Abstract
Pectinase is an important kind of enzyme with many industrial applications, among which pectinases produced by bacteria were scarce compared with fungal sources. In this study, a novel bacterium which produced extracellular pectinase was firstly isolated from flue-cured tobacco leaves and identified as Bacillus subtilis PB1 according to its 16S rRNA gene. The pectinolytic enzyme was purified by ammonium sulfate precipitation, ion-exchange and gel filtration chromatography, after which molecular weight was determined as 43.1 ± 0.5 kDa by SDS–PAGE. Peptide mass fingerprinting of the pectinase by MALDI-TOF MS showed that the purified enzyme shared homology with pectate lyase and was designated as BsPel-PB1. The optimal temperature for BsPel-PB1 was 50 °C. The optimal pH was pH 9.5 for BsPel-PB1 while it had a broad pH stability from 5 to 11. The values of K m and V max were 0.312 mg/mL and 1248 U/mL, respectively. Accordingly, the BsPel-PB1 was a novel alkaline pectate lyase which could find potential application as a commercial candidate in the pectinolytic related industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzymes have been extensively used in numerous industrial processes owing to their significant biotechnological potential with desirable biochemical characteristics, low production cost and waste reduction (Jegannathan and Nielsen 2013), which are considered as environment-friendly process compared with the conventional ones (Hoondal et al. 2002). Among the available catalog of enzymes, pectinase is of great significance with tremendous commercial applications (Jayani et al. 2005) and its production contributes about 10% share of the overall manufacturing of enzyme preparations (Pedrolli et al. 2009).
Pectinase is the collective name for an array of enzymes hydrolyzing pectic substances, the ubiquitous polysaccharides present in plants. It is classified as polygalacturonase, pectin esterase, pectin lyase and pectate lyase depending on their mode of action (Sharma et al. 2013), which can be further subdivided into acidic and alkaline pectinase based on the optimal pH for enzymatic activity (Chiliveri and Linga 2014). Acidic pectinase has been extensively used in food industry for extraction and clarification of juice and wine (Jayani et al. 2005). It has been estimated that microbial pectinases account for 25% of the global food enzymes sales (Sharma et al. 2013). Compared to acidic pectinases, alkaline pectinase is relatively under-explored. However, recent work has indicated that alkaline pectinase has potential applications in degumming of plant fibers (Liang et al. 2015), coffee fermentations (Ouattara et al. 2011), and bioenergy (Delabona Pda et al. 2013).
Nowadays, pectinases are mainly produced by microbes via large-scale process of fermentation. In nature, many microorganisms have evolved repertoires of enzyme activities which act in tandem to decompose pectin, such as fungi (Shi et al. 2015), bacteria (Liang et al. 2015; Rehman et al. 2015) and actinomycetes (Yuan et al. 2012). Most of the currently used pectinase are from fungal species, however bacteria are likely to be an important contributor in future biotechnology strategies because of the wide functional diversity and broad array of terminal electron acceptors as well as their ability to degrade lignin (Rosnow et al. 2016). Moreover, bacteria are more amenable to manipulation using molecular genetics and protein engineering, as well as easier to implement efficient submerge fermentation process in industrial scale to increase the yield of production. Therefore, it is of great significance to screen for bacteria strains producing pectinases from novel ecological environments (Hoondal et al. 2002).
The aging fermentation of tobacco is one of the natural ecological environments which involve in the degradation of pectin. It is well-known that tobacco generally contains 10–15% pectin. Nevertheless, high pectin content will generate toxic and harmful substances during tobacco burning, and moreover, has a negative effect on tobacco aroma and other qualities (Wang et al. 2013). Hence, the aging process of tobacco is an essential and crucial manufacturing process to reduce the content of pectin, thus producing tobacco product with desirable qualities. Many microorganisms are confirmed to play very important roles in the process (Huang et al. 2010) and thus make flue-cured tobacco an excellent candidate in isolation of pectinase-producing strains. To the best of our knowledge, the bacteria which contribute to the yield of pectinolytic enzymes in the flue-cured tobacco have not yet been reported.
In this study, the pectinolytic bacteria were firstly isolated from flue-cured tobacco leaves and the bacterium with strongest activity was identified by 16S rRNA gene. Then the pectinase production process was optimized by response surface methodology (RSM). Further, its secreted pectinase was purified via chromatography and identified by matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Finally, its biochemical and molecular properties were characterized.
Materials and methods
Isolation and identification of pectinolytic bacteria
The cut aged flue-cured tobacco (FCT) leaves (10 g), collected from Yunnan, Guizhou, Hunan and Shaanxi province (four major tobacco production regions of China), were added into sterile liquid LB medium and spread on selective medium plates (consisted of 5.0 g/L pectin, 1.0 g/L K2HPO4, 0.5 g/L MgSO4, 3.0 g/L (NH4)2SO4, 0.01 g/L FeSO4, 20 g/L agar). After incubation at 37 °C for 72 h, the plates were stained with 0.2% Congo red for 4 h and washed with 1 M NaCl. The colonies with transparent circle were preliminarily isolated as potential pectinolytic bacteria. The isolates were inoculated into liquid LB medium at 37 °C, 150 rpm for 24 h and their pectinase activities were assayed. Finally, one strain with the maximum pectinase activity designated as PB1 was selected for further investigation.
