Abstract
A new strain of Bacillus coagulans CGMCC 9551, which has a broad range of antibacterial activities against six main pathogenic bacteria including Escherichia coli O8, Staphylococcus aureus, Salmonella enterica subsp. enterica serovar enteritidis, Streptococcus suis, Listeria monocytogenes and Pasteurella multocida, was isolated from healthy piglet feces. In adhesion assay, the isolate exhibited a stronger adhesion to pig intestinal mucus than that of B. subtilis JT143 and L. acidophilus LY24 respectively isolated from BioPlus®2B and FloraFIT® Probiotics (P < 0.05). The adhesion activity reached 44.5 ± 3.2, 48.9 ± 2.6, 42.6 ± 3.3 and 37.6 ± 2.4 % to jejunum, ileum, transverse colon and sigmoid colon, separately. The survival rate of B. coagulans CGMCC 9551 was reduced by only 20 % at 4 h exposure under 0.9 % w/v bile salt. The strain was fully resistant to pH 2 for 2 h with 90.1 ± 3.5 % survival and susceptible to 15 antibiotics commonly used in veterinary medicine. Additionally, the bacteria showed amylase, protease and cellulase activities. The safety assessment demonstrated the lack of toxicity potential in B. coagulans CGMCC 9551 by ligated rabbit ileal loop assay, acute and subchronic toxicity test. These results implied that that the new strain of B. coagulans CGMCC 9951 isolated from healthy piglet feces has promising probiotic characteristics and offers desirable opportunities for its successful commercialization as one excellent candidate probiotic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
China is now the world’s largest producer—and consumer—of animal products. The outputs of meat and egg in 2010 have reached 79.25 million tons and 27.63 million tons respectively. The amount of Chinese pigs, sheep, chicken, ducks are taken the largest percentage of the world livestock. However, the use of antibiotics in animal husbandry has not been banned in China. Animal husbandry is the biggest application sector for antibiotics, covering above 50 % of total antibiotic consumption (Hvistendahl 2012). There is a compelling link between heavy use of antibiotics in animal husbandry and bacterial resistance in farm animals, and resistance in humans in china (Zhu et al. 2013). Thus, in this context, probiotics, as one of alternatives to antibiotics, could be possible solutions. However, in China animal husbandry, the majority of probiotics from overseas do not show the expected probiotic effects, such as nutritional effect characterized by reduction of metabolic reactions that produce toxic substances, stimulation of indigenous enzymes and production of vitamins and antimicrobial substances; health effect, distinguished by increase in colonisation resistance, competition for gut surface adhesion and stimulation of the immune response. Thus, development of native probiotics to improve the domestic livestock industry has attracted more and more attention.
Microorganisms studied and commercialized as probiotics are mainly Gram-positive bacteria belonging to the genera of Lactobacillus, Bifidobacterium (O’Sullivan et al. 2005), and Bacillus (Cutting 2011). Bacillus species, as one of probiotics, have been in market for at least 50 years with the Italian product known as Enterogermina registered 1958 in Italy. However, there has been increasing attention to Bacillus species as probiotics in the last 20 years (Hong et al. 2005; Cutting 2011). Of the species that have been extensively evaluated are Bacillus subtilis, Bacillus clausii, Bacillus cereus, Bacillus coagulans and Bacillus licheniformis (Cutting 2011). Compared with non-spore formers, such as Lactobacillus spp., some advantages of the bacterial spores are their resistance to heat, allowing the storage at room temperature in a desiccated form without any deleterious effect on viability (Soccol et al. 2010). A second advantage is that the spore is able to reach small intestine since they survive the gastric pH of the stomach (Spinosa et al. 2000; Barbosa et al. 2005) which is not the case for all species of Lactobacillus (Tuohy et al. 2007). Moreover it can survive animal feed manufacturing conditions and ensures long term viability (De Vecchi and Drago 2006).
Bacillus coagulans is a lactic acid-forming bacterial species within the genus Bacillus. This species exhibits characteristics of both genera Lactobacillus and Bacillus. It has been reported that B. coagulans has function as a probiotic in animal trials and there is significant interest in the potential of this bacterium as a pro-biotic in humans (Drago and De Vecchi 2009). Moreover, B. coagulans has been approved for veterinary purposes as generally regarded as safe (GRAS) by the U.S. Food and Drug Administration’s Center for Veterinary Medicine and has been added by the European Food Safety Authority (EFSA) to their Qualified Presumption of Safety list (Andreoletti et al. 2008), and is listed by the Association of American Feed Control Officials (AAFCO) for use as a direct-fed microbial in livestock production (Flint and Garner 2009). Up to now, only a few strains of B. coagulans has been commercialized as probiotics besides B. coagulans strain GBI-30 (Keller et al. 2010) and Unique1S2 (GRAS Notice (GRN) No. 526 2014). The present study describes that a new strain of B. coagulans CGMCC 9951 isolated from healthy piglet feces has promising characteristics with reference to its survival in gastrointestinal conditions in the in vitro tests and offers desirable opportunities for its successful commercialization as one excellent candidate probiotic.
Materials and methods
Isolation, screening and identification of candidate probiotic
Isolation of Bacillus strains
Fecal samples of healthy piglets (15–40 days old) were collected from a swine farm in the suburbs of Luoyang City. 1.5 g of each fecal sample was dissolved in 10 mL sterile saline solution (0.85 % NaCl w/v), and the suspensions were incubated at 80 °C for 30 min. Subsequently, ten-fold serial dilutions of the suspensions were spread on plates containing nutrient agar to isolate Bacillus strains. Plates were cultured at 37 °C for 24 h and colonies were picked and purified further by streaking on tryptic soy agar (TSA) (15 g L−1 enzymatic digest of casein, 5 g L−1 enzymatic digest of soya, 5 g L−1 NaCl and 15 g L−1 agar). Isolates were stored at −80 °C with 20 % sterile glycerol until further analysis.
