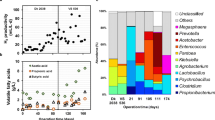
Abstract
Bioprocesses in conventional second generation biorefineries are mainly based on the fermentation of sugars obtained from lignocellulosic biomass or agro-industrial wastes. An alternative to this process consists in gasifying those same feedstocks or even other carbon-containing materials to obtain syngas which can also be fermented by some anaerobic bacteria to produce chemicals or fuels. Carbon monoxide, carbon dioxide and hydrogen, which are the main components of syngas, are also found in some industrial waste gases, among others in steel industries. Clostridium carboxidivorans is able to metabolise such gases to produce ethanol and higher alcohols, i.e. butanol and hexanol, following the Wood–Ljungdahl pathway. This does simultaneously allow the removal of volatile pollutants involved in climate change. The bioconversion is a two step process in which organic acids (acetate, butyrate, hexanoate) are produced first, followed by the accumulation of alcohols; although partial overlap in time of acids and alcohols production may sometimes take place as well. Several parameters, among others pH, temperature, or gas-feed flow rates in bioreactors, affect the bioconversion process. Besides, the accumulation of high concentrations of alcohols in the fermentation broth inhibits the growth and metabolic activity of C. carboxidivorans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most fuels and a wide range of platform chemicals produced in industrialized countries have traditionally been obtained from petroleum in oil refineries. For environmental reasons and because of the near shortage of crude oil reserves, modern societies need to develop new production processes and alternative fuels (Gowen and Fong 2011; Abdehagh et al. 2014). Biorefineries have recently emerged as a potential solution to such problem. Ethanol and longer chain alcohols such as butanol are suitable substitutes of fossil fuels such as gasoline. They can also be used as chemicals and solvents. Mixtures of alcohols such as butanol and ethanol can potentially be produced from wastes and renewable sources in bioreactors, which is an advantage compared to alcohols obtained from non renewable fossil sources. The most extensively studied bioprocess is based on the fermentation of carbohydrates, available from lignocellulosic biomass or similar feedstocks, using anaerobic bacteria, usually clostridia. This is commonly known as the ABE fermentation yielding a mixture of acetone, butanol and ethanol. Clostridium acetobutylicum has most often been used as biocatalyst for such bioconversion, metabolizing sugars and producing the aforementioned three solvents as end metabolites. Other substrates such as glycerol and other clostridial species have more recently been used as well. Another possible bioconversion process for the production of (bio)alcohols has emerged much more recently. It can use similar feedstocks as for the ABE fermentation, such as biomass, but also municipal solid waste, agro-industrial wastes and a broader range of some other carbon containing materials, which represents a clear advantage (Mohammadi et al. 2011). The feedstock is then gasified in order to obtain syngas on one side, which is a mixture of CO, CO2 and H2 mainly and, on the other side, some inert solid residue (ash) is formed as well. This is different from the ABE process, in which the starting material does first undergo pretreatments and hydrolytic treatments to extract simple sugars from the polymeric lignocellulosic structure. This gas mixture (i.e., syngas) is not only obtained through gasification of biomass or waste, it is also found in some industrial gaseous effluents, among others in steel producing processes. Similarly to sugars, syngas and the aforementioned industrial waste gases can be fermented by clostridia and a few other acetogenic bacteria (Drake et al. 2008). In a few strains, this may yield ethanol and, occasionally, higher alcohols. Contrary to the first generation biorefinery processes which are based on the use of sugar containing food crops as feedstock and lead to food-fuel competition (Abubackar et al. 2011; Kennes et al. 2016), the present alternative uses lignocellulosic biomass or wastes mainly or even waste gases and does not generate such food-fuel dilemma. Besides, this gas fermentation technology can reduce the emissions of gaseous pollutants and greenhouse gases such as carbon dioxide and it gives some commercial use to industrial pollutants and agricultural wastes. Only very few strains have so far been proven to be able to convert syngas and CO-rich waste gases into ethanol and higher alcohols such as butanol and hexanol. The best known and most studied species is Clostridium carboxidivorans, which will be the focus of this review paper.
Clostridium carboxidivorans: major morphological and metabolic characteristics
Clostridium carboxidivorans P7 (= ATCC BAA-624 = DSM 15243) is a Gram positive, mesophilic and obligate anaerobic carboxydotroph, originally isolated from an agricultural settling lagoon in Oklahoma, USA (Liou et al. 2005). Its cells are mobile, with rod shape (0.5 × 3 μm) and can present sporulated forms, which appear like a terminal or subterminal protuberance (Liou et al. 2005) (Fig. 1). Its main morphological, metabolic and growth characteristics are summarized in Table 1 and described more in details below.