The genomic DNA of PB1 was extracted by genome DNA isolation kit (Omega Bio-Tek, USA) and 16S rRNA gene of PB1 was amplified using the U-F (AGAGTTTGATCCTGGCTCAG) and U-R (GGTTACCTTGTTACGACTT). PCR amplification program was conducted as previously described (Lü et al. 2014). Amplified gene fragment was separated by electrophoresis using 1% (w/v) agarose gel, recovered and sequenced by Sangon Biotech (Shanghai, China). Strain PB1 was deposited in China General Microbiological Culture Collection Center (Beijing, China), and the accession number was CGMCC No. 12324. The 16S rRNA gene of strain PB1 was submitted to GenBank under the accession numbers KU904502.
Pectinase activity and protein content assay
Pectinase activity was determined by mixing 1 ml of enzyme solution (being appropriately diluted) and 5 ml citrus pectin solution (0.4%, w/v) in 0.05 M Gly-NaOH buffer (pH 9.4) at 50 °C for 30 min. The release of galacturonic acid was measured using the 3,5-dinitrosalicylic acid colorimetric (DNS) method (Uzuner and Cekmecelioglu 2015) in which galacturonic acid was used as the standard. The boiled crude enzyme solution was used as the control. One unit of pectinase was defined as the amount of enzyme required to release 1 μmol of d-galacturonic acid ml− 1 min− 1 under the assay conditions. Protein content was determined by the Bradford method with bovine serum albumin (BSA) as the standard.
Optimization of pectinase production
The Plackett-Burman design was employed to identify the significant variables, in which 6 nutritional parameters (pectin, starch, peptone, MnCl, MgSO4 and K2HPO4), 2 physical variables (pH and inoculum dose) and 3 dummies (to evaluate the error in design) were analyzed (Table 1). The upper and the lower limits of each variable were chosen by preliminary investigations. The response was the mean value of the pectinase production by PB1.
Based on the results of Plackett–Burman experiments, significant factors were selected for further optimization by central composite design (CCD). The experimental design included 16 factorial points and 4 replications at the center point (Table 2). The response value was the pectinase production by PB1. The regression model utilized in the factorial planning included the interaction terms could be described by the following quadratic polynomial Eq. (1):
where Y represented response variable, β0, βi, βii, βij were intercept coefficient, linear term, quadratic term and interaction term, respectively. x i , x j were coded levels of independent variables.
Analysis of variance (ANOVA) was performed to validate the statistical model. The experimental design, statistical analyses and response surface plotting were carried out by using ‘Design Expert 8.0.6’ (Stat-Ease, Inc., Minneapolis, USA). All experiments were performed in three replicates and the results were reported as mean values.
Purification of pectinase
An overnight growth of the PB1 strain was inoculated (inoculum size: 4%) into 50 ml production medium optimized by RSM in 60 conical flasks. The flasks were incubated at 37 °C in a rotary shaker (150 rpm) for 24 h. Then the culture was centrifuged (7878×g, 4 °C, 20 min) and the cell-free supernatant was used for purification.
Ammonium sulfate precipitation
The proteinaceous substances in the cell-free supernatant were precipitated by serial saturated ammonium sulfate (20, 40, 60, 80 and 100%). The precipitation with highest pectinase activity was dialyzed against the same buffer for 24 h at 4 °C with the buffer replacement every 2 h and used for further purification.
Ion exchange chromatography
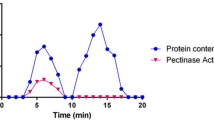
The dialyzed crude pectinase was purified by the AKTA purifier 100 (GE, Amersham Biosciences, Sweden) equipped with a Q XL 16/10 column (GE Healthcare Life Sciences, Sweden), in which the system was equilibrated with 0.02 M Gly-NaOH buffer (pH 9.4) and 1 M NaCl was used for elution at 1.0 ml/min. Fractions (1 ml) were collected automatically and the process was monitored by UV detector at 280 nm. Fractions with absorbance peak were pooled, followed by the pectinase activity and protein content measurements.
Gel filtration chromatography
The fraction showing pectinase activity was purified by Superdex G-75 10/300 column (GE, Amersham Biosciences, Sweden), in which the system was equilibrated and eluted with 0.02 M Gly-NaOH buffer (pH 9.4) at 0.5 ml min−1. Fractions (1 ml) were collected automatically and the process was monitored by UV detector at 280 nm. Finally, the fraction with activity was obtained for the following research.
Peptide mass fingerprinting analysis
The homogeneity and molecular weight of the enzyme were determined by SDS–PAGE with 12% separating gel and 5% stacking gel and the protein bands were stained with Coomassie Brilliant Blue G-250. Then, the clear target band was cut off and subjected to peptide mass fingerprinting (PMF) analysis by using a matrix-assisted laser desorption ionization-time of flight mass spectrometry (5800 Plus MALDI-TOF MS, ABI, USA). Databank searching was performed with the MASCOT search engine (Matrix Science, MA) to query NCBI databases with a tolerance of 100 ppm for the MS analysis and 0.3 Da for the MS/MS analysis.
