Abstract
Six biosurfactant-producing bacteria were isolated from hydrocarbon contaminated soils in Sfax, Tunisia. Isolates were screened for biosurfactant production by different conventional methods including hemolytic activity, surface tension reduction, drop-collapsing and oil displacement tests. All these screening tests show that all the isolates behave differently. Among the isolated bacteria, DCS1 strain was selected for further studies based on its highest activities and it was identified as Bacillus methylotrophicus DCS1. This strain was found to be a potent producer of biosurfactant when cultivated in mineral-salts medium supplemented with diesel oil (2 %, v/v) as a sole carbon source. Physicochemical properties and stability of biosurfactants synthesized by B. methylotrophicus DCS1 were investigated. The produced biosurfactants DCS1, from Landy medium, possess high surface activity that could lower the surface tension of water to a value of 31 from 72 mN m−1 and have a critical micelle concentration (CMC) of 100 mg L−1. Compared with SDS and Tween 80, biosurfactants showed excellent emulsification activities against different hydrocarbon substrates and high solubilization efficiency towards diesel oil. Biosurfactants DCS1 showed good stability in a wide range of temperature, pH and salinity. These results suggested that biosurfactants produced by B. methylotrophicus DCS1 could be an alternative to chemically synthesized surfactants for use in bioremediation processes to enhance the solubility of hydrophobic compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are amphiphilic surface-active molecules with hydrophobic and hydrophilic moieties. They can be categorized as chemical and biological surfactants or biosurfactants. Biosurfactants are synthesized by a wide variety of microorganisms during growth on water-immiscible substrates (Desai and Banat 1997). They are classified in varied structures, ranging from glycolipids, lipopeptides and lipoproteins to fatty acids, neutral lipids, phospholipids, polymeric and particulate biosurfactants. Compared with synthetic surfactants, biosurfactants offer a lot of advantages including their biodegradability, low toxicity, tolerance to extreme conditions such as high temperature value, extreme pH and high salinity. Moreover, they reduce the surface and the interfacial tension with very low critical micelle concentration (Muthusamy et al. 2008) and show better environmental compatibility (Menezes et al. 2011; Zheng et al. 2012). Furthermore, biosurfactants offer numerous biological activities for application in many fields (pharmaceutical, food industry, cosmetic) (Felnagle et al. 2008). For these reasons, in the past few decades, they showed great economic interest, specifically, in environmental field as bio-control agent, in bioremediation for their role in hydrocarbon biodegradation and sequestering (Banat et al. 2010). Bioremediation is a process that utilizes the natural capability of microorganisms to degrade toxic wastes and due to their diverse metabolic capacities, is emerging as a promising technology for the treatment of soil and groundwater contamination (Milic et al. 2009). In fact, biosurfactants facilitate the process of hydrocarbon emulsification in aqueous phase by forming micelles thereby enhancing their availability for microbial uptake and degradation. Hence they have potential application in the field of bioremediation of persistent and recalcitrant organic pollutants (Noudeh et al. 2005).
The aims of the present study were the isolation of new biosurfactant-producing bacterial strains from hydrocarbon contaminated soils and the evaluation of the physicochemical properties and stability at different environmental conditions of the produced biosurfactants.
Materials and methods
Materials
Diesel oil used in this study was obtained from a local petrol pump (Sfax, Tunisia). SDS (anionic surfactant) and Tween 80 (non-ionic surfactant) were purchased from Sigma and dissolved in distilled water. They were tested as synthetic chemical surfactants to perform the physicochemical tests in parallel with the biosurfactants preparation.
Bacterial isolation
Biosurfactant-producing strains were screened from hydrocarbon contaminated soil from area ‘Nakta’ (near the company ‘British Gaz’, Sfax, Tunisia) and from diesel contaminated soil near an automobile workshop at Sfax city, where the oil spilled in the soil. Approximately 1 g of soil sample was suspended in 9 mL of saline water. The direct isolation of the microorganisms was carried out using serial dilutions (up to 10−8) of soil samples in 0.9 % sterile saline. A 0.1 mL from each diluted samples was plated onto the surface of nutrient agar medium. The plates were then incubated at 37 °C for 1–5 days. Pure cultures with different morphological properties were obtained by picked repetitive streaking and stored in Luria–Bertani (LB) agar medium at 4 °C.
Culture media and cultivation conditions
The isolates were inoculated into a 250 mL shake flask containing 25 mL LB medium (g L−1): peptone, 10.0; yeast extract, 5.0; and NaCl, 5.0; and cultivated at 37 °C with shaking at 200 rpm for 18 h as inoculums.
