Abstract
Dissimilatory metal reducing bacteria can exchange electrons extracellularly and hold great promise for their use in simultaneous wastewater treatment and electricity production. This study investigated the role of riboflavin, an electron carrier, in the decolourisation of Congo red in microbial fuel cells (MFCs) using Shewanella oneidensis MR-1 as a model organism. The contribution of the membrane-bound protein MtrC to the decolourisation process was also investigated. Within the range of riboflavin concentrations tested, 20 µM was found to be the best with >95% of the dye (initial concentration 200 mg/L) decolourised in MFCs within 50 h compared to 90% in the case where no riboflavin was added. The corresponding maximum power density was 45 mW/m2. There was no significant difference in the overall decolourisation efficiencies of Shewanela oneidensis MR-1 ΔMtrC mutants compared to the wild type. However, in terms of power production the mutant produced more power (Pmax 76 mW/m2) compared to the wild type (Pmax 46 mW/m2) which was attributed to higher levels of riboflavin secreted in solution. Decolourisation efficiencies in non-MFC systems (anaerobic bottles) were similar to those under MFC systems indicating that electricity generation in MFCs does not impair dye decolourisation efficiencies. The results suggest that riboflavin enhances both decolourisation of dyes and simultaneous electricity production in MFCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Azo dyes have been one of many synthetic dyes used in the textile industry. To this day, more azo dyes are synthesized, not only for colouring purposes but also for biological-medical imaging and other optical applications (Mohammadi et al. 2015). Research focusing on azo decolourisation has always been in demand, focusing on novel ways to degrade this dye, the danger always lying in the azo group (–N=N–). Methods may depend on physicochemical removal such as adsorption on magnetic biocomposites (Sivashankar et al. 2015) or could rely on biological removal depending on enzyme induction such as peroxidases, azo reductases etc. Biological removal is considered more favourable for complete degradation of azo compounds (Imran et al. 2016).
Biologically, the azo bond is thought to be cleaved under anaerobic conditions leading to decolourisation but the mechanism is not clear. Since azo dyes are large and usually charged, they are likely to be reduced extracellularly. It has been suggested that the decolourisation process is a fortuitous one where azo dyes might act as an electron acceptor supplied by carriers of the electron transport chain. There are also suggestions that decolourisation is due to non-specific extracellular reactions occurring between reduced compounds of anaerobic metabolism e.g. sulphides and the azo dyes. Others suggest that anaerobic reductive cleavage of the azo bond is aided by azoreductases, the electron shuttling being aided by soluble redox mediators e.g. flavins (Fernando 2014; Saratale et al. 2011).
Rau et al. 2002 observed enhanced anaerobic reduction of azo dyes using Escherichia coli in presence of quinoid redox mediators e.g. Anthraquinone −2-sulphonate and lawsone and attributed this to induction of azoreductases. High concentrations though of lawsone (>200 µM) did not increase reduction rate possibly due to toxicity.
The use of microbial fuel cells (MFCs) has been considered a promising technology for both decolourisation of azo dyes and electricity production (Hou et al. 2011; Pandey et al. 2016). MFCs utilise electrochemically active bacteria to catalyse an oxidation reaction at the anode. Electrons generated are channelled via an external circuit to a cathode electrode where they recombine with protons and a terminal electron acceptor e.g. oxygen. The extracellular reduction of azo moieties in the anode chamber is likely to occur using electrons coming from a co-substrate e.g. glucose. Furthermore, the ability of MFCs to use oxygen or ferricyanide indirectly as the terminal electron acceptor also confers MFCs an additional advantage over conventional anaerobic systems in terms of faster microbial metabolic rates and growth rates that could potentially be beneficial in achieving faster azo dye degradation kinetics in MFC anodes.