Effect of pH, temperature on pectinase
The optimum pH for PB1 pectinase was investigated at different pH buffer solutions ranging from 3.0 to 12.0. The pH stability of PB1 pectinase activity was investigated by pre-incubating pectinase at certain pH ranging from 3.0 to 12.0 for 2 h, afterwards, the residual pectinase activity was evaluated. The optimum temperature for PB1 pectinase activity was measured at temperatures ranging from 40 to 60 °C with 5 °C intervals. The thermal stability of PB1 pectinase was estimated by pre-incubating the enzyme at 40, 45, 50, 55 and 60 °C for 2 h prior to the residual pectinase activity measurement.
Effects of metal ions and reagents on pectinase
The effects of metal ions and chemical reagents on the enzyme activities were determined by incubating the enzymes in the presence of 1 mM of CaCl2, CoCl2, NiCl2, CuCl2, MgCl2, MnCl2, ZnCl2, BaCl2, SDS and ethylenediaminetetraacetic acid (EDTA). The residual activity was measured and compared with that of control without additive.
Substrate specificity and kinetic determinations
The substrate specificity of purified enzyme was assayed by incubating the enzyme solution with 0.4% (w/v) substrate (polygalacturonic acid and citrus pectin, Sigma-Aldrich) under standard conditions. The kinetic parameters, namely Michaelis–Menten constants (K m), maximum velocity (V max), deduced turnover number (k cat) and catalytic efficiency (k cat/ K m) were determined by performing the enzyme assay at different concentrations of pectin (0.1–10 mg/ml) and calculated using Lineweaver–Burk plot (1/[S] versus 1/[v]).
Results
Isolation and identification of pectinolytic bacteria
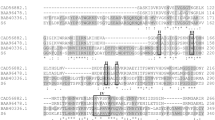
Ninety-eight strains were isolated from aged FCT samples and thirty-two strains were selected based on the hydrolysis circle. According to pectinase activity, PB1 strain was selected because of its highest pectinase activity. According to 16S rRNA gene sequence (GenBank Accession Number: KU904502), PB1 was identified as Bacillus subtilis by performing BLAST search on NCBI and the phylogenetic tree was constructed accordingly (Fig. 1).
Optimization of pectinase production
Table 1 shows the effect of selected variables on the production of pectinase. It was shown that the p values of pectin, peptone and pH were 0.010, 0.010 and 0.009, respectively. Thereby, they were identified as significant factors and selected for further optimized by CCD. The experimental and predicted values for optimization of selected variables by CCD are presented in Table 2.
After eliminating the statistically insignificant terms (p > 0.05), the step-wise regression model for pectinase production was shown in the Eq. (2) in terms of coded values.
The ANOVA of the regression model for pectinase production is summarized in Table 3. The F value of the model was 48.92, suggesting that the model was highly statistically significant (p < 0.0001). Meantime, the lack of fits was found to be not significant, implying the model was adequate to predict the pectinase production.
The three dimensional graphs of the effects of peptone, pH and pectin on the pectinase production are plotted in Fig. 2. It was observed that the pectinase production increased linearly when pectin increased (Fig. 2a), whereas it increased initially and then declined as peptone and pH increased (Fig. 2b, c). Combined with the contour plots and the second order regression equation, the maximum pectinase production was predicted to occur at pectin 12 g/L, pH 9.0 and peptone 20 g/L.
To verify the optimization results, experiment was done under the optimal condition in triplicates. The results indicated that the experimental pectinase activity was 19.50 ± 0.28 U/ml, which was nearing the predicted value of 20.20 U/ml.
Purification and identification of pectinase
The pectinase produced by Bacillus subtilis PB1 was purified to 18.67-fold with a specific activity of 1252.82 U/mg (Table 4) by a combination of titration with ammonium sulfate and chromatographic procedures. After precipitation by 80% saturation ammonium sulfate, the specific activity was increased to 67.09 U/mg. The retentate obtained post dialyzation was subjected to Q XL column where one peak (P2, Fig. 3a) showed pectinase activity with a recovery of 40.11% (Table 4). The fraction (P2) was further purified by Superdex G-75 column where only the third peak (P23, Fig. 3b) had pectinase activity. The homogeneity of P23 was confirmed by SDS–PAGE and the molecular weight of P23 was approximately 43.1 ± 0.5 kDa according to SDS–PAGE (Fig. 3c).
The P23 was cut from the gel and digested by trypsin, after which the fragments were identified by MALDI-TOF MS for peptide mass fingerprint (PMF) analysis (Fig. 4). Twelve peptides were detected as follows, Peptide 1: AYDPSTWGK; Peptide 2: SNVINQAGAGK; Peptide 3: KEPSGTQEEAR; Peptide 4: ASSSNVYTVSNR; Peptide 5: VIAPESFQYLK; Peptide 6: NPNANVYVSAYK; Peptide 7: IYAQNNVIDVPGLSAAK; Peptide 8: ETAFGNGQNQLGFSILR; Peptide 9: VMVDIPANTTIVGSGTNAK; Peptide 10: DLWMTEVYYPNSDNNSADR; Peptide 11: GTIDMNVDDNLKPLGLNDYKDPEYDLDK; Peptide 12: FGQVHVYNNYYEGSTSSSSYPFSYAWGIGK. Particularly, MS/MS analysis confirmed that five peptides (Peptide 2, Peptide 4, Peptide 7, Peptide 9 and Peptide12) are unique peptides of B. subtilis pectate lyase (BsPel). The secondary mass spectrum of one representative P2 (3395.55 Da) is shown in Fig. 4b. After MASCOT searching of NCBI protein database, the enzyme was matched with the BsPel-A (NCBI Accession: AFP43211.1, molecular weight: 43.5 kDa) by 93% sequence identity and a total score of 249, accordingly P23 was considered as a pectate lyase and named as BsPel-PB1.
