Abstract
Eighty endophytic bacteria were isolated from healthy tissues of roots, stems, leaves and fruits of tomato plants (Lycopersicon esculentum). Four strains, named BL1, BT5, BR8 and BF11 were selected for their antagonism against Botrytis cinerea, a phytopathogenic fungus responsible of gray mold in several important crops, with growth inhibitory activity ranging from 27 to 53 %. Morphological, biochemical, and molecular parameters as 16S rDNA sequencing demonstrated that the selected bacterial strains were related to Bacillus species which are known to produce and secrete a lot of lipopeptides with strong inhibitory effect against pathogen mycelial growth. Electrospray mass spectrometry analysis showed that these strains produced heterogeneous mixture of antibiotics belonging to fengycin and surfactin for BL1 and BT5, to iturin and surfactin for BR8, to bacillomycin D, fengycin and surfactin for BF11. Furthermore, these bacteria exhibited biocontrol potential by reducing the disease severity when tested on detached leaflets. Based on their antifungal activity against Botrytis cinerea, these strains could be used for biological control of plant diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botrytis cinerea is a necrotrophic pathogen that infects more than 200 plant species resulting in significant yield losses (Elad et al. 2004). It is the causal agent of gray mold diseases in many economically important fruits, vegetables, and flowers. Control of plant diseases still relies on the use of synthetic fungicides. However, the emergence of fungicide resistance that conducts to the loss of effectiveness of these compounds (Rosslenbroich and Stuebler 2000), the potential harmful effects of fungicides on the environment as well as on human health and the increasing public demands for reduction in pesticide use (Ippolito and Nigro 2000), emphasize the need for alternative disease control strategies. Among explored alternatives, the use of microbial biocontrol agents has shown significant potential.
Endophytic bacteria colonize healthy plant tissues intercellularly and/or intracellularly without causing any obvious symptoms of infections or diseases (Fisher and Petrini 1987). Plants constitute vast and diverse niches that can be exploited by an extensive variety of microorganisms including endophytes (Azevedo et al. 2000). Both Gram-positive and Gram-negative bacteria endophytes have been isolated from a large diversity of plants, e.g., citrus (Kalai-Grami et al. 2014), grapevines (Bell et al. 1995), maize (Araújo et al. 2000), potato (Sessitsch and Berg 2004), rice (Stolzfus et al. 1997), tomato (Pillay and Nowak 1997) and wheat (Coombs and Franco 2003). Such bacteria are well adapted to live inside the plant and therefore could behave as efficient biological control agents (Lin et al. 2013). Many endophytic bacteria are able to produce bioactive compounds that exert antifungal activity against plant pathogenic fungi such as Fusarium, Botrytis cinerea, Phoma tracheiphila and bacterial genuses such as Lactobacillus, Xanthomonas, Pseudomonas and Bacillus.
Bacillus species are ubiquitous and involved in the degradation of organic polymers in soil (Emmert and Handelsman 1999). Several strains belonging to this genus have shown great promises in the control of a wide range of phytopathogenic fungi owing to their production of lipopeptides as iturin, surfactin and fengycin, or proteases and chitinase that degrade fungal structural polymers, and antifungal volatiles (Katz and Demain 1977). Moreover, this genus forms endospores that are resistant to desiccation, heat, UV irradiation, and organic solvents (Sadoff 1972) and constitutes therefore an ideal candidate for use in biocontrol.
In the present study, endophytic bacteria that inhibit the growth of Botrytis cinerea were isolated from healthy tomato plants and characterized by biochemical, physiological and molecular tools. The lipopeptide antibiotics were extracted from the culture filtrate of these strains and characterized by electrospray ionization–mass spectrometry (ESI–MS). The effect of endophytic bacteria on disease severity reduction was tested on detached tomato leaflets.