Screening of candidate probiotic
Six pathogenic bacteria including Escherichia coli O8, Staphylococcus aureus ATCC 29213, Salmonella enterica subsp. enterica serovar enteritidis ATCC 13076, Streptococcus suis ATCC 43765, Listeria monocytogenes ATCC 7644 and Pasteurella multocida ATCC 12947 were used as test organisms. These test organisms were grown on the TSA plates at 37 °C. The isolates were cultured in 250 mL Erlenmeyer flasks containing 50 mL of TSB (17 g L−1 enzymatic digest of casein, 3 g L−1 soy peptone, 5 g L−1 NaCl, 2.5 g L−1 K2HPO4, 2.5 g L−1 d-glucose) at 37 °C for 48 h in a rotary shaking incubator at 200 rpm. The cells were removed from the broth by centrifugation at 10,000×g for 10 min, and the resultant supernatants were sterilized by filtration through a 0.22 μm membrane. The 5 mm paper disc impregnated with the supernatant described above was placed on the agar plate which was spread with a test organism for antibacterial activity assay. After 48 h of incubation at 37 °C, the antibacterial activity of the candidate probiotic was determined by measuring the diameter of the clear zones around the paper disks. Experiments were done in three parallels and three replicates.
Identification of candidate probiotic
The purified Bacillus spp. BC158 and BC361 were identified based on Gram staining, motility, and biochemical assay according to Bergey’s Manual of Systematic Bacteriology Volume 3: The Firmicutes (Vos et al. 2009).
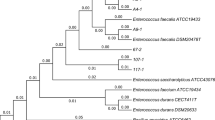
Genetic identification was carried out by 16S rDNA sequencing according to Sambrook et al. (1989). Genomic DNA (100 ng) isolated from Bacillus sp. BC361 was amplified by PCR using 5′-GAGTTTGATCMTGGCTCAG-3′, and 5′-CTAHAGGGTATCTAATCCT-3′ primers. The same operation was performed on BC158 using universal bacterial primers 27F and 1492R. Amplified 16S rDNA fragments as the sequencing templates were sequenced by Sangon Biotech (Shanghai, China). The 16S rRNA gene sequence of the isolates and their closest match were retrieved from GenBank and aligned using Clustal W with MEGA software version 4.1. Neighbor-joining method (with the Jukes-Cantor correction) was employed to construct the phylogenetic tree with 1000 bootstrap replications to assess nodal support in the tree. Nucleotide sequence data reported for the 16S rDNA of Bacillus spp. BC158 and BC361 are available in the GenBank database under the accession number KF494191 and KP003985. The two isolates have been preserved in the China general microbiological culture collection center with a preservation number of CGMCC 7433 and CGMCC 9951.
In vitro evaluation of its potential probiotic properties
Mucus adhesion assay
Mucus preparation Pig intestinal mucus was isolated from different regions of the small and large intestines as described earlier (Collado et al. 2005, 2007). In brief, the intestine was cut and washed gently in PBS containing 0.01 % (w/v) gelatin until no contents were visible. Mucus was collected from part of the washed material and placed in a small amount of 10 mM HEPES-Hanks buffer (HH; pH 7.4) by gently scraping the mucosa with a rubber spatula. The mucus was centrifuged at 10,000×g for 10 min to remove cell debris and bacteria, and determined by a modification of the method as described previously (Ohland and Macnaughton 2010). The intestine mucus was stored at −70 °C until use.
Radiolabeled bacteria Three strains were tested for their adhesion to the isolated intestinal mucus: test strain CGMCC 9951 (i.e. Bacillus sp. BC361), reference strain B. subtilis JT143 (isolate from BioPlus®2B, Christian Hansen Hoersholm, Denmark), and L. acidophilus LY24 (isolate from HOWARU Balance FloraFIT® Probiotics, DuPont Danisco, USA). The strains were radiolabeled with 3H according to the method described previously (Juntunen et al. 2001; Collado et al. 2007). CGMCC 9951 and B. subtilis JT143 were grown overnight at 37 °C in Luria Broth medium (10 g L−1 peptone, 5 g L−1 yeast extract, 5 g L−1 NaCl). LY24 was cultured in MRS media, composed by casein peptone (10 g L−1), meat extract (10 g L−1), yeast extract (5 g L−1), glucose (20 g L−1), Tween 80 (1 g L−1), sodium acetate tri-hydrate (5 g L−1), ammonium citrate (2 g L−1), Na2HPO4 (2 g L−1), MgSO4·7H2O (0.2 g L−1), MnSO4·H2O (0.05 g/L), at 37 °C overnight. In addition, the both medium contains 10 μl of methyl-1,2-[3H]thymidine (6.7 Ci/mmol; PerkinElmer, Inc., USA) per mL broth to metabolically radiolabel the bacteria. Radiolabeled bacteria were harvested by centrifugation at 5000×g for 10 min, and washed twice with HH buffer and resuspended in HH. The absorbance (A600 nm) of resuspension solution was adjusted to 0.25 ± 0.05 in order to standardize the bacterial concentration (107 to 108 CFU mL−1).
In vitro adhesion assay For determination of the level of adhesion to intestine mucus, a method modified from that of Collado et al. (2005) was employed. In short, 100 mL bacteria was added to the wells and incubated for 1 h at 37 °C. The wells were then washed twice with 200 mL of HH to remove unattached bacteria. Adhering bacteria were released and lysed with 1 % (w/v) sodium dodecyl sulfate (SDS) in 0.1 M NaOH (200 mL per well) by incubation at 60 °C for 1 h. The radioactivity was measured by liquid scintillation (Xi’an Nuclear Instrument Factory, China). Adhesion was expressed as the percentage of radioactivity recovered after adhesion relative to the radioactivity of the bacterial suspension added to the immobilized mucus. Each experiment was performed in triplicate.
