Abstract
α-Toxin, a pore-forming toxin secreted by most Staphylococcus aureus, plays critical role in the pathogenesis associated with various infectious diseases. The USA300 which is a major international epidemic methicilin-resisrant S. aureus has spread rapidly to multiple countries and become an emerging public health concern. In this study, the in vitro efficacy of Dracorhodin Perochlorate (DP) against USA300 virulence was evaluated. Using susceptibility testing, immunoblots, rabbit blood haemolytic assay and real-time RT-PCR, we observed that the α-toxin production was decreased when USA300 was co-cultured with different sub-inhibitory concentration of DP. Further, the protective effect of DP against USA300-mediated injury of human alveolar epithelial cells (A549) and MH-S cells was evaluated by cytotoxicity assays, and the result revealed that DP, at final concentration of 16 µg/ml, is a potent antagonist for USA300-mediated cell damage. Importantly, those beneficial effects might partially correlate with hla and RNAIII suppression by DP, leading to the inhibition of α-toxin production in culture supernatant. Overall, these results suggest that DP could attenuate the virulence of USA300 by decreasing α-toxin production without inhibiting bacterial growth, and this compound may represent an ideal candidate for the development of anti-virulence agent combating S. aureus infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus (S. aureus), an ubiquitous opportunistic pathogen, has been a serious threat to public health with persistent colonization of approximately 20 % of the human population. This gram positive bacterium can lead to both superficial infections including skin and soft-tissue infections, and invasive and life-threatening infectious diseases with significant mortality, such as necrotizing pneumonia, bacteremia and sepsis (Foster et al. 2014). Ever since S. aureus was first described by Sir Alexander Ogston over 130 years ago, it has continued to decimate millions of patients and rapidly spread internationally (Rigby and DeLeo 2012). Due to this bacteria’s remarkable ability to acquire resistance to various antibiotics, numerous lineages of methicilin-resisrant Staphylococcus aureus (MRSA) have emerged on every continent and growing prevalence, most notably strain and well-documented strain is USA300, which makes S. aureus infection diseases more difficult to treat (Mediavilla et al. 2012).
MRSA was first reported only 2 years after methicillin was recommended to treat S. aureus which is resistant to penicillin late 1960s and this resistant strain has wildly disseminated in many counties since the early 2000s, such as Western Australia, Europe, India, and United States (Enright et al. 2002; Stefani et al. 2012). Molecular-based epidemiologic studies have demonstrated that among clinically significant S. aureus infectious diseases, about 51 % were identified as MRSA and the most common strain is USA300. The USA300 genotype, with increasingly accumulated resistance to a variety of commonly prescribed antibiotics, is a major cause of heath care-associated blood stream infections. Meanwhile, previous studies have described that MRSA is associated with 72 % of community-onset S. aureus skin and soft-tissue infections, almost 87 % of which were caused by USA300 (Diekema et al. 2014; Seybold et al. 2006; Strommenger et al. 2014). Furthermore, corresponding with the large number of S. aureus infections, MRSA is also responsible for long-term hospital staying and the most abundant cause of hospital-associated infections. For example, an international prospective cohort study showed that compared with Meticillin-susceptible S. aureus, MRSA bacteremia can lead to almost double odds of 30-day mortality (Gould et al. 2012). What’s more, another study showed that there was approximately half a million patients were infected by S. aureus in USA every year, and the cost for S. aureus healthcare-associated infection diseases was more than 14 billion dollars in 2003 (Rigby and DeLeo 2012). Furthermore, the widespread clinical use of antimicrobial therapy for S. aureus infections has already contributed to high selective pressure for S. aureus, leading to the rapid development of antibiotic-resistant strain, like MRSA, even mutidrug-resistant strains. Taken together, the development of alternative treatment represents an urgent unmet medical need.