Materials and methods
Fungal pathogen culture
A strain of Botrytis cinerea, provided by Dr. Hajlaoui (National Institute of Agronomic Research, Tunisia), was used in these experiments. It was routinely grown on potato dextrose agar (PDA, FLUKA) for 10 days at 25 °C under light. Conidial suspension was obtained by flooding the fungal culture with sterile distilled water containing 0.01 % tween 80, gentle rubbing of the mycelium and filtering through four layer cheesecloth. Conidia concentration was determined with a haemacytometer and adjusted to 106 conidia ml−1 with sterile distilled water.
Isolation of endophytic bacteria
Endophytic bacterial strains were isolated from various organs of healthy tomato plants. Leaves, roots, stems and fruits were sterilized for 5 min with 1 % sodium hypochlorite solution and 70 % ethanol for 1 min and washed 3 times with sterile distilled water. Each sample was aseptically cut into small pieces and deposited on Petri dishes containing Luria–Bertani medium (LB). After plates’ incubation for 2 days at 30 °C, representative colonies were streaked onto new LB plates. Bacteria were stored at −20 °C in LB broth containing 25 % glycerol.
Dual culture assay
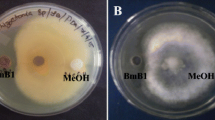
Each isolate was streaked at the middle of a Petri plate containing PDA medium and a mycelial disk (4 mm) from the peripheral region of 7 days old Botrytis cinerea’s culture was placed at approximately 2 cm from the bacteria. Plates were incubated at 25 °C for 7 days and bioactivity evaluated by measuring the percentage of growth inhibition (Korsten et al. 1995) as indicated below:
R1 is the distance of fungal growth from the point of inoculation to the colony on control plates, and R2, the distance of fungal growth from the point of inoculation to the direction of the antagonist. Selected strains were identified using biochemical and molecular tools. Control plates were not inoculated with bacteria.
Antifungal activity of cell-free supernatant
To evaluate the antifungal activity of cell-free supernatant, the well diffusion method was used (Kalai-Grami et al. 2013). Disks of 4 mm diameter of 7 days old Botrytis cinerea culture was cut and placed in the center of PDA plates. Four wells of 4 mm diameter were punctured around the fungus. One hundred µl of twofold serial dilutions (1, 1/2, 1/4, 1/8) of cell-free supernatant or LB broth (control) were deposited in each well. Plates were incubated at 25 °C for 7 days. The antifungal activity titer (AU ml−1) was defined as the reciprocal of the highest dilution that produced a detectable inhibition zone (Ben Slimene et al. 2012).
Morphological and biochemical identification of bacterial strains
Morphological characteristics and motility were assessed using light microscopy, 50 µl of bacterial culture were deposited on glass slide and then observed with an optical microscope (Olympia) 100× magnification.
The 4 selected bacteria were tested for catalase and oxidase activities and Gram stained. Strains were then identified with the analytical profile index API (BioMérieux, Marcy l’Etoile, France). The fermentation of 49 carbohydrates was tested with API 50 CHB/E medium for Bacillus and related genera. Acids produced, decreased pH, and changes in colours either to yellow, blue or green were observed after 24 and 48 h. Enzymes activities (β-galactosidase, arginine dihydrolase, lysine and ornithine decarboxylase, urease, tryptophane desaminase and gelatinase), citrate utilisation, glucose fermentation and H2S, indole and acetoin production were carried out with API 20E for Gram negative bacteria. Colour reactions were scored against a chart provided by the manufacturer and results analyzed with API WEB (BioMérieux).
