Abstract
Shewanella decolorationis S12 was able to reduce various azo dyes in a defined medium with formate, lactate, and pyruvate or H2 as electron donors under anaerobic conditions. Purified membranous, periplasmic, and cytoplasmic fractions from strain S12 analyzed, respectively, only membranous fraction was capable of reducing azo dye in the presence of electron donor, indicating that the enzyme system for anaerobic azoreduction was located on cellular membrane. Respiratory inhibitor Cu2+, dicumarol, stigmatellin, and metyrapone inhibited anaerobic azoreduction by purified membrane fraction, suggesting that the bacterial anaerobic azoreduction by strain S12 was a biochemical process that oxidizes the electron donors and transfers the electrons to the acceptors through a multicompound system related to electron transport chain. Dehydrogenases, cytochromes, and menaquinones were essential electron transport components for the azoreduction. The electron transport process for azoreduction was almost fully inhibited by O2, 6 mM of \({\text{NO}}^{ - }_{3} \), and 0.9 mM of \({\text{NO}}^{ - }_{2} \), but not by 10 mM of Fe3+. The inhibition may be a result from the competition for electrons from electron donors. These findings impact on the understanding of the mechanism of bacterial anaerobic azoreduction and have implication for improving treatment methods of wastewater contaminated by azo dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Azo dyes are almost all xenobiotic compounds, which are characterized by containing one or more azo groups (–N = N–). They are the largest and most versatile class of dyes and are widely used in textiles, leather, plastics, cosmetics, and food industries (Selvam et al. 2003). Because they are toxic, highly persistent, and ubiquitously distributed in the environment (Pearce et al. 2003; Maguire 1992; Zollinger 1991; Selvam et al. 2003), azo dyes are environmental pollutants (Stolz 2001). The discharge in open waters not only presents an aesthetic problem, but also causes a toxic impact on aquatic life and eventually affect human health (Brown and DeVito 1993; Chung and Cerniglia 1992).
Normally, upon reductive cleavage of the azo bond, azo dyes are reduced and amines are formed. Bacteria can subsequently mineralize some aromatic amines aerobically (Stolz 2001; Pearce et al. 2003; Haug et al. 1991). A variety of microorganisms can reduce azo dyes, including purely anaerobic strains (e.g., Bacteroides sp., Eubacterium sp., and Clostridium sp.) (Rafii et al. 1990; Bragger et al. 1997), facultative anaerobic strains (e.g., Sphingomonas sp. strain BN6, Pseudomonas luteola sp., Proteus vulgaris, and Streptococcus faecalis) (Kudlich et al. 1997; Hu 1994; Dubin and Wright 1975; Scheline et al. 1970), and some intestinal anaerobes (Brown 1981; Chung et al. 1978). The most generally accepted hypothesis is that anaerobic bacterial azoreduction is a nonspecific reduction process by redox mediator shuttling electrons from bacteria to azo dyes (Keck et al. 1997; Rau and Stolz 2003; Rau et al. 2002). It is generally considered that azoreduction by bacteria was occurred under anaerobic condition (Stolz 2001; Kudlich et al. 1997; Rau and Stolz 2003). Anaerobic azo dye reduction was considered an important reaction in the process of degradation of azo dyes. Therefore, how to improve the rate of anaerobic azoreduction is a key factor to the treatment of wastewater contaminated by azo dyes.
Because various electron donors and alternative electron acceptors are almost always present in the environment (He and Sanford 2003; Jan et al. 1999), it is important to study the effects of different electron donors and acceptors on bacterial azoreduction. However, there have been few studies in which azoreduction by pure cultures under different electron donor and acceptor conditions was examined. In this paper we describe that S. decolorationis S12, previously isolated from activated sludge of a textile-printing wastewater treatment plant in Guangzhou, China (Xu et al. 2005), can reduce azo dyes with various organic substances and H2 as electron donors. Moreover, the azoreduction of strain S12 was affected by several typical electron acceptors. These findings are significant for understanding the mechanism of bacterial anaerobic azoreduction in-depth and for gaining sufficient control over azoreduction process based on the activity of strain S12 when it is used in situ or off site for the treatment of soil or water contaminated by azo dyes.