S. aureus is a well-armed pathogen that can produce a broad range of virulence factors which is essential for adhesion, invasion, immune evasion, and cell damage, such as adhesins, α-toxin, Panton-Valentine leukokcidin (PVL) etc. Among these virulence factors, α-toxin is a versatile water-soluble virulence factor that plays an important role in skin and soft tissue infections, necrotizing pneumonia, and fatal sepsis. Several studies have demonstrated that α-toxin mutants displayed less virulence in animal models of pneumonia, dermonecrotic skin infection, sepsis, peritonitis, and infection of the cornea, central nervous system, endocardium, and the mammary gland (Berube and Bubeck Wardenburg 2013). The gene encoding α-toxin is hla which is mainly controlled by the Agr two-component system and also modulated by other regulator systems, such as SaeR, SarZ, Rot, Sart etc. Cellular lysis is the typically biological activity of α-toxin. After being expressed at the exponential phase of growth as a water-soluble monomer, α-toxin bind on the surface of susceptible host cell membrane, and fully assembled oligomeric structure to form a beta-barrel pore, finally leading to cell death and lysis (Tavares et al. 2014). Alarmingly, the current epidemic clone-USA300 appears to exhibit more virulent as well as be more capable of colonizing multiple body sites and stronger resistance to the environmental surfaces (Chen et al. 2015; DeLeo et al. 2010; Strommenger et al. 2014). The essential role of α-toxin in the pathogenicity of S. aureus infection suggests that targeting this virulence factor would be a promising strategy for the discovery of anti-virulence agents against USA300 infection.
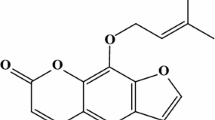
Dracorhodin Perochlorate (DP, Fig. 1), a natural compound isolated from the fruit named Daemonorops draco, has been demonstrated to be capable of inducing apoptosis in Hela cells and human breast cancer MCF-7 cells (Xia et al. 2004; Yu et al. 2013). In the present work, we discovered that DP, at final concentration of 16 µg/ml, significantly inhibits α-toxin expression by down-regulating the transcription of hla and RNAIII, the effector of the Agr two-component system. Furthermore, the addition of DP effectively protects A549 cells and MH-S cells from cell injury induced by S. aureus. Our results may offer a new agent and novel strategy for the development of anti-virulence agents against MRSA infection.
Materials and methods
Bacterial strains, culture condition and regents
MRSA strain USA300, purchased from the American Type Culture Collection (ATCC), was used for all experiments. UAS300 cells were grown in 2 ml of tryptic soy broth (TSB, Sigma-Aldrich) at 37 °C for 12 h, subsequently; all pre-culture bacterium were transferred to 150 ml TSB medium in a 250-ml flask. For immunoblots, rabbit blood haemolytic assay and real-time RT-PCR testing, USA300 cells were cultured at 37 °C in TSB medium with the indicated concentrations of DP to post-exponential growth phase (about OD600 nm of 2.5) and pelleted (2 min, 1000×g, room temperature). For the cell infection, 100 ml USA300 cells was grown at 37 °C in TSB in a 250-ml flask to OD600 nm of 1.0 and washed twice with PBS; subsequently, 10 ml of the cells was harvested as described above and resuspended with Dulbecco’s modified Eagle’s medium (DMEM) or RPMI 1640 medium to 5 × 107 CFU/ml without antibiotic. DP, which was commercially obtained from Chengdu Herbpurify CO, LTD (Chengdu, China), was prepared by dissolution in DMSO (Sigma-Aldrich) to make a stock solution.
Susceptibility testing
The broth microdilution method was employed as previously described (Zhou et al. 2015) to determine the minimal inhibitory concentration (MIC) of DP for USA300.
Growth curve assay
UAS300 cells were grown in 2 ml of TSB overnight and transferred to 150 ml TSB in a 250-ml flask 2 h to reach OD600 of 0.3 at 37 °C with shaking. For each sample, 10-ml aliquots of the bacterial suspensions were placed in a 50-ml flask without DP or with various concentrations of DP, and all the cells were further grown at 37 °C with shaking. The bacterial growth was determined at OD600 nm per 60 min.
Rabbit blood haemolytic assay
200 μl cell-free culture supernatants as described above coupled with 25 μl defibrinated rabbit red cells were mixed with 775 μl PBS and the mixture system was incubated for 10 min at 37 °C. Following concentration (2 min, 10,000×g, room temperature), the haemolytic activity of each sample were qualified by measuring the absorbance of the supernatant at OD543 nm. PBS treatment sample and 1 % Triton-X 100 treatment sample were used as the negative control (0 % haemolysis) and positive control (100 % haemolysis), respectively.
