Abstract
Bipolaris sorokiniana synthesizes the 1,8-dihydroxynaphthalene (DHN) melanin via pentaketide pathway and promotes the development of aerial mycelia and conidia. A melanin biosynthesis inhibitor Tricyclazole (TCZ), brought changes when applied at 5–100 μg ml−1 concentration in the colony morphology, radial growth, mycelia weight, melanin content, antioxidant enzymes (SOD and CAT) and extracellular hydrolytic enzymes (cellulase, pectinase, amylase and protease) in black, mixed and white isolates of B. sorokiniana. A significant alteration was recorded in antioxidant enzymes in black and mixed isolates; however, non-significant alteration was recorded in white isolate. Isolates of B. sorokiniana exposed to 100 µg ml−1 TCZ showed significantly increased formation of superoxide radical (O ·−2 ) and hydrogen peroxide (H2O2)·H2O2 was detected significantly high in hyphae and conidia while, O ·−2 was found primarily in the conidia. Microscopic results suggest that TCZ damages not only the cell wall but also the cell membrane. The foliar application of TCZ (25, 50 and 100 µg ml−1) decreases the area under disease progress curve, lesion development and spore formation on barley leaves thereby reducing potential for the disease development. In conclusion TCZ influences the pathogenic ability by damaging the cell structure of hyphae and conidia and also alters the antioxidant enzyme levels in B. sorokiniana. TCZ may therefore, works against to pathogen for better management of spot blotch disease in barley infected with B. sorokiniana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bipolaris sorokiniana is a well known plant pathogen and causative agent of several diseases of cultivated and wild plants including barley (Chand et al. 2003). A significant yield reduction was reported for spot blotch disease caused by B. sorokiniana in South Asian countries, and therefore it is considered a serious pathogen (Duveiller et al. 1998; Saari 1998). The infected cereal seeds are considered to be a major source of spot blotch disease (Chand et al. 2002; Chowdhury et al. 2013; Pandey et al. 2008). The risk of spot blotch epidemics are high in areas characterized by average temperature above17 °C during the cropping season with high relative humidity (Chaurasia et al. 1999).
During its development, the fungi interect with environmental factors and are constantly subjected to physical and chemical stresses. Environmental factors like ionizing radiation (α, β, γ, and X-rays), UV radiation, temperature shifts and mechanical damage etc. significantly influences fungal development. In nature, the involvement of oxygen in metabolic processes of living organisms is coupled to its activation and formation of a number of highly reactive oxygen species (ROS) i.e. singlet state of oxygen (1O2), superoxide anion (O ·−2 ), hydroxyl radical (OH·), peroxide radical (HO ·2 ), peroxide ion (HO2 −), hydrogen peroxide (H2O2) and nitric oxide (NO·). At present, the main source of O ·−2 in the cell is the partial reduction of oxygen, releasing a H2O molecule during the respiration process (Dröge 2002; Skulachev 1996). The toxicity of radicals and their role in pathological processes as well as aging are well documented (Dröge 2002; Longo et al. 2005; Zenkov et al. 2001). Reports are available on ROS mediated regulation on proliferation, differentiation, extracellular signal transduction, ion transport and immune response in organism (Chand et al. 2014; Dröge 2002). The population of B. sorokiniana produces black to mixed or white fluffy mycelia and belongs to black, mixed and white sub-populations (Bashyal et al. 2010; Chand et al. 2014; Poloni et al. 2009; Pandey et al. 2008).
Melanin is one of the most stable and resistant biochemical moieties insoluble in water (Dixon et al. 1991). Melanins are structurally very diverse and carry three types of polymers viz- eumelanin (DOPA)n, pheomelanin (Cysteinyl DOPA)n and allomelanin (DHN)n. Melanins are often complexed with protein and less often with carbohydrates (Cheng et al. 2004). In many phytopathogenic fungi melanin plays an important role in persistence of hyphae, conidia and formation of appressoria (Butler and Day 1998; Henson et al. 1999). Melanin is reported to be important for conidiogenesis in B. sorokiniana (Bashyal et al. 2010). The protective role of allomelanin against strong oxidants (O2, H2O2, O3, OH·, HO2, etc), free radicals (NO and NO2) and ROS is required for the virulence of several phyto-pathogenic fungi including Magnaporthe grisea, Colletotrichum lagenarium, Paracoccidioides brasilensis, Sporothrix schenckii and Exophialia (Wangiella) dermatitidis (Dixon et al. 1991; Gomez et al. 2001; Romero-Martinez et al. 2000; Schnitzler et al. 1999). The regulation of peg penetration during infection is prevented due to high turgor pressure exerted by the melanized appressoria (Deising et al. 2000). Melanin also limits the secretions of lytic enzymes necessary for host tissue degradation and pathogenesis in Verticillium dahliae, Alternaria alternata, Cochliobolus heterostrophus (Bell and Wheeler 1986; Henson et al. 1999; Tanabe et al. 1995). Extracellular hydrolytic enzymes produced by melanin deficient white isolate of B. sorokiniana play important role in pathogenesis and disease development (Chand et al. 2014).