Effect of pH, temperature on BsPel-PB1
As shown in Fig. 5a, the BsPel-PB1 reached maximum activity at pH 9.5. The enzyme remained stable within a wide range of pH from 5 to 11 since almost 80% activity remained after incubation for 2 h (Fig. 5b). However, it lost most of activity after treated at pH 3 or pH 12. It was observed that the optimum temperature for BsPel-PB1 was 50 °C (Fig. 5c). After treatment at 40, 45 and 50 °C for 2 h, 90% activity remained, while the activity obviously decreased after treated at 55 and 60 °C (Fig. 5d).
Effects of metal ions and reagents on BsPel-PB1
The enzyme activity was stimulated by Ca2+, Mg2+, Mn2+ and Ba2+, whereas Cu2+ and EDTA strongly inhibited it (Table 5). In addition, the presence of Co2+, Zn2+, Ni2+ and SDS had little or no significant effect on the enzymatic activity. From the enhancement of enzyme activity by Ca2+ (257.5%) and reduction of enzyme activity by EDTA (16.65%), it can be concluded that the enzyme requires calcium for its activity.
Substrate specificity and kinetic determinations
The BsPel-PB1 was highly active against polygalacturonic acid (PGA) (1248 U/mg), and to a lesser extent, against citrus pectin (1063 U/mg). The apparent values of K m and V max towards PGA were observed to be 0.312 mg/ml and 1248 U/mg, respectively (Fig. 6). The deduced turnover number (kcat) and catalytic efficiency (kcat/Km) values were 2300 s− 1 and 7372 L/(s·g), respectively.
Discussion
Isolation and identification of pectinolytic bacteria
Pectinases play a key role in the fermentation process of plant raw material when microorganisms invade plants (Ochiai et al. 2007). Pectin is also a major component of the tobacco primary cell walls (5–13%) (Wang et al. 2013), thus the fermentation of FCT has been linked to the pectinolytic actions of abundant microbial communities (Huang et al. 2010). Therefore, the pectinolytic strains are presumably abundant on the surface of tobacco leaves which urged our interest to isolate pectinolytic strains from a hitherto unexplored source, after which PB1 was isolated and selected. Actually various strains of the genus Bacillus from diverse ecological niches had the capability to degrade pectic substances, such as sugar beet pulp (Berensmeier et al. 2004), soil (Basu et al. 2008), river (Chiliveri and Linga 2014) and cocoa bean fermentation (Ouattara et al. 2010). However, scarce report was found to investigate the pectinase-producing strains from tobacco leaves to our knowledge.
Optimization of pectinase production
RSM is widely employed in various process optimizations (Wang and Lü 2014). In order to boost the output of the enzyme production, RSM method was performed for the optimization of fermentation conditions. As observed in Fig. 2, the pectinase production increased linearly when pectin increased. This could be explained by the fact that pectin, as well as polygalacturonic acid and d-galacturonic acid are able to induce the expression of pectinase encoding genes (Yadav et al. 2009b). After optimization, a 9.4-fold increase in the pectinase production of PB1 was achieved, which was higher than those reported for production improvement of pectinase from Bacillus subtilis (Uzuner and Cekmecelioglu 2015).
Purification and Identification of pectinase
The purified BsPel-PB1 was different with previous reported pectate lyases produced by different Bacillus sp. based on it’s molecular weight, such as pectate lyases form Bacillus pumilus (37 kDa) (Klug-Santner et al. 2006), Bacillus clausii (35 kDa) (Li et al. 2012), Bacillus subtilis (45 kDa) (Liu et al. 2012) and Bacillus tequilensis (43 kDa) (Chiliveri and Linga 2014). Moreover, BsPel-PB1 showed a specific activity of 1252.82 U/mg (Table 4), which was apparently higher than those previously reported enzymes’ activities of Bacillus pumilus (909 U/mg) (Basu et al. 2008), Bacillus clausii (297 U/mg) (Li et al. 2012) and Bacillus subtilis WB600 (445 U/mg) (Liu et al. 2012).