Different bacteria have the ability to degrade azo dyes, among which are those belonging to family Shewanellaceae which is considered a model bacteria for studying electron transfer (Carmona-Martinez et al. 2011). The electron transport process in Shewanella is thought to be through one of two mechanisms: direct electron transfer which depends on the direct contact and facilitated electron transfer which usually takes place via electron shuttles or mediators (Chen et al. 2013). Direct electron transfer from the cytoplasm to an electron acceptor (e.g. azo dye) is thought to be aided by a number of multi-heme cytochromes (CymA, MtrA, MtrB, MtrC, OmcA) that span the inner membrane, periplasm and outer membrane. Electron shuttles are low molecular weight organic molecules that are chemically stable, can easily diffuse into the medium to pass on the electron to external electron acceptors and thus catalyse the reduction/oxidation reactions (Velasquez-Orta and Head. 2010). Some studies have suggested that 75% of the total electron transfer in Shewanella is due to shuttling by Flavin-based redox mediators (Kotloski and Gralnick 2013) leaving 25% for direct electron transfer.
There are different applications for electron shuttling in biotechnology, those include azo dye reduction, enhancing electricity production in MFCs as well as halogenated organic compound degradation. The electron shuttling molecules are usually derivatives of the following compounds: Cobalamin, Phenazine, Naphthoquinone, Anthraquinone and Viologen (Watanaeb et al. 2009).
One of the electron shuttle compounds is riboflavin, which is secreted by Shewanella oneidensis MR-1 and is confirmed as an electron shuttle compound (Marsili et al. 2008). Flavins in general play different roles for Shewanella; they were reported to be released to act, not only as growth accelerators but also as electron shuttles during Fe (III) oxides reduction (von Canstein et al. 2008). Riboflavin is a commonly known redox mediator (Hsueh et al. 2014) and its presence in Shewanella biofilm was correlated to its electron transfer ability (Marsili et al. 2008).
It would be expected that supplementation of Shewanella oneidensis cells in MFCs with Riboflavin would aid decolourisation. However, the situation in an MFC anode chamber may be complicated by the presence of an anode which can also accept electrons depending on its redox potential relative to that of the dye / electron donor. Co-substrate conversion in an MFC is however, expected to be higher than in typical anaerobic systems as oxygen (or ferricyanide) is used indirectly (in the cathode) as a terminal electron acceptor. It would also be expected that a ΔMtrC mutant of Shewanella oneidensis would not decolourise azo dyes to the same extent as the wild type strain because the direct electron transfer mechanism would not be in operation.
The aim of the present work was to assess the role of riboflavin in azo dye decolourisation as the main dominant form of electron shuttling in Shewanella oneidensis MR-1 and to determine whether the decolourisation process is competitive with electricity production. The contribution of the MtrC cytochrome in the decolourisation process was also investigated.
Materials and methods
Chemicals used
Congo red (C.I Direct red 28), riboflavin and chemicals used for GC (purity ≥96%) were purchased from Sigma Aldrich, UK. For the chemical oxygen demand (COD) test, Ficodox Plus™ mixed reagent was purchased from Fisher Scientific UK.
Microorganisms and media used
Microbial cultures used in this study were Shewanella oneidensis MR-1 (ATCC 700550) and Shewanella oneidensis MR-1 ∆mtrC; the latter was kindly provided by Marcus Edwards, School of Biological Sciences, University of East Anglia.
A loopful of strain seed was pre-cultured in 50 ml of LB media (Sigma Aldrich) overnight at 37 °C and 150 rpm. Then 20% (v/v) of the pre-cultured broth was inoculated into an MFC system for cell propagation to stimulate dye decolourisation and/or bioelectricity generation.
Congo red decolourisation (50, 100, 200 and 400 mg/l), riboflavin addition (10, 20, 30, 40 µM), use of 10 mM of different inhibitors (sodium nitrate, copper sulphate, sodium azide and ferrous sulphate) and cell entrapment in alginate beads were all performed in 20 ml bottles containing 5 ml LB media. Cultures were incubated at 30 °C overnight after purging with nitrogen gas for 10 min through 0.22 µm pore size diameter filter. The used inoculum was 20%v/v.
OD and Riboflavin concentration measurement
The bacterial growth was monitored by measuring OD at 600 nm. Riboflavin concentration was performed according to Xu et al. (2015) by measuring the absorbance at 444 nm.