To evaluate whether DP could directly neutralize α-toxin-induced haemolysis, 200 μl cell-free culture supernatant of USA300 was incubated with various concentrations of DP for 20 min at 37 °C and mixed with 775 μl PBS and 25 μl defibrinated rabbit red cells. The haemolytic activity of each sample was determined as described above.
Immunoblot analysis
Immunoblot analysis was performed to test the impact of DP on α-toxin production in the presence of graded concentrations of DP as previous study. Briefly, 20 μl of culture supernatant was mixed with 5 μl 5 × concentrated sample buffer, heated at 100 °C for 10 min and resolved via SDS-PAGE (12 % acrylamide). Proteins were transferred to PVDF membrane, incubated with primary antibody to α-toxin (1:8000; Sigma-Aldrich) and secondary horseradish peroxidase-conjugated anti-rabbit antiserum (1:4000; Sigma-Aldrich) and revealed with ECL chemiluminescence reagents.
Real-time RT-PCR
The RNA from pelleted S. aureus USA300 was isolated with Qiagen RNeasy Maxi columns and the cDNA was generated from isolated RNA by using the Takara RNA PCR kit (AMV), ver. 3.0 (Takara, Kyoto, Japan) as described previously (Zhou et al. 2015). Each PCR reaction was conducted in 50 μl volumes by using SYBR Pre-mix Ex Taq TM (Takara), according with the manufacturer’s instructions. The PCR amplification of each sample was carried out using the 7000 Sequence Detection System (Applied Biosystems, Courtaboeuf, France). All samples were analyzed in triplicate, and the 16S rRNA was used as an endogenous control to normalize the expressional levels between samples. The relative expression levels of hla and RNAIII of each sample were assessed using the ∆∆C T method (Tavares et al. 2014). The primer sequences used in this assay as following: hla: Forward primer TTGGTGCAAATGTTTC, reverse primer TCACTTTCCAGCCTACT; RNAIII: forward primer TTCACTGTGTCGATAATCCA, reverse primer GGAAGGAGTGATTTCAATGG; 16sRNA: forward primer GCTGCCCTTTGTATTGTC, reverse primer AGATGTTGGGTTAAGTCCC.
Cytotoxicity assays
Human alveolar epithelial cells (A549) were maintained in DMEM (Sigma-Aldrich) containing 10 % fetal calf serum (Biological Industries), plated in 96-well cell culture dishes with a density of 2.0 × 104 cells per well and infected with 200 μl of bacterial suspension as described above at 37 °C for 5 h with the indicated concentrations of DP. Following concentration (10 min, 1000×g, room temperature), the LDH released into supernatants was determined by using Cytotoxicity Detection Kit (Roche) according to the manufacturer’s directions. Additionally, A549 cells were stained by live/dead (green/red) reagent (Roche) and the morphology was captured using a confocal laser scanning microscope (Nikon, Tokyo, Japan).
The protective effect of DP against USA 300-induced MH-S cells injury in RPMI 1640 medium was also determined by examining the level of LDH in supernatants as described above.
Statistical analysis
Data were represented as mean values ± standard error of the mean (SEM) and analyzed by an independent Student’s t test. P values were considered as significant if P values were lower than 0.5, indicating a significant difference between two samples.
Results
DP has no influence on USA300 growth
Susceptibility testing and growth curve assay were developed to determine the anti-S. aureus activity of DP. The minimal inhibitory concentration (MIC) value of DP against USA300 was 256 µg/ml. No visible effect was observed between DP-treatment groups (sub-MIC levels of DP, ranging from 2 to 16 µg/ml) and DP-free group for bacterial growth curve (Fig. 2). Taken together, our result established that DP, with weak anti-S. aureus activity, almost has no inhibitory effects on USA300 growth at the concentrations of 2–16 µg/ml.
DP inhibits the production of α-toxin by USA300
The haemolytic activity is one of the most represented biology properties referred to α-toxin, and multiple cell types are target by this pore-forming toxin, including erythrocyte (rabbit). Furthermore, the haemolytic activity of bacterial culture supernatants is largely depending on α-toxin. Therefore, the potential inhibitors of α-toxin were identified using haemolysis assay. Here, we found that after co-cultured with DP, the haemolytic activity of culture supernatants of S. aureus USA300 was decreased in a dose-dependent manner (Fig. 3a). Importantly, at the concentrations required for such inhibition, DP has no influence on S. aureus growth, indicating that DP-induced the decrease of the haemolytic activity of culture supernatants may be caused by an inhibition of α-toxin production or a neutralization of α-toxin function by this compound. However, in the mix systems of USA300 culture supernatants directly co-incubated with DP for 20 min, no inhibitory effect of DP against the haemolytic activity induced by culture supernatants was observed, suggesting that DP can not directly neutralize the haemolytic activity of USA300 culture supernatants (Fig. 3b). Thus, the production of α-toxin may be reduced by DP.