Materials and methods
Azo dyes
Amaranth, a typical azo dye used in this study, was purchased from Sigma-Aldrich. Other azo dyes were purchased from a dyestuff factory in Guangzhou, China. Chemical structures of azo dyes are depicted in Fig. 1.
Organism, media, and cultivation
S. decolorationis S12 (Xu et al. 2005), a new species of the genus Shewanella, was isolated from activated-sludge of textile-printing wastewater treatment plant, Guangzhou, China. The strain S12 was facultative anaerobic capable of growing under both aerobic and anaerobic conditions. Luria–Bertani (LB) medium was used for aerobic cultivation at 32°C with shaking at 150 rpm. The bacteria were cultivated under anaerobic condition in the defined medium (pH 7.5) containing the following (mM): succinate (10), Na2HPO4 (5.7), KH2PO4 (3.3), NH4Cl (18.0), MgSO4 (1.01), l-cysteine (20 μg ml−1), vitamin solution, and trace element solution (Wolin et al. 1963). A modified Hungate technique (Miller and Wolin 1974) was used throughout the study for anaerobic cultivation. All batch experiments were conducted in 50-ml serum bottles. The medium was prepared by adding all the components from concentrated stock solutions in O2-free distilled water and equilibrated the preparation with N2–CO2 (4:1), which was passed through a filter to remove bacteria. After flushed with the O2-free gas for 5 min, the serum bottles were sealed with butyl rubber stoppers and incubated in an anaerobic station (Ruskinn C0105). H2 was provided with 101 kPa unless otherwise noted. The initial concentration of cells was 3.0 ~ 3.8 × 105 CFU ml−1 unless indicated otherwise.
Electron donor experiments
Aerobically grown cells in LB medium were harvested by centrifugation, washed twice, and resuspended in the fresh defined medium (initial pH 7.5) containing 1 mM of azo dye and supplying 10 mM of different organic substance or H2 as electron donors, then dispensed into 50-ml serum bottles (25 ml per bottle), and incubated as described above. Control experiments were conducted to test the reduction of azo dyes in the absence of external electron donors or using heat killed cells (incubated at 95°C for 30 min). All cultures were incubated statically under anaerobic condition at 32°C.
Electron acceptor experiments
For experiments in which azoreduction by strain S12 was studied in the presence of different electron acceptors, different volumes of sterile aqueous stock solutions of KNO3, NaNO2, and ferric citrate were added to 30 ml of cultures, resulting in final concentrations of 3, 6, and 9 mM of \({\text{NO}}^{ - }_{3} \); 0.6, 0.9, and 1.2 mM of \({\text{NO}}^{ - }_{2} \); and 5 and 10 mM of Fe3+. The experiments were conducted in the defined medium (initial pH 7.5) supplemented with 1.0 mM of amaranth and 20 mM of formate at 32°C. To study the effects of O2 on azoreduction, 30 ml of the defined medium supplemented with 1.0 mM of amaranth and 20 mM of formate was incubated for 33 h under an aerobic condition with shaking at 150 rpm for 33 h and followed anaerobic incubation for 2 h.
Membranous, cytoplasmic, and periplasmic preparation of proteins
The buffer used throughout was 50 mM of Tris–HCl buffer (pH 8.0), containing 2 mM of sodium dithionite (buffer A). Cell-free extracts of S. decolorationis S12 were prepared by suspending 5 g (wet weight) of frozen cells in 50 ml of buffer A. The cell suspension was sonicated in an ice bath (3 s, 40% output, ×80) using a sonification unit (SONICS VC-505, USA), with monitoring of the cells’ breaking progress under the microscope. Unbroken cells were removed by centrifugation at 10,000×g for 15 min. The crude extract was then centrifuged at 150,000×g for 2.0 h. The supernatant contained cytoplasmic proteins and the pellet was membrane. The membrane fraction was resuspended in buffer A. The periplasmic fraction was prepared using a modified method of Osborn and Munson (1974). Protein concentrations were determined by using Bradford reagent (Bradford 1976), with bovine serum albumin as standard.