Determination of tolerances of acid and bile salt
The determination of tolerances of acid and bile salt was performed as described previously (Thirabunyanon and Thongwittaya 2012). For evaluation of acid tolerance, B. coagulans CGMCC 9951 was grown in TSB at 37 °C for 2 days. To ensure only spores were present, the cultured liquid was heat treated at 80 °C for 12 min and then chilled in ice. Subsequently, spore suspensions were diluted to 106 CFU mL−1 in fresh TSB adjusted to different pH values (1, 2, 3, 4, and 5) using 2 M hydrochloric acid. After incubation for 2 h at 37 °C, the cells were serially diluted in a phosphate buffer (0.1 M, pH 6.2) to neutralize the medium acidity, and the viable cells on the TSA plate were counted after 48 h of incubation at 37 °C. The survival rate was calculated as the percentage of B. coagulans CGMCC 9951 colonies grown on the TSA plate compared to the initial bacterial concentration. For determination of bile tolerance, 15 μL of condiment the cells grown in TSB at 37 °C for 48 h (equivalent to 106 CFU mL−1) was spotted onto the TSA plates containing bile salt (Sigma Chemical Co., USA) at different concentration (0.1–0.9 % w/v). The plates were incubated at 37 °C for 48 h. The survival rate was tested as the above method. These experiments were done in triplicate.
Susceptibility test
Antibiotic susceptibility of B. coagulans CGMCC 9951 was carried out by the disc diffusion method recommended by the National Committee for Clinical Laboratory Standards (NCCLS, 2012). Fifteen antibiotics commonly applied in veterinary medicine were used to determine antibiotic susceptibility. Bacteria from 18 h cultures were used at approximately 108 CFU mL−1. The suspensions were seeded onto Mueller–Hinton agar (Bejing Unique Biotechnology Co., Ltd. China) plates using glass spreading rod. Antibiotic-impregnated discs were placed on the seeded plates and incubated at 37 °C for 18 h. The inhibition zone diameters were measured and the results were expressed as: susceptibility, S (diameter ≥20 mm); moderate susceptibility, M (diameter 15–19 mm); and resistance, R (diameter ≤14 mm) according to Performance Standards for Antimicrobial Disk Susceptibility Tests (NCCLS, 2012). The assay was performed in triplicate.
Assay of extracellular hydrolase activity
To investigate the extracellular hydrolase activity in B. coagulans CGMCC 9951 strain, the following methods were used: Decimal dilutions of the fresh overnight culture were spread on agar plate (0.5 g L−1 yeast extract, 4.5 g L−1 (NH4)2SO4, 0.1 g L−1 Cacl2·2H2O, 0.1 g L−1 MgSO4·7H2O, 0.1 g L−1 NaCl, 0.7 g L−1 K2HPO4, 0.01 g L−1 MnSO4·H2O, 0.01 g L−1 FeSO4·7H2O) added with 1 % (w/v) skimmed milk. Cultures were incubated at 37 °C for 48 h. Proteolytic activity was shown by formation of clear zone surrounding colonies. Amylolytic activity of B. coagulans CGMCC 9951 was evaluated by a similar method except the medium was mixed with 1 % (w/v) commercial cassava starch instead of skimmed milk. Hydrolysis zone around the colonies were detected by iodine staining solution (15 g L−1 KI and 15 g L−1 I2 in distilled water). Confirmation of cellulose-degrading ability of B. coagulans CGMCC 9951 was performed by streaking on the cellulose Congo-Red agar media with the following composition: K2HPO4 0.5 g L−1, MgSO4 0.25 g L−1, carboxymethyl cellulose 5 g L−1, agar 15 g L−1, Congo-Red 0.2 g L−1, gelatin 2 g L−1 and at pH 6.8–7.2. Colonies showing discoloration of Congo-Red were taken as positive cellulose-degrading bacterial colonies (Lu et al. 2004).
Safety assessment of B. coagulans CGMCC 9951
Enterotoxicity studies
To investigate the ability of B. coagulans CGMCC 9951 produce enterotoxins and emetic toxin, the ligated rabbit ileal loop experiment, as one of the classic method of biological assays, was adopted. The basic procedure of ligated rabbit ileal assays used was that of De and Chatterje (Turnbull 1976). B. coagulans CGMCC 9951 was cultured in 200 mL of TSB broth at 37 °C for 24 h. Test sample was prepared from the culture media followed by centrifugation (4000×g) and filtrates concentration (approximately l0-fold) by dialysis with carbowax. A heat-labile enterotoxin (LT) obtained from Sigma Chemical Co. (St. Louis, MO,UAS) was dissolved in sodium phosphate buffer (1 μg mL−1), and used as a positive control, sodium phosphate buffer (25 mM) and TSB without inoculation served as negative controls. The rabbits were fasted for 48 h and anaesthetized followed by a midline incision approximately 5 cm long along the abdominal linea alba of each animal. The ileum was then drawn out of the abdomen, and three 6 cm segments of the ileum (ileal loops) separated from each other by 2 cm segments were isolated. Into each loop were injected 2 mL aliquots of test sample, LT solution, TSB medium without inoculation, or sodium phosphate buffer. The abdomen of the animals was then closed and the animals were euthanized 4 h post-treatment, followed by observation of the loops for gross pathological signs of toxicity. A final loop volume: length (V/L) ratio of >0.3 was regarded as evidence of net fluid secretion into the lumen and termed a ‘positive loop’.
