trends Microbiol 16(3):115–125. https://doi.org/10.1016/j.tim.2007.12.009
Miao JY, Guo HX, Ou YW, Liu G, Fang X, Liao ZL, Ke C, Chen YJ, Zhao LC, Cao Y (2014) Purification and characterization of bacteriocin F1, a novel bacteriocin produced by Lactobacillus paracasei subsp. tolerans FX-6 from Tibetan kefir, a traditional fermented milk from Tibet, China. Food Control 42:48–53. https://doi.org/10.1016/j.foodcont.2014.01.041
Midura-Nowaczek K, Markowska A (2014) Antimicrobial peptides and their analogs: searching for new potential therapeutics. Perspect Medicin Chem 6:73–80. https://doi.org/10.4137/PMC.S13215
Migliolo L, Felício MR, Cardoso MH, Silva ON, Xavier ME, Nolasco DO, Oliveira AS, Roca-Subira I, Estape JV, Teixeira LD, Freitas SM, Otero-Gonzalez AJ, Gonçalves S, Santos NC, Franco OL (2016) Structural and functional evaluation of the palindromic alanine-rich antimicrobial peptide Pa-MAP2. BBA-Biomembrane 1858(7):1488–1498. https://doi.org/10.1016/j.bbamem.2016.04.003
Miyoshi N, Saito T, Ohmura T, Kuroda K, Suita K, Ihara K, Isogai E (2016) Functional structure and antimicrobial activity of persulcatusin, an antimicrobial peptide from the hard tick Ixodes persulcatus. Parasites Vectors 9:85. https://doi.org/10.1186/s13071-016-1360-5
Mookherjee N, Hancock REW (2007) Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci 64:922–933. https://doi.org/10.1007/s00018-007-6475-6
Niu LL, Bi Y, Bai XD, Zhang SG, Xue HL, Li YC, Wang Y, Calderón-Urrea A (2016) Damage to Trichothecium roseum caused by sodium silicate is independent from pH. Can J Microbiol 62(2):161–172. https://doi.org/10.1139/cjm-2015-0657
Nunes CA (2012) Biological control of postharvest diseases of fruit. Eur J Plant Pathol 133(1):181–196. https://doi.org/10.1007/s10658-011-9919-7
Onaizi SA, Leong SSJ (2011) Tethering antimicrobial peptides: current status and potential challenges. Biotechnol Adv 29(1):67–74. https://doi.org/10.1016/j.biotechadv.2010.08.012
Plácido A, Bragança I, Marani M, A AR, Vasconcelos AG, Batziou K, Domingues VF, Eaton P, Leite JRSAA., Delerue-Matos C (2017) Antibacterial activity of novel peptide derived from Cry1Ab16 toxin and development of LbL films for foodborne pathogens control. Mat Sci Eng C-Mater 75(1):503–509. https://doi.org/10.1016/j.msec.2017.02.027
Rahman MS, Choi YH, Choi YS, Yoo JC (2017) Glycin‑rich antimicrobial peptide YD1 from B. amyloliquefaciens, induced morphological alteration in and showed affinity for plasmid DNA of E. coli. AMB Expr 7(8):1–11. https://doi.org/10.1186/s13568-016-0315-8
Regmi S, Choi YH, Choi YS, Kim MR, Yoo JC (2017) Antimicrobial peptide isolated from Bacillus amyloliquefaciens K14 revitalizes its use in combinatorial drug therapy. Folia Microbio 62(2):127–138. https://doi.org/10.1007/s12223-016-0479-2
Rishad KS, Rebello S, Shabanamol PS, Jisha MS (2017) Biocontrol potential of Halotolerant bacterial chitinase from high yielding novel Bacillus Pumilus MCB-7 autochthonous to mangrove ecosystem. Pestic Biochem Phys 137:36–41. https://doi.org/10.1016/j.pestbp.2016.09.005
Shali A, Ghasemi S, Ahmadian G, Ranjbar G, Dehestani A, Khalesi N, Motallebi E, Vahed M (2010) Bacillus pumilus SG2 chitinases induced and regulated by chitin, show inhibitory activity against Fusarium graminearum and Bipolaris sorokiniana. Phytoparasitica 38(2):141–147. https://doi.org/10.1007/s12600-009-0078-8
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. https://doi.org/10.1093/molbev/msm092
Tang YM, Xue HL, Bi Y, Li YC, Wang Yi, Zhao Y, Shen KP (2014) A method of analysis for T-2 toxin and neosolaniol by UPLC-MS/MS in apple fruit inoculated with Trichothecium roseum. Food Addit Contam Part A 32(4):480–487. https://doi.org/10.1080/19440049.2014.968884
Thompson AH, Bjourson AJ, Orr DF, Shaw C, McClean S (2007) A combined mass spectrometric and cDNA sequencing approach to the isolation and characterization of novel antimicrobial peptides from the skin secretions of Phyllomedusa hypochondrialis azurea. Peptides 28(7):1331–1343. https://doi.org/10.1016/j.peptides.2007.05.001
Wang G, Yu MZ, Dong F, Shi JR, Xu JH (2017) Esterase activity inspired selection and characterization of zearalenone degrading bacteria Bacillus pumilus ES-21. Food Control 77:57–64. https://doi.org/10.1016/j.foodcont.2017.01.021
Acknowledgements
This study was funded by the financial support of the Nature Science Foundation of China (NSFC) (No. 31360405), and the Project of Gansu Major Science and Technology Plans (No. 2016GS09742).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Yan, H., Yun, J., Ai, D. et al. Two novel cationic antifungal peptides isolated from Bacillus pumilus HN-10 and their inhibitory activity against Trichothecium roseum . World J Microbiol Biotechnol 34, 21 (2018). https://doi.org/10.1007/s11274-017-2392-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2392-5