Analysis methods
Formate, nitrate, and nitrite concentration was measured by ion chromatograph analyzer (Metrohm 761 Compont IC) equipped with a polysulfonate ion exclusion column (Metrosep A Supp 5). The eluent contained the following: 3.2 mM of Na2CO3, 0.8 mM of NaHCO3, and 3% MeOH. The experiment was performed under the condition at 25°C, 7.3 MPa. Fe(II) in the samples was analyzed by using the HCl extraction ferrozine assay as previously described (Lovley and Phillips 1988).
The double bond of the azo linkage together with the conjugated-bond system of the aromatic compounds constitutes the chromophore of azo dyes. If the double bond of the azo compounds was reduced, the azo compounds were decolorized. So the azoreduction was quantified according to the change of OD value measured at a specific wavelength. The azoreduction activity was calculated according to the formulation as follows at maximum absorbance, and all assays were done in duplicate.
Results
Effect of electron donors on azoreduction
Several organic substances and H2 were chosen to determine their effects on reduction of azo dyes. To study the effect of different electron donors on azoreduction, cells of strain S12 were inoculated in the defined medium supplemented with 10 mM of organic substance or H2 and 1 mM of azo dyes under anaerobic condition. Experimental results showed that H2, formate, lactate, and pyruvate were able to serve as sole electron donor for reduction of various azo dyes by S. decolorationis S12, but acetate, propionate, salicylate, glycerin, ethanol, citrate, and succinate were not effective electron donors for azoreduction (Table 1). Single azo bond azo dye amaranth, methyl red, orange G, orange II, acid red 13, and xylidine ponceau were almost completely reduced within 36 h with H2, formate, and lactate as electron donor in this experiment, but the extent of reduction of acid violet and metanil yellow was lower than other single azo bond azo dyes under the same condition. The extents of reduction of double azo bond azo dye brilliant crocein MOO with H2, formate, and lactate as electron donor were 68.4, 75.3, and 80.4%, respectively. The difference of reducing rate may be resulted from the different structures of azo dyes. When the cells of S12 were incubated at 95°C for 30 min or electron donors was omitted, almost no reduction could be measured over a period of 36 h.
To quantify the electron transfer from electron donor to azo dyes, the oxidation of formate and the reduction of amaranth were determined simultaneously in the incubation containing 5 mM of formate and 1 mM of amaranth. Within 40 h, complete disappearance of 1.0 mM of amaranth was accompanied stoichiometrically consuming 2.0 mM of formate over time. When 1.0 mM of amaranth was reduced completely, the consumption of formate was stopped simultaneously (Fig. 2a). The result in Fig. 2b revealed there was a linear relation between consumption of formate and reduction of amaranth with excess amaranth (2.0 mM) and limiting formate. When formate was exhausted, the reduction of amaranth stopped. These results clearly showed that azoreduction by strain S12 completely depend on the consumption of formate. The ratio of the number of moles of formate oxidized to the number of moles of amaranth reduced was 1.92. Because one molecular formate can provide two electrons and one molecular amaranth (one azo bond) can accept four electrons for reduction, the ratio is 2.0 in theory. It can be concluded that the amount of electrons accepted by amaranth were almost completely supplied from formate. Formate consumption and amaranth reduction was in agreement with the reaction according to: \({\text{Ar}}_{1} - {\text{N}} = {\text{N}} - {\text{Ar}}_{2} + 2{\text{COOH}}^{ - } + 2{\text{H}}_{2} {\text{O}} \to {\text{Ar}}_{1} - {\text{NH}}_{2} + {\text{H}}_{2} {\text{N}} - {\text{Ar}}_{2} + 2{\text{HCO}}^{ - }_{3} + 2{\text{H}}^{ + } .\)
Stoichiometry of azoreduction coupled to the oxidation of electron donor by S. decolorationis S12. Samples were incubated at 32°C for 36 h. a The consumption of formate and reduction of amaranth were simultaneously determined. b Reduction of amaranth under different concentration formate. Data are averages from duplicate experiments. Error bars represent standard deviations of duplicate incubations
Effect of O2 on azoreduction
To investigate the effect of different electron acceptors on azoreduction by strain S12, amaranth and formate were chosen as model azo dye and electron donor, respectively. O2 is a very effective electron acceptor for energy conservation due to its high redox potential. To evaluate the effect of O2 on azoreduction, two experiments under aerobic and anaerobic condition were performed. Within 33 h, 1.0 mM of amaranth was reduced under anaerobic condition with formate as electron donor in the defined medium, but almost no reduction was observed from the culture under aerobic condition at 32°C with shaking at 150 rpm. The result indicates that azoreduction was strongly inhibited by oxygen. After 33 h of cultivation, the biomass produced in aerobic condition was around tenfold more than that produced in anaerobic condition. If the aerobic culture was switched to anoxic static condition, color disappeared within 2 h due to fast consumption of the DO by the large biomass previously built up under aerobic condition (Fig. 3), indicating that anaerobic condition would be essential to achieve an effective azoreduction. The biomass was an essential but not a sufficient condition for azoreduction by strain S12.