Decolourisation in MFC systems
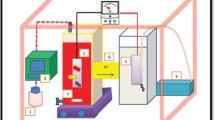
MFC systems were H-type two chambered system with two identical Duran bottles held together with external metal clamps. The electrodes were made of carbon fibre and were cut to 4 cm x 4 cm. The anode and the cathode compartments were separated with a cation-exchange membrane CMI-7000 (Membranes International USA). The anaerobic anode compartment contained 200 ml working volume was purged with nitrogen gas for 10 min through 0.22 µm pore size diameter filter prior to inoculation, the media used was MSM prepared according to Fernando et al. 2012, which contained the following (g/L): NH4Cl 0.46, KCl 0.225, MgSO4.7H2O 0.117, NaH2PO4 2.5, Na2HPO4 4.11, (NH4)2SO4 0.225, a vitamin mixture and trace mineral solution was added (1%), 500 mg/L casein hydrolysate and 2.2 g/L sodium pyruvate were also added. Air- saturated cathode contained 200 ml working volume of 0.1 M potassium ferricyanide in 50 mM sodium phosphate buffer (pH 7). MFC systems were incubated at 30 °C in a Stuart 160 Incubator (Fisher Scientific UK). Congo red (200 mg/l) was added to the anodic chamber for all experiments, riboflavin concentrations used for the first experiment were in the following concentrations: 10, 20 and 40 µM. Shewanella oneidensis MR-1 was used to inoculate the first experiment. As for the second experiment, 4 MFC bottles were used, the first was inoculated with Shewanella oneidensis MR-1, the second contained Shewanella oneidensis MR-1 and 40 µM riboflavin, the third was inoculated with Shewanella oneidensis MR-1 ∆mtrC and the fourth (control) Shewanella oneidensis MR-1 and contained no dye. External resistance for all experiments was 1000 Ω.
Electrochemical measurements
Voltage output data was collected using a Picolog ADC-24 (Pico Technology, UK) online data logging system. Polarization tests were done by connecting different values of external resistances once voltage had stabilized.
COD tests
The chemical oxygen demand removal was determined using the closed reflux titrimetric method as described by Westwood (2007). COD was calculated as follows:
where Vb was the ferrous ammonium sulphate titrant of the blank, Vs was the ferrous ammonium sulphate titrant of the sample, DF was the dilution factor, M was the molarity of the ferrous ammonium sulphate titrant.
The percentage of COD removal was calculated as follows:
where CODI and CODT were the initial and final calculated COD values, respectively.
Detection of by-products using gas chromatography
To detect the by-products remaining at the end of the MFC operation, samples were withdrawn from each system centrifuged at 14,000 rpm for 15 min, filtered and placed in a 2 ml glass vial tube for metabolite detection using a Varian 3900 GC system. The mobile phase consisted of a carrier gas (helium) with a flow rate of 2 mL/min; the column was a 30 mm length x 0.320 mm diameter x 0.50 µm film-HP INNOWax (Agilent J&W GC columns), injector temperature was 260 °C. The oven was initially set at 35 °C for 5 min and then ramped up to 170 °C for the subsequent 10 min. Detector temperature was 250 °C. The presence of degradation metabolites ethanol, acetic acid and butyric acid was detected using the retention time of the respective standard compounds.
Decolourisation and UV–Visible scans
Decolourisation of Congo red dye was calculated as follows:
where AI is the absorbance of the dye at zero time, AF is the absorbance of the dye at the end of the operation process. The absorption maxima of Congo Red was 497 nm. UV–Visible scans for the samples before and after decolourisation were performed using Perkin-Elmer Lambda-35 UV–Visible spectrophotometer; scans were performed within a range from 280 to 700 nm. Data was collected using Perkin-Elmer UV Winlab 6.0.4.
Toxicity assay
The confocal microscope was used to study the impact of using riboflavin to enhance the degradation of Congo red. A VL-17A cell line was used (HepG2 cells), About 250 µl was added to wells in a 6 well plate. DMEM media with 10% FCS (3 ml) was added to each well and incubated at 37 °C overnight. The media was removed and replaced with 100 µl of the following samples: (1) dye before decolourisation, (2) dye after decolourisation, and (3) positive control cells. Samples were left to incubate overnight, after which, 300 µl of freshly prepared Calcein fluorescent dye dissolved in DMSO (excitation and emission wavelength of 495/515 nm, respectively) was added and the plate was left to incubate in darkness for another 45 min at 37 °C. Samples were then studied using confocal scanning laser microscope (CSML). Images were acquired by sequential sequencing using Leica TCS SP2 confocal system (Leica Microsystems, Milton Keynes, UK) and a X63 ceramic dipping objective at 1024 × 1024 format and scanning speed of 400 Hz with a line average of 2.