To overcome the problems of disease development, Tricyclazole (TCZ; C9H7N3S) [5-methyl-1, 2,4,-triazolo (3,4,-b) (1,3) benzothiazole], is commonly used as fungicide in many countries (Chaube and Pundhir 2005; Chida and Sisler 1987; Thines et al. 2004). TCZ inhibits the DHN-melanin biosynthesis, but does not significantly affect the mycelia growth (Bashyal et al. 2010; Bashyal and Aggarwal 2013; Kunova et al. 2013), however it blocks the melanin biosynthesis pathway namely the reduction of 1, 3, 6, 8-THN to scytalone and 1, 3, 8-THN to vermelone (Huang 1981; Huang et al. 1993). TCZ is shown to have no inhibitory effect on the enzymatic dehydration of scytalone or vermelone (Wheeler 1982). Chattopadhyaya et al. (2013) reported that the foliar application of TCZ reduces the disease development caused by B. sorokiniana. The direct effect of TCZ on the capability of germinating conidia to penetrate host epidermis is well documented (Mares et al. 2004; Inoue et al. 1987; Woloshuk et al. 1983), whereas reduced secondary infection observed in the field was inferred to be due to reduced production of conidia or to lower virulence of conidia produced on tricyclazole-treated lesions (Kurahashi 2001; Kunova et al. 2013; Okuno et al. 1983; Zhang and Zhou 2004). The present work therefore, aims to study the effect of TCZ on B. sorokiniana with respect to colony morphology, melanin content, antioxidant, extracellular hydrolytic enzymes, infection process and damage to the cell structure for the management of spot blotch disease in barley caused by B. sorokiniana. Further, to evaluate the impact of TCZ treatment on barley spot blotch progress, the possible effects of TCZ on the infection efficiency of conidia, germination, AUDPC, lesion developments and spore formations on barley leaves have been studied.
Materials and methods
Experimental material
The three isolates of B. sorokiniana maintained on Sorghum grain (Chand et al. 2013), belonging to black (WPB-23), mixed (WPM-29) and white (WPR4 (87) subpopulations were used in this study. To study the various concentrations of TCZ (0, 5, 10, 25, 50 and 100 μg ml−1) as food poison in medium for isolates were performed (Elliott 1995). 4 days old culture mycelia plug (5 mm diameter) inoculated and incubated at 25 ± 1 °C and data were recorded for colony color/morphology, radial growth (mm) and mycelia weight (mg) 7 days after inoculation. Experiment was laid in complete randomized block design in triplicate.
Extraction and quantification of melanin
The melanin pigment was extracted from 10 days old culture of B. sorokiniana according to the method of Gadd (1982). Melanin content (µg/g of mycelium) was determined by using standard melanin (Sigma Chemicals Co., St. Louis, USA).
Assay of extracellular hydrolytic enzymes
The cultures were grown in liquid media (6 g NaNO3, 0.5 g KCl, 1.5 g KH2PO4, 0.5 g MgSO4, 0.01 g ZnSO4, 0.01 g FeSO4, one litre distilled H2O) by adding different concentration of TCZ i.e. 0, 5, 10, 25, 50 and 100 μg ml−1 and substrates according to the assay of enzymes by a slightly modified method of Poloni et al. (2009). A single mycelia plug (5.0 mm diameter) from 4 days old cultures of B. sorokiniana isolates were transferred individually in 20 ml liquid media supplemented with enzyme specific substrates carboxymethyl cellulose (CMC) (1 %), pectin (1 %), starch (1 %) and gelatin (4 %) for cellulase, pectinase, amylase and protease respectively in triplicate and incubated at 25 ± 1 °C for 10 days. The culture was filtered through Buchner funnel using Whatman No.1 filter paper, centrifuged at 10,000×g for 10 min at 4 °C and supernatant transferred in fresh tubes for further study. Protein concentration in all the enzyme preparations was determined by the method of Bradford (1976), while the activities of extracellular hydrolytic enzymes (cellulase, pectinase, amylase and protease) were estimated according to previous studies (Miller 1959; Barnett and Fergus 1971; Dubey et al. 2010; Sarao et al. 2010).
Assay of antioxidant enzymes
Superoxide dismutase (SOD) activity was measured based on the inhibition of nitroblue tetrazolium chloride (NBT) reduction by O ·−2 under light. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of the rate of NBT reduction measured at 560 nm (Beauchamp and Fridovich 1971).
Catalase (CAT) activity was assayed by the method of Beers and Sizer (1952). About 200 mg of 10 days old mycelia were homogenized in 5 ml of 50 mM Tris–HCl buffer (pH 8.0) (0.5 mM EDTA, 0.5 % (v/v), Triton X-100 and 2 % (w/v) polyvinyl pyrrolidone using chilled mortar and pestle. The homogenates were centrifuged at 12,000×g for 10 min at 4 °C; supernatant was transferred in fresh tubes. The CAT activity was measured at 240 nm by decomposition of H2O2 (extinction coefficient of 0.036 mM−1 cm−1) by observing decrease in absorbance using a double beam UV–VIS spectrophotometer (ELICO-SL 191). Enzyme specific activity is expressed as µmol H2O2 oxidized mg−1protein min−1.
Histochemical study for O ·−2 and H2O2 in B. sorokiniana exposed to Tricyclazole
NBT was used as a dye to localize superoxide anion (O ·−2 ) according to method of Frahry and Schopfer (2001). About 100 mg, 10 days old mycelia were scraped by sterilized blade and put in test tube. One ml of 6 mM NBT solution (prepared in 10 mM Na-Citrate buffer (pH 6.0) was added in tube and exposed under light for 8 h., washed thrice in PBS buffer (pH 7.2). Stains react with O ·−2 and formed blue coloured insoluble formazan deposits which were visualized under light microscope (model Nikon Eclipse E200MV R, Nikon Instruments Inc.) using a combination of eye piece and objective (12.5 × 25).