Acute and subchronic toxicity
To assess the acute toxicity of B. coagulans CGMCC 9951, groups of male KM mice (five males per group) were administered a single dose of a B. coagulans CGMCC 9951 saline suspension via intraperitoneal injection at doses of 0, 1 × 102, 1 × 104, 1 × 106, or 1 × 108 spores per animal, approximately equivalent to 5 × 103, 5 × 105, 5 × 107, and 5 × 109 spores per kg body weight. Following injection of the spore suspensions, the mice in each group were observed for clinical signs of toxicity and mortality for 72 h. And, blood samples were collected from each animal 3 days after the injection to determine if there’s a bacterial infection caused by test strain. In the subchronic toxicity study, groups of KM mice (a group consists of 5 males and 6 females) were administered a saline suspension of B. coagulans CGMCC 9951 at 1 × 106, 1 × 108, and 1 × 1010 spores per kg body weight via gavage daily for 4 weeks. The animals were observed daily for clinical signs of toxicity and mortality. Meanwhile, daily feed intake and body weight of mice were recorded. Four weeks after the gavage, the animals were euthanized by cervical dislocation and their heart, liver, spleen, lungs, kidneys, thymuses, testes, ovaries were stripped out and weighed accurately. The present study was conducted in accordance with the principles outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (http://grants1.nih.gov/grants/olaw/) and was approved by the local animal ethics committee at Henan University of Science and Technology.
Statistical analysis
Results are presented as mean ± SEM. One-way ANOVA followed by Tukey’s post hoc test was used to assess the statistical significance between groups. P < 0.05 was considered significant. All statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Isolation, screening and Identification of isolates with antimicrobial activity
Isolation and screening of antibacterial-producing isolates
A total of 35 fecal samples of healthy piglets were collected from a swine farm in the suburbs of Luoyang City. Approximately 150 strains of Bacillus species were isolated from these samples. Antibacterial activities of the isolated Bacillus strains against six pathogenic bacteria were tested. Seven isolates, designated as Bacillus spp. BC46, BC59, BC104, BC158, BC236, BC238 and BC361, displayed a broad range of antibacterial activities (as shown in Table 1). Bacillus spp. BC361 and BC158 possessed much higher inhibitory activity against all the indicator bacteria compared with other isolates. Moreover, all seven strains showed antibacterial activities against Salmonella enteritidis and S. suis known as the main pathogens causing most commonly diarrheal and meningitis in animals and man (Sanchez et al. 2002; Choi et al. 2012 ). Further research is needed to evaluate the potential probiotic properties of Bacillus spp. BC158 and BC361.
Identification of antimicrobical-producing bacteria
The physiological and biochemical characteristics of Bacillus spp. BC158 and BC361 showed in Table 2. Comprehensive Bacillus sp. BC158 morphological characteristics, physiological and biochemical characteristics and 16S rDNA nucleotide sequence homology analysis (as shown in Fig. 1), the strain was identified as B. cereus. Because of its involvement in food-borne diseases, the use of B. cereus as a probiotic raises safety problems (Hong et al. 2005; Cutting 2011). Recently, one B. cereus product, Paciflor, used in animal feed has been withdrawn from use in the EU (SCAN, 2001). Thus BC158 was abandoned as a candidate probiotic in this study.
Based on the comparison of physiological and biochemical properties of Bacillus sp. BC361 versus B. coagulans type strain NRIC 1005 (= ATCC 7050) or B. coagulans as presented in the Bergey’s Manual of Systematic Bacteriology 2nd edn, the isolate was characterized and initially identified as the endospore-forming, Gram-positive B. coagulans. Moreover, it shared 99.79 % homology with B. coagulans ATCC 7050T in its 16S rRNA gene sequences. In the NJ trees (Fig. 1), Bacillus sp. BC361 and B. coagulans ATCC 7050T formed a clade with a bootstrap value of 100 %. The 16S rRNA gene analysis supported the result of physiological and biochemical identification.
Probiotic properties of B. coagulans CGMCC 9951
Adhesion properties of B. coagulans CGMCC 9951
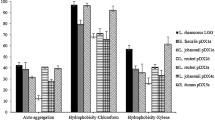
The adhesion of B. coagulans CGMCC 9951 to pig intestinal mucus was shown in Fig. 2. The strain showed a stronger adhesion to pig intestinal mucus than that of B. subtilis JT143 and L. acidophilus LY24 respectively isolated from BioPlus®2B and FloraFIT® Probiotics (P < 0.05). It reached 44.5 ± 3.2, 48.9 ± 2.6, 42.6 ± 3.3 and 37.6 ± 2.4 % of the adhesion activity to jejunum, ileum, transverse colon and sigmoid colon, separately. The result implied that B. coagulans CGMCC 9951 has desirable adhesion characteristic as one of candidate probiotic.
Adhesion of B. coagulans CGMCC 9951 to intestinal pig mucus preparations from four different intestinal areas. Results are expressed as the percentage of radioactivity recovered from immobilized mucus compared to radioactivity added to mucus. The figures share the same small letter has no significant difference at 5 %
Resistance to Acid and bile salt
In vitro test protocols can be readily adopted to examine the maintenance of a strain’s ability to tolerate acidic conditions, and test the survival and growth of candidate bacteria in the presence of bile. B. coagulans CGMCC 9951′s resistance level to acid and bile salt are shown in Fig. 3. A survival rate of 90.1 ± 3.5 % (pH 2.0 for 2 h) was observed for B. coagulans CGMCC 9951 (as shown in Fig. 3a). Even under the lower pH conditions, the survival rate was still higher than 80 %. In addition, B. coagulans CGMCC 9951 was resistant to high concentration bile salt (as shown in Fig. 3b); viability was reduced by only 20 %, even at 4 h exposure under 0.9 % w/v bile salt. Few studies have previously reported that selected Bacillus probiotics could grow at more acidic than pH 2.0. The strain demonstrated a significant level of resistance to low acidic and high bile salt conditions.