Effect of O2 on azoreduction by S. decolorationis S12. Diamonds, aerobic incubation without cells of S12. Squares, anaerobic incubation for 33 h. Triangles, after aerobic incubation for 33 h, then anaerobic incubation for 2 h. The experiments were performed at 32°C by adding 1 mM of amaranth and 10 mM of formate
Effect of nitrate and nitrite on anaerobic azoreduction
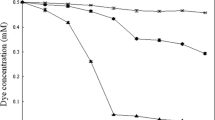
The impact of different concentrations of nitrate and nitrite on reduction of amaranth was also studied. After 36 h incubation, 1 mM of amaranth was completely reduced in the culture containing no \({\text{NO}}^{ - }_{3} \), but the extent of azoreduction was only 50% in the culture containing 3 mM of nitrate at 36 h. This result showed that the reduction of amaranth was obviously inhibited by 3 mM of \({\text{NO}}^{ - }_{3} \). When the concentration of nitrate was more than 6 mM, azoreduction was inhibited completely within 36 h (Fig. 4a). The amounts of \({\text{NO}}^{ - }_{3} \) and amaranth were measured simultaneously in the anaerobic culture containing 6 mM of \({\text{NO}}^{ - }_{3} \) and 1 mM of amaranth. Until around 90% of \({\text{NO}}^{ - }_{3} \)was consumed after 60 h of incubation, azoreduction began to occur and was completed within 20 h. The inhibition by \({\text{NO}}^{ - }_{3} \) can be relieved if the concentration of \({\text{NO}}^{ - }_{3} \) drops to lower than 1.5 mM (Fig. 5a).
The effect of nitrite on anaerobic azoreduction was similar to the effect of nitrate on the reduction; 0.6 mM of nitrite had no obvious impact on the rate of anaerobic azoreduction, while 0.9 and 1.2 mM of nitrite strongly inhibited the reduction. Within 36 h, no reduction could be detected in the culture added with 0.9 mM of \({\text{NO}}^{ - }_{2} \) under anaerobic condition (Fig. 4b). The amounts of \({\text{NO}}^{ - }_{3} \) and amaranth were measured simultaneously in the anaerobic culture containing 1 mM of \({\text{NO}}^{ - }_{2} \) and 1 mM of amaranth. The result in Fig. 5b shows that amaranth was not reduced until the concentration of nitrite dropped to 0.2 mM.
Effect of Fe3+ on anaerobic azoreduction
The result from Fig. 4c revealed that the rate of azoreduction by strain S12 was improved at ratio about 20% in the presence of 5 or 10 mM of Fe3+ citrate. Because the typical rate of Fe3+ reduction by S. decolorationis S12 would have resulted in depletion of Fe3+ within several hours, we performed an experiment in which reduction of amaranth and reduction of Fe3+ were measured simultaneously. The result showed that azoreduction and Fe3+ reduction occurred simultaneously in the culture containing 10 mM of Fe3+ and 1 mM of amaranth (Fig. 5c). There is weak competition for accepting electrons between Fe3+ and azo dyes.