Hydrogen peroxide’s localization (H2O2) in TCZ treated cultures was performed using 3, 3-diaminobenzidine (DAB; Amresco, Solon, OH, USA) according to Kumar et al. (2001), Thordal-Christensen et al. (1997). The mycelia were washed with distilled water and submerged in a solution containing 1 mg ml−1 DAB (dissolved in acidified water with HCl (pH 3.8) and incubated for 8 h to allow DAB uptake and its reaction with H2O2. The treated mycelia were further washed in saturated chloral hydrate solution. Visualization of processed samples under light microscope showed reddish brown color indicating the localization of H2O2.
Electron microscopy and ultrastructure of cell wall of B. sorokiniana
Melanin deposition and pores in cell wall were determined by transmission electron microscopy (TEM) (Money 1990; Brendan et al. 2003). The ultra structure for deposition of melanin contents in hyphae and conidial wall was investigated using 4-day-old TCZ (25 μg ml−1) treated cultures of black and white isolates from PDA over the controls. The hyphae were fixed in 2.5 % glutaraldehyde solution. Sample mounting and viewing was performed at AIRF JNU, the images were examined on a JEOL USA JEM-2100F Transmission Electron Microscope.
Effect of Tricyclazole on aggressiveness of B. sorokiniana on barley
The experiment was conducted in polyhouse under 80–95 % humidity at 27 ± 2 °C. 40 days old barley plants (cv. RD-2508) were inoculated by B. sorokiniana spore suspension (104 spores/ml) for the disease reactions and after specific time intervals (0, 24, 48 and 72 h.) inoculated plants were treated by spraying of TCZ (25, 50 and 100 μg ml−1). The control plant sets were also maintained by sterile water treatments. Initial data was collected 72 h. after inoculation and then subsequently three readings were taken after every 4 days. Percent disease severities were calculated according to Saari and Prescott (1975). The AUDPC was calculated using percent disease severity value corresponding to the disease ratings according to Shaner and Finney (1977).
where Yi = disease level at time ti, (ti + 1)−ti = time (days) between two lesion scores, n = no of observations (score).
10 days after inoculation, the infected leaves were taken and lesions were excised (1 cm2) from five leaves randomly selected and used for: (a) visualization of spore positions on the infected portions directly placing under light microscope and (b) numbers of spores/leaf by dislodging the spore from infected leaves pieces in 1 ml of water and visualized under light microscope. The infection process and spore germination of B. sorokiniana on barley leaves from above treatments were determined by histopathological studies according to Sillero and Rubiales (2002). The suitable photographs were taken under a combination of eye piece and objective (12.5 × 25) by the Nikon Eclipse E200MV R microscope (Nikon Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis was carried out using one-way ANOVA for determination of significant differences (separately for radial growth, mycelia weight, melanin, spore production, number of septa and spore size) by statistical analysis software (SAS) using PROC GLM and PROC CORR (version 9.2; SAS Institute Inc., Cary, NC 2010). All the experiments were carried out in triplicate. P values ≤0.05 were considered as statistically significant.
Results
Effect of Tricyclazole on B. sorokiniana
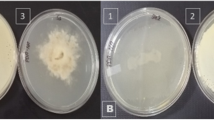
TCZ treated black and mixed isolates turned reddish brown or whitish with increasing concentration of TCZ in the growth media as compared to control (Fig. 1). The significant variation among black, mixed and white isolates of B. sorokiniana were recorded for radial growth, mycelia weight, melanin content, antioxidant enzymes (SOD and CAT) and extracellular hydrolytic enzymes (cellulase, pectinase, amylase and protease) under the TCZ exposure (5–100 µg ml−1). The mean melanin content (0.58 µg/g), SOD (12.29 U mg−1 protein) and CAT (21.59 µmol H2O2 oxidized mg−1 protein min−1) were significantly (P ≥ 0.05) higher in the black isolate followed by mixed and white. However, the mean radial growth (55.33 mm), mycelia weight (71.72 mg), cellulase (15.17 U mg−1 proteins), pectinase (10.98 U mg−1 proteins), amylase (7.20 U mg−1 proteins) and protease (3.49 U mg−1 protein) were significantly (P ≥ 0.05) higher in the white isolate followed by mixed and black (Table 1).
The effect of increasing concentrations of Tricyclazole (5–100 µg ml−1) on colony morphology (a: upper plates) and conidia (b: lower plates) of black isolate (WPB-23). The microscopic view b indicates reduced conidia size with increasing Tricyclazole levels as compared to controls (scale bar 100 µm)
In black isolate the significant growth reduction was recorded at 100 µg ml−1 TCZ, in mixed isolate it was recorded at 25–100 µg ml−1 TCZ, however in white isolate the growth reduction was noted at 10–100 µg ml−1 TCZ compare to controls (Table 2). Similarly, the significant reduction in mycelia weight was recorded when TCZ was applied in black and mixed isolates, but in white isolate a non-significant increase in mycelia weight was noted (Table 2). Black and mixed isolates produced melanin in the culture media and were drastically affected by the application of TCZ. The melanin content was affected on exposure of increasing concentrations of TCZ (5–100 µg ml−1) by 2.04–45.00 folds in back and 2.19–11.25 folds in mixed isolate (Table 2).