DNA extraction, 16S rDNA amplification and sequencing
Each strain was inoculated into 5 ml of LB broth medium and incubated at 30 °C for 18 h on a rotary shaker at 150 rpm min−1. DNA extraction was done using DNeasy Blood and Tissue kit (QIAGEN) following the manufacturer’s standard protocol. 16S rDNA was amplified by standard PCR using universal primers FD1 (5′-AGAGTTTGATCATGGCTCAG-3′) and RD1 (5′-AAGGAGGTGATCCAGCCGCA-3′) (Edwards et al. 1989). The PCR product was purified using MinElute Gel Extraction kit (QIAGEN) and used directly for sequencing. Sequences similar to the 16S rDNA were carried out using the NCBI BLAST. Sequences from the selected bacteria and those identified by BLAST were aligned with ClustalW program (Thompson et al. 1994).
Extraction and identification of lipopeptides
Extraction of lipopeptides was carried out using the method of Kim et al. (2004) with minor modifications. After cell free medium centrifugation at 10,000g for 15 min and 4 °C, the collected supernatant was acidified with 3 N HCl to pH 2 and stored overnight at 4 °C, for lipopeptide precipitation. The precipitates were then collected by centrifugation at 10,000g for 20 min at 4 °C and dissolved in chloroform/methanol (2:1, v:v). After evaporation of solvent at 50 °C using a rotary vacuum evaporator RE 200 (BIBBY, Sterilin Ltd., UK), the precipitate was weighed and solubilized in methanol at a concentration of 50 mg ml−1.
Each lipopeptide sample was tuned by direct injection into QTRAP mass spectrometer (Applied Biosystem) equipped with an electrospray ionization interface, at a flow rate of 10 µl min−1. MS analysis was performed in positive ion mode. The ion source parameters were as follows: ion spray voltage: 5500 V; nebulizer gas (gas 1): 20 psi, curtain gas (nitrogen 99.9 %):10 psi.
Paper disk-agar assay method
For paper disk-agar diffusion assay, the method described by Raahave (1974) was used. Conidial suspension was spread on PDA plates and twofold serial dilutions of 1000, 500, 250, 125, 62.5, 31.25, 15.62 and 7.81 µg ml−1 of the lipopeptide crude extracts. Twenty microliters of each solution were deposited on 5 mm sterile Whatman paper disk. Control disk amended with 20 µl methanol only was tested. Disks were allowed to dry then applied on the agar surface. Results were recorded after 72 h at 25 °C by measuring the diameter of the clear zone around the paper disks.
Detached leaflets assay
Detached leaflets free from wounds and lesions, from 4-week-old tomato plants were surface-sterilized by soaking in 2 % aqueous sodium hypochlorite for 3 min. Leaflets were then thoroughly rinsed with sterile distilled water, dried and placed into Petri dishes containing water-soaked filter paper. Bacterial suspension was deposited at a final concentration of 108 CFU ml−1 and sprayed onto leaflets. Three needle-prick wound were applied to each leaflet. These wounds were then inoculated with 20 µl of Botrytis cinerea conidial suspension (106 conidia ml−1). Inoculated leaflets were maintained at 25 °C in the dark for 5 days under 95 % relative humidity. Experiments were performed in triplicate, each consisting in nine leaflets excised from three plants. At the end of the incubation period, percent lesion area was assessed visually according to the method of Rajkumar et al. (2005), based on a 0-4 scale: 0; no symptoms, 1; 1–12 %, 2; 13–25 %, 3; 26–50 %, 4; 51–100 % of leaflet area covered with brown lesions. The disease severity was calculated based on the following formula:
Statistical analysis
All experiments were carried out in triplicate and data expressed as mean ± SD. The experimental data were established using Duncan’s Multiple Range tests (p = 0.05) following the one-way analysis of variance (ANOVA). All statistical analysis was performed using “Statistica v 5.1” software (Statsoft 1998).
Results
Antagonistic activity
Eighty bacterial strains were screened for their antagonistic activity against Botrytis cinerea that was evaluated using the diameter of the inhibition zone (Fig. 1). Four antagonistic strains called BL1, BT5, BR8 and BF11 were the most active, inhibiting fungal mycelia growth by 46, 42, 27 and 53 % over control, respectively (Table 1). All subsequent experiments were conducted using these strains. Cell-free supernatant from BL1, BT5 and BF11 strains exhibiting an antifungal activity of 40 (AU ml−1) inhibited the growth of the fungal pathogen as well (Table 1).