Pectate lyase cleaves pectin using a β-elimination mechanism. It has been reported that the catalytic sites of pectate lyases from orthologous species show a comparable topology, with an identical arrangement of the conserved functional residues. According to the phylogenetic tree of Bacillus subtilis PB1 (Fig. 1), it was inferred that Bacillus subtilis PB1 showed close relationships with Bacillus licheniformis and Bacillus cereus. Pectate lyases from Bacillus licheniformis (Berensmeier et al. 2004) and Bacillus cereus (Ouattara et al. 2011) have been reported and both of them belonged to pectate lyase family 1 (PL1). Therefore, BsPel-PB1 might also belong to PL1. Pectate lyases from PL1 generally adopt a similar mechanism of action. It involves a basic residue, usually Arg, Lys or His, acting as a Brønstead base to abstract the proton at position C-5 of the galacturonate residue (Hugouvieux-Cotte-Pattat et al. 2014). The enzyme activity also involves a divalent cation (Ca2+ in most cases) that binds to the substrate carboxylate and promotes acidification at the C-5 position. Although the pectate lyases from PL1 share resemble topology folds, there will be some variations in the amino acids as the result of horizontal gene transfers or another rearrangement. That would be a reason that they show different characteristics.
Effects of pH, temperature on BsPel-PB1
Genus Bacillus predominantly produce alkaline pectate lyases, whose optimum pH ranged from 8.0 to 10.5 (Chiliveri and Linga 2014). The optimum pH of the BsPel-PB1 was 9.5, which was higher than the value of pectate lyase from Bacillus subtilis (Klug-Santner et al. 2006; Liang et al. 2015). This would be possibly due to the abstraction of the proton from C5 of the galacturonate residue by amino acid variations, which improved the alkali resistance of BsPel-PB1 (Seyedarabi et al. 2010). When treated at pH 3 (Fig. 5), the BsPel-PB1 activity fell away dramatically. It is assumed that the protonation of aspartates leads to the loss of the two catalytic calcium-ions causing a profound failure to correctly organize the Michaelis complex (Ali et al. 2015).
Effects of metal ions and reagents on BsPel-PB1
BsPel-PB1 had great resistance to most metal ions and chemical reagents (Table 5), which was different with pectate lyase produced by Bacillus clausii (Li et al. 2012). The dependance on Ca2+ of BsPel-PB1 was similar to other microbial pectate lyases (Basu et al. 2008; Klug-Santner et al. 2006; Yuan et al. 2011). The enzyme was also activated by Mg2+, Mn2+ and Ba2+, which was similarly as pectate lyases from Bacillus pumilus and Bacillus tequilensis (Basu et al. 2008; Chiliveri and Linga 2014) probably because of their similar participation in enzyme binding or formation of salt bridges between polygalacturonic chains (Yuan et al. 2011). The variation in pectate lyase sequence among the same genus Bacillus sp. strains may be responsible for the difference in sensitivity towards metal ions and reagents (Basu et al. 2008).
Substrate specificity and kinetic determinations
The physiological efficiency value (V max/K m) provides a model to determine the enzyme efficiency on the aspect of industrial application (Basu et al. 2008). Bacillus subtilis PB1 shows an apparently better catalytic efficiency [4000 U (mg PGA ml− 1)−1] than the previously reported the values of other pectate lyases from Bacillus pumilus DKS1 [2206 U (mg PGA ml− 1)−1] (Basu et al. 2008) and Bacillus tequilensis [1453 U (mg PGA ml− 1)−1] (Chiliveri and Linga 2014). The kcat value of BsPel-PB1 was 2300 S− 1, which was higher than the Pels from Bacillus pumilus (514.1 S− 1) (Liang et al. 2015) while lower than the Pels from Bacillus sp. Strain N16-5 (3319 S− 1) (Zhou et al. 2015). The kcat/Km value of BsPel-PB1 was relatively low when compared with recombinant Pels (Li et al. 2014), which indicating that there is lot of scope for improving the catalytic efficiency using modern molecular techniques such as protein engineering. In the case of substrate specificity, PGA was found to be the preferred substrate over pectin. Similar observations have been reported that the pectate lyases from Bacillus strains show high specificity for PGA (Chiliveri and Linga 2014; Klug-Santner et al. 2006; Rehman et al. 2015), which was possibly because pectate lyase has three central subsites showing a profound preference for PGA but only the distal subsites can accommodate pectin (Seyedarabi et al. 2010).
Implications
As one of the most widely used commercial enzymes, alkaline pectate lyase has many industrial applications: (1) Food industry: food additives (Min et al. 2010), wine quality improvement (Radoi et al. 2005), coffee and tea fermentation (Chandini et al. 2011; Ouattara et al. 2011), functional food development (Bonnin et al. 2014; Gullón et al. 2013; Khan et al. 2013), oil production (Ricochon et al. 2011; Servili et al. 1992); (2) Textile industry: degumming (Liang et al. 2015), retting (Yadav et al. 2009a), bioscouring (Klug-Santner et al. 2006); (3) Lignocellulosic biofuels production (Delabona Pda et al. 2013; Zhou et al. 2017): reducing the recalcitrance and accelerating saccharification of lignocellulose biomass; (4) Paper and pulp industry: substitution for chemical bleaching (Ahlawat et al. 2008); (5) Aminal feed industry: as enzyme cocktail component to reduce the feed viscosity and the amount of faeces (Igbasan et al. 1997), increasing absorption of nutrients, and as functional and prebiotic component (Tu et al. 2014); and so on.