Inhibition of α-toxin expression by DP in culture supernatants. a In the mix system of DP co-cutured with USA300, DP decreased the haemoglobin release of USA300 in culture supernantants. b DP can not directly neutralize α-toxin-mediated haemolytic activity. c DP inhibited α-toxin production in the culture supernatants revealed by immunoblots analysis. d Attenuation the transcription of hla and RNAIII in USA300 grown with indicated concentrations of DP by RT-PCR assay. All columns in (a), (b) and (d) stand for the average values from three independent experiments (*P < 0.05, **P < 0.01)
Immunoblots analysis were further employed to directly determine whether an inhibition effect of α-toxin production by DP. As expected, compared with control group without DP, α-toxin production in the DP-supplemented samples was remarkable reduced. Importantly, no band was detected for α-toxin in the sample treated with 16 µg/ml of DP (Fig. 3c). These results were consistent with the haemolysis assay performed above, suggesting that DP diminished the production of α-toxin in culture supernatants and, subsequently, decreased the haemolytic activity of culture supernatants of USA300 in the experiment condition.
Real-time RT-PCR assay was performed to further elucidate the mechanism by which DP decreases the production of α-toxin. In addition, previous reports have demonstrated the gene encoding α-toxin is hla, which is positively regulated by Agr two-component system. Thus, the transcriptional levels of hla and RNAIII, the effector of the Agr two-component system, were both examined. Consistent with haemolysis and immunoblots analysis assay, when USA300 was exposed to the indicate concentrations of DP, the transcriptional levels of hla and RNAIII were significantly down-regulated by this compound. Following the treatment with 16 µg/ml of DP, the transcriptional levels of hla and RNAIII were significant decreased by DP in MRSA USA300, versus their respective controls (Fig. 3d). Taken together, these results indicate that treatment with DP effective decreased α-toxin production via down-regulating the transcription of hla and RNAIII.
DP alleviates USA300-mediated cell injuries
α-Toxin has long been well demonstrated as an essential virulence factor for S. aureus pneumonia, as indicated by the fact that α-toxin mutant failed to induce cell injuries and pneumonia (Berube and Bubeck Wardenburg 2013; Hua et al. 2014). Therefore, a co-culture system of S. aureus and A549 cells or MH-S cells was used to evaluate the impact of DP-treatment on USA300-mediated cell injuries.
A549 cells were infected with USA300 at multiplicity of infection (MOI) of 500 in the presence of variable concentrations of DP for 5 h and stained with live/dead (green/red) reagent. A549 cells without USA300 infection were all stained in green (Fig. 4a), indicating that no cell injuries were observed. In contract, major A549 cells were stained with red (Fig. 4b), suggesting that infection with USA300 for 5 h would lead to serious cell death. However, the addition of DP in the co-culture system offered a robust protection against such cell injuries with a dose-dependent manner (Fig. 4c–e). Importantly, almost no cell death in the sample treated with 16 µg/ml of DP (Fig. 4e).
DP effectively alleviated cell injuries caused by USA300. A549 cells were exposed with USA300 at MOI of 500 for 5 h with or without DP and stained by calcein AM and ethidium homodimer-1 (EthD-1) from live/dead reagents. Representative images of fluorescent imaging (100× magnification objective) were presented with the live cells labeled with fluorescent green and death cells labeled with fluorescent red. a A549 cells cultured in serum-free DMEM; b A549 cells infected with USA300; A549 cells infected with USA300 treated with 2 μg/ml (c), 4 μg/ml (d), 8 μg/ml (e), and 16 μg/ml (f) of DP; g the LDH release in supernatants of the co-culture system of USA300 and A549 cells or MH-S cells at MOI of 500 in the presence of various concentrations of DP. The columns in (g) stand for the average values from three independent experiments. While *P < 0.05 and **P < 0.01, compared with the DP-free culture
Furthermore, S. aureus-induced cytotoxictiy was qualified by examining LDH release in culture supernatants of A549 cells or MH-S cells in the presence of indicated concentrations of DP. Consistent with the results presented as above, treatment with DP provided a dose-dependent protection at concentrations ranging from 2 to 16 µg/ml for S. aureus-induced cytotoxictiy (Fig. 4g). Taken together, these results clearly demonstrated that DP, when added in the co-culture system, inhibited USA300-mediated cell injuries for both A549 cells and MH-S cells.