Identification of the endophyte strains
The four endophyte strains belonged to the genus Bacillus (Table 2) according to their morphological and biochemical properties and to the standardized API strip system. Bacillus species are mostly rhizospheric and endophytic bacteria, Gram’s reaction, catalase and oxidase positive, exhibit motility and endospore formation in liquid culture, and UV fluorescence negative. DNA homology studies carried by comparison with GenBank database of the 16S rDNA gene sequence, indicated that BL1 (accession number KR071854) shared 99.71 % homology with Bacillus mojavensis NBRC 15718T strain, that BT5 (accession number KR071855) exhibited 99.93 % homology with Brevibacterium halotolerans DSM 8802T strain, that BR8 (accession number KR071856) showed up to 99 % homology with Bacillus subtilis, and that BF11 (accession number KR071857) shared 99 % homology with Bacillus amyloliquefaciens BCRC 11601T strain.
Antifungal activity of lipopeptide crude extract
As Bacillus species produced lipopeptides responsible of their antifungal activity, we focused on their inhibitory effect against phytopathogenic fungi using the paper disk-agar diffusion method. The in vitro antifungal activity of lipopeptides is presented in Table 3. The cell-free supernatant containing lipopeptide from the four strains significantly inhibit the growth of B. cinerea versus control and BF11 strain exhibited the strongest activity at a crude extract concentration of 7.81 µg ml−1. The three other strains namely BL1, BT5 and BR8 elicited the same level of inhibition at 62.50 µg ml−1 an eightfold higher concentration.
Lipopeptide identification
Lipopeptides from cell-free supernatant of endophyte strains were identified by ESI–MS. Comparative analysis of mass spectra obtained from BL1, BT5, BR8 and BF11 strains revealed very clear peak clusters at m/z values between 1008–1092, 1031–1097 and 1435–1505 which could be attributed to surfactins (C13–C16 and C19), bacillomycin D (C14–C16) and fengycins (C14–C19) respectively. This analysis revealed also the presence of compounds at m/z values 1085–1141 which were assigned as iturins C17–C21 (Table 4). All strains produced surfactins C13 to C15. Iturins were solely produced by BR8. BL1, BT5 and BF11 strains produced fengycins and bacillomycin D C14, C15 and C16 were produced by BF11. The high antifungal activity of BF11 is likely due to the presence of both fengycin and bacillomycin D antibiotics whereas the lower activity of BL1 and BT5 is probably linked to the sole fengycin and that of BR8 to iturin production.
Tomato leaflets assay
When applied on detached leaflets, all four endophyte strains were able to inhibit the lesions induced by fungal infection (Fig. 2). BF11 was the most protective strain as it reduced disease severity till 11 % when compared to positive control of B. cinerea on its own which induced 94 % of disease severity. The three other bacteria provided a moderate protection that reached 50 % of disease severity (Fig. 3).
Protective effect of endophytic strains against necrotic lesions induced by Botrytis cinerea. Negative control untreated leaflets (a), leaflets treated with endophytic bacteria BL1 (b), BT5 (c), BR8 (d) and BF11 (e). Positive control leaflets infected with Botrytis cinerea (f), leaflets pre-treated with BL1 (g), BT5 (h), BR8 (i) and BF11 (j) and infected with pathogen
Discussion
Endophytes are microorganisms isolated from surface sterilized plant organs, which colonize the same environment than various pathogens and as such could constitute convenient biocontrol agents in the fight against multiple plant diseases caused by soil borne pathogens (Manso and Nunes 2011). Numerous studies have demonstrated the potential of endophytic bacteria in the control of pathogens in cultured plants. Paul et al. (2013) demonstrated that endophytic bacteria, isolated from healthy tissues of chili pepper plants (Capsicum annum L.) belonged to three bacteria genus as Pseudomonas, Bacillus and Burkholderia and exhibited antifungal activity against Alternaria panax, Botrytis cinerea, Colletrotichum acutatum, Fusarium oxysporum and Phytophtora capsici.