Location of the system for anaerobic azoreduction and effect of respiratory inhibitors on the azoreduction
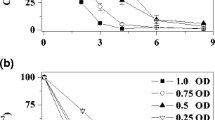
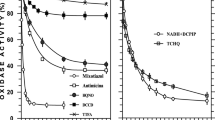
To locate the enzymic system for anaerobic azoreduction, periplasmic, cytoplasmic, and membranous proteins of S12 were prepared. Different fractions with equal protein concentration were resuspended in equal volumes of buffer A containing 10 mM of formate as electron donors and 1 mM of amaranth. Figure 6a showed that the proteins or enzymes from the cytoplasm or periplasm had no activity of azoreduction, but the membranous fraction was capable of efficiently catalyzing azoreduction with formate as electron donors. The specific azoreduction activity of membranous fraction was 12.5 μM min−1 mg−1 protein. These results demonstrated the system of anaerobic azoreduction of strain S12 is located on the cellular membrane (Fig. 6b). The effect of respiratory inhibitors on the azoreduction by membranous fraction of S12 is shown in Fig. 6b. The reduction by membranous fraction was strongly inhibited by 5 μM of CuP2+ ions, 200 μM of dicumarol, 100 μM of stigmatellin, and 100 μM of metyrapone. CuP2+ ions, a membrane-impermeable dehydrogenase inhibitor (Fernandez et al. 1989; Louie and Mohn 1999), inhibit anaerobic azoreduction when HB2B or formate serve as electron donor, indicating that hydrogenase and formate dehydrogenase are important components for electron transfer. Stigmatellin, which are quinone analogs that are able to bind to the cytochrome b (Gerencsér et al. 2004; Ouchane et al. 2002), inhibited anaerobic azoreduction, suggesting that a low-potential cytochrome b is involved in the electron transport from electron donor to azo dyes. It is likely that the cytochrome b shuttle electrons between primer dehydrogenases and menaquinone (MK). Dicumarol, which was thought to inhibit electron transport of MK in bacteria (Ghiorse and Ehrlich 1976; Arnold et al. 1986), also inhibited azoreduction. This observation supports that MK is an essential compound of electrons transport for azoreduction. Furthermore, anaerobic azoreduction was sensitive to metyrapone, a specific cytochrome P450 inhibitor (Williams et al. 2004), indicating that P450 cytochrome plays important roles in the anaerobic azoreduction of strain S12.
Anaerobic azoreduction by membranous vesicle of S. decolorationis S12 and the effect of respiratory inhibitors on the azoreduction. The experiments were performed in the defined medium under anaerobic condition at 32°C with formate as electron donors. a Anaerobic azoreduction by proteins from cytoplasm, periplasm, and membrane of cells. SI Periplasmic fraction, SII cytoplasmic fraction, SIII membranous fraction. b Anaerobic azoreduction by membranous vesicle in the presence of various respiratory inhibitors: 5 μM of Cu2+, 100 μM of metyrapone, 200 μM of dicumarol, and 100 μM of stigmatellin. All measurements were obtained by calculating the average values for two independent incubations and error bars represent standard deviations of duplicate incubations
Discussion
Bacterial anaerobic azoreduction was explored by a number of investigators and previously reviewed in documents (Stolz 2001; Bumpus 1995; Chung et al. 1992), but the mechanism of the subject is not completely known. It was generally considered that bacterial anaerobic azoreduction is a nonspecific reduction process, either a direct enzymatically catalyzed reaction or a reaction with enzymatically reduced redox mediator (Stolz 2001). To date, the correlation between primary electron donors and azo dyes is not clear. In this paper, we describe that azoreduction of strain S12 is a process of transferring the reducing equivalents originating from the oxidation of organic substance or H2 to the azo dyes under anaerobic condition. Moreover, the azoreduction is accompanied by stoichiometric consumption of electron donors. Pure culture microorganism that is capable of oxidizing formate, lactate, pyruvate, or H2 coupled to azoreduction has apparently not been described previously. Only mixed culture in anaerobic granular sludge studies have been shown, indicating that azoreduction can be stimulated when volatile fatty acids were present as electron donor (Van der Zee et al. 2001).