Statistical analysis
All experimental data indicated on the graphs and table are the means of duplicate experiments unless otherwise stated and the error bars in the graphs represent the standard deviation of the mean (SD). Statistical analysis of data was conducted by one way analysis of variance (ANOVA) using Microsoft Excel statistics package.
Results
Effect of riboflavin concentration on decolourisation and current production
Different amounts of riboflavin were added to Shewanella oneidensis MR-1 culture supplemented with 200 mg/l Congo red in anaerobic serum bottles. The relationship between decolourization and growth with the different concentrations is illustrated in Fig. 1. The figure shows that both decolourisation of Congo red dye increased with the increase in riboflavin concentration; there were no changes in the decolourisation for concentrations above 40 µM. On the other hand, the addition of riboflavin enhanced the growth profile. As the concentration of riboflavin increased, the OD of bacterial growth showed an increase as well.
Figure 2a represents the variation of current production with time in response to the addition of different riboflavin concentrations. The Figure shows that the maximal current was produced when 20 µM riboflavin was added to the MFC culture. The decrease in voltage in Fig. 2a when 20 µM Riboflavin was supplemented could be due to depletion of easy to use substrate (sodium pyruvate) with the microorganisms starting to use fermentation end products e.g. butyrate. The addition of 40 µM riboflavin resulted in less current production (perhaps due to toxicity of riboflavin an high concentrations) and the minimal current production resulted after adding 10 µM riboflavin to the MFC culture, which was less than that obtained for control (no riboflavin). The same result was obtained for both the voltage and power density curves (Fig. 2b, c), where the addition of 20 µM riboflavin resulted in the highest voltage (510 mV) and power density (45 mW/m2).
Deducing the pathway for electron transfer for decolourisation
To investigate the mechanism by which Shewanella oneidensis MR-1 cells transfer electrons, different approaches were tested and decolourisation was calculated. Figure 3 shows that decolourisation increased from 81% for control (freely suspended cells, no riboflavin or inhibitor) to 96.7% when riboflavin was added to the media. On the other hand, decolourisation decreased when sodium nitrate (46.8%), copper sulphate (43.7%), sodium azide (52%), ferrous sulphate (60.8%) and when cells were entrapped in sodium alginate beads (32%). Entrapment was used to preclude direct contact between the bacteria and the azo dye in the media.
Decolourisation of congo red using Shewanella oneidensis MR-1 in non-MFC conditions (anaerobic bottles) under the following conditions: riboflavin for increasing decolourisation, sodium nitrate and ferrous sulphate for competitive terminal electron acceptor, copper sulphate and sodium azide for respiratory inhibition, entrapped cell to prevent direct electron transfer
The role of MtrC in decolourisation and current production
To determine the role of MtrC, an MtrC knock out strain was used to compare its performance to that for Shewanella oneidensis MR-1 wild type in the presence and absence of riboflavin. The results shown in Fig. 4a represent the decolourisation profile over 72 h. The figure clearly shows that the rate of decolourisation increased gradually in the first 24 h, after which there was a steady state that was maintained. Supplementation of the cultures with riboflavin gave a decolourisation efficiency of almost 100% (Fig. 4a). As for the MtrC knock out, there was an initial lag in decolourisation which was maintained for the first 24 h, followed by a compensated decolourisation that was more or less the same as that for Shewanella oneidensis MR-1 wild type. As a negative control, the dye was added to the media without adding any microbial cells, the results show an average of 8% decolourisation which accounts for the percentage of non-biological degradation.
Figure 4b represents the riboflavin concentration at the end of the MFC system. The figure shows that the highest riboflavin was produced by the MtrC knock out (15.1 µM) and that when the dye was added to the media there was a decrease in riboflavin production (11.9 µM).
As for the bioelectricity production, Fig. 5a shows that the MtrC knockout exhibited the highest voltage, this was followed by that obtained when riboflavin was added to Shewanella oneidensis MR-1 culture. The figure also shows that the least current was obtained from Shewanella oneidensis MR-1 culture in the absence of riboflavin. Both voltage and power density followed the same trend for the culture as shown in Fig. 5b, c, the highest belonging to MtrC knock out (500 mV) and power density reaching 78 mW/m2. The remaining COD was shown to be the least when riboflavin was added to the media (41%), as compared to that without riboflavin (52%) and MtrC knock out culture which showed 46% residual COD (Fig. 5d).