In the present study, several bacterial strains were isolated from various organs of healthy tomato plants cultivated in an area regularly infected with B. cinerea. Four isolates exhibiting high antifungal activity against B. cinerea were selected and analyzed. Sequencing of the 16S rDNA indicated that the four selected isolates were phylogenetically related to the Bacillus genus i.e., BL1 to B. mojavensis, BT5 to B. halotolerans, BR8 to B. subtilis and BF11 to B. amyloliquefaciens. Several bacterial strains belonging to the Bacillus sp. displaying biocontrol activities against fungal pathogens have been isolated from various plants such as B. subtilis strain Lu144 from mulberry leaves (Xianling et al. 2008), Bacillus endoradicis sp. nov. from soybean root (Zhang et al. 2012) or Bacillus amyloliquefaciens BZ6-1 from peanut (Wang and Liang 2014).
As Bacillus species secrete a large variety of secondary metabolites, cell-free supernatants were tested for their antifungal activity against B. cinerea. The antifungal compounds purified from the culture broth strongly inhibited the growth of B. cinerea. Bacillus species are well known producers of biologically active compounds as lipopeptides with potent antifungal activities (Yun-Feng et al. 2012). Our data are in agreement with those of Yoshida et al. (2001), who demonstrated the inhibition of mulberry anthracnose by secreted antifungal compounds from B. amyloliquefaciens RC-2 strain. Other studies reported about the antifungal activity of Bacillus pumilus 3PPE and B. amyloliquefaciens 2TOE strain against B. cinerea (Mari et al. 1996) or on the antifungal activity of B. amyloliquefaciens against Phoma tracheiphila (Kalai-Grami et al. 2013).
ESI–MS analysis of the secreted lipopeptides allowed the identification of peptides belonging to surfactin, fengycin and iturin families. Iturin exhibited strong antifungal activity, fengycin inhibited the growth of filamentous fungi (Touré et al. 2004) and surfactin enhanced the antifungal activity of iturin A but is not fungitoxic on its own (Maget-Dana et al. 1992). The present strains produce various lipopeptide isoforms. Thus, BL1 (B. mojavensis) and BT5 (B. halotolerans) expressed fengycin and surfactin whereas BR8 (B. subtilis) produced iturin and surfactin. BF11 (B. amyloliquefaciens) secreted bacillomycin D, fengycin and surfactin. The potential of the selected bacteria to produce multiple antimicrobial peptides is obviously at the basis of their effectiveness in the inhibition of B. cinerea growth. Moreover they afford the opportunity to investigate the mechanism of action of the lipopeptides in particular the synergism that could occur between the various isoforms.
The production of surfactin and iturin by B. subtilis strain has been reported previously by Ben Slimene et al. (2012). Asaka and Shoda (1996) showed that B. subtilis BR14 strain which produced iturin A and surfactin was effective in the control of damping-off caused by Rhizoctonia solani in tomato plants. The high protection offered by this strain is likely due to the synergism between surfactin and iturin (Maget-Dana et al. 1992) which are able to exert significant pressure on pathogens (Phae et al. 1990).