Discussion
S. aureus infection remains a daunting challenge to public health. Furthermore, treatment of S. aureus infection is increasingly being hampered due to it remarkable ability of acquiring antibiotics resistance. Antibiotic therapies using vancoycin, linezolid, and daptomycin were considered as the mainstay against MRSA infection. However, MRSA resistance to those antibiotics has been described in clinical settings, further worsening this situation (Skov et al. 2012). Meanwhile, the poor therapeutic outcome about antibiotic therapies have been increasingly reported (Abdelhady et al. 2013; van Hal and Fowler 2013). The inevitability and increasingly development of S. aureus resistance to commonly prescribed antibiotics coupled with the dwindling development of new antibiotics on behalf of an urgent need for alternatives or adjuncts to traditional antibiotic therapies.
Currently, numerous studies have addressed the promising role of anti-virulence strategy plays in better combating the antibiotic resistance crisis (Sully et al. 2014; Yu et al. 2014). Anti-virulence strategy that aiming at decreasing bacterial virulence through interfering with virulence factors relaies on host immune system to defense bacterial infection, which would lessen the burden of increasingly infection caused by antibiotic-resistant bacterium (Daly et al. 2015; Khodaverdian et al. 2013). Differing from currently antibiotic therapy, which often killing bacteria or suppressing bacterial growth, anti-virulence therapies disarmed the disease-causing virulence factors which were not essential for bacterial survival, might slowing down the development of drug-resistant bacterium. Consist with the feature of anti-virulence agents, the MIC value of DP on USA300 was 256 µg/ml and DP had no influence on S. aureus growth at the concentrations of 2–16 µg/ml, indicating that DP, as an agent with weak anti-S. aureus activity, might put less survival pressure on USA300. More importantly, some of bactericidal effects also exerted on other non-pathogenic or commensal bacterium, antibiotics, especially the broad-spectrum antibiotics, might disrupt the balance between host and commensal microbiology. Anti-virulence agents interfere with virulence factors would supply less impact on other bacterium and facility innate immune system to eradicate invasion bacterium, suggesting a positive impact on bacterial infection and resistance development (Watkins et al. 2012).
The critical role of α-toxin for S. aureus virulence has rendered this virulence factor a promising target for developing agents for S. aureus infections, especially MRSA. Kobayashi et al. have demonstrated that α-toxin contributes prominently to the skin and soft tissue infection, and compared with wild-type USA300, rabbit abscesses caused by α-toxin deletion strain had less volume and size in experiment condition (Kobayashi et al. 2011). In another animal model of S. aureus-induced pneumonia, the mutant lacking of hla was avirulent and failed to cause pneumonia (Bubeck Wardenburg et al. 2007), indicating that targeting this virulence factor was a potentially strategy for S. aureus infection. Here, DP, a natural compound with weak anti-S. aureus activity, was identified as an effective inhibitor of α-toxin by decreasing the production of α-toxin when this agent was co-cultured with USA300. Furthermore, the transcriptional level of hla and RNAIII was significantly inhibited in the presence of DP. Moreover, DP afford significant cell survival benefits to A549 cells, as well as MH-S cells during USA300 infections, suggesting that anti-virulence agents, such as DP, might be developed as alternative treatment for currently used antibiotic, which were viable and represented an urgent unmet medical need.