For biosurfactant production, three percentage (v/v) inoculums was transferred into a 1000 mL shake flasks containing 100 mL of Landy medium (Landy et al. 1948) which contains: glucose, 20 g L−1; l-glutamic acid, 5 g L−1; yeast extract, 1 g L−1; K2HPO4, 1 g L−1; MgSO4, 0.5 g L−1; KCl, 0.5 g L−1; CuSO4, 1.6 mg L−1; Fe2(SO4)3, 0.4 mg L−1; MnSO4, 1.2 mg L−1. Culture was incubated in an orbital shaker at 30 °C and 150 rpm for 72 h.
Mineral-salts medium (MSM) contained (g L−1): (NH4)2SO4, 1; KH2PO4, 0.5; MgSO4, 0.25; KCl, 0.25; CaCl2, 0.01; FeSO4, 0.0152; MnSO4, 0.00151; CuSO4, 0.00016; ZnSO4, 0.00016. Culture was grown in 250 mL Erlenmeyer flask containing 25 mL of culture medium and inoculated with 3.0 % (v/v) of seed culture [OD600 = 8]. Culture was incubated in an orbital shaker at 30 °C and 150 rpm for 18 days.
The initial pH values of the media were adjusted to 7.0. Culture samples were taken at regular intervals (every 3 days) and analyzed for cell growth (Okoh et al. 2001) and surface tension. The determination of cell growth in mineral-salts medium was done by taking 1 ml of the medium, then centrifugation at (1200 rpm, 10 min), discarding the supernatant and adding the same volume with distilled water to the biomass and measure OD600 nm.
Screening assays for potential biosurfactant producing strains
The screening of the most potent biosurfactant-producing strains was assayed by qualitative test namely drop collapse, and quantitative tests using blood hemolysis, oil displacement tests and measurement of surface tension of cell-free culture supernatants by the Du Nouy ring method using a digital surface-tensiometer (Gibertini). All the experiments were done in triplicate.
Hemolytic activity
The hemolytic activity of isolates was tested on blood agar plate containing 5 % of human blood (v/v). The plates were incubated at 37 °C for 3 days and observed for the zone of clearance around the colonies which is correlated to the biosurfactants production (Carrillo et al. 1996).
Drop collapsing test
Diesel oil (2 μL) was added to each well of 96-well microtitre plate and allowed to equilibrate for 1 h at 37 °C. Then 5 μL of culture broth supernatant, of culture conducted in Landy medium, were added at the centre of the wells over the oil layer. The shape of the oil drop was examined after 1 min. Biosurfactants producing cultures giving flat drops were scored as positive ‘ + ’. Those cultures that gave rounded drops were scored as negative ‘−’, indicative of the lack of biosurfactants production. Distilled water was used as negative control (Youssef et al. 2004).
Oil displacement test
The oil displacement test was done by adding 40 mL of distilled water to a Petri dish with a diameter of 15 cm. After that 20 μL of diesel oil was dropped onto the surface of the water, followed by the addition of 10 μL of culture broth supernatant. The area of the clear halo on the oil surface was measured and compared with 10 μL of distilled water as negative control (Rodrigues et al. 2006).
Identification of bacteria by 16S rDNA gene sequencing
Bacterial isolate which displayed high biosurfactant production was selected in order to be identified. PCR amplification of the 16S rDNA gene with the universal primers of forward F (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse R (5′-ACCAGGGTATCTAATCCTGT-3′) was carried out and was directly sequenced. PCR amplification conditions were as follows: preheated at 94 °C for 3 min then 35 cycles of 94 °C for 45 s, annealed at 55 °C for 1 min and extend at 72 °C for 2 min. Final extension was performed at 72 °C for 7 min.
The resulting sequences were aligned compared with sequences in the GenBank database of the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov) using the nucleotide–nucleotide blast (BLASTn) network service (Zhang et al. 2000). Phylogenetic tree was draw using the software MEGA version 6 by the neighbor joining method. The 16S rDNA gene sequence was submitted to Genbank under the accession number (KX376319.1).
Biosurfactant recovery
The culture broth of DCS1 strain from Landy medium was centrifuged at 8000 rpm for 20 min at 4 °C. The cell free supernatant was acidified to pH 2.0 by adding 6 N HCl and incubated overnight at 4 °C for the precipitation of biosurfactants products. The precipitated biosurfactants were then collected by centrifugation at (8000 rpm/4 °C/20 min) and dissolved in distilled water. The pH was adjusted to 8.0 with 1.0 N NaOH and the extract was lyophilized (Abouseoud et al. 2008a).
Physicochemical characterization of biosurfactant
Critical micelle concentration (CMC) determination
The CMC represents a critical value above which increasing concentration of that component forces the formation of micelles and no further effect is expected in the surface activity (Macdonald et al. 1981). The CMC was determined by measuring the surface tension at different concentrations of diluted biosurfactants in distilled water up to a constant value of surface tension.