Among them, the application in lignocellulosic biofuels is a burgeoning and promising but poorly explored field. The importance of pectin in lignocellulosic biomass has long been underestimated, and so do the pectinases. However, pectinases have been reported to reduce the recalcitrance and accelerate the lignocellulose saccharification of herbaceous plants (Lionetti et al. 2010), Arabidopsis (Francocci et al. 2013), switchgrass (Chung et al. 2014) and woody biomass (Biswal et al. 2014, 2015). In fact, pectinases play important roles in the deconstruction of lignocellulosic biomass. As one of the plant cell wall components, pectin embeds in the cellulose–hemicellulose network of the cell wall and regulates intercellular adhesion like glues. It is the complex matrix of pectin that masks cellulose and/or hemicellulose through hydrogen bonding interactions, and blocks their accessibility to degradative enzymes, thus resulting in plant biomass that is less susceptible to degradation and more recalcitrant to deconstruction. Therefore, pectinases have multiple benefits in the efficient hydrolysis of lignocellulosic material and applications in the lignocellulosic biofuels. For more details, please refer to our recently published work (Zhou et al. 2017).
As described in Introduction, the pectinase from bacterial origins has many favorable attributes. Therefore, as the novel use of pectinase in lignocellulosic biofuels and increasing demands from various industries, the discovery of novel pectinase from bacterial source has big significance. Here, a novel pectinolytic bacterium, Bacillus subtilis PB1, was firstly isolated from aged tobacco leaves. According to the results of SDS–PAGE and PMF, pectinase was a novel one and was identified as pectate lyase. Moreover, BsPel-PB1 had distinct characteristics different with other reported pectinases produced by genus Bacillus.
There are some implications. First, this study confirmed the degradation of pectin in flue-cured tobacco leaves, which would provide insights into the production and qualities improvement of tobacco industry. Second, the distinct characteristics of BsPel-PB1 gave us a hint that various Bacillus species from diverse ecological niches might have different genetic mechanisms in producing pectinases. Third, the novel pectate lyase will expand the arsenal of pectinases originated from bacterial source as well as the potential application in numerous industrial processes.
Abbreviations
- MALDI-TOF MS:
-
Matrix assisted laser desorption ionization-time of flight mass spectrometry
- FCT:
-
Flue-cured tobacco
- DNS:
-
3, 5-Dinitrosalicylic acid colorimetric
- BSA:
-
Bovine serum albumin
- CCD:
-
Central composite design
- RSM:
-
Response surface methodology
- PMF:
-
Peptide mass fingerprinting
- SDS–PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- BsPel:
-
Bacillus subtilis pectate lyase
- PGA:
-
Polygalacturonic acid
- PL1:
-
Pectate lyase family 1
References
Ahlawat S, Mandhan RP, Dhiman SS, Kumar R, Sharma J (2008) Potential application of alkaline pectinase from Bacillus subtilis SS in pulp and paper industry. Appl Biochem Biotechnol 149:287–293. doi:10.1007/s12010-007-8096-9
Ali S, Sondergaard CR, Teixeira S, Pickersgill RW (2015) Structural insights into the loss of catalytic competence in pectate lyase activity at low pH. FEBS Lett 589:3242–3246. doi:10.1016/j.febslet.2015.09.014
Basu S, Ghosh A, Bera A, Saha MN, Chattopadhyay D, Chakrabarti K (2008) Thermodynamic characterization of a highly thermoactive extracellular pectate lyase from a new isolate Bacillus pumilus DKS1. Bioresour Technol 99:8088–8094. doi:10.1016/j.biortech.2008.03.032
Berensmeier S, Singh SA, Meens J, Buchholz K (2004) Cloning of the pelA gene from Bacillus licheniformis 14A and biochemical characterization of recombinant, thermostable, high-alkaline pectate lyase. Appl Microbiol Biotechnol 64:560–567. doi:10.1007/s00253-003-1446-9
Biswal AK et al (2014) Aspen pectate lyase Ptxt PL1-27 mobilizes matrix polysaccharides from woody tissues and improves saccharification yield. Biotechnology for biofuels 7:11. doi:10.1186/1754-6834-7-11
Biswal AK et al (2015) Downregulation of GAUT12 in Populus deltoides by RNA silencing results in reduced recalcitrance, increased growth and reduced xylan and pectin in a woody biofuel feedstock. Biotechnol Biofuels 8:41. doi:10.1186/s13068-015-0218-y
Bonnin E, Garnier C, Ralet MC (2014) Pectin-modifying enzymes and pectin-derived materials: applications and impacts. Appl Microbiol Biotechnol 98:519–532. doi:10.1007/s00253-013-5388-6
Chandini SK, Rao LJ, Gowthaman MK, Haware DJ, Subramanian R (2011) Enzymatic treatment to improve the quality of black tea extracts. Food Chem 127:1039–1045. doi:10.1016/j.foodchem.2011.01.078