Antibiotic susceptibility
The profile of antibiotic susceptibility for B. coagulans CGMCC 9951 was shown in Table 3. The strain was susceptible to twelve antibiotics, including Amikacin, Bacitracin, Cephalothin, Chloramphenical, Penicillin G, Erythromycin, Gentamicin, Lincomycin hydrochloride, Neomycin, Streptomycin, Tetracycline, Vancomycin. It was intermediately susceptible to Amoxicillin, Flavomycin and Oxytetracyclin. The result suggested that B. coagulans CGMCC 9951 was sensitive to all clinically important antibiotics in veterinary use.
Hydrolytic activity to protein, starch and cellulose
In this study, extracellular hydrolytic enzyme activities of B. coagulans CGMCC 9951 to protein, starch and cellulose was observed. Apparent Hydrolysis zones on the media contained commercial cassava starch were found (as shown in Fig. 4a). The same phenomenon appeared on the medium contained skimmed milk or carboxymethyl cellulose (as shown in Fig. 4b, c). Observation of extracellular hydrolytic enzyme activities of CGMCC 9951 to protein, starch and cellulose implied that the isolate may generate a synergistic-mediated improvement of the production performance and nutrient digestibility of livestock.
Safety of B. coagulans CGMCC 9951
To evaluate the ability of B. coagulans CGMCC 9951 produce toxins
Due to under certain specific conditions, some Bacillus species have shown to be able to produce both enterotoxins and emetic toxin, there is a growing need to evaluate the safety of individual Bacillus strains as well as species on a case-by-case basis (Hong et al. 2005; Cutting 2011). Compared with mass-spectrometry and immunological assays, the biological assays, including rabbit ileal loop, vascular permeability reaction and cytotoxicity assays, are generally employed in determining the enterotoxins, resulting in the advantage of detecting all biologically active toxins with high sensitivity (Ceuppens et al. 2012). To ensure the safety of B. coagulans CGMCC 9951 strain, assessment of bacterial toxins in rabbit ileum was carried out. Analysis of the data (as shown in Table 4) revealed swelling with intra-luminal fluid accumulation in the loops injected with the heat-labile enterotoxin solution. No fluid accumulation was observed in the loops injected with concentrated cell-free filtrates of B. coagulans CGMCC 9951, TSB medium without inoculation and sodium phosphate buffer, respectively. These experiments provided the indirect evidence for the loss of ability to produce toxins in B. coagulans CGMCC 9951.
Assessment of acute and subchronic toxicity
Probiotics, intended for livestock feed supplement, in vivo safety is one of the safety assessment criteria. The aim of safety test is to evaluate for the animals the risk of an accidental overdosing. In the acute toxicity test, there were no deaths in any of the treated groups or control group. B. coagulans CGMCC 9951 was not detected in the blood of the strain treated mice at any of the doses tested. In a subchronic toxicity study, no morbidity and mortality were observed in these groups. Four weeks after the gavage, mice were killed by cervical dislocation and their heart, liver, spleen, lungs, kidneys, thymuses, testes, ovaries were stripped out and weighed accurately. Due to the significant differences in organ weight between treated and control animals may occur in the absence of any morphological changes, organ weight usually acts as the most sensitive indicator of an effect of an experimental compound (Bailey et al. 2004). Organ weight data for mice in a 28-day subacute toxicity test were shown in Table 5. The organ weights of heart, liver, spleen, lungs, kidneys, testes and ovaries were not significantly different between control and treated animals, except for the spleen and thymus. Moreover, feed intake and weight gain of treatment groups remarkably increased compared with the control group (data not shown in this paper). The results indicated that CGMCC 9951 was safe at dose up to 1 × 1010 spores/kg bw/day. However, the spleen index and thymus index of KM mice evidently enhanced when dose of probiotics was up to 1 × 108 spores/kg bw/day (P < 0.05). This case implied B. coagulans CGMCC 9951 may involve in the modulation of immune system of KM mice.
Discussion
Bacterial spore formers are being used extensively in humans as dietary supplements, in animals as growth promoters and lastly in aquaculture for enhancing the growth and disease-resistance of cultured shrimps (Cutting 2011). The present study identified a new isolate B. coagulans CGMCC 9951 from healthy piglet feces. Besides antibacterial activity of the isolate against pathogens, a number of probiotic properties have been examined including bile and low pH tolerance, mucus adherence, safety assessment as well as extracellular hydrolase activity. The results implied that the strain had a promising potential as a non-toxigenic and non-pathogenic candidate probiotic.
In fact, there is an important selection criteria for probiotic strains, which is safety of the strains, except for antibacterial activity against enteric pathogens, survival during passage through the gastrointestinal tract, and the ability to adhere to and colonize the epithelial cell surface of the host gastrointestinal tract (Petsuriyawong and Khunajakr 2011). Some reports show that commensal bacteria may play a specific role in both receiving and transferring antibiotic resistance genes; hence bacteria used as probiotics should not contain any transferable antimicrobial resistance genes (von Wright 2005). As a result, antibiotic susceptibility is an essential prerequisite for its approval as a probiotic. The other hand, a number of reports have shown that isolates of some Bacillus species occur toxigenic properties, for example, one or more genes that could encode an enterotoxin have been found in species of the ‘B. cereus group’ (From et al. 2005). Therefore, in the case of Bacillus species, the safety assessment of individual Bacillus strains as well as species become particularly important (Cutting 2011). In this work, the result of antimicrobial susceptibility testing implied that B. coagulans CGMCC 9951 does not carry transferable antibiotic-resistance genes. Furthermore, the safety assessment revealed the lack of toxicity potential in the candidate probiotic by ligated rabbit ileal loop assay, acute and subchronic toxicity test. Actually, the safety of Bacillus species has been extensively reported and most incidences of illness associated with Bacillus appear to result for opportunistic infections or miss-diagnosis (Cutting 2011). Extensive animal trials including acute and sub-chronic toxicity testing as well as in vitro studies have been carried out on many species, including B. subtilis var. Natto (Cutting 2011), B. indicus (Hong et al. 2008), B. coagulans (Endres et al. 2009), B. subtilis 2335 (Sorokulova et al. 2008), B. licheniformis 2336 (Sorokulova et al. 2008), and B. cereus var. toyoi (Gueimonde et al. 2013). These studies suggest that all the tested strains of Bacillus species show no indicators of adverse effects. Moreover, B. coagulans, as GRAS strain, is recommended as a direct-fed microbial in livestock production by AAFCO (2006), and has been added by EFSA to their Qualified Presumption of Safety list (Andreoletti et al. 2008). In this study, the data of different toxicity studies and susceptibility test clearly indicated that B. coagulans CGMCC 9951, as one novel candidate probiotic, has a high level of safety.