Measurement of emulsification activity
The emulsifying activity of biosurfactants DCS1 was measured according to the method of Bodour et al. (2004). Briefly, 2 mL of the studied hydrocarbons was mixed with 2 mL (5 mg mL−1) of biosurfactants preparation, SDS or Tween 80 and subsequently incubated at 25 °C for 24 h. The emulsification index (E24) was calculated according to the following formula:
where He and Ht are the height of emulsion and total height of the mixture respectively. All measurements were performed in triplicate.
Effect of physicochemical factors on emulsification activity
Studies were carried out by using (5 mg mL−1) biosurfactants solutions. The effect of pH on the biosurfactants emulsification activity was assayed at different pH values from 2.0 to 10.0. Emulsification index (E24) was then determined. To investigate thermal stability, biosurfactants DCS1 were incubated in water bath at different temperatures (20–100 °C) for 15 min. After cooling to room temperature, the emulsification index (E24) was calculated. The effect of salinity on emulsification activity was determined by adjusting the concentration of added NaCl to 0.5, 1, 2, 3 and 4 g L−1. Values presented are the mean of triplicate analyses.
Application of biosurfactants in enhancing solubilization of diesel oil
Test tube solubilization assay
Solubilization assay was done as described by Barkay et al. (1999). Briefly, 2.0 % of diesel oil was distributed into glass test tube, followed by addition of a volume of assay buffer (20 mM Tris–HCl, pH 7.0) to attain 10 mL final volume with graded amounts of biosurfactants/chemical surfactants (SDS and Tween 80) (from 0.1 to 2 g L−1). Tubes were incubated in a vertical position overnight at 30 °C with shaking (150 rpm) in the dark. 4.0 mL of the phase containing the soluble diesel was removed in a clean tube to which 4.0 mL of hexane was added for the extraction of soluble diesel by vortexing for 2 min. Then, tubes were centrifuged at 8000 rpm for 15 min to separate the aqueous and hexane phases. Concentration of diesel in the hexane extracts was measured spectrophotometrically at 295 nm. The concentration of the solubilized diesel was determined by using the calibration curve of the crude diesel in hexane. Assay buffers containing emulsifiers at various concentrations without diesel, were extracted with hexane as described above and served as blanks. Control experiments were also run in parallel where no emulsifiers were added to the assay sample before extraction with hexane. Results are expressed as percentage of solubilization calculated according to the following formula:
where Dassay is the concentration of diesel in the hexane extracts of assay samples; Dcontrol is the concentration of diesel in control experiments and Dinitial is the initial concentration of diesel before solubilization test.
Gas phase chromatography analysis of residual diesel
The organic solvent extract containing the solubilized diesel was also subjected to gas phase chromatography analysis equipped with a flame ionization detector and 60 m fused silica capillary column. The injector and detector temperatures were set at 280 °C and 300 °C respectively with 5 ml min−1 of hydrogen as the carrier gas. The column temperature was initially set at 150 °C for 6 min then raised to 280 °C at the rate of 7 °C min−1 and finally set at 280 °C for 6 min.
Effects of physicochemical factors on diesel oil solubilization
To study the effect of different physicochemical factors on solubilization capacity, experiments were performed as described above for the test solubilization assay using 1 g L−1 concentration of biosurfactants. The effect of diesel concentrations on the yield of solubilization was evaluated by using 1, 2, 3 and 4 % of diesel oil. The effect of pH buffer on solubilization activity at pH values ranging from 3.0 to 10.0 was evaluated using glycine–HCl buffer (pH 2.0–3.0), acetate buffer (pH 4.0–5.0), phosphate buffer (pH 6.0–8.0) and glycine–NaOH buffer (pH 9.0–10.0) (all at a final concentration of 20 mmol L−1). The effect of temperature was achieved by placing the assay tubes at 20, 30, 37 and 45 °C. The effect of salinity on diesel solubility was determined by keeping neutral pH value while adjusting the concentration of added NaCl to 5, 10, 15, 20, 25 and 30 g L−1.
Results
Screening for biosurfactants producing strains
The isolation of new biosurfactant-producing strains was carried out from soil contaminated by hydrocarbons collected from Sfax city, Tunisia. Six morphologically distinct microbial strains were isolated. Data presented in Table 1 show the results of the screening methods for biosurfactants producing isolated bacteria. Inoculation of the isolates on blood agar plates produced a clear zone around the colonies indicating the biosurfactants activity by the surface active molecule produced.