Effect of Tricyclazole on antioxidant enzyme activity and release of extracellular hydrolytic enzymes
A greater impact of TCZ on antioxidant enzymes (SOD and CAT) were recorded in black, mixed and white isolates. The inhibition in activity of SOD was recorded to be 1.07–3.97 folds in black, 1.08–2.64 folds in mixed and 1.04–1.35 folds in white isolate upon TCZ exposure (5–100 μg ml−1) in the culture media. Similarly, the activity of CAT also declined significantly in 10–100 μg ml−1 TCZ treatments; however a 5 μg ml−1 TCZ treatment led to a non-significant reduction in black, mixed and white isolate (Table 2).
The exposure of TCZ (5–100 μg ml−1) in culture media significantly enhanced the production of cellulase in black (2.72–6.50 folds) and mixed (1.59–1.55 folds) isolates over the controls, however in white isolate the cellulase production declined by 1.35–1.55 folds and it may be due to the toxic effect of TCZ. An increase in pectinase activity was recorded in black isolate by 5.89–11.64 folds but, declined in mixed 1.01–1.52 folds and 1.21–1.75 folds in white isolate upon 5–100 µg ml−1 TCZ exposures. Similarly, the amylase was increased by 1.41–1.92 folds in black isolate. However, decline in mixed and white isolates, but a non-significant alteration was recorded in mixed isolate upon exposure of TCZ. The release of protease was also recorded increased in white isolate by 1.13–1.41 folds in black and 1.43–2.01 folds in mixed isolates; whereas in white isolate a significant decline was recorded by 1.14–1.40 folds (Table 3).
Table 4 shows the effect of TCZ on all of the 9 parameters on black, mixed and white isolates of B. sorokiniana. The activities of extracellular hydrolytic enzymes and mycelia weight were positive correlated with radial growth upon application of TCZ. However, melanin content and antioxidant enzymes (SOD and CAT) were negative correlated with radial growth.
Effect of Tricyclazole on formation of H2O2 and O ·−2 in black isolate of B. sorokiniana
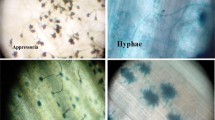
The microscopic studies was done for formation of H2O2 and O ·−2 in B. sorokiniana grown under 5–100 µg ml−1 TCZ-PDA media 7 day after inoculation (Fig. 2). The localization of O ·−2 was carried out using NBT that resulted in a dark blue formazan product (Fig. 2). TCZ treatments significantly increased the dark blue spots as compared to untreated wherein the stain for O ·−2 levels was much intense in B. sorokiniana exposed to 100 µg ml−1 TCZ. Formation of H2O2 in B. sorokiniana was detected as reddish brown stain resulting from reaction between DAB and H2O2. The 100 µg ml−1 TCZ treated B. sorokiniana had more H2O2 by the appearance of dense reddish-brown colour (Fig. 2). H2O2 was largely located in the conidia and hyphae, while O ·−2 was found primarily in the conidia (Fig. 2).
Localization of O ·−2 and H2O2 stained by NBT and DAB in hyphae and conidia in black isolate (WPB-23) of B. sorokiniana. The conidia size drastically reduced by the exposure of increasing concentrations (5–100 µg ml−1) of Tricyclazole, without staining hyphae and conidia serve as control for the formation of O ·−2 and H2O2 (scale bar 100 µm)
Electron microscopy for Tricyclazole treated isolates
Figure 3 shows the transmission electron micrograph for cell wall of B. sorokiniana. The TCZ treatments showed damage in cell wall and cell membrane of the B. sorokiniana isolates, the thick, deeply corrugated cell wall contained two distinct layers in non treated black isolate (Fig. 3a). However in white isolate these layers were not clear and showed pores in cell wall. These pores may be helpful to release of the extracellular hydrolytic enzymes (Fig. 3b). In TCZ (25 μg ml−1) treated cultures the cell wall and cell membrane integrity were affected and damaged (Fig. 3c, 4d).
Transmission electron micrograph of longitudinal section showing instance zones of melanin content with smooth surface in black isolate (a) and pores in diffused membrane visualized in white isolate (b), the cell wall and cell membrane damage in black (c) and white (d) isolate of B. sorokiniana under Tricyclazole (25 μg ml−1) exposure (scale bar 100 nm)
The white isolate hyphae are melanin deficient on outermost cell wall, while black isolate hyphae and conidia contained melanin contents. The melanin pigment decreased in TCZ (100 µg ml−1) treated hyphae and conidia (Fig. 3). Variations were found in the pore size of black and white isolate and varied from 4 to 50 nm in white isolate, while pore sizes were relatively smaller and less in number in black isolate. No effect was recorded in pore size in the cell wall of white isolate after the TCZ treatment. Although, the melanin erosion from cell wall and cell membrane in B. sorokiniana isolates were also visualized (Fig. 3).