To be effective, probiotic strains must retain the fundamental characteristics. These characteristics include the ability to survive transit through the stomach and small intestine and to colonize the animal gastrointestinal tract (Tuomola et al. 2001). Thus, low pH and high bile salt tolerance are considered as important characteristics of the probiotics which enable them to survive, grow, and exert their probiotic effects in gastrointestinal tract (Bao et al. 2010). Tolerance seems to vary amongst strains of B. coagulans, as Hyronimus et al. (2000) examined 3 different strains (BCI4 LMAB, CIP5264 and CIP6625) and none of them showed detectable survival after 3 h at pH 2.5. However, B. coagulans Unique 1S2, as one of a probiotic used in the food, appeared one log cycle reduction at pH 2.0 (Sudha et al. 2010). In this study, B. coagulans CGMCC 9951 exhibited amazing survivability at low pH and high bile concentration conditions which are similar to host gastrointestinal tract. The high degree of survivability of B. coagulans under gastrointestinal condition could be attributed to the spore forming ability of the bacteria that prevents the bacteria from severe conditions. Another hand, the isolate may be endowed with the strong adaptation to the harsh environment of the gastrointestinal tract owing to the strains originated from livestock or their feces.
As an effective probiotic, two requirements have also been identified as attractive properties, these include the ability to adhere and then to consequently colonize mucous surfaces. Adhesion has been related to shortening the duration of diarrhea, immunogenic effects, competitive exclusion, and other health effects (Salminen et al. 1996; Saavedra et al. 1994). Thus, the intestinal mucosal adhesion properties may be another important criterion used to select probiotic microbes, as well as assess gut barrier effects (Tuomola et al. 2001). Mucus layer is the first physical barrier to host-cell stimulation by bacteria in the gut (Ohland and Macnaughton 2010). Adhesion to mucus is therefore the first step required for the probiotic to interact with host cells and evoke any particular response. Although Donskey et al. (2001) reported that B. coagulans lacks the ability to adhere to the intestinal epithelium, B. coagulans CGMCC 9951 exhibited stronger adhesion to pig intestinal mucus than that of B. subtilis JT143 and L. acidophilus LY24 which were separately isolated from two different types of commercial Probiotics (BioPlus®2B and FloraFIT® Probiotics). As a matter of fact, the recent work has confirmed that the genome of B. coagulans contains determinants involved in the adhesion (i.e. fibronectin- and mucus-binding proteins) (Orrù et al. 2014). Bidle et al. (1993) ever found that surface-associated proteins of B. coagulans are involved in the adhesion. Moreover, Sánchez et al. (2009) also illustrated that some proteins including S-layer components, flagellin and cell-bound proteases might play important roles in the interaction of this probiotic Bacillus strain within the gastrointestinal tract. These results verify that B. coagulans has indisputable adhesion ability to the host cells despite of its adhesion ability could vary amongst different strains of B. coagulans.
In the animal experiment, we observed that the relative weights of the thymus and spleen of treatment groups were much higher than that of control group. Kabir et al. (2004) also noted that significant differences appeared in the weight of spleen and bursa due to probiotics supplementation. Moreover, Awad et al. (2009) found that the absolute and relative weight of spleen and thymus of broiler chicken tended to be greater for the probiotic-supplemented group compared with the synbiotic-supplemented group. Babar et al. (2012) demonstrated that probiotic strain B. coagulans shows a significant stimulation of the cell mediated immunity and humoral immunity. Indeed, modulation of the immune system play a key role in the benefits associated with the consumption of probiotics (Gill and Prasad 2008).
Additionally, it is well known that the primary components of animals feed originated cereals are protein, starch and cellulose. The use of exogenous enzyme products to improve feed utilization by animals has attracted growing attention. However, enzyme supplementation substantially increases the cost of feed and is only used for a short-term solution in enhancing digestion of cereals (Liu et al. 2005). A less-expensive strategy might be to develop probiotics with the capacity of producing hydrolytic enzymes to assist the digestive process (Cho et al. 2000). Therefore, screening of probiotic strains with hydrolase activity has already aroused more and more attention. In this work, the isolate B. coagulans CGMCC 9951 showed the capacity to hydrolyze protein, starch and cellulose. B. coagulans CMB3 isolated from sea water is able to produce more extracellular enzyme, including amylase, protease, pectinase, chitinase and lecithinase (Bal et al. 2009). Wang et al. (2012) also evaluated the effects of B. coagulons as a diet additive on digestive enzymes of the white shrimp Litopenaeus vannamei, and higher activities of protease, amylase, and lipase were also found in treatment groups. Furthermore, dietary supplementation with B. subtilis, which secretes protease, amylase, and lipase, distinctly improves growth performance in broiler chicks (Santoso et al. 1995). Liu et al. (2007) demonstrated that Lactobacillus reuteri strain expressing rumen fungal xylanase could improve the digestion in chickens. Thus, activities of extracellular hydrolytic enzyme of B. coagulons CGMCC 9951 to primary components of animals feed originated cereals suggested that the isolate may bring out a synergistic-mediated improvement of the nutrient digestibility and production performance of animals.