Because azo dyes containing a sulfonate group is hydrophilic, it is difficult for the dyes to enter into cells across cell membrane (Kudlich et al. 1997). For intact cells, a membrane transport system would be more suited to the reduction of azo dyes. Our study showed that the azoreduction enzyme system of strain S12 is completely located on cellular membrane fraction. Periplasmic and cytoplasmic fractions of S12 have no azoreduction activity. Similar studies suggest that azoreduction by whole cells of Sphingomonas sp. strain BN6 was mainly related to membrane fraction (presumably an NADH–ubiquinone oxidoreductase), but azoreduction activity was also found in the cytoplasm (a soluble FAD-dependent enzyme) (Kudlich et al. 1997). According to an azoreduction character of strain S12, we presume that anaerobic azoreduction by strain S12 was catalyzed by a multicompound system linking to electron transport chain. Respiratory inhibitor experiments confirm the deduction. Dehydrogenase, cytochrome b, MK, P450 type cytochrome, and a deduced terminal azoreductase are important components in the multicompound system for azoreduction. These evidences indicate that the bacterial anaerobic azoreduction is an electron transport process depending on electron transport chain system. Azo compounds can serve as electron acceptor of accepted electrons transported through an electron transport chain from a primary electron donor. Whether this electron transport from electron donors to terminal electron acceptor azo dyes can conserve energy for growth is a more interesting subject to be illustrated in the future.
S. decolorationis S12 displays remarkable anaerobic respiratory plasticity, which can conserve energy using a variety of terminal electron acceptors, including O2, \({\text{NO}}^{ - }_{3} \), \({\text{NO}}^{ - }_{2} \), and Fe3+ (Xu et al. 2005), it provides a unique opportunity to study the competitive effects of different electron acceptors on azoreduction in a single organism. In the simultaneous presence of O2 and azo dyes, electrons prioritize to transport to O2 because of its high redox potential (+820 mV); therefore, the azoreduction was fully inhibited by O2. The reduction of amaranth by S12 was also fully inhibited by 3 mM of \({\text{NO}}^{ - }_{3} \) and 0.9 mM of \({\text{NO}}^{ - }_{2} \). Possibly because redox potential of \({\text{NO}}^{ - }_{3} \) (+360 mV) and \({\text{NO}}^{ - }_{2} \) (+440 mV) is higher than amaranth (−180 mV) (Kudlich et al. 1997), electrons are favorable to flow toward \({\text{NO}}^{ - }_{3} \) or \({\text{NO}}^{ - }_{2} \), resulting in the inhibition. Moreover, the inhibition by \({\text{NO}}^{ - }_{3} \) or \({\text{NO}}^{ - }_{2} \) could be the result of the production of NO as a product of \({\text{NO}}^{ - }_{3} \) and \({\text{NO}}^{ - }_{2} \) reduction. NO is capable of binding to heme and iron-sulfur to form metal–nitrosyl complexes and to inhibit the ability of the metalloprotein to transfer electrons (Satoh 1984; Zumft 1993). Because dehydrogenase and cytochrome contain iron-sulfur and heme, it is possible that NO inhibits the activity of dehydrogenase and cytochrome to transfer electrons. In addition, it is very interesting that Fe3+ can stimulate azoreduction because its redox potential (+770 mV) is also higher than amaranth. The stimulation may be due to azoreduction and Fe3+ reduction using different electron transport pathway; therefore, there might be weak competition between azoreduction and Fe3+ reduction. The mechanism needs to be further investigated in depth.