Effect of Tricyclazole on aggressiveness of B. sorokiniana
Tricyclazole influenced the disease development in term of AUDPC, lesions developments/leaf and spore formation/leaf at different inoculation hours in black (WPB23), mixed (WPM29) and white (WPR4 (87)) isolates of barley. A significant reduction was noted in AUDPC, lesion formation/development and per leaf spore production under TCZ applications (25–100 µg ml−1) in comparison to controls. Table 5 shows that the significant difference in AUDPC value with respect to LSD (5 %) for the fungicide dose signified that 100 µg ml−1 (AUDPC value = 106.22) was most effective after spore inoculation on host at 0 h. as compared to 24, 48 and 72 h. The AUDPC was maximum for white isolate and minimum for black isolate while, mixed isolate scored medium AUDPC. A significant difference in the AUDPC by a particular isolate for different fungicide dose at a constant time of inoculation after spraying. There appeared to be a non significant interaction between isolate × fungicide doses (Table 5). Similar observations were also made for lesion development and number of spores formed per leaf. The isolates, isolates × inoculation hours and fungicide dose nonsignificantly affected the lesion development. The inoculation hours isolates × fungicide dose and isolates × inoculation hours × fungicide dose depicted the significant effects lesion numbers after TCZ spraying on barley plants (Table 5). The isolates, inoculation hours and isolates × inoculation hours are significantly affected by TCZ. However, the fungicide dose, inoculation hours and isolates × inoculation hours × fungicide dose depicted nonsignificant effect on sporulation after TCZ treatment on barley plants (Table 5). The TCZ inhibited the spore germination, growth and their stability on the infected portion of the host surface (Fig. 4).
Discussion
B. sorokiniana synthesizes 1,8- Dihydroxynaphthalene (DHN)-melanin via pentaketide pathway (Bell and Wheeler 1986) and melanin has been reported to promote the development of aerial mycelia and conidia of the fungus (Butler and Day 1998; Frederick et al. 1999; Henson et al. 1999). Similarly, a melanin deficient mutant of A. alternata had smaller conidia and reduced number of septa/conidia (Kawamura et al. 1999). Bashyal et al. (2010) reported the role of melanin in differentiation of secondary hyphae, coniodiophores and conidia. The lower dose of TCZ had non-significant effect on spore germination, but the higher dose became toxic to fungus (Chattopadhyaya et al. 2013). Eliahu et al. (2007) reported that C. heterostrophus mutant produces contrast albino conidia over the melanized wild type C. heterostrophus. The increasing doses of TCZ, reduced the melanin content, sporulation and number of septa/conidia over the control (Bashyal et al. 2010; Chattopadhyaya et al. 2013).
In this study the activity of antioxidant enzymes and damage in ROS system was recorded by the application of TCZ. Results of present study indicate dual role of TCZ, first on antioxidant enzymes and second on melanin content in B. sorokiniana. According to the earlier reports melanin has the property to bind with the drugs and anti-fungal compounds and reduces the diffusion of toxic chemical inside the hyphae (Eisenman et al. 2005; Nosanchuk and Casadevall 2006). Thus, ROS production in the TCZ treated isolates reduced to the antioxidant activities and melanin content in B. sorokiniana. The earlier reports shows that the melanin inhibitors based fungicides i.e. TCZ, Pthalide and Probenazole are nontoxic to fungi and stimulate O ·−2 production (Aver’Yanov et al. 1997). Melanin in fungal cell resists ROS and also plays an important role in disease development (Jacobson et al. 1995; Jacobson 2000). In A. nidulans the H2O2 is considered to be one of the most important metabolites in all respiring cells and provoked gene transcription (Pocsi et al. 2005), as well as sclerotial differentiation in Sclerotium rolfsii (Sidery and Georgiou 2000), increased expression of genes of carotenogenesis in N. crassa (Iigusa et al. 2005), and promoted transition of filamentous growth and development of its pathogenicity in U. maydis (Leuthner et al. 2005). Melanin in the conidial wall restricted the influx of water and solutes from the external environment, thus reducing spore germination (Chand et al. 2002). Contrary to this, our result suggests that TCZ leads to damage the melanin deposition in cell wall and facilitates the release of extracellular hydrolytic enzymes (Chand et al. 2014).
TCZ minimizes the pathogenesis in fungi and triggers a series of deleterious processes, including lipid peroxidation, degradation of proteins and DNA damage in the cell (Scandalios 1993). The over production of ROS as observed herein causes oxidative damage as also evident from electron microscopic studies of membrane, release of extracellular hydrolytic enzymes, etc. SOD and CAT play an important role in the defence of aerobic organisms against oxidative stress by converting ROS into nontoxic molecules (Tosi et al. 2010; Apel and Hirt 2004). Among these enzymes, SOD plays an important role in scavenging of O ·−2 by catalyzing the dismutation of two molecules of O ·−2 into O2 and H2O2 and serve as first line of defence against toxic O ·−2 (Wang et al. 2005). We found that increasing concentrations of TCZ (>10 µg ml−1) declined the activity of SOD and CAT. Changes in the activity of SOD are used as an indicator of changes in O ·−2 production (Wang et al. 2005). The significant difference in the lesion development was reported by the application of TCZ in black, mixed and white isolate Chattopadhyaya et al. 2013). White isolate is more aggressive in causing infection than black isolate (Chand et al. 2014). Results of this work also indicate that the application of TCZ on white and black isolate resulted in similar infection.