References
Andreoletti O, Budka H, Buncic S et al (2008) Scientific opinion of the panel on biological hazards on a request from EFSA on the maintenance of the QPS list of microorganisms intentionally added to food or feed. EFSA J 923:1–48
Awad WA, Ghareeb K, Abdel-Raheem S, Böhm J (2009) Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci 88:49–56
Babar V, Thomas R, Bhaskar M (2012) Immunomodulatory activity of lactobacillus sporogenes. Int J Ther Appl 3:32–38
Bailey SA, Zidell RH, Perry RW (2004) Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol 32:448–466
Bal S, Mishra RR, Rath B, Sahu HK, Thatoi HN (2009) Characterization and extracellular enzyme activity of predominant marine Bacillus spp. isolated from sea water of Orissa Coast, India. Malays J Microbiol 5:87–93
Bao Y, Zhang YC, Zhang Y, Liu Y, Wang SQ, Dong XM, Wang YY, Zhang HP (2010) Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 2:695–701
Barbosa TM, Serra CR, La Ragione RM, Woodward MJ, Henriques AO (2005) Screening for Bacillus isolates in the broiler gastrointestinal tract. App Environ Microbiol 71:968–978
Bidle K, Wickman HH, Fletcher M (1993) Attachment of a Pseudomonas like bacterium and Bacillus coagulans to solid surfaces and adsorption of their S-layer proteins. J Gen Microbiol 139:1891–1897
Ceuppens S, Rajkovic A, Hamelink S, Van de Wiele T, Boon N, Uyttendaele M (2012) Enterotoxin production by Bacillus cereus under gastrointestinal conditions and their immunological detection by commercially available kits. Foodborne Pathog Dis 9:1130–1136
Cho JS, Choi YJ, Chung DK (2000) Expression of Clostridium thermocellum endoglucanase gene in Lactobacillus gasseri and Lactobacillus johnsonii and characterization of the genetically modified probiotic Lactobacilli. Curr Microbiol 40:257–263
Choi SM, Cho BH, Choi KH, Nam TS, Kim JT, Park MS, Kim BC, Kim MK, Cho KH (2012) Meningitis caused by Streptococcus suis: case report and review of the literature. J Clin Neurol 8:79–82
Collado MC, Gueimonde M, Hernández M, Sanz Y, Salminen S (2005) Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. J Food Prot 68:2672–2678
Collado MC, Grześkowiak Ł, Salminen S (2007) Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr Microbiol 55:260–265
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28:214–220
De Vecchi E, Drago L (2006) Lactobacillus Sporogenes or Bacillus Coagulans: misidentification or mislabelling? Int J Probiotics Prebiotics 1:3–10
Donskey CJ, Hoyen CK, Das SM, Farmer S, Dery M, Bonomo RA (2001) Effect of oral Bacillus coagulans administration on the density of vancomycin-resistant enterococci in the stool of colonized mice. Lett Appl Microbiol 33:84–88
Drago L, De Vecchi E (2009) Should Lactobacillus sporogenes and Bacillus coagulans have a future. J Chemother 21:371–377
Endres JR, Clewell A, Jade KA, Farber T, Hauswirth J, Schauss AG (2009) Safety assessment of a proprietary preparation of a novel probiotic, Bacillus coagulans, as a food ingredient. Food Chem Toxicol 47:1231–1238
Flint JF, Garner MR (2009) Feeding beneficial bacteria: a natural solution for increasing efficiency and decreasing pathogens in animal agriculture. J Appl Poult Res 18:367–378
From C, Pukall R, Schumann P, Hormazábal V, Granum PE (2005) Toxin-producing ability among Bacillus spp. outside the Bacillus cereus group. Appl Environ Microbiol 71:1178–1183
Gill H, Prasad J (2008) Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol 606:423–454
GRAS Notice (GRN) No. 526 (2014) GRAS Notification for Bacillus coagulans Unique 1S2. 1–52
Gueimonde M, Sánchez B, G de Los Reyes-Gavilán C, Margolles A (2013) Antibiotic resistance in probiotic bacteria. Front Microbiol 4:202
Hong HA, le Duc H, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835
Hong HA, Huang JM, Khaneja R, Hiep LV, Urdaci MC, Cutting SM (2008) The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J Appl Microbiol 105:510–520
Hvistendahl M (2012) China takes aim at rampant antibiotic resistance. Science 336:795
Hyronimus B, Le Marrec C, Sassi AH, Deschamps A (2000) Acid and bile tolerance of spore-forming lactic acid bacteria. Int J Food Microbiol 61:193–197
Juntunen M, Kirjavainen PV, Ouwehand AC, Salminen SJ, Isolauri E (2001) Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin Diagn Lab Immunol 8:293–296
Kabir SML, Rahman MM, Rahman MB, Rahman MM, Ahmed SU (2004) The dynamics of probiotics on growth performance and immune response in broilers. Int J Poult Sci 3:361–364
Kaneko T, Kozaki M, Komagata K (1987) Morphological, biochemical and physiological characteristics of sporeforming lactic acid bacteria. J Gen Appl Microbiol 33:33–45
Keller D, Farmer S, McCartney AL, Gibson G (2010) Bacillus coagulans as a probiotic. Food Sci Technol Bull Funct Foods 7:103–109
Liu JR, Yu B, Liu FH, Cheng KJ, Zhao X (2005) Expression of rumen microbial fibrolytic enzyme genes in probiotic Lactobacillus reuteri. Appl Environ Microbiol 71:6769–6775
Liu JR, Lai SF, Yu B (2007) Evaluation of an intestinal Lactobacillus reuteri strain expressing rumen fungal xylanase as a probiotic for broiler chickens fed on a wheat-based diet. Br Poult Sci 48:507–514