Substrates, nutrients and products
Clostridium carboxidivorans P7 is able to grow autotrophicaly with syngas and chemoorganotrophically with a great variety of sugars such as glucose, xylose, fructose, cellobiose and arabinose. It is able to ferment all those carbon sources to produce acids, mainly acetic acid, butyric acid, and hexanoic acid, and alcohols (Liou et al. 2005; Liu et al. 2014; Phillips et al. 2015). Lactic acid, propionic acid and formic acid have also recently been detected in glucose fermentation (Fernández-Naveira et al., non published data). Suitable carbon and energy sources and their main metabolites are listed in Table 2. Recent interest in that species has mainly been focused on its ability to produce alcohols from syngas and waste gases. C. carboxidivorans is one of the few bacteria able to grow autotrophically on syngas, using the gaseous CO, CO2, H2 compounds as carbon or energy source to produce short chain alcohols such as ethanol as well as longer chain alcohols such as butanol and hexanol (Bruant et al. 2010; Dürre 2016; Fernández-Naveira et al. 2016a; Hemme et al. 2010; Liou et al. 2005; Phillips et al. 2015). That organism uses CO and CO2 as carbon source whereas H2 is used as source of electrons by means of the enzyme “hydrogenase” (Krasna 1979). Under conditions of inhibition of the hydrogenase enzyme, the bacteria will need another source of electrons, which can then be obtained from CO. As described more in detail below, this is the case in presence of compounds such as NO, which can appear as minor compound in syngas, and has been shown to inhibit the hydrogenase enzyme. However, this provokes also a limitation in the use of CO for the formation of desired metabolites and does consequently result in a less efficient fermentation process (Ahmed et al. 2006).
So far, in terms of solvents, the highest end product concentration has always been found for ethanol followed by butanol and finally hexanol. Those alcohols are produced in that same chronological order during carbon monoxide or syngas fermentation, with short chain alcohols appearing first while longer chain ones appear later on. C. carboxidivorans has the typical “biphasic fermentation pattern” of many acetogens producing alcohols, and usually the gas fermentation process takes place in two stages; initially carboxylic acids are produced from the gaseous substrates followed by the subsequent conversion of those acids and remaining gases into alcohols. Besides, exponential biomass growth and acidogenesis (with production of acids) are two related processes and take place simultaneously. The solventogenic phase in clostridia has been considered to start when the conditions are not favourable anymore for growth, i.e. low pH, low ATP levels, accumulation of high concentrations of organic acids, sporulation, low level of availability of reducing energy (Dürre et al. 1995; Dürre and Hollergschwandner 2004; Guedon et al. 1999; Meyer and Papoutsakis 1989). When alcohols are the desired end products, it is necessary to identify the optimum medium composition and conditions for an efficient conversion of accumulated organic acids with the concomitant production of solvents. The suitable range of conditions depends on the bacterial species, and such conditions are shown in Table 1 for C. carboxidivorans.
Besides the main carbon and energy sources, several nutrients and trace compounds may be needed as well. In case of C. carboxidivorans, a recent study was published in which the effect of different media compositions were analyzed for their effect on growth and butanol production. Removing copper (Cu) from the culture medium and increasing the molybdate (Mo) concentration allowed to improve the production of butanol (Phillips et al. 2015). It was concluded that Mo can be considered to be analogous to tungsten (W), which is related with the enzyme AOR (aldehyde:ferredoxin oxidoreductase), an enzyme involved in the conversion of acids to alcohols. Similarly, the presence of W had previously been proven to stimulate the conversion of carbon monoxide and acetic acid into ethanol in C. autoethanogenum (Abubackar et al. 2015). Micronutrients, trace metals or vitamins play a key role in the activity of specific enzymes and in favouring a given metabolic route. Other parameters, described below, such as temperature and pH, will also affect growth, the metabolic behavior and the bioconversion process.