As seen in Table 1, drop collapse and oil displacement tests were highly positive for the culture broth supernatant of DCS1 strain than commercial surfactants SDS and Tween 80 used with a concentration of 5 g L−1. These results confirmed that this strain is a potential producer of surface-active molecules. The biosurfactants producers were also screened based on the reduction in surface tension during growth in Landy medium. The best result in decreasing surface tension was observed with isolate DCS1 (31 mN m−1). Therefore, based on these results, DCS1 strain was selected as the highest biosurfactants producer for subsequent study.
Identification of the isolate
The DCS1 strain was Gram-positive, rod-shaped, motile and strictly aerobic. Based on biochemical analysis, in accordance with Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994), the isolate belonged to the genus Bacillus (Table 2). In order to precisely identify this bacterium, 16S ribosomal DNA gene sequencing was determined. Primers used for the amplification of the 16S rDNA gene yielded a fragment of about 1.5 kb. The sequence obtained of about 1316 bp for the DCS1 strain was compared with those available in the GenBank database, and the results obtained showed 99 % sequence identity with Bacillus methylotrophicus. Therefore, DCS1 strain was identified as B. methylotrophicus and has the GenBank accession number (KX376319.1). The 16S rDNA gene comparative analysis showed that B. methylotrophicus strain DCS1 form a clade with the species Bacillus amyloliquefaciens, Bacillus subtilis, Bacillus vallismortis, Bacillus atrophaeus, Bacillus mojavensis and Bacillus licheniformis (Fig. 1). This bacterium was closely related to the species in genus Bacillus, in the phylum Firmicutes is taxonomically assigned to Bacilli class and the Bacillaceae family.
Kinetics of biomass growth and biosurfactants production in mineral salts-medium
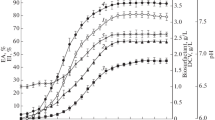
The potential of the isolate to utilize diesel oil as a sole source of carbon at aerobic condition was evaluated in a MSM containing diesel oil at a final concentration of 2.0 % (v/v). Under these conditions, biosynthesis of biosurfactants by B. methylotrophicus strain DCS1 was evaluated through determination of surface tension. The evolution of the cell growth and surface tension reduction are presented in Fig. 2. The strain was able to grow in mineral medium supplemented with 2 % diesel and maximum cell growth was observed after 15 days of incubation. In the following 3 days, the biomass decreased slightly. As can be seen, biosynthesis of biosurfactants was growth related. In fact, a significant reduction in the surface tension was observed after inoculation reaching its lowest value (36 mN m−1) after about 9 days and then remained nearly constant up to the end of cultivation (18 days).
Physicochemical characterization of biosurfactants
Critical micelle concentration (CMC) determination
CMC is an important characteristic of biosurfactants. Upon reaching the CMC, the surface tension remains relatively constant due to the interface saturation with the surfactants. The profile of surface tension as a function of biosurfactants concentration is illustrated in Fig. 3. CMC value of biosurfactants produced from B. methylotrophicus DCS1 was about 100 mg L−1.
Emulsification activity
The biosurfactants obtained from the culture broth of B. methylotrophicus DCS1 were tested for their ability to emulsify and stabilize oil-in-water emulsion. Figure 4 shows the emulsification index of biosurfactants DCS1 at a concentration of 5 mg mL−1 compared with those of SDS and Tween 80. As it is expected, all surfactants showed different degrees of emulsification of hydrocarbon substrates tested (diesel, kerosene, hexane and vegetable oil). The highest emulsification activity of biosurfactants DCS1 was observed with diesel oil (60 %) followed by kerosene and hexane (50 %) and the lowest activity was observed with vegetable oil (45 %). Moreover, the emulsification activities of the produced biosurfactants DCS1 were similar to those of the synthetic surfactants (Tween 80 and SDS).
Effects of temperature, pH and salinity on biosurfactants activity
Figure 5a shows the effect of temperature on emulsification index E24 of the biosurfactants produced by B. methylotrophicus strain DCS1. The results show that there were no differences in emulsification index values at the temperatures ranging from 20 to 80 °C, while a slight decrease of E24 was observed at 100 °C. Thus, the emulsification activity was not affected by heat treatment.
The effect of pH on emulsification activity of biosurfactants DCS1 activity is shown in Fig. 5b. The emulsification activity was low at acidic pH values. The highest emulsification index (60 %) was observed at pH 8.0 and 10.0.
Figure 5c represents the effect of NaCl on E24 of biosurfactants DCS1. The results show that emulsification activity of biosurfactants was affected by the addition of NaCl. E24 values in the presence of 0.5 and 1 g L−1 were 57.5 and 51.43 %, respectively, while that in the absence of NaCl was 60 %. Above 1 g L−1 NaCl concentration, a significant decrease in emulsification index was observed with biosurfactants.