This study is significant for the treatment of wastewater contaminated by azo dyes. Normally, azo dyes are reduced under anaerobic condition and the reducing products are subsequently mineralized under aerobic condition (Stolz 2001; Haug et al. 1991). Therefore, the anaerobic/aerobic treatment is a very effective method to decolorize azo dye-containing wastewaters (Seshadri et al. 1994; Chang and Lin 2000). Based on our study, the conditions of electron donors and acceptors have a remarkable effect on azoreduction. Therefore, the concentration of electron donors and acceptors is an important factor for anaerobic treatment of azo dye-containing wastewaters. Under a general condition, there is abundance of organic substance in the active sludge, so azoreduction can occur with some organic substance as electron donors. But if there is a lack of electron donor in the treatment reactor, the azoreduction will not occur. Therefore, adding an exogenous electron donor is essential to realize the effective reduction of azo dyes. Because of the competition for electrons, electron acceptors can inhibit the anaerobic azoreduction. Especially if a high concentration of \({\text{NO}}^{ - }_{3} \) and \({\text{NO}}^{ - }_{2} \) is present in the environment, microbial anaerobic azoreduction may be greatly repressed. So how to decrease the concentration of \({\text{NO}}^{ - }_{3} \) and \({\text{NO}}^{ - }_{2} \) is a very important strategy to effectively decolorize azo dyes under anaerobic condition.
In summary, the results of this study with S.decolorationis S12 have demonstrated that azo dyes can be used as terminal electron acceptor accepting electrons from primary electron donors through electron transport chain. This reduction was affected by other electron acceptors that can be used by strain S12. This strain provides a novel model organism with which to study organic substances or H2 metabolism coupled to azoreduction under a defined condition.
References
Arnold RG, DiChristina TJ, Hoffmann MR (1986) Inhibitor studies of dissimilative Fe(III) reduction by Pseudomonas sp. strain 200 (“Pseudomonas ferrireductans”). Appl Environ Microbiol 52:281–289
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bragger JL, Lloyd AW, Soozandehfar SH, Bloomfield SF, Marriott C, Martin GP (1997) Investigations into the azo reducing activity of a common colonic microorganism. Int J Pharm 157:61–71
Brown JP (1981) Reduction of polymeric azo and nitro dyes by intestinal bacteria. Appl Environ Microbiol 41:1283–1286
Brown MA, DeVito SC (1993) Predicting azo dye toxicity. Crit Rev Environ Sci Technol 23:249–324
Bumpus JA (1995) Microbial degradation of azo dyes. Prog Ind Microbiol 32:157–176
Chang JS, Lin YC (2000) Fed-batch bioreactor strategies for microbial decolorization of azo dye using a Pseudomonas luteola strain. Biotechnol Prog 16:979–985
Chung KT, Cerniglia CE (1992) Mutagenicity of azo dyes: structure-activity relationships. Mutat Res 77:201–220
Chung KT, Fulk GE, Egan M (1978) Reduction of azo dyes by intestinal anaerobes. Appl Environ Microbiol 35:558–562
Chung KT, Stevens SEJ, Cerniglia CE (1992) The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol 18:175–197
Dubin P, Wright KL (1975) Reduction of azo food dyes in cultures of Proteus vulgaris. Xenobiotica 5:563–571
Fernandez VM, Rua ML, Reyes P, Cammack R, Hatchikian EC (1989) Inhibition of Desulfovibrio gigas hydrogenase with copper salts and other metal ions. Eur J Biochem 185:449–454
Gerencsér L, Rinyu L, Kálmán L, Takahashi E, Wraight CA, Maróti P (2004) Competitive binding of quinone and antibiotic stigmatellin to reaction centers of photosynthetic bacteria. Acta Biologica Szegediensis 48:25–33
Ghiorse WC, Ehrlich HL (1976) Electron transport components of the MnO2 reductase system and the location of the terminal reductase in a marine Bacillus. Appl Environ Microbiol 31:977–985
Haug W, Schmidt A, Nortemann B, Hempel DC, Stolz A, Knackmuss HJ (1991) Mineralization of the sulfonated azo dye mordant yellow 3 by a 6-aminonaphthalene-2-sulfonate-degrading bacterial consortium. Appl Environ Microbiol 57:3144–3149
He Q, Sanford RA (2003) Characterization of Fe(III) reduction by chlororespiring Anaeromxyobacter dehalogenans. Appl Environ Microbiol 69:2712–2718