Temperature
The suitable growth temperature of C. carboxidivorans ranges between 24 and 42 °C, but its optimum temperature was found to be 37–40 °C (Liou et al. 2005). “Acid crash”, which is the accumulation of undissociated acids and can inhibit the solventogenic stage, is a phenomenon that depends on temperature (Maddox et al. 2000). Therefore, it is useful to identify temperature conditions that prevent acid crash and allow an efficient solvent production while maintaning a near optimum temperature for growth. The incubation of C. carboxidivorans at suboptimal temperature of 25 °C (which is still within the suitable temperature range for growth) was shown to allow to avoid acid crash (Ramió-Pujol et al. 2015). However, a lag phase and a slower bacterial growth were observed than under optimal temperature conditions, while the concentrations of alcohols were somewhat higher than when incubating the same strain at 37 °C. An overview of the production yields of alcohols obtained at different temperatures and pH is presented in Table 3.
pH
The pH range of C. carboxidivorans is between 4.4 and 7.0, but its optimum pH was found to be between 5.0 and 7.0. A few studies have focused on the effect of pH in syngas fermentation. Fernández-Naveira et al. (submitted) studied its effect using the bacterium C. carboxidivorans as biocatalyst and a mixture of CO, CO2, H2 and N2 supplied to a continuous gas-fed bioreactor, using two different operating conditions. In a first experiment, a near optimum pH of 5.75 was used and maintained constant during the study. The second experiment was started at pH 5.75, but pH was not regulated in that case and natural acidification took place as a result of the production of organic acids; and once it reached pH 4.75 its value was maintained constant in order to avoid any inhibition at lower pH. The results of that study showed that the highest concentrations of alcohols were observed at pH 5.75, with 2.7 g/L ethanol, 1.9 g/L butanol and 0.85 g/L hexanol; whereas the highest production rates of alcohols were obtained at pH 4.75, reaching 0.048 g ethanol/h g biomass, 0.036 g butanol/h g biomass, and 0.026 g hexanol/h g biomass. Those data, and other related information of production rates, are summarized in Table 3. However, a negative effect on bacterial growth and on the accumulation of acids was observed at lower pH, in the experiment with natural acidification. As a result of the lower accumulation of acids in the first step of that fermentation, lower amounts of alcohols were obtained in the experiment at lower pH compared to the study at a higher, constant, pH of 5.75. Growth rates of 0.0057 and 0.072 h− 1 were observed at pH 4.75 and pH 5.75, respectively. It was concluded that the pH is a critical factor for growth, the accumulation of acids as well as the efficient production of alcohols. Although it has often been assumed that stress conditions, such as a low pH, are necessary for solventogenesis based on data of the ABE fermentation in C. acetobutylicum, strong acidification does not seem to be a prerequisite for solventogenesis in the conversion of organic acids into alcohols in hexanol–butanol–ethanol (HBE) fermentation with C. carboxidivorans, as a slightly acidic environment (pH 5.75) allowed the efficient conversion of organic acids into alcohols, compared to lower pH values (e.g., pH 4.75).
Metabolic pathway
Clostridium carboxidivorans uses a variation of the Wood–Ljungdahl pathway for the bioconversion of gaseous substrates to end metabolites, where the eastern branch of its pathway involves the enzymes in charge of the conversion of the C1 substrates (CO, CO2) to formate, and later on methyl-tetrahydrofolate; and the western branch is composed of the enzymes catalyzing the direct conversion of C1 compounds into acetyl-CoA (Ragsdale and Pierce 2008). Acetyl-CoA is a common intermediate of both branches, and it can either be converted to acetate or to ethanol. Alternatively, acetyl-CoA can also be converted to butyryl-CoA and subsequently into butyrate and/or butanol, or into hexanoyl-CoA and then hexanoate and/or hexanol. Although acetate is a common product of autotrophic acetogens, butyrate and hexanoate are quite more unusual among the acetogenic bacteria isolated so far; the same holds true for butanol and hexanol which are still less common than long chain (C4, C6) fatty acids. Examples of acetogens producing long chain fatty acids (butyric acid, hexanoic acid) and alcohols (butanol, hexanol) from volatile substrates (CO, CO2, H2) are listed in Table 4, confirming that the production of alcohols is less common than organic acids in such bacteria. So far, ethanol does generally always appear and has been detected in all acetogenic cultures in which higher alcohols (butanol and/or hexanol) are produced. Acetic acid is present in all cases during the gas fermentation, although its presence may often be transient, as it can further be converted to alcohols, mainly ethanol. The Wood–Ljungdahl pathway does hardly yield any energy. One mole of ATP is generated per mole of acetic acid produced. As explained above, biomass growth and acetic acid production are concomitant. Later on, that organic acid can be converted into acetaldehyde which yields ethanol in turn, but with no generation of ATP and no biomass growth detected. The Wood–Ljungdahl pathway generating ethanol, butanol and hexanol, besides volatile fatty acids and typical in C. carboxidivorans, is shown in Fig. 2.