B. mojavensis and B. halotolerans strains, which belong to the B. subtilis group, are surfactin and fengycin producers. However the antifungal activity is mostly linked to fengycins which are able to create pores that drastically affects the cell membrane permeability (Deleu et al. 2005) emphasizing the relevance of fengycins in the biocontrol of phytopathogenic fungi (Chan et al. 2009). Concerning surfactin they rather acted synergistically with iturin A (Akpa et al. 2001). B. mojavensis strain isolated from maize secreted inhibitory compounds into the medium that exhibited antifungal activity against Fusarium monilifrome (Bacon and Hinton 2002) and B. mojavensis strain AB1 isolated from coffee twigs produced thermostable compounds with strong antifungal activity against a wide range of phytopathogenic fungi (Nair et al. 2002). However little is known about B. halotolerans as a biocontrol agent. To our knowledge, the present study describes for the first time the isolation of an endophytic B. halotolerans strain from healthy tomato plants. Further studies to assess the antifungal activities of purified lipopeptide compounds and their mode of action are in progress.
B. amyloliquefaciens specie which is closely related to B. subtilis, has been reported as an antagonist in various plant diseases (Yoshida et al. 2001). Yin et al. (2011), evaluating the antifungal activity of endophytic B. amyloliquefaciens from poplar against B. dothidea, suggested that this effect was due to cyclic lipopeptides. The ability in producing bioactive lipopeptides against fungal pathogen has also been reported by Arrebola et al. (2010), who further showed that the control of postharvest fungal pathogens by B. amyloliquefaciens PPCB004 mainly result from the synergism between iturin A, surfactin and fengycin. Similarly the highly efficient antagonistic activity of BF11 could likely result from the synergism between bacillomycin D, surfactin and fengycin.
Our selected endophytic bacteria were also effective in reducing the disease severity of detached leaflets inoculated with the pathogen in a similar fashion as described for tomato leaves and cucumber seedling cotyledons (Wang et al. 2009). This effect may be due to the ability of the antagonistic isolates to inhibit hyphal growth of the pathogen; it is indeed established that abolition of the pathogen development contributes to the reduction disease incidence. In this respect, Daayf et al. (2003) reported a higher protection on whole plant than on detached leaves. This latter experimental assay is a model of choice for the determination of the potential biocontrol of antagonistic strains before in planta assays particularly when testing the BF11 strain, which is the most efficient one exerting an almost complete reduction of the disease.
The production of a large array of lipopeptides by endophytic bacteria is an interesting feature with potential practical applications. In this context, B. amyloliquefaciens BF11 strain is a promising candidate for the biological control of gray mold of tomatoes caused by B. cinerea. The current research is so a substantial contribution to the preservation of the environment by means of an alternative to chemical fungicides by bioactive molecules as lipopeptides-producing endophytic bacteria.
References
Akpa E, Jacques P, Wathelet B, Paquot M, Fuchs R, Budzikiewicz H, Thonart P (2001) Influence of culture conditions on lipopeptide production by Bacillus subtilis. Appl Biochem Biotechnol 91:551–561
Araújo JM, Silva AC, Azevedo JL (2000) Isolation of endophytic actinomycetes from roots and leaves of maize (Zea mays L.). Braz Arch Biol Technol 43:447–451
Arrebola E, Jacobs R, Korsten L (2010) Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J Appl Microbiol 108:386–395
Asaka O, Shoda M (1996) Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol 62:4081–4085