In an attempt to assess the decolourisation to be removal or degradation, a UV–Visible scan was performed for the samples, the results show that both MtrC knock out culture and riboflavin supplemented cultures (20 and 40 µM) exhibited a complete breakdown of dye as indicated by the complete disappearance of the peak at 480 nm as compared to Shewanella oneidensis MR-1 without riboflavin which exhibited a decrease in the peak absorbance (Fig. 6).
Analysis of the metabolites produced
The analysis of the metabolites at the end of the MFC system showed no distinctive peaks for both acetic acid and ethanol, on the other hand butyric acid was detected in the media (Table 1). The concentrations were highest for Shewanella oneidensis MR-1 (88.9 mg/l) and decreased dramatically when the dye was added. The addition of riboflavin resulted in a slight increase in butyric acid concentrations from 29.7 mg/l for control samples to 32 mg/l for samples supplemented with riboflavin. On the other hand, MtrC knock out produced the lowest butyric acid concentration (9.7 mg/l).
Comparison of dye removal in the presence or absence of MFC
In order to determine if an MFC system is required to achieve better decolourisation, MFC systems were prepared as mentioned in the materials and methods section. The results shown in Fig. 7 represent decolourisation in MFC and non-MFC systems in the presence and absence of riboflavin. The results clearly show that it is not the closed MFC system that contributes to decolourisation but the presence of riboflavin in the system.
Toxicity test
To assess the effect of dye on the human Hep G2 cells, we incubated the dye before and after decolourisation with the cells and visualised the live cells using calcein dye. The images shown in Fig. 8 show that the fluorescence was minimal when the dye was added before it was treated, on the other hand, the cells incubated with dye after treatment showed fluorescence almost the same as that obtained for control cells.
Images of HepG2 cells exposed to different treatments. Images are taken using confocal scanning laser microscopy (CSLM) and represent cells treated with congo red prior to decolorization (b), cells after incubation with treated dye under MFC conditions (c) as compared to control cells grown under regular conditions (a)
Discussion
Shewanella oneidensis MR-1, a classic model exoelectrogen used in bioremediation and electricity production, was used in this study and the effect of added riboflavin as electron shuttle compound was evaluated in terms of azo dye decolourisation and electricity production. The increase in both decolourisation and growth indicates that riboflavin was used by Shewanella oneidensis MR-1 wild type to transfer the electrons and reduce the azo dye in a matter of hours. The variation of the optimal riboflavin concentration required to achieve high decolourisation and that required to obtain highest current indicates that both decolourisation and electricity production are not necessarily a simultaneous process and that there might be a competition in both functions. There is currently no agreement on the mode of action of bacterial electron transfer. While Chen et al. (2011) and Hsueh et al. (2014) indicated that the two electron transfer processes go in parallel, our results indicate that the process is competitive. Considering that all the mentioned studies used azo dyes as a common chemical structure, this might suggest that the difference could be attributed to the type of organisms used, since they are not the same. Chen et al. (2011) used Proteus hauseri, Hsueh et al. 2014 used Enterobacter cancerogenus, while in our study we used Shewanella oneidensis MR-1 wild type.
The electron transport process for azo reduction indicated different inhibition levels when nitrate, copper, azide and ferrous ions were present. The results suggest that inhibition in decolourisation may have occurred as a result of competition for electrons from electron donors. This is in agreement with Hong et al. (2007) who studied the effect of electron donors to understand the mechanism of bacterial anaerobic azo reduction to improve the treatment of azo dye contaminated waste water.