Recent genetic studies have been done on the genomic characterization of novel solventogenic microorganisms such as C. carboxidivorans by sequencing the genome and comparing the results with the genome of various other solventogenic bacteria. Bruant et al. (2010) sequenced the entire genomic material of C. carboxidivorans and compared that with other major ethanol and butanol producing strains. They found that C. carboxidivorans has a complete gene cluster associated with the Wood–Ljungdahl pathway, including the genes involved in CO and CO2 fixation and conversion to acetyl-CoA, but with the exception of the acetone pathway, as no acetoacetate decarboxylase genes were found in that species. Therefore, the authors concluded that C. carboxidivorans is closely related to C. acetobutylicum and C. beijerinckii, in terms of ABE fermentation pathways for volatile fatty acids, ethanol and butanol, but that it lacks the acetone production pathway. Both C. carboxidivorans and C. acetobutylicum encode an NADPH-dependent butanol dehydrogenase that allows the conversion of acetyl-CoA into butanol. Other clostridia have been shown to grow on syngas or waste gases (CO, CO2, H2), such as C. autoethanogenum, C. ljungdahlii, C. drakei but, to the best of our knowledge, none has yet been found to possess such butanol dehydrogenase. The same happens for hexanol, which has so far only been found to be produced in C. carboxidivorans. That organism is thus unique in that respect. As shown in Table 5, among the few gas fermenting solventogenic anaerobic bacteria isolated so far, C. carboxidivorans, is basically the only species found to be able to produce higher alcohols such as butanol and hexanol.
Solvent inhibition
Alcohols such as ethanol and butanol are known to exert inhibitory effects on strains such as C. acetobutylicum during the ABE fermentation. Besides, it is worth reminding that the inhibitory effect may be different depending on the bacterial species and type of alcohol. Therefore, toxicity levels should be evaluated in each specific case. Recently, the toxic effect of different concentrations of ethanol, butanol or their mixtures was estimated in C. carboxidivorans grown on carbon monoxide as single carbon source in bottle batch assays (Fernández-Naveira et al. 2016b). No information is available in the literature on hexanol, but that alcohol is generally produced at lower concentrations during HBE fermentation than its C2 and C4 counterparts. The experiments showed that butanol causes a significantly higher inhibitory effect than ethanol in terms of the bacterial growth rate, the final biomass density and the CO consumption rate. That inhibitory effect was quantified by means of the IC50 (i.e., the half maximal inhibitory concentration), which reached 14.5 g/L for butanol and 35 g/L for ethanol (Fernández-Naveira et al. 2016b). Mixtures (1:1) of both alcohols have intermediate toxic effects compared to each alcohol individually. The authors concluded that both alcohols have an inhibitory effect on C. carboxidivorans at high concentrations. Besides, inhibition is higher in the case of butanol than for ethanol, as a lower IC50 value was found for the former than the latter (ethanol). These values are rather similar to those found during ABE fermentation of sugars by C. acetobutylicum.
Trace compounds in syngas
Although CO, H2 and CO2 are the main components of some industrial waste gases and syngas, they may also contain trace amounts of additional compounds, which could have some inhibitory effect on the biocatalyst. The influence of those trace compounds is barely considered in lab-scale research as prepared gas mixtures are generally used, mimicking the composition of only the major compounds of syngas. Other compounds that can be formed during the gasification process include products such as methane, ethylene, ethane, acetylene, NH3, sulphur compounds and NO, among others (Ahmed et al. 2006; Haryanto et al. 2009).
Although no research has been reported on this with C. carboxidivorans, some other alcohol producing species have been studied. Some of those trace compounds have been shown to be potential inhibitors of the fermentation process and bacterial growth. For example acetylene and NO may inhibit the activity of the hydrogenase enzyme, which catalizes the generation of electrons from H2 (Xu and Lewis 2012). When NO inhibits the hydrogenase enzyme, the cells must generate electrons from CO, using the CODH enzyme. Sulphur compounds and ammonia (NH3) are other compounds that may appear in syngas. A negative effect on the bacterial growth in presence of sulphur compounds has been reported in the ethanol producing species C. ljungdahlii (Klasson et al. 1993). Besides, Xu and Lewis (2012) found that the presence of ammonia can lead to the accumulation of ammonium ions (NH4 +) in the medium, which was observed to inhibit the hydrogenase activity and bacterial growth of C. ragsdalei.