Azevedo JL, Maccheroni WJ, Pereira JO, Araújo WL (2000) Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electron J Biotechnol 3:40–65
Bacon CW, Hinton DM (2002) Endophytic and biological control potential of Bacillus mojavensis and related species. Biol Control 23:274–284
Bell CR, Dickie GA, Harvey WLG, Chan JWYF (1995) Endophytic bacteria in grapevine. Can J Microbiol 41:46–53
Ben Slimene I, Tabbene O, Djebali N, Cosette P, Schmitter JM, Jouenne T, Urdaci MC, Limam F (2012) Putative use of a Bacillus subtilis L194 strain for biocontrol of Phoma medicaginis in Medicago truncatula seedlings. Res Microbiol 163:388–397
Chan Y, Savard M, Reid L, Cyr T, McCormick W, Seguin C (2009) Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat. Biol Control 54:567–574
Coombs JT, Franco CMM (2003) Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl Environ Microb 69:5603–5608
Daayf F, Adam L, Fernando WGD (2003) Comparative screening of bacteria for biological control of potato late blight (strain US-8), using in vitro, detached-leaves, and whole-plant testing systems. Can J Plant Pathol 25:276–284
Deleu M, Paquot M, Nylander T (2005) Fengycin interaction with lipid monolayers at the air–aqueous interface—implications for the effect of fengycin on biological membranes. J Colloid Interface Sci 283:358–365
Edwards U, Rogall T, Blockerl H, Emde M, Bottger EC (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17:7843–7853
Elad Y, Williamson B, Tudzynski P, Delen N (eds) (2004) Botrytis spp. and diseases they cause in agricultural systems—an introduction. In: Botrytis: biology, pathology and control. Kluwer Academic Publishers, Dordrecht, pp 1–8
Emmert EAB, Handelsman J (1999) Biocontrol of plant disease: a grampositive perspective. FEMS Microbiol Lett 171:1–9
Fisher PJ, Petrini O (1987) Location of fungal endophytes in tissues of Suaeda fruiticosa: a preliminary study. Trans Br Mycol Soc 89:246–249
Ippolito A, Nigro F (2000) Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot 19:715–723
Kalai-Grami L, Ben Slimane I, Mnari-Hattab M, Rezgui S, Aouani MA, Hajlaoui MR, Limam F (2013) Protective effect of Bacillus amyloliquefaciens against infections of Citrus aurantium seedlings by Phoma tracheiphila. World J Microbiol Biotechnol 30:529–538
Kalai-Grami L, Saidi S, Bachkouel S, Ben Slimene I, Mnari-Hattab M, Hajlaoui MR, Limam F (2014) Isolation and characterization of putative endophytic bacteria antagonistic to Phoma tracheiphila and Verticillium albo-atrum. Appl Biochem Biotechnol 174:365–375
Katz E, Demain AL (1977) The peptide antibiotics of Bacillus: chemistry biogenesis and possible functions. Bacteriol Rev 41:449–474
Kim PI, Bai H, Bai D, Chae H, Chung S, Kim Y, Park R, Chi YT (2004) Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J Appl Microbiol 97:942–949
Korsten L, De Jager ES, De Villers EE, Lourens A, Kotze JM, Wehner FC (1995) Evaluation of bacterial epiphytes isolated from avocado leaf and fruit surfaces for biocontrol of avocado postharvest diseases. Plant Dis 79:1149–1156
Lin T, Zhao L, Yang Y, Guan Q, Gong M (2013) Potential of endophytic bacteria from Sophora alopecuroides nodule in biological control against Verticillium wilt disease. Aust J Crop Sci 7:139–146
Maget-Dana R, Thimon L, Peypoux F, Ptack M (1992) Surfactin/iturin A interactions may explain the synergistic effect of surfactin on the biological properties of iturin A. Biochimie 74:1047–1051
Manso T, Nunes C (2011) Metschnikowia andauensis as a new biocontrol agent of fruit postharvest diseases. Postharvest Biol Technol 61:64–71
Mari M, Guizzardi M, Pratella GC (1996) Biological control of gray mold in pears by antagonistic bacteria. Biol Control 7:30–37
Nair JR, Singh G, Sekar V (2002) Isolation and characterization of a novel Bacillus strain from coffee phyllosphere showing antifungal activity. J Appl Microbiol 93:772–780