Diesel oil solubilization assays
As can be seen in Fig. 6, a high rate of solubilization was observed (about 35 %) with a low concentration of all surfactants tested (0.1 g L−1). Thereafter, solubilization rate remained nearly constant for SDS. Above a concentration of 0.1 g L−1, the solubilization rate using biosurfactants DCS1 and Tween 80 increased slightly reaching their maximum (about 50 and 53 %, respectively) at a concentration of 2 g L−1. After that, the increase in surfactant concentration did not raise the solubilization rate.
The enhancement of water solubility of diesel by biosurfactants DCS1 was confirmed by GC analysis. The chromatograms of diesel oil (which is a complex mixture of hydrophobic compounds) solubilized in aqueous phase in the presence and absence of biosurfactants DCS1 are shown in Fig. 7. Results showed that biosurfactants DCS1 are able to solubilize compounds present in diesel oil.
Effects of physicochemical factors on diesel oil solubilization
The effects of some environmental factors (including initial oil concentration, pH, temperature and NaCl concentration) that might affect the effectiveness in oil solubilization were carried out. A series of solubilization tests were carried out.
The water solubility of diesel by biosurfactants DCS1, at a concentration of 1 g L−1, in the presence of different concentrations of diesel is shown in Fig. 8a. Solubilization of oil decreased with increasing oil concentration. In fact at 1 % diesel, solubilization percentage reached a value of 84.74 % with biosurfactants DCS1, which is 2.0-fold higher to that obtained by using 2 % diesel.
Diesel oil solubilization was also studied at different pH values. High solubilization efficiency was obtained at pH 7.0, while the lowest diesel solubilization was observed at pH 3.0 (Fig. 8b).
Regarding the effect of temperature, as shown in Fig. 8c, no significant differences were observed for the percentage of the solubilization at 30, 37 and 45 °C, although the solubilization increased slightly with the increase of temperature.
The effect of NaCl at different concentrations on solubilization efficacy was tested. As shown in Fig. 8d, solubilization efficiency increased with increasing NaCl concentration, and reached a maximum (80.2 %) with 10 g L−1 of NaCl. Above this concentration the solubilization rate of diesel oil noted a decrease but remain significant.
Discussion
Isolates from soil contaminated by hydrocarbons were screened for their ability to produce biosurfactants based on their hemolytic activity. In fact, inoculation of isolates on blood agar plate produced a clear zone around colonies, indicating biosurfactants activity. Results were in line to that reported by Ben Ayed et al. (2014) who showed that hemolytic activity appears to be a good screening criterion for surfactant-producing strains. In this context, Arima et al. (1968) showed that the amphiphilic properties of biosurfactants allow them to interfere with biological membranes and destructuring them.
Different other conventional screening methods including drop collapsing and oil displacement tests realized with culture supernatant broth of isolates are also used to confirm biosurfactants production. In fact, the drop collapsing and oil displacement tests are also indicatives of the surface and wetting activities (Youssef et al. 2004). From the results, the culture broth supernatant of DCS1 strain showed the highest area with oil displacement test toward diesel oil and a positive result with drop collapsing test. Surface activities were highly positive than commercial surfactants (SDS and Tween 80). The isolates were also screened based on the reduction in surface tension during growth in Landy medium. The best result in decreasing surface tension of the production medium from its initial value of 72 mN m−1 was observed with the isolate DCS1 (31 mN m−1). Similar results obtained by Sriram et al. (2011) who isolated Bacillus cereus NK1 strain which showed the ability to reduce culture-broth surface tension to 38 mN m−1 from its initial value of 64 mN m−1. According to Ruggeri et al. (2009), a microorganism can be considered promising for biosurfactant production, when it is able to reduce the surface tension to values below 40 mN m−1.
Therefore, among the isolated bacteria, DCS1 strain was selected for further studies as the highest biosurfactants producer based on their primary abilities to hemolytic activity, drop collapse, oil displacement and surface tension reduction.
As the strain DCS1 was found to be highly producer of biosurfactants, the 16S rDNA gene sequence analysis of this strain was determined and it was identified as Bacillus. methylotrophicus. Its ability to degrade diesel oil and to use it as a sole source of carbon was conducted in a MSM containing diesel at a final concentration of 2.0 % (v/v). In this context, Chandankere et al. (2014) studied the biodegradation of 2.0 % w/v crude oil by B. methylotrophicus USTBa in MSM under aerobic conditions. Turbidity of the culture medium and results on bacterial cell growth clearly showed that B. methylotrophicus DCS1 utilized certain components of diesel oil as a sole source of carbon and energy, which was evident from the maximum cell growth observed after 15 days of incubation. A significant reduction in the surface tension of the supernatant (36 mN m−1) was observed in the first 9 days of growth. Biosurfactants produced facilitated the access of diesel oil to cells through solubilization and accelerated bacterial cell growth in diesel medium. The mechanism proposed for improving solubility of hydrophobic organic compound is the decrease of the surface resistance at the hydrocarbon-water interface by the presence of biosurfactants and thus the increase of the bioavailability and the surface area of insoluble hydrophobic substances in water. When the concentration of biosurfactants reached or surpassed CMC, the surfactant micelles were formed and insoluble diesel members were wrapped in the micelle hydrophobic center. Thus the water solubility of diesel was significantly improved and also the contacts of bacterial cells with diesel were increased (Sun et al. 2008).