Present and future industrial perspectives
The production of ethanol and higher alcohols, such as butanol or hexanol, by acetogenic bacteria from C1 gases is not a favourable process from an energetic point of view (Latif et al. 2014). However, although it was originally considered that reaching concentrations approaching one gram per liter in wild type bacteria would be impossible or, at least, challenging, recent data confirm that the production of several g/L of butanol and hexanol mixtures, besides ethanol is feasible through this hexanol–butanol–ethanol (HBE) fermentation process. Optimization of the bioreactor operating conditions would allow to further improve such values. The use of metabolically engineered strains is another possible alternative for the improvement of yields and of the end concentrations of metabolites. Some research has been performed in that respect for butanol production with recombinant strains grown on carbon monoxide (Köpke and Liew 2011). However, improvements are necessary and higher butanol concentrations would still need to be reached from C1 gases with such engineered clostridia. Other bacterial strains are able to produce ethanol as single alcohol from syngas/waste gas, sometimes together with butanediol, but with no accumulation of either butanol or hexanol (Table 4). This is the case of C. autoethanogenum, C. ljungdahlii, and C. ragsdalei, among others (Abubackar et al. 2011). Recent studies undertaken at pre-commercial stage confirmed that such a process may be cost-effective (van Groenestijn et al. 2013). Some demonstration plants have recently allowed to produce ethanol, either from syngas or from waste gases from steel producing industries, with such acetogenic bacteria, reaching promising results. In terms of public safety, it is worth mentioning that, with the exception of only four or five species, most clostridia are non pathogenic at all and do not cause any diseases in humans. Some clostridia can even be used for therapeutic purposes (Kubiak and Minton 2015). Concerning the environmental benefit, it is worth to remind that this HBE fermentation process consumes carbon dioxide, a greenhouse gas, but does also allow to remove carbon monoxide. Although carbon monoxide as such has only a very weak greenhouse effect, it contributes to tropospheric ozone generation, the formation of carbon dioxide, and reacts with hydroxyl radicals in the atmosphere. Those OH radicals would otherwise be involved in reducing the concentration of greenhouse gases such as methane.
The gas fermentation process has attracted interest of some industries and, as indicated above, some demonstration plants have been build recently. The technology has reached pre-commercial stage for the production of ethanol, but not yet for other routes such as the HBE fermentation, and an exhaustive overview of the industrial landscape, among others for the HBE process, would thus be behind the scope of this review. Information on the industrial lansdscape, mainly for ethanol production, can be found in other recent literature (van Groenestijn et al. 2013; Latif et al. 2014). One of the major companies developing this technology is, among others, LanzaTech which produces ethanol using waste gases from industry or using syngas obtained through the gasification of biomass or wastes. In 2013, that company started pre-commercial operation of a plant in China. Similarly, Coskata, in the US, was using a large variety of biomass sources to obtain syngas and ferment it into fuels and chemicals. Finally, INEOS Bio focuses largely on ethanol production through sugar fermentation, but has also evaluated possible commercialization of the biomass gasification process and its subsequent fermentation.
Syngas fermentation vs other biological and non-biological alternatives
Biomass, agro-industrial waste or other related feedstocks can be used to obtain either carbohydrates or syngas as potential fermentable substrates, which can both be metabolized by clostridia to produce ethanol and higher alcohols such a butanol. Expensive pretreatments are needed in order to extract carbohydrates from cellulose and hemicellulose, two major polymers of lignocellulosic feedstocks. However, lignin which is the third polymer found in such feedstocks, does not yield any carbohydrates and is thus useless for this fermentation process. Conversely, all three major polymers of lignocellulosic materials can be gasified to yield syngas, resulting in a better use of the complete feedstock in the gas fermentation process (Liew et al. 2016).
When comparing the biological and the non-biological syngas conversion routes, the former does also present some technical and economical advantages compared to the latter. The biological conversion, through the Wood–Ljungdahl pathway, takes place at near room temperature and atmospheric pressure, or if needed with slight overpressure. Conversely, the chemical Fischer Tropsch (FT) process for the production of chemicals is more complex and requires higher temperatures (150–350 °C) and elevated pressures (e.g., 30 bar). Besides, for the FT process, a specific H2:CO ratio close to 2:1 is needed (de Klerk et al. 2013), while syngas composition does generally not reach such ratio. A pretreatment consisting in a water–gas shift reaction is then required in order to adjust the gas ratio, with the concomitant increased process costs (Liew et al. 2016). On the other side, C. carboxidivorans and some other clostridia can metabolize different gas compositions to produce ethanol or higher alcohols, including pure CO, mixtures of CO2/H2, or mixtures of all three gases, among others. Syngas fermentation is thus simpler and less restrictive. Finally, although the possible inhibitory effect of some trace compounds on bioconversion processes has been mentioned above, the FT process is much more sensitive to some chemicals such as sulphur compounds and has a lower tolerance to their presence than the Wood–Ljungdahl process (Michael et al. 2011; Mohammadi et al. 2011).