Paul NC, Ji SH, Deng JX, Yu SH (2013) Assemblages of endophytic bacteria in chili pepper (Capsicum annum L.) and their antifungal activity against phytopathogens in vitro. POJ 6:441–448
Phae CG, Shoda M, Kubota H (1990) Suppressive effect of Bacillus subtilis and its products to phytopathogenic microorganisms. J Ferment Bioeng 69:1–7
Pillay VK, Nowak J (1997) Inoculum density, temperature, and genotype effects on in vitro growth promotion and epiphytic and endophytic colonization of tomato (Lycopersicon esculentum L.) seedlings inoculated with a Pseudomonas bacterium. Can J Microbiol 43:354–361
Raahave D (1974) Paper disk-agar diffusion assay of penicillin in the presence of streptomycin. Antimicrob Agents Chemother 6:603–605
Rajkumar M, Lee WH, Lee KJ (2005) Screening of bacterial antagonists for biological control of Phytophthora blight of pepper. J Basic Microbiol 45:55–63
Rosslenbroich HJ, Stuebler D (2000) Botrytis cinerea—history of chemical control and novel fungicides for its management. Crop Prot 19:557–561
Sadoff HL (1972) Sporulation antibiotics of Bacillus species. In: Halvorson HO, Hanson R, Campbell LL (eds) Spores V. American Society for Microbiology, Bethesda, pp 157–166
Sessitsch ARB, Berg G (2004) Endophytic bacterial communities of field-grown potato plants and their plant growth promoting and antagonistic abilities. Can J Microbiol 50:239–249
Statsoft (1998) STATISTICA for windows (computer program electronic manual). StatSoft Inc, Tulsa
Stolzfus JR, So R, Malarvithi PP, Ladha JK (1997) Isolation of endophytic bacteria from rice and assessment of their potential for supplying rice with biologically fixed nitrogen. Plant Soil 194:25–36
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Touré Y, Ongena M, Jacques P, Guiro A, Thonart P (2004) Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J Appl Microbiol 96:1151–1160
Wang X, Liang G (2014) Control efficacy of an endophytic Bacillus amyloliquefaciens strain BZ6-1 against seanut sacterial wilt, Ralstonia solanacearum. BioMed Res Int 2014:1–11
Wang S, Hu T, Jiao Y, Wei J, Cao K (2009) Isolation and characterization of Bacillus subtilis EB-28, an endophytic bacterium strain displaying biocontrol activity against Botrytis cinerea Pers. Front Agric China 3:247–252
Xianling J, Guobing L, Yingping G, Chengchao Z, Zhimei M (2008) Biological control against bacterial wilt and colonization of mulberry by an endophytic Bacillus subtilis strain. FEMS Microbiol Ecol 65:565–573
Yin XT, Xu LN, Xu L, Fan SS, Liu ZY, Zhang XY (2011) Evaluation of the efficacy of endophytic Bacillus amyloliquefaciens against Botryosphaeria dothidea and other phytopathogenic microorganisms. Afr J Microbiol Res 5:340–345
Yoshida S, Hiradate S, Tsukamoto T, Hatakeda K, Shirata A (2001) Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology 91:181–187
Yun-Feng YE, Qi-Qin LI, Gang FU, Gao-Qing Y, Jian-Hua M, Wei L (2012) Identification of antifungal substance (Iturin A2) produced by Bacillus subtilis B47 and its effect on southern corn leaf blight. J Integr Agric 1:90–99
Zhang YZ, Chen WF, Li M, Sui XH, Liu HC, Zhang XX, Chen WX (2012) Bacillus endoradicis sp. nov., an endophytic bacterium isolated from soybean root. Int J Syst Evol Microbiol 62:359–363
Acknowledgments
We are grateful to Dr. Mohamed Rabeh Hajlaoui for kindly providing Botrytis cinerea strain. This work was supported by Ministère de l’Enseignement Supérieur et de la Recherche Scientifique of Tunisia. We would like to thank Professor Ezzedine Aouani for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kefi, A., Slimene, I.B., Karkouch, I. et al. Characterization of endophytic Bacillus strains from tomato plants (Lycopersicon esculentum) displaying antifungal activity against Botrytis cinerea Pers. World J Microbiol Biotechnol 31, 1967–1976 (2015). https://doi.org/10.1007/s11274-015-1943-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1943-x