In an attempt to understand the role of direct electron transfer in the azo reduction process, Shewanella oneidensis MR-1 was entrapped in alginate beads to preclude the direct contact. The results showed that the decolourisation decreased by almost threefold as compared to control cells which were in direct contact with the dye. This result confirms the involvement of both direct and electron mediated decolourisation process. Bacterial electron transport in Shewanella oneidensis MR-1 involves either direct transfer via outer membrane c-type cytochromes (OMCs), nanowires or indirect electron transfer via endogenously secreted flavins (Yong et al. 2013). Most studies focus on the involvement of membrane bound cytochromes in the electron transfer process (Carmona-Martinez et al. 2011). MtrC is one of the membrane bound cytochromes. Edwards et al. (2015) stated that redox-linked flavin sites in extracellular decaheme proteins are involved in microbe-mineral electron transfer under anaerobic conditions. MtrC combines with flavin mononucleotide to produce flavocytochromes, a semi-reduced flavin that is located at the biofilm-electrode interface (Edwards et al. 2015). From this stand point, an MtrC knock out strain was used to study azo decolourisation and electricity production. The results show that initial decolourisation within the first 24 h was faster in wild types, both with and without riboflavin, the latter being faster. MtrC knock out strain lagged in decolourisation but the rate quickly increased to match that for Shewanella oneidensis MR-1 wild type (Fig. 4a). Results showing residual riboflavin concentrations in the media revealed that riboflavins were endogenously secreted in the media for the MtrC knock out strain. This result suggests that in order for the cells to compensate the lack of MtrC as the end protein in the electron transport chain, the cells resorted to over secretion of Flavin to act as a mediator in the process. The results are the same for both decolourisation and electricity production. While Geobacter species were reported to lack the ability to secrete flavins by themselves, Shewanella oneidensis MR-1 have been reported to produce quinone-like compounds and flavins which act as electron shuttling compounds (Watanabe et al. 2009). A study focusing on the kinetic measurements of purified OmcA and MtrC revealed that those cytochromes were slower in direct reduction of metal oxides and that this was rapidly increased after the addition of flavins to the medium (Ross et al. 2009). Exploring metabolic pathways can shed light on optimal strategies to maximize the performance of microbial fuel cells (Hsueh et al. 2014). The results obtained indicated that only butyric acid was produced under the employed culture conditions. This suggests the possibility of reusing the butyric acid again by the cells which sustained the electricity production. Identification of metabolites and/or degraded azo dye could shed light on how to increase the decolourisation process in terms of rate and efficiency (Solanki et al. 2013). The debate about MFC Vs. non-MFC in rate and efficiency of decolourisation is still not concluded. Some studies confirm that MFC conditions are required for faster and significant colour removal (Hsueh et al. 2014), while our study indicates that the addition of riboflavin is the key to rapid decolourisation as indicated by the complete disappearance of the dye peak. For a comparison of dye decolourisation rates in MFC and non-MFC conditions, see Fernando (2014).
In order to claim that we can re-use the treated coloured wastewater, a toxicity test was performed to assess the quality of the resulting treated water. The results obtained indicate that there is no obvious difference between treated and control cells in viability, as opposed to dead cells resulting from incubation with the dye before treatment. This is a preliminary test that proposes the possibility to reuse. However, more tests are required for confirmation.
Conclusion
Deciphering the role of electron shuttling in bioremediation and electricity production reveals information about how to enhance these processes through fine tuning of microbial metabolism. In the current study, there were two main findings; the first results showed that flavin addition plays a role in the decolourisation process which indicates that this process is mediated. The second indicates that electricity production in an MFC does not affect simultaneous dye decolourisation. The study also shows that MtrC was not the responsible protein in both electricity production and decolourisation, confirming that riboflavin is the key player. A question still outstanding is which gene is responsible for decolourisation and which one is responsible for electricity production? We are currently studying the exact role of Mtr pathway genes for both decolourisation and energy production using synthetic biology to answer this question.