However, some potential drawback needs also to be discussed. The most important one is the low aqueous solubility of the volatile compounds of the syngas mixture, when working with bioreactors in which the bioconversion takes place in liquid phase. This results in a poor gas–liquid mass transfer and in limiting rates of supply of the gaseous substrates to the microbial cells, which limits the alcohols production yields. Mass transfer of the volatile substrates can be improved when using microbubble spargers. Using pressurized bioreactors would be another alternative to improve the gas solubility and mass transfer, although this will also increase operating costs. Packed-bed bioreactors, such as biofilters or biotrickling filters, with a reduced amount of water and a small water layer between the gas phase and the biofilm (Kennes et al. 2009), have also been suggested to improve the microbial use of substrates such as carbon monoxide in gas-phase bioreactors (Jin et al. 2009).
Conclusions
Clostridium carboxidivorans is a unique acetogenic bacterium in that it has proven to be basically the only organism isolated so far able to produce a mixture of alcohols, i.e. ethanol, butanol and hexanol, at significant concentrations, from syngas or waste gases which are composed mainly of carbon monoxide, carbon dioxide and hydrogen. From a metabolic point of view, the process does hardly yield any energy; still total concentrations of alcohols of several g/L have already been obtained in stirred tank bioreactors. From a chronological point of view, organic acids (C2, C4, C6, mainly) appear first, followed by the production of the corresponding alcohols. Optimizing the operating conditions, in terms of parameters such as pH, temperature or bioreactor configuration and flow rates, among others, allow to maximize the production of alcohols. The process still needs to be further improved in order to increase yields and productivity, taking into account that the accumulation of high concentrations of alcohols in the fermentation broth will end up inhibiting biomass growth and bioconversion, which may require their removal in-situ from the medium in bioreactors.
References
Abdehagh N, Tezel FH, Thibault J (2014) Separation techniques in butanol production: challenges and developments. Biomass Bioenerg 60:222–246
Abubackar HN, Veiga MC, Kennes C (2011) Biological conversion of carbon monoxide-rich syngas or waste gases to bioethanol. Biofuels Bioprod Biorefin 5:93–114
Abubackar HN, Veiga MC, Kennes C (2015) Carbon monoxide fermentation to ethanol by Clostridium autoethanogenum in a bioreactor with no accumulation of acetic acid. Bioresour Technol 186:122–127
Ahmed A, Cateni BG, Huhnke RL, Lewis RS (2006) Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7(T). Biomass Bioenerg 30:665–667
Bruant G, Lévesque MJ, Peter C, Guiot SR, Masson L (2010) Genomic analysis of carbon monoxide utilization and butanol production by Clostridium carboxidivorans strain P7T. PLoS One 5:13033
Cotter JL, Chinn MS, Grunden AM (2009) Ethanol and acetate production by Clostridium ljungdahlii and Clostridium autoethanogenum using resting cells. Bioprocess Biosyst Eng 32:369–380
de Klerk A, Li Y-W, Zennaro R (2013) Fischer-tropch technology (Chap 3). In: Maitlis PM, de Klerk A (eds) Greener Fischer–Tropsch processes for fuels and feedstocks. Wiley-VCH, Weinheim, pp 53–79
Drake HL, Gossner AS, Daniel SL (2008) Old acetogens, new light. Ann NY Acad Sci 1125:100–128
Dürre P (2016) Butanol formation from gaseous substrates. FEMS Microbiol Lett 363:1–7
Dürre P, Hollergschwandner C (2004) Initiation of endospore formation in Clostridium acetobutylicum. Anaerobe 10:69–74
Dürre P, Fischer R-J, Kuhn A, Lorenz K, Schreiber W, Sturzenhofecker B, Ullmann S, Winzer K, Sauer U (1995) Solventogenic enzymes of Clostridium acetobutylicum: catalytic properties, genetic organization, and transcriptional regulation. FEMS Microbiol Rev 17:251–262