In a recent report it was found that the melanin deficient white isolate of B. sorokiniana produce much more extracellular hydrolytic enzymes and play an important role in pathogenecity than melanized black and mixed isolates (Chand et al. 2014). Another study on A. alternata also supported that melanin is not essential for the pathogenicity, because melanin deficient mutants have the same capacity to produce necrotic lesions on host tissue as the parent wild-type strain (Kawamura et al. 1999; Tanabe et al. 1995). However, the pigment does protect conidia from UV radiation and is likely to promote survival of the pathogen in field conditions (Kawamura et al. 1999). In the present study TCZ treated isolates also produce more extracellular hydrolytic enzymes (cellulose, pectinase, amylase and protease) in black isolate. The increased production of cellulose and protease was recorded under increasing concentrations of TCZ treated mixed isolate. However, the decline production of extracellular hydrolytic enzymes (cellulose, pectinase, amylase and protease) was noted in white isolate under increasing concentrations of TCZ exposure. The reduced aggressiveness in black isolate on barley leaf tissues was perhaps due to more damage caused due to reduced antioxidative enzyme activity with increased concentrations of TCZ.
Chattopadhyaya et al. 2013 reported a significant reduction in disease development in term of area under lesion progress curve at different fungicide dose for black, mix and white isolates suggest that varying degree of aggressiveness. Similarly the AUDPC is reduces by the application of TCZ. This indicated that TCZ affect the disease progress and colonization in pathogen on plant tissue. Kunova et al. (2013) reported that the efficacy of TCZ towards inhibition of sporulation and secondary infection indicates an additional possible mode of action of this fungicide that is different from inhibition of melanin biosynthesis. Herein, TCZ behave as fungicidal molecule in plant system by restricting pathogen growth. The previous report suggests that the TCZ deposited on the host leaves by their foliar applications and provides the defence to the host against pathogen (Ishiguro et al. 1992, Kunova et al. 2013).
Earlier studies showed that the melanised cells arrested cell growth and provide resistance to the fungi against lysis and protect the pathogens against environmental, chemical and physical stress (Bloomfield and Alexander 1967). TCZ damage to the infection behaviour and pathogenicity of A. alternata and also reported that the electron dense materials (melanin) accumulated in the spore of cell wall of parent spore but not in albino (Tanabe et al. 1995). We also report that TCZ damages to the key enzymes of melanin biosynthesis pathway in B. sorokiniana (Chand et al. 2014).
Although the application of TCZ has been studied for many years, most of the studies have been reported by the foliar application of TCZ on disease control in rice and other cereal crops. This study is one of the pioneer studies wherein TCZ has been applied to culture media to study its direct toxic effects on B. sorokiniana. From the results it can be concluded that TCZ influences the pathogenic ability of B. sorokiniana by damaging the cell structure of hyphae and conidia and reducing the melanin content concurrent with the altered antioxidant enzyme activities and can therefore be employed for management of the disease in barley plants.
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Aver’yanov AA, Pasechnik TD, Lapikova VP, Gaivoronskaya LM (2001) Fungitoxic responses of rice callus culture as an expression of inheritable resistance to blast. Implication of active oxygen. Plant Physiol Biocehm 39:415–424
Barnett EA, Fergus CL (1971) The relation of extracellular amylase, mycelium and time in some thermophilic and mesophilic Humicola species. Mycopathol Mycol Appl 44:131–141
Bashyal BM, Aggarwal R (2013) Molecular identification of Fusarium species associated with bakanae disease of rice (Oryza sativa) in India. Indian J Agric Sci 83:71–76
Bashyal BM, Chand R, Kushwaha C, Sen D, Prasad LC, Joshi AK (2010) Association of melanin content with coniodiogenesis in Bipolaris sorokiniana of barley (Hordeum vulgare L.). World J Microbiol Biotechnol 26:309–316
Beauchamp CO, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44:176–287
Beers RF, Sizer IW (1952) Colorimetric method for estimation of catalase. J Biol Chem 195:133–139
Bell AA, Wheeler MH (1986) Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol 24:411–451
Bloomfield BJ, Alexander M (1967) Melanins and resistance to fungi to lysis. J Bacteriol 93:1276–1280
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brendan J, Foran, Kastenmeier B, Bright DS (2003) Determination of pore-size distributions in low-k dielectric films by transmission electron microscopy. CP683, characterization and metrology for VLSI technology: international conference
Butler MJ, Day AW (1998) Fungal melanins: a review. Can J Microbiol 44:1115–1136
Chand R, Singh HV, Joshi AK, Duveiller E (2002) Physiological and morphological aspects of Bipolaris sorokiniana on wheat straw. Plant Pathol J 18:328–332