References
Carmona-Martinez AA, Harnisch F, Fitzgerald LA, Biffinger JC, Ringeisen BR, Schroder U (2011) Cyclic voltammetric analysis of the electron transfer of Shewanella oneidensis MR-1 and nanofilament and cytochrome knock-out mutants. Bioelectrochemistry 81:74–80
Chen BY, Wang YM, Ng IS (2011) Understanding interactive characteristics of bioelectricity generation and reductive decolourisation using Proteus hauseri. Bioresour Technol 102:1159–1165
Chen BY, Hong J, Ng IS, Wang YM, Liu SQ, Lin B, Ni C (2013) Deciphering simultaneous bioelectricity generation and reductive decolourisation using mixed-culture microbial fuel cells in salty media. J Taiwan Inst Chem Eng 44:446–453
Edwards MJ, White GF, Norman M, Fernandez AT, Ainsworth E, Shi L, Fredrickson JK, Zachara JM, Butt JN, Richardson DJ, Clarke TA (2015) Redox linked flavin sites in extracellular decaheme proteins involved in microbe-mineral electron transfer. Sci Rep 5:11677. doi:10.1038/srep11677
Fernando E (2014). Treatment of azo dyes in industrial wastewater using microbial fuel cells. PhD thesis, University of Westminster, London, UK
Hong Y, Chen X, Guo J, Xu Z, Xu M, Sun G (2007) Effects of electron donors and acceptors on anaerobic reduction of azo dyes by Shewanella decolorationis S12. Appl Microbiol Biotechnol 74:230–238
Hou B, Sun J, Hu S (2011) Effect of enrichment procedures on performance and microbial diversity of microbial fuel cell for Congo red decolourisation and electricity generation. Appl Microbiol Biotechnol 90:1563–1572
Hsueh CC, Wang YM, Chen BY (2014) Metabolite analysis on reductive biodegradation of reactive green 19 in Enterobacter cancerogenus bearing microbial fuel cell (MFC) and non-MFC cultures. J Taiwan Inst Chem Eng 45:436–443
Imran M, Arshad M, Negm F, Khalid A, Shaharoona B, Hussain S, Nadeem SM, Crowley DE (2016) Yeast Extract promotes decolourisation of azo dyes by stimulating azoreductase activity in Shewanella sp strain IFN4. Ecotoxicol Environ Saf 124:42–49
Kotloski N.J, Gralnick J.A. (2013). Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. mBio 4(1):e00553–12.
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. PNAS 105:7698–7973
Mohammadi A, Khalili B, Tahavor M (2015) Novel push–pull heterocyclic azo disperse dyes containing piperazine moiety: Synthesis, spectral properties, antioxidant activity and dyeing performance on polyester fibres. Spectrochim Acta Part A 150:799–805
Pandey P, Shinde V, Deopurkar JL, Pant D (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723
Rau J, Knackmuss H-J, Stolz A (2002) Effect of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ Sci Technol 36:1497–1502
Ross DE, Brantley SL, Tien M (2009) Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1. Appl Environ Microbiol 75:5218–5226
Saratale RG, Saratale GD, Chang JS, Govindar SP (2011) Bacterial decolourisation and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42(1):138–147
Sivashankar R, Sathya AB, Krishnakumar U, Sivasubramanian V (2015) Synthesis of magnetic biocomposite for efficient adsorption of azo dye from aqueous solution. Ecotoxicol Environ Saf 121:149–153
Solanki K, Subramanian S, Basu S (2013) Microbial fuel cells for azo dye treatment with electricity generation: a review. Bioresour Technol 131:564–571
Velasquez-Orta SB, Head IM (2010) The effect of flavin electron shuttles in microbial fuel cells current production. Appl Microbiol Biotechnol 85:1373–1381
Von Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Watanabe K, Manefield M, Lee M, Kouzuma A (2009) Electron shuttles in biotechnology. Curr Opin Biotechnol 20:633–641
Xu Z, Lin Z, Wang Z, Chen T (2015) Improvement of the riboflavin production by engineering the precursor biosynthesis pathways in Escherichia coli. Chin J Chem Eng 23:1834–1839
Yong YC, Cai Z, Yu YY, Chen P, Jian R, Cao B, Sun JZ, Wang JY, Song H (2013) Increase of riboflavin biosynthesis underlies enhancement of extracellular electron transfer of Shewanella in alkaline microbial fuel cells. Bioresour Technol 130:763–768
Acknowledgements
The authors would like to thank the British Council for facilitating the travel of the first author to conduct this work at the department of Life Sciences at University of Westminster, London, UK through the travel grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomaa, O.M., Fapetu, S., Kyazze, G. et al. The role of riboflavin in decolourisation of Congo red and bioelectricity production using Shewanella oneidensis-MR1 under MFC and non-MFC conditions. World J Microbiol Biotechnol 33, 56 (2017). https://doi.org/10.1007/s11274-017-2223-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2223-8