Fernández-Naveira Á, Abubackar HN, Veiga MC, Kennes C (2016a) Efficient butanol–ethanol (B–E) production from carbon monoxide fermentation in Clostridium carboxidivorans. Appl Microbiol Biotechnol 100:3361–3370
Fernández-Naveira Á, Abubackar HN, Veiga MC, Kennes C (2016b) Carbon monoxide bioconversion to butanol-ethanol by Clostridium carboxidivorans: kinetics and toxicity of alcohols. Appl Microbiol Biotechnol 100:4231–4240
Fernández-Naveira Á, Veiga MC, Kennes C Production of higher alcohols through anaerobic H–B–E fermentation of syngas or waste gas (submitted)
Gößner AS, Picardal F, Tanner RS, Drake HL (2008) Carbon metabolism of the moderately acid-tolerant acetogen Clostridium drakei isolated from peat. FEMS Microbiol Lett 287:236–242
Gowen CM, Fong SS (2011) Applications of systems biology towards microbial fuel production. Trends Microbiol 10:516–524
Guedon E, Payot S, Desvaux M, Petitdemange H (1999) Carbon and electron flow in Clostridium cellulolyticum grown in chemostat culture on synthetic medium. J Bacteriol 181:3262–3269
Haryanto A, Fernando SD, Pordesimo LO and Adhikari S. 2009. Upgrading of syngas derived from biomass gasification: a thermodynamic analysis. Biomass Bioenerg 33:882–889
Heiskanen H, Virkajärvi I, Viikari L (2007) The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzyme Microb Tech 41:362–367
Hemme CL, Mouttaki H, Lee Y-J, Zhang G, Goodwin L, Lucas S, Copeland A, Lapidus A, Glavina del Rio T, Tice H, Saunders E, Brettin T, Detter JC, Han CS, Pitluck S, Land ML, Hauser LJ, Kyrpides N, Mikhailova N, He Z, Wu L, Van Nostrand JD, Henrissat B, He Q, Lawson PA, Tanner RS, Lynd LR, Wiegel J, Fields MW, Arkin AP, Schadt CW, Stevenson BS, McInerney MJ, Yang Y, Dong H, Xing D, Ren N, Wang A, Huhnke RL, Mielenz JR, Ding S-Y, Himmel ME, Taghavi S, van der Lelie D, Rubin EM, Zhou J (2010) Sequencing of multiple clostridial genomes related to biomass conversion and biofuel production. J Bacteriol 192:6494–6496
Jansen M, Hansen TA (2001) Non-growth-associated demethylation of dimethylsulfonopropionate by (homo)acetogenic bacteria. Appl Environ Microbiol 67:300–306
Jeong J, Bertsch J, Hess V, Choi S, Choi I-G, Chang IS, Volker M (2015) Energy conservation model based on genomic and experimental analyses of a carbon monoxide-utilizing, butyrate-forming acetogen, Eubacterium limosum KIST612. Appl Environ Microbiol 84:4782–4790
Jin Y, Guo L, Veiga MC, Kennes C (2009) Optimization of the treatment of carbon monoxide-polluted air in biofilters. Chemosphere 74:332–337
Kane MD, Breznak (1991) Acetonema longum gen. Nov. Sp. Nov., and H2/CO2 acetogenic bacterium from the termite, Pterotermes occidentis. Arch Microbiol 156:91–98
Kennes C, Rene E, Veiga MC (2009) Bioprocesses for air pollution control. J Chem Technol Bioetchnol 84:1419–1436
Kennes D, Abubackar HN, Diaz M, Veiga MC, Kennes C (2016) Bioethanol production from biomass: carbohydrate vs syngas fermentation. J Chem Technol Bioetchnol 91:304–317
Klasson KT, Ackerson CMD, Clausen EC, Gaddy JL (1993) Biological conversion of coal and coal-derived synthesis gas. Fuel 72:1673–1678
Köpke M, Liew FM (2011) Recombinant microorganism and methods of production thereof. Patent US 20110236941 A1
Köpke M, Straub M, Dürre P (2013) Clostridium difficileis an autotrophic bacterialpathogen. PLoS One 8(4):e62157
Krasna AI (1979) Hydrogenase: properties and applications. Enzyme Microb Tech 1(3):165–172
Kubiak AM, Minton NP (2015) The potential of clostridial spores as therapeutic delivery vehicles in tumor therapy. Res Microbiol 166:244–254
Küsel K, Dorsch T, Acker G, Stackebrandt E, Drake HL (2000) Clostridium scatologenes strain SL1 isolated as an acetogenic bacterium from acidic sediments. Int J Syst Evol Microbiol 50:537–546