Biosurfactants produced by B. methylotrophicus strain DCS1 could effectively emulsifying and stabilizing emulsions with diesel. The emulsification index was found to be 60 %. Emulsification activities of biosurfactants DCS1 were similar to those of synthetic surfactants (Tween 80 and SDS). However, several factors including temperature, pH and salinity could influence the effectiveness of biosurfactants. Therefore, the effect of these parameters was studied. The emulsification activity of biosurfactants DCS1 was not influenced by temperature between 20 and 80 °C, while it was little affected at 100 °C suggesting that these biosurfactants are thermostables and might be useful in extreme environments such as industrial systems where extremes of temperature are integral elements. Our results are in accordance with those of Chandran and Das (2010) who reported that biosurfactant isolated from Trichosporon asahii showed emulsification activity at temperatures ranging from 10 to 100 °C.
Biosurfactants DCS1 display high emulsifying capacity for pH values ranging between 8.0 and 10.0. However, the E24 value decreased by decreasing the pH values, which may be due to partial precipitation of biosurfactants (Abouseoud et al. 2008b).
Emulsification activity of biosurfactants DCS1 was affected by the addition of NaCl. In fact, above 1 g L−1 NaCl concentration, a significant decrease in emulsification index was observed. Our findings are in contrast with reports on some biosurfactants produced by bacteria showing stability of emulsification activity in presence of high salt concentration (Obayori et al. 2009; Sarubbo et al. 2007).
Many previous reports described the utilization of chemical surfactants (SDS and Tween 80) in enhancing oil recovery and aqueous solubility of hydrocarbon in water, and remediation of hydrophobic organic compounds from soil (Urum et al. 2006; Torres et al. 2007), but their toxicity to living microorganisms reduced their use in aquatic and soil environments. In the second part of this paper, we showed that biosurfactants produced by B. methylotrophicus DCS1 could be a good alternative to synthetic emulsifiers (SDS and Tween 80) in enhancing the solubility of diesel hydrocarbon and promoting its biodegradation in the aquatic environment. Further, biosurfactants have clear advantage over synthetic surfactants due to their low toxicity, biocompatibility and biodegradability. In fact, the solubilization rate with increasing concentrations using biosurfactants DCS1 and Tween 80 increased slightly reaching their maximum (about 50 and 53 %, respectively) at a concentration of 2 g L−1. After that, the increase in surfactant concentration did not raise the solubilization rate, which can be explained by the fact that interactions between biosurfactant and hydrocarbon become weaker than biosurfactant–biosurfactant interactions, hence biosurfactants molecules tend to form a sort of complex structure or aggregate and no increase in solubility should be observed above such concentration (Abouseoud et al. 2010). Our findings are in accordance to the previous reports of Darvishi et al. (2011) and Thavasi et al. (2011) indicating that biosurfactants act as efficient enhancers for hydrocarbon biodegradation. It may be due to increase in the surface area of hydrophobic water-insoluble substrates and increase in the bioavailability of hydrophobic compounds (Perfumo et al. 2010a, b).
Diesel oil solubilization was firstly studied at different diesel concentrations. Results showed that solubilization rate of oil decreased with increasing oil concentration. Our results are in accordance with those of Mnif et al. (2012) who reported a loss of 20.8 % in solubilization using 5 % diesel in comparison to 2 % diesel for SPB1 biosurfactant. So, too high hydrocarbon concentration reduces the solubilization rate. However, our findings are in contrast to those of Ben Ayed et al. (2014) who reported that diesel solubility, by biosurfactants produced by Bacillus mojavensis A21, increased with increasing oil concentration.
The applicability of biosurfactants for bioremediation also depends on their stability at extreme conditions. Therefore, diesel oil solubilization was studied at different pH and temperature values. High solubilization efficiency was obtained at neutral pH. This is similar to the report published by Ben Ayed et al. (2014) who reported that biosurfactant A21 preserve higher activities at pH between 6.0 and 10.0 and the minimum solubilization was observed at pH 3.0. Our results correlate with the pronounced effects of pH on the emulsification activity. The activities of biosurfactants DCS1 were found to decrease by decreasing the pH from basic to acidic values.