References
Abdelhady W et al (2013) Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:1447–1454. doi:10.1128/AAC.02073-12
Berube BJ, Bubeck Wardenburg J (2013) Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins 5:1140–1166
Bubeck Wardenburg J, Patel RJ, Schneewind O (2007) Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75:1040–1044. doi:10.1128/IAI.01313-06
Chen Y et al (2015) Basis of virulence in a Panton-Valentine leukocidin-negative community-associated methicillin-resistant Staphylococcus aureus strain. J Infect Dis 211:472–480. doi:10.1093/infdis/jiu462
Daly SM et al (2015) omega-Hydroxyemodin limits Staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob Agents Chemother 59:2223–2235. doi:10.1128/AAC.04564-14
DeLeo FR, Otto M, Kreiswirth BN, Chambers HF (2010) Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi:10.1016/S0140-6736(09)61999-1
Diekema DJ et al (2014) Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from a nationwide surveillance study. Infect Control Hosp Epidemiol 35:285–292. doi:10.1086/675283
Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG (2002) The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci USA 99:7687–7692. doi:10.1073/pnas.122108599
Foster TJ, Geoghegan JA, Ganesh VK, Hook M (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi:10.1038/nrmicro3161
Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, Peters G (2012) New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents 39:96–104. doi:10.1016/j.ijantimicag.2011.09.028
Hua L et al (2014) Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58:1108–1117. doi:10.1128/AAC.02190-13
Khodaverdian V et al (2013) Discovery of antivirulence agents against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:3645–3652. doi:10.1128/AAC.00269-13
Kobayashi SD et al (2011) Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 204:937–941. doi:10.1093/infdis/jir441
Mediavilla JR, Chen L, Mathema B, Kreiswirth BN (2012) Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr Opin Microbiol 15:588–595. doi:10.1016/j.mib.2012.08.003
Rigby KM, DeLeo FR (2012) Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34:237–259. doi:10.1007/s00281-011-0295-3
Seybold U et al (2006) Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis 42:647–656. doi:10.1086/499815
Skov R, Christiansen K, Dancer SJ, Daum RS, Dryden M, Huang YC, Lowy FD (2012) Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents 39:193–200. doi:10.1016/j.ijantimicag.2011.09.029
Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H, Mackenzie FM (2012) Meticillin-resistant Staphylococcus aureus (MRSA): global epidemiology and harmonisation of typing methods. Int J Antimicrob Agents 39:273–282. doi:10.1016/j.ijantimicag.2011.09.030
Strommenger B et al (2014) Evolution of methicillin-resistant Staphylococcus aureus towards increasing resistance. J Antimicrob Chemother 69:616–622. doi:10.1093/jac/dkt413
Sully EK et al (2014) Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog 10:e1004174. doi:10.1371/journal.ppat.1004174
Tavares A et al (2014) Insights into alpha-hemolysin (Hla) evolution and expression among Staphylococcus aureus clones with hospital and community origin. PLoS One 9:e98634. doi:10.1371/journal.pone.0098634
van Hal SJ, Fowler VG Jr (2013) Is it time to replace vancomycin in the treatment of methicillin-resistant Staphylococcus aureus infections? Clin Infect Dis 56:1779–1788. doi:10.1093/cid/cit178
Watkins RR, David MZ, Salata RA (2012) Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. J Med Microbiol 61:1179–1193. doi:10.1099/jmm.0.043513-0
Xia M, Wang D, Wang M, Tashiro S, Onodera S, Minami M, Ikejima T (2004) Dracorhodin perchlorate induces apoptosis via activation of caspases and generation of reactive oxygen species. J Pharmacol Sci 95:273–283
Yu JH et al (2013) Dracorhodin perchlorate induced human breast cancer MCF-7 apoptosis through mitochondrial pathways. Int J Med Sci 10:1149–1156. doi:10.7150/ijms.6275
Yu G, Kuo D, Shoham M, Viswanathan R (2014) Combinatorial synthesis and in vitro evaluation of a biaryl hydroxyketone library as antivirulence agents against MRSA. ACS Comb Sci 16:85–91. doi:10.1021/co400142t
Zhou X et al (2015) Phloretin derived from apple can reduce alpha-hemolysin expression in methicillin-resistant Staphylococcus aureus USA300. World J Microbiol Biotechnol 31:1259–1265. doi:10.1007/s11274-015-1879-1
Acknowledgments
This work was supported by the National Basic Research Program of China (Grant 2013CB127205) and the National Nature Science Foundation of China (Grant 31130053). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Liu, Y., Shi, D., Guo, Y. et al. Dracorhodin Perochlorate attenuates Staphylococcus aureus USA300 virulence by decreasing α-toxin expression. World J Microbiol Biotechnol 33, 17 (2017). https://doi.org/10.1007/s11274-016-2129-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2129-x