Lu WJ, Wang HT, Nie YF, Wang ZC, Huang DY, Qiu XY, Chen JC (2004) Effect of inoculating flower stalks and vegetable waste with ligno-cellulolytic microorganisms on the composting process. J Environ Sci Health B 39:871–887
NCCLS (2012) Performance standards for antimicrobial disk susceptibility tests. Approved standard M02-A11, 11th (edn) National Committee for Clinical Laboratory Standards, Wayne PA
Ohland CL, Macnaughton WK (2010) Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol-Gastr L 298:807–819
Orrù L, Salvetti E, Cattivelli L, Lamontanara A, Michelotti V, Capozzi V, Spano G, Keller D, Cash H, Martina A, Torriani S, Felis GE (2014) Draft genome sequence of Bacillus coagulans GBI-30, 6086, a Widely Used Spore-Forming Probiotic Strain. Genome Announc 2 pii: e01080–14
O’Sullivan GC, Kelly P, O’Halloran S, Collins C, Collins JK, Dunne C, Shanahan F (2005) Probiotics: an emerging therapy. Curr Pharm Des 11:3–10
Petsuriyawong B, Khunajakr N (2011) Screening of probiotic lactic acid bacteria from piglet feces. Kasetsart J Nat Sci 45:245–253
Saavedra JM, Bauman N, Oung I, Perman J, Yolken R (1994) Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046–1049
Salminen S, Isolauri E, Salminen E (1996) Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful strains and future challenges. Antonie Van Leeuwenhoek 70:347–358
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sanchez S, Hofacre CL, Lee MD, Maurer JJ, Doyle MP (2002) Animal sources of salmonellosis in humans. J Am Vet Med Assoc 221:492–497
Sánchez B, Arias S, Chaignepain S, Denayrolles M, Schmitter JM, Bressollier P, Urdaci MC (2009) Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology 155:1708–1716
Santoso U, Tanaka K, Ohtani S (1995) Effect of dried Bacillus subtilis culture on growth, body composition and hepatic lipogenic enzyme activity in female broiler chicks. Br J Nutr 74:523–529
SCAN (2001) Assessment by the scientific committee on animal nutrition of the safety of product paciflor for use as feed additive. European Commission, Health and Consumer Protection Directorate-General. (SCAN) Scientific Committee on Animal Nutrition
Soccol CR, Vandenberghe LPS, Spier MR, Medeiros ABP, Yamaguishi CT, Lindner JDD, Pandey A, Thomaz-Soccol V (2010) The potential of probiotics: a review. Food Technol Biotech 48:413–434
Sorokulova IB, Pinchuk IV, Denayrolles M, Osipova IG, Huang JM, Cutting SM, Urdaci MC (2008) The safety of two Bacillus probiotic strains for human use. Dig Dis Sci 53:954–963
Spinosa MR, Braccini T, Ricca E, De Felice M, Morelli L, Pozzi G, Oggioni MR (2000) On the fate of ingested Bacillus spores. Res Microbiol 151:361–368
Sudha RM, Chauhan P, Dixit K, Babu S, Jamil K (2010) Molecular typing and probiotic attributes of a new strain of Bacillus coagulans Unique IS-2: a potential therapeutic agent. Genetic Eng Biol J 2010:GEBJ-7
Thirabunyanon M, Thongwittaya N (2012) Protection activity of a novel probiotic strain of Bacillus subtilis against Salmonella Enteritidis infection. Res Vet Sci 93:74–81
Tuohy KM, Pinart-Gilberga M, Jones M, Hoyles L, McCartney AL, Gibson GR (2007) Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract of healthy human volunteers and its impact on the faecal microflora. J Appl Microbiol 102:1026–1032
Tuomola E, Crittenden R, Playne M, Isolauri E, Salminen S (2001) Quality assurance criteria for probiotic bacteria. Am J Clin Nutr 73:393–398
Turnbull PC (1976) Studies on the production of enterotoxins by Bacillus cereus. J Clin Pathol 29:941–948
von Wright A (2005) Regulating the safety of probiotics—the European approach. Curr Pharm Des 11:17–23
Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB (2009) Bergey’s manual of systematic bacteriology, Vol 3 The Firmicutes., 2nd edn, Springer, Berlin, pp 21–127
Wang YB, Fu LL, Lin JD (2012) Probiotic (Bacillus coagulans) cells in the diet benefit the white shrimp Litopenaeus vannamei. J Shellfish Res 31:855–860
Zhu Y-G, Johnson TA, Su J-Q, Qiao M, Guo G-X, Stedtfeld RD, Hashsham SA, Tiedje JM (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci 110:3435–3440
Acknowledgments
We are grateful to Dr. Ke Ding for generous gifts of pathogenic bacteria and Dr. Bin Zhang for helpful discussions and critical reading of the manuscript. This work was supported by The Key Scientific and Technological Project of Henan Province under Grant No. 122102110034 and National University Student Innovation Training Program No. 111416090109.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical strandard
The present study was conducted in accordance with the principles outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (http://grants1.nih.gov/grants/olaw/) was approved by the local animal ethics committee at Henan University of Science and Technology (HUST). All procedures performed in studies involving animals were in accordance with the ethical standards of the local animal ethics committee at HUST.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, SB., Zhao, LN., Wu, Y. et al. Potential probiotic attributes of a new strain of Bacillus coagulans CGMCC 9951 isolated from healthy piglet feces. World J Microbiol Biotechnol 31, 851–863 (2015). https://doi.org/10.1007/s11274-015-1838-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1838-x