Chiliveri SR, Linga VR (2014) A novel thermostable, alkaline pectate lyase from Bacillus tequilensis SV11 with potential in textile industry. Carbohydr Polym 111:264–272. doi:10.1016/j.carbpol.2014.04.065
Chung D, Pattathil S, Biswal AK, Hahn MG, Mohnen D, Westpheling J (2014) Deletion of a gene cluster encoding pectin degrading enzymes in Caldicellulosiruptor bescii reveals an important role for pectin in plant biomass recalcitrance. Biotechnol Biofuels 7:147. doi:10.1186/s13068-014-0147-1
Delabona Pda S et al (2013) Understanding the cellulolytic system of Trichoderma harzianum P49P11 and enhancing saccharification of pretreated sugarcane bagasse by supplementation with pectinase and alpha-l-arabinofuranosidase. Bioresour Technol 131:500–507. doi:10.1016/j.biortech.2012.12.105
Francocci F, Bastianelli E, Lionetti V, Ferrari S, De Lorenzo G, Bellincampi D, Cervone F (2013) Analysis of pectin mutants and natural accessions of Arabidopsis highlights the impact of de-methyl-esterified homogalacturonan on tissue saccharification. Biotechnol Biofuels 6:163. doi:10.1186/1754-6834-6-163
Gullón B, Gómez B, Martínez-Sabajanes M, Yáñez R, Parajó JC, Alonso JL (2013) Pectic oligosaccharides: manufacture and functional properties. Trends Food Sci Technol 30:153–161. doi:10.1016/j.tifs.2013.01.006
Hoondal GS, Tiwari RP, Tewari R, Dahiya N, Beg QK (2002) Microbial alkaline pectinases and their industrial applications: a review. Appl Microbiol Biotechnol 59:409–418. doi:10.1007/s00253-002-1061-1
Huang J et al (2010) Bacterial diversities on unaged and aging flue-cured tobacco leaves estimated by 16S rRNA sequence analysis. Appl Microbiol Biotechnol 88:553–562. doi:10.1007/s00253-010-2763-4
Hugouvieux-Cotte-Pattat N, Condemine G, Shevchik VE (2014) Bacterial pectate lyases, structural and functional diversity. Environ Microbiol Rep 6:427–440. doi:10.1111/1758-2229.12166
Igbasan FA, Guenter W, Slominski BA (1997) The effect of pectinase and α-galactosidase supplementation on the nutritive value of peas for broiler chicken Canadian. J Anim Sci 77:537–539
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40:2931–2944. doi:10.1016/j.procbio.2005.03.026
Jegannathan KR, Nielsen PH (2013) Environmental assessment of enzyme use in industrial production—a literature review. J Clean Prod 42:228–240. doi:10.1016/j.jclepro.2012.11.005
Khan M, Nakkeeran E, Umesh-Kumar S (2013) Potential application of pectinase in developing functional foods. Annu Rev Food Sci Technol 4:21–34. doi:10.1146/annurev-food-030212-182525
Klug-Santner BG, Schnitzhofer W, Vrsanska M, Weber J, Agrawal PB, Nierstrasz VA, Guebitz GM (2006) Purification and characterization of a new bioscouring pectate lyase from Bacillus pumilus BK2. J Biotechnol 121:390–401. doi:10.1016/j.jbiotec.2005.07.019
Li ZM et al (2012) Purification and characterization of alkaline pectin lyase from a newly isolated Bacillus clausii and its application in elicitation of plant disease resistance applied. Biochem Biotechnol 167:2241–2256. doi:10.1007/s12010-012-9758-9
Li X, Wang H, Zhou C, Ma Y, Li J, Song J (2014) Cloning, expression and characterization of a pectate lyase from Paenibacillus sp. 0602 in recombinant Escherichia coli. BMC Biotechnol 14:18
Liang C, Gui X, Zhou C, Xue Y, Ma Y, Tang SY (2015) Improving the thermoactivity and thermostability of pectate lyase from Bacillus pumilus for ramie degumming. Appl Microbiol Biotechnol 99:2673–2682. doi:10.1007/s00253-014-6091-y
Lionetti V et al. (2010) Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc Natl Acad Sci 107:616–621
Liu Y et al (2012) Efficient expression of an alkaline pectate lyase gene from Bacillus subtilis and the characterization of the recombinant protein. Biotechnol Lett 34:109–115. doi:10.1007/s10529-011-0734-1
Lü X, Yi L, Dang J, Dang Y, Liu B (2014) Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ 32 for inhibiting bacterial foodborne pathogens including antibiotic-resistant. Microorg Food Control 46:264–271. doi:10.1016/j.foodcont.2014.05.028
Min B, Bae IY, Lee HG, Yoo SH, Lee S (2010) Utilization of pectin-enriched materials from apple pomace as a fat replacer in a model food system. Bioresour Technol 101:5414–5418. doi:10.1016/j.biortech.2010.02.022
Ochiai A, Itoh T, Kawamata A, Hashimoto W, Murata K (2007) Plant cell wall degradation by saprophytic Bacillus subtilis strains: gene clusters responsible for rhamnogalacturonan depolymerization. Appl Environ Microbiol 73:3803–3813. doi:10.1128/AEM.00147-07
Ouattara HG, Reverchon S, Niamke SL, Nasser W (2010) Biochemical properties of pectate lyases produced by three different Bacillus strains isolated from fermenting cocoa beans and characterization of their cloned genes. Appl Environ Microbiol 76:5214–5220. doi:10.1128/AEM.00705-10