Chand R, Pandey SP, Singh HV, Kumar S, Joshi AK (2003) Variability and its probable cause in natural populations of spot blotch pathogen (Bipolaris sorokiniana) of wheat (T. aestivum L.) in India. J Plant Disease Prod 110:27–35
Chand R, Yadav OP, Bashyal BM, Prasad LC, Joshi AK (2013) Technique for the maintenance of heterokaryotic isolates of Bipolaris sorokiniana. Indian Phytopathol 66:61–65
Chand R, Kumar M, Kushwaha C, Shah K, Joshi AK (2014) Role of melanin in release of extracellular enzymes and selection of aggressive isolates of Bipolaris sorokiniana in Barley. Curr Microbiol 69:202–211
Chattopadhyaya A, Kushwaha C, Chand R, Srivastava JS (2013) Differential mode of action of tricyclazole in vitro and in planta on Bipolaris sorokiniana causing spot blotch in barley. Indian Phytopathol 66:155–158
Chaube HS, Pundhir VS (2005) Crop disease and their management (Google e-book). PHI Learning Pvt. Ltd, Mumbai
Chaurasia S, Joshi AK, Dhari R, Chand R (1999) Resistance to foliar blight of wheat: a search. Genet Res Crop Evol 46:469–475
Cheng Q, Kinney KA, Whitman CP, Szaniszlo PJ (2004) Characterization of two polyketide synthase genes in Exophiala lecanii-corni, a melanized fungus with bioremediation potential. Bioorg Chem 32:92–108
Chida T, Sisler HD (1987) Effect of inhibitors of melanin biosynthesis on appressorial penetration and reductive reactions in Pyricularia oryzae and Pyricularia grisea. Pestic Biochem Physiol 29:244–251
Chowdhury AK, Singh G, Tyagi BS, Ojha A, Dhar T, Bhattacharya PM (2013) Spot blotch disease of wheat- a new thrust area for sustaining productivity. J Wheat Res 5:1–11
Deising HB, Werner S, Wernitz M (2000) The role of fungal appressoria in plant infection. Microbes Infect 2:1631–1641
Dixon DM, Szaniszlo PJ, Polak A (1991) Dihydroxynaphthalene (DHN) melanin and its relationship with virulence in the early stages of phaeohyphomycosis. In: Cole GT, Hoch HC (eds) The fungal spore and disease initiation in plants and animals. Plenum Press, N.Y, pp 297–318
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Dubey R, Adhikary S, Kumar J, Sinha N (2010) Isolation, production, purification, assay and characterization of alkaline protease enzyme from Aspergillus niger and its compatibility with commercial detergents. Dev Microbiol Mol Biol 1:75–94
Duveiller E, Garcia I, Franco J, Toledo J, Crossa J, Lopez F (1998) Evaluating spot blotch resistance of wheat: Improving disease assessment under controlled condition and in the field. In: Duveiller E, Dubin HJ, Reeves J, McNab A (eds) Helminthosophism diseases of wheat: spot blotch and tan spot. CIMMYT, Mexico, pp 63–66
Eisenman HC, Nosanchuk JD, Webber JB, Emerson RJ, Camesano TA, Casadevall A (2005) Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 44:3683–3693
Eliahu N, Igbaria A, Rose MS, Horwitz BA, Lev S (2007) Melanin biosynthesis in the maize pathogen Cochliobolus heterostrophus depends on two mitogen-activated protein kinases, Chk1 and Mps1 and the transcription factor Cmr1. Eukaryot Cell 6:1–42
Elliott ML (1995) Effect of melanin biosynthesis inhibiting compounds on Gaeumannomyces species. Mycologia 87:370–374
Frahry G, Schopfer P (2001) NADPH stimulated, cyanide-resistant superoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta 212:175–183
Frederick BA, Caesar-TonThat TC, Wheeler M, Sheehan KB, Edens WA, Henson JM (1999) Isolation and characterization of Gaeumannomyces graminis var. graminis melanin mutants. Mycol Res 10:99–110
Gadd GM (1982) Effects of media composition and light on colony differentiation and melanin synthesis in Microdochium bolleyi. Trans Br Mycol Soc 78:115–122
Gomez BL, Nosanchuk JD, Diez S, Youngchim S, Aisen P, Cano LE, Restrepo A, Casadevall A, Hamilton A (2001) Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccicioides brasiliensis in vitro and during infection. Infect Immun 69:5760–5767
Henson JM, Butler MJ, Day AW (1999) The dark side of the mycelium: melanins of phytopathogenic fungi. Annu Rev Phytopathol 37:447–471
Huang HC (1981) Tan sclerotia of Sclerotinia sclerotiorum. Can J Plant Pathol 3:136
Huang HC, Kokko EG, Kozub GC, Saito I, Tajimi A (1993) Effect of tricyclazole and pyroquilon on cell wall melanization of sclerotia of Sclerotinia sclerotiorum and S. minor. Trans Mycol Soc Jpn 34:77
Iigusa H, Yoshida Y, Hasunuma K (2005) Oxygen and hydrogen peroxide enhance light-induced carotenoid synthesis in Neurospora crassa. FEBS Lett 579:4012–4016
Inoue S, Kato T, Jordan VWL, Brent KJ (1987) Inhibition of appressorial adhesion of Pyricularia oryzae to barley leaves by fungicides. Pestic Sci 19:145–152
Ishiguro K, Takechi S, Hashimoto A (1992) Behavior of tricyclazole residue on rice leaves and its efficacy for rice leaf blast control in the field. Ann Phytopathol Soc Jpn 58:259–266
Jacobson ES (2000) Pathogenic roles for fungal melanins. Clin Microbiol Rev 13:708–717
Jacobson ES, Hove E, Emery HS (1995) Antioxidants function of melanin in black fungi. Infect Immun 63:4944–4945
Kawamura C, Tsujimoto T, Tsuge T (1999) Targeted disruption of a melanin biosynthesis gene affects conidial development and UV tolerance in the Japanese pear pathotype of Alternaria alternata. Mol Plant Microbe Interact 12:59–63