Regarding the effect of temperature, the solubilization rate increased slightly with the increase of temperature. Also the effect of salinity on solubilization efficacy was tested and results showed that solubilization efficiency increased with increasing NaCl concentration, and reached a maximum with 10 g L−1 of NaCl. Above this concentration, we noted a slight decrease in solubilization percentage. Our results are in accordance with those published by Huszcza and Burczyk (2003), and Ben Ayed et al. (2015) who reported that the addition of salts enhanced the surface activity of biosurfactants produced by Bacillus coagulans and Bacillus amyloliquefaciens An6, respectively. Mnif et al. (2012) reported that the maximum solubilizing activity by B. subtilis SPB1 biosurfactant was achieved when 15 % NaCl was added. The higher tolerance to high salt concentration is of great interest. These findings suggested that B. methylotrophicus DCS1 biosurfactant is a good candidate for use in marine environments and other systems where salt concentration is above physiological level. The effects of salinity on solubilization using biosurfactants were also reported by Wang et al. (2007) who suggested that the presence of electrolytes causes a decrease in the critical micelle concentration (CMC) and therefore increases the solubility of hydrocarbons.
Conclusion
In the present study, biosurfactant-producing potential of six strains isolated from hydrocarbon contaminated soils has been demonstrated by different methods of screening. DCS1 strain was selected as the highest biosurfactant producer and identified as B. methylotrophicus. The biosurfactants produced by this strain were able to reduce the surface tension and to emulsify a variety of hydrocarbons. These results suggested that produced biosurfactants by B. methylotrophicus DCS1 could be an alternative to chemically synthesized surfactants for use in bioremediation processes to enhance the solubility of hydrophobic compounds.
References
Abouseoud M, Maachi R, Amrane A, Boudergua S, Nabi A (2008a) Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 223:143–151
Abouseoud M, Yataghene A, Amrane A, Maachi R (2008b) Biosurfactant production by free and alginate entrapped cells of Pseudomonas fluorescens. J Ind Microbiol Biotechnol 35:1303–1308
Abouseoud M, Yataghene A, Amrane A, Maachi R (2010) Effect of pH and salinity on the emulsifying capacity and naphthalene solubility of a biosurfactant produced by Pseudomonas fluorescens. J Hazard Mater 180:131–136
Arima K, Kakinuma A, Tamura G (1968) Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem Biophys Res Commun 31:488–494
Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444
Barkay T, Navon-Venezia S, Ron EZ, Rosenberg E (1999) Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Appl Environ Microbiol 65:2697–2702
Ben Ayed H, Jridi M, Maalej H, Nasri M, Hmidet N (2014) Characterization and stability of biosurfactant produced by Bacillus mojavensis A21 and its application in enhancing solubility of hydrocarbon. J Chem Technol Biotechnol 89:1007–1014
Ben Ayed H, Jemil N, Maalej H, Bayoudh A, Hmidet N, Nasri M (2015) Enhancement of solubilization and biodegradation of diesel oil by biosurfactant from Bacillus amyloliquefaciens An6. Int Biodeterior Biodegradation 99:8–14
Bodour AA, Gerrero-Barajas C, Jiorle BV, Malcomson ME, Paull AK, Somogyi A, Trinh LN, Bates RB, Maier RM (2004) Structure and characterization of flavolipids, a novel class of biosurfactants produced by Flavobacterium sp. strain MTN11. Appl Environ Microbiol 70:114–120
Carrillo PG, Mardaraz C, Pitta-Alvarez SI, Giulietti AM (1996) Isolation and selection of biosurfactant production bacteria. World J Microbiol Biotechnol 12:82–84
Chandankere R, Yao J, Cai M, Masakorala K, Jain AK, Choi MMF (2014) Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel 122:140–148
Chandran P, Das N (2010) Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahii isolated from petroleum hydrocarbon contaminated soil. Int J Eng Sci Technol 2:6942–6953
Darvishi P, Ayatollahi S, Mowlaa D, Niazi A (2011) Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids Surf B Biointerfaces 84:292–300
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Felnagle EA, Jackson EE, Chan YA, Podevels AM, Berti AD, McMahon MD, Thomas MG (2008) Non ribosomal peptide synthetases involved in the production of medically relevant natural products. Mol Pharm 5:191–211
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. Lippincott, William & Wilkins, Baltimore, Md
Huszcza E, Burczyk B (2003) Biosurfactant production by Bacillus coagulans. J Surfactants Deterg 6:61–64
Landy M, Warren GH, Rosenman SB, Colio LG (1948) Bacillomycin: an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med 67:539–541