Hu TL (1994) Decolourization of reactive azo dyes by transformation with Pseudomonas luteola. Bioresour Technol 49:47–51
Jan G, Oliver D, Geert K, Mischa K, Luit PW, Roger H, Matthew DC, Jan CG (1999) Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl Environ Microbiol 65:5212–5221
Keck A, Klein J, Kudlich M, Stolz A, Knackmuss HJ, Mattes R (1997) Reduction of azo dyes by redox mediators originating in the naphthalenesulfonic acid degradation of Sphingomonas sp. strain BN6. Appl Environ Microbiol 63:3684–3690
Kudlich M, Keck A, Klein J, Stolz A (1997) Localization of the enzyme system involved in the anaerobic degradation of azo dyes by Sphingomonas sp. BN6 and effect of artificial redox mediators on the rate of azo reduction. Appl Environ Microbiol 63:3691–3694
Louie TM, Mohn WW (1999) Evidence for a chemiosmotic model of dehalorespiration in Desulfomonile tiedjei. DCB-1. J Bacteriol 181:41–46
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Maguire RJ (1992) Occurrence and persistence of dyes in a Canadian river. Water Sci Technol 25:265–270
Miller TL, Wolin ML (1974) A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol 27:985–987
Osborn MJ, Munson R (1974) Separation of the inner (cytoplasmic) and outer membranes of gram-negative bacteria. Methods Enzymol 31:642–653
Ouchane S, Agalidis I, Astier C (2002) Natural resistance to inhibitors of the ubiquinol cytochrome c oxidoreductase of Rubrivivax gelatinosus: sequence and functional analysis of the cytochrome bc1 complex. J Bacteriol 184:3815–3822
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigm 58:179–196
Rafii F, Franklin W, Cerniglia CE (1990) Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol 56:2146–2151
Rau J, Stolz A (2003) Oxygen-insensitive nitroreductases NfsA and NfsB of Escherichia coli function under anaerobic conditions as lawsone-dependent azo reductases. Appl Environ Microbiol 69:3448–3455
Rau J, Knackmuss H-J, Stolz A (2002) Effects of different quinoide redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ Sci Technol 36:1497–1504
Satoh T (1984) Inhibition of electron transfer through the cytochrome b-c 1 complex by nitric oxide in a photodenitrifer, Rhodopseudomonas sphaeroides forma sp. denitrificans. Arch Microbiol 139:179–183
Scheline RR, Nygaard RT, Longberg B (1970) Enzymatic reduction of the azo dye, acid yellow, by extracts of Streptococcus faecalis, isolated from rat intestine. Food Cosmet Toxicol 8:55–58
Seshadri S, Bishop PL, Agha AM (1994) Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manag 14:127–137
Selvam K, Swaminathan K, Keo-Sang C (2003) Microbial decolorization of azo dyes and dye industry effluent by Fomes lividus. World J Microbiol Biotechnol 19:591–593
Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol 56:69–80
Van der Zee FP, Lettinga G, Field JA (2001) Azo dye decolourisation anaerobic granular sludge. Chemosphere 44:1169–1176
Williams PA, Cosme J, Vinkovic DM, Ward A, Angove HC, Day PJ, Vonrhein C, Tickle IJ, Jhoti H (2004) Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science 305:683–686
Wolin EA, Wolin MJ, Wolfe RS (1963) Formation of methane by bacterial extracts. J Biol Chem 238:2882–2886
Xu M, Guo J, Cen Y, Zhong X, Cao W, Sun G (2005) Shewanella decolorationis sp. nov., a dye-decolorizing bacterium isolated from an activated-sludge of wastewater treatment plant. Int J Syst Evol Microbiol 55:363–368
Zollinger H (1991) Color chemistry: syntheses, properties and applications of organic dyes and pigments, 2nd edn. Wiley, New York
Zumft WG (1993) The biological role of nitric oxide in bacteria. Arch Microbiol 160:253–264
Acknowledgements
This research was supported by Chinese National Programs for High Technology Research and Development (2003AA214040), Guangdong Provincial Programs for Natural Science Foundation Group (015017), and Guangdong Provincial key Programs for Science and Technology Development (05100365).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, Y., Chen, X., Guo, J. et al. Effects of electron donors and acceptors on anaerobic reduction of azo dyes by Shewanella decolorationis S12. Appl Microbiol Biotechnol 74, 230–238 (2007). https://doi.org/10.1007/s00253-006-0657-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0657-2