Kumar J, Hückelhoven R, Beckhove U, Nagarajan S, Kogel KH (2001) A compromised Mlo pathway affects the response of barley to the necrotrophic fungus Bipolaris sorokiniana (teleomorph: cochliobolus sativus). Phytopathology 91:127–133
Kunova A, Pizzatti C, Cortesi P (2013) Impact of tricyclazole and azoxystrobin on growth, sporulation and secondary infection of the rice blast fungus, Magnaporthe oryzae. Pest Manag Sci 69:278–284
Kurahashi Y (2001) Melanin biosynthesis inhibitors (MBIs) for control of rice blast. Pestic Outlook 12:32–35
Leuthner A, Ichinder C, Oechmen E, Koopmann E, Muller E, Kahmann R, Bolker M, Schreier PH (2005) A H2O2-producing glyoxal oxidase is required for filamentous growth and pathogenicity in Ustilago maydis. Mol Gen Genomics 272:639–650
Longo VD, Mitteldorf J, Skulachev VP (2005) Programmed and altruistic ageing. Nat Rev Genet 6:866–872
Mares D, Romagnoli C, Andreotti E, Manfrini M, Vicentini CB (2004) Synthesis and antifungal action of new tricyclazole analogues. J Agric Food Chem 52:2003–2009
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Money NP (1990) Measurement of pore size in the hyphal cell wall of Achlya bisexualis. Exp Mycol 14:234–242
Nosanchuk JD, Casadevall A (2006) Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother 50:3519–3528
Okuno T, Kitamura Y, Matsuura K (1983) Mechanism of inhibitory effect of tricyclazole on secondary infection by spores of Pyriculariaoryzae. J Pestic Sci 8:361–362
Pandey SP, Sharma S, Chand R, Shahi P, Joshi AK (2008) Clonal variability and its relevance in generation of new pathotypes in the spot blotch pathogen, Bipolaris sorokiniana. Curr Microbiol l56:33–41
Pocsi I, Miskei M, Karanyi Z, Emri T, Ayoubi P, Pusztahelyi T, Balla G, Prade RA (2005) Comparison of gene expression signatures of diamide, H2O2 and menadione exposed Aspergillus nidulans cultures—linking genome-wide transcriptional changes to cellular physiology. BMC Genomics 6:182
Poloni A, Pessi IS, Frazzon APG, Van-Der Sand ST (2009) Morphology, physiology and virulence of Bipolaris sorokiniana isolates. Curr Microbiol 59:267–273
Romero-Martinez R, Wheeler MH, Guerrero-Plata A, Rico G, Torres-Guerrero H (2000) Biosynthesis and functions of melanin in Sporothrix schenckii. Infect Immun 68:3696–3703
Saari EE (1998) Leaf blight diseases and associated soil borne fungal pathogens of wheat in north and south East Asia. In: Duveiller E, Dubin HJ, Reeves J, McNab A (eds) Helminthosophism diseases of wheat: spot blotch and tan spot. CIMMYT, Mexico, pp 37–51
Saari EE, Prescott JM (1975) A scales for appraising the foliar intensity of wheat diseases. Plant Dis Reptr 59:377–380
Sarao L, Arora M, Sehgal VK, Bhatia S (2010) Production of protease by submerged fermentation using Rhizopus microsporus var oligospous. Int J. Microbiol 9:1–10
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Schnitzler N, Peltrochela-Llacsahuanga H, Bestier N, Zundorf J, Lutticken R, Haase G (1999) Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst and killing by human neutrophils. Infect Immun 67:94–101
Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. Phytopathol 67:1051–1056
Sidery M, Georgiou ChD (2000) Differentiation and hydrogen peroxide production in Sclerotium rolfsii are induced by the oxidizing growth factors light and iron. Mycologia 92:1033–1042
Sillero JC, Rubiales D (2002) Histological characterization of resistance to Uromyces viciae-fabae in faba bean. Phytopathology 92:294–299
Skulachev VP (1996) Soros Edu J ISSEP 2(3):4–10
Tanabe K, Park P, Tsuge T, Kohmoto K, Nishimura S (1995) Characterization of the mutants of Alternaria alternata Japanese pear pathotype deficient in melanin production and their pathogenicity. Ann Phytopathol Soc Jpn 61:27–33
Thines E, Anke H, Weber RWS (2004) Fungal secondary metabolites as inhibitors of infection-related morphogenesis in phytopathogenic fungi. Mycol Res 108:14–25
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J 11:1187–1194
Tosi S, Kostadinova N, Krumova E, Pashova S, Dishliiska V, Spassova B, Vassilev S, Angelova M (2010) Antioxidant enzyme activity of Wlamentous fungi isolated from Livingston Island, Maritime Antarctica. Polar Biol 33:1227–1237
Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA (2005) Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol 162:465–472
Wheeler MH (1982) Melanin biosynthesis in Verticillium dahliae: dehydration and reduction reactions in cell-free homogenates. Exp Mycol 6:171–179
Woloshuk CP, Sisler HD, Vigil EL (1983) Action of the antipenetrant, tricyclazole, on appressoria of Pyricularia oryzae. Physiol Plant Pathol 22:245–259
Zenkov NK, Lankin VZ, Menshchikova EB (2001) Oxidation Stress. Biochemical and Patophysiological Aspects. MAIK, Moscow, p 343
Zhang CQ, Zhou MG (2004) Effect of tricyclazole on secondary infection by Magnaporthe grisea Barr. Chin J Pestic Sci 6:23–27
Acknowledgement
This work was financially supported by the Council of Scientific and Industrial Research, New Delhi, in the form of major research project. The authors are also thankful to Ms. Prerna Singh, Research scholar, Department of Biochemistry, Faculty of Science, Banaras Hindu University, Varanasi