Ouattara HG, Reverchon S, Niamke SL, Nasser W (2011) Molecular identification and pectate lyase production by Bacillus strains involved in cocoa fermentation. Food Microbiol 28:1–8. doi:10.1016/j.fm.2010.07.020
Pedrolli DB, Monteiro AC, Gomes E, Carmona EC (2009) Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol J 3:9–18
Radoi F, Kishida M, Kawasaki H (2005) Characteristics of wines made by Saccharomyces mutants which produce a polygalacturonase under wine-making conditions. Biosci Biotechnol Biochem 69:2224–2226
Rehman HU, Aman A, Nawaz MA, Qader SAU (2015) Characterization of pectin degrading polygalacturonase produced by Bacillus licheniformis KIBGE-IB21. Food Hydrocoll 43:819–824. doi:10.1016/j.foodhyd.2014.08.018
Ricochon G, Elfassy A, Pages X, Piffaut B, Girardin M, Muniglia L (2011) Correlation between the release of sugars and uronic acid and free oil recovery following enzymatic digestion of oil seed cell walls. Bioresour Technol 102:9599–9604. doi:10.1016/j.biortech.2011.06.097
Rosnow JJ, Anderson LN, Nair RN, Baker ES, Wright AT (2016) Profiling microbial lignocellulose degradation and utilization by emergentomics technologies. Crit Rev Biotechnol. doi:10.1080/07388551.2016.1209158
Servili M, Begliomini AL, Montedoro G, Petruccioli M, Federici F (1992) Utilisation of a yeast pectinase in olive oil extraction and red wine making processes. J Sci Food Agric 58:253–260
Seyedarabi A et al (2010) Structural insights into substrate specificity and the anti beta-elimination mechanism of pectate lyase. Biochemistry 49:539–546. doi:10.1021/bi901503g
Sharma N, Rathore M, Sharma M (2013) Microbial pectinase: sources, characterization and applications. Rev Environ Sci Bio-Technol 12:45–60. doi:10.1007/s11157-012-9276-9
Shi A, Hu H, Zheng F, Long L, Ding S (2015) Biochemical characteristics of an alkaline pectate lyase PelA from Volvariella volvacea: roles of the highly conserved N-glycosylation site in its secretion and activity. Appl Microbiol Biotechnol 99:3447–3458. doi:10.1007/s00253-014-6146-0
Tu T et al (2014) Molecular characterization of a thermophilic endo-polygalacturonase from Thielavia arenaria XZ7 with high catalytic efficiency and application potential in the food and feed industries. J Agric Food Chem 62:12686–12694. doi:10.1021/jf504239h
Uzuner S, Cekmecelioglu D (2015) Enhanced pectinase production by optimizing fermentation conditions of Bacillus subtilis growing on hazelnut shell hydrolyzate. J Mol Catal B 113:62–67. doi:10.1016/j.molcatb.2015.01.003
Wang X, Lü X (2014) Characterization of pectic polysaccharides extracted from apple pomace by hot-compressed water. Carbohydr Polym 102:174–184. doi:10.1016/j.carbpol.2013.11.012
Wang ZY, Shao Y, Zhou QM, Chen G, Li J (2013) The pectin and its influence on tobacco physiology and quality. J Agric Sci Technol 15:130–135
Yadav S, Yadav PK, Yadav D, Yadav KD (2009a) Purification and characterization of pectin lyase produced by Aspergillus terricola and its application in retting of natural fibers. Appl Biochem Biotechnol 159:270–283. doi:10.1007/s12010-008-8471-1
Yadav S, Yadav PK, Yadav D, Yadav KDS (2009b) Pectin lyase: a. review. Process Biochem 44:1–10. doi:10.1016/j.procbio.2008.09.012
Yuan P et al (2011) A novel low-temperature active alkaline pectate lyase from Klebsiella sp. Y1 with potential in textile industry. Process Biochem 46:1921–1926. doi:10.1016/j.procbio.2011.06.023
Yuan P et al (2012) An alkaline-active and alkali-stable pectate lyase from Streptomyces sp. S27 with potential in textile industry. J Ind Microbiol Biotechnol 39:909–915. doi:10.1007/s10295-012-1085-1
Zhou C, Ye J, Xue Y, Ma Y (2015) Directed evolution and structural analysis of alkaline pectate lyase from the Alkaliphilic Bacterium Bacillus sp. strain N16-5 to improve its thermostability for efficient ramie degumming. Appl Environ Microbiol 81:5714–5723. doi:10.1128/AEM.01017-15
Zhou M et al (2017) Metagenomic mining pectinolytic microbes and enzymes from an apple pomace-adapted compost microbial. Community Biotechnol Biofuels 10:198. doi:10.1186/s13068-017-0885-y
Acknowledgements
The authors gratefully acknowledge the financial assistance supported by the Special Fund for Agro-scientific Research in the Public Interest [Grant Number 201503135], Ministry of Agriculture, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, M., Wu, J., Wang, T. et al. The purification and characterization of a novel alkali-stable pectate lyase produced by Bacillus subtilis PB1. World J Microbiol Biotechnol 33, 190 (2017). https://doi.org/10.1007/s11274-017-2357-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2357-8