Latif H, Zeidan AA, Nielsen AT, Zengler K (2014) Trash to treasure: production of biofuels and commodity chemicals via syngas fermenting microorganisms. Curr Opin Biotechnol. 27:79–87
Liew F, Martin ME, Tappel RC, Heijstra BD, Mihalcea C, Köpke M (2016) Gas fermentation—a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front Microb 7:694
Liou JS, Balkwill DL, Drake GR, Tanner RS (2005) Clostridium carboxidivorans sp. nov., a solvent-producing Clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int J Syst Evol Microbiol 55:2085–2091
Liu K, Atiyeh HK, Tanner RS, Wilkins MR, Huhnke RL (2012) Fermentative production of ethanol from syngas using novel moderately alkaliphilic strains of Alkalibaculum bacchii. Bioresour Technol 104:336–341
Liu K, Atiyeh HK, Stevenson BS, Tanner RS, Wilkins MR, Huhnke RL (2014) Mixed culture syngas fermentation and conversion of carboxylic acids into alcohols. Bioresour Technol 152:337–346
Maddox IS, Steiner E, Hirsch S, Wessner S, Gutierrez NA, Gapes JR, Schuster KC (2000) The cause of ‘‘acid-crash’’ and ‘‘acidogenic fermentations’’ during the batch acetone-butanol-ethanol (ABE-) fermentation process. J Mol Microbiol Biotechnol 2:95–100
Meyer CL, Papoutsakis ET (1989) Increased levels of ATP and NADH are associated with increased solvent production in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 30: 450–459
Michael K, Steffi N, Peter D (2011) The past, present, and future of biofuels–biobutanol as promising alternative. In: dos Santos Bernades MA (ed) Biofuel production-recent developments and prospects. InTech, Rijeka, pp 451–486
Mohammadi M, Najafpour GD, Younesi H, Lahijani P, Uzir MH, Mohamed AR (2011) Bioconversion of synthesis gas to second generation biofuels: a review. Renew Sustain Energy Rev 15:4255–4257
Phillips JR, Atiyeh HK, Tanner RS, Torres JR, Saxena J, Wilkins MR, Huhnke RL (2015) Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: medium development and culture techniques. Bioresour Technol 190:114–121
Ragsdale SW, Pierce E (2008) Acetogenesis and the Wood–Ljungdahl pathway of CO(2) fixation. Biochim Biophys Acta. 1784:1873–1898
Ramió-Pujol S, Bañeras L, Ganigué R Colprim J (2015) Impact of formate on the growth and productivity of Clostridium ljungdahlii PETC and Clostridium carboxidivorans P7 grown on syngas. Int Microbiol 17:195–204
Richter H, Molitor B, Wei H, Chen W, Aristilde L, Angenent LT (2016) Ethanol production in syngas-fermenting Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression. Energy Environ Sci 9:2392
Shen GJ, Shieh JS, Grethlein AJ, Jain MK, Zeikus JH (1999) Biochemical basis for carbon monoxide tolerance and butanol production by Butyribacterium methylotrophicum. Appl Microbiol Biotechnol 51:827–832
Ukpong MN, Atiyeh HK, De Lorme MJM, Liu K (2012) Physiological response of Clostridium carboxidivorans during conversion of synthesis gas to solvents in a gas-fed bioreactor. Biotechnol Bioeng 109:2720–2728
Van Groenestijn JW, Abubackar HN, Veiga MC, Kennes C (2013) Bioethanol (Chap. 18). In: Kennes C, Veiga MC (eds) Air pollution prevention and control: bioreactors and bioenergy. Wiley, Chichester, pp 431–463
Xu D, Lewis RS (2012) Syngas fermentation to biofuels: effects of ammonia impurity in raw syngas on hydrogenase activity. Biomass Bioenerg 45:303–310
Acknowledgements
The authors thank Prof. I. Maddox and the WJMB for inviting them to prepare this manuscript. AFN acknowledges a pre-doctoral fellowship from the Xunta de Galicia (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández-Naveira, Á., Abubackar, H.N., Veiga, M.C. et al. Production of chemicals from C1 gases (CO, CO2) by Clostridium carboxidivorans . World J Microbiol Biotechnol 33, 43 (2017). https://doi.org/10.1007/s11274-016-2188-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2188-z