Macdonald CR, Cooper DG, Zajic JE (1981) Surface-active lipids from Nocardia erythropolis grown on hydrocarbons. Appl Environ Microbiol 41:117–123
Menezes CTB, Barros EC, Rufino RD, Lun JM, Sarubbo LA (2011) Replacing synthetic with microbial surfactants as collectors in the treatment of aqueous effluent produced by acid mine drainage, using the dissolved air flotation technique. Appl Biochem Biotechnol 163:540–546
Milić JS, Beškoski VP, Ilić MV, Ali SAM, Gojgić-Cvijović GD, Vrvić MM (2009) Bioremediation of soil heavily contaminated with crude oil and its products: composition of the microbial consortium. J Serb Chem Soc 74:455–460
Mnif I, Ellouze-Chaabouni S, Ghribi D (2012) Economic production of Bacillus subtilis SPB1 biosurfactant using local agro-industrial wastes and its application in enhancing solubility of diesel. J Chem Technol Biotechnol 88:779–787
Muthusamy K, Gopalakrishnan S, Ravi TK, Sivachidambaram P (2008) Biosurfactants: properties, commercial production and application. Curr Sci 94:736–747
Noudeh GD, Housaindokht M, Bazzaz BSF (2005) Isolation, characterization and investigation of surface and hemolytic activities of a lipopeptide biosurfactant produced by Bacillus subtilis ATCC 6633. J Microbiol 43:272–276
Obayori OS, Ilori MO, Adebusoye SA, Oyetibo GO, Omotayo AE, Amund OO (2009) Degradation of hydrocarbons and biosurfactant production by Pseudomonas sp strain LP1. World J Microbiol Biotechnol 25:1615–1623
Okoh A, Ajisebutu S, Babalola G, Trejo-Hernandez MR (2001) Potential of Burkholderia cepacia RQ1 in the biodegradation of heavy crude oil. Int Microbiol 4:83–87
Perfumo A, Rancich I, Banat IM (2010a) Possibilities and challenges for biosurfactants use in petroleum industry. In: Sen R (ed) Biosurfactants, vol 672. pp 135–157
Perfumo A, Smyth TJP, Marchant R, Banat IM (2010b) Production and roles of biosurfactants and bioemulsifiers in accessing hydrophobic substrates. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, Germany, pp 1501–1512
Rodrigues LR, Teixeira JA, Van der Mei HC, Oliveira R (2006) Physiochemical and functional characterization of a biosurfactant produced by Lactococcus lactis 53. Colloids Surf B Biointerfaces 49:79–86
Ruggeri C, Franzetti A, Bestetti G, Caredda P, La Colla P, Pintus M, Sergi S, Tamburini E (2009) Isolation and characterization of surface active compound-producing bacteria from hydrocarbon-contaminated environments. Int Biodeterior Biodegradation 63:936–942
Sarubbo LA, Farias CB, Campos-Takaki GM (2007) Co-utilization of canola oil and glucose on the production of a biosurfactant by Candida lipolytica. Curr Microbiol 54:68–73
Sriram MI, Kalishwaralal K, Deepak V, Gracerosepat R, Srisakthi K, Gurunathan S (2011) Biofilm inhibition and antimicrobial action of lipopeptide biosurfactant produced by heavy metal tolerant strain Bacillus cereus NK1. Colloids Surf B Biointerfaces 85:174–181
Sun N, Wang H, Chen Y, Lu S, Xiong Y (2008) Effect of surfactant SDS, Tween 80, Triton X-100 and rhamnolipid on biodegradation of hydrophobic organic pollutants. In: The 2nd international conference, ICBBE2008, bioinformatics and biomedical engineering, pp 4730–4734
Thavasi R, Jayalakshmi S, Banat IM (2011) Application of biosurfactant produced from peanut oil cake by Lactobacillus delbrueckii in biodegradation of crude oil. Bioresour Technol 102:3366–3372
Torres LG, Climent M, Saquelares J, Bandala ER, Urquiza G, Iturbe R (2007) Characterization and treatability of a contaminated soil from an oil exploration zone. Int J Environ Sci Technol 4:311–322
Urum K, Grigson S, Pekdemir T, McMenamy S (2006) A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere 62:1403–1410
Wang Q, Fang X, Bai B, Liang X, Shuler PJ, Goddard WA III, Tang Y (2007) Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol Bioeng 98:842–853
Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ (2004) Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Methods 56:339–347
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comp Biol 7:203–214
Zheng C, Wang M, Wang Y, Huang Z (2012) Optimization of biosurfactant-mediated oil extraction from oil sludge. Bioresour Technol 110:338–342
Acknowledgment
This work was funded by the Ministry of Higher Education and Scientific Research, Tunisia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jemil, N., Ben Ayed, H., Hmidet, N. et al. Characterization and properties of biosurfactants produced by a newly isolated strain Bacillus methylotrophicus DCS1 and their applications in enhancing solubility of hydrocarbon. World J Microbiol Biotechnol 32, 175 (2016). https://doi.org/10.1007/s11274-016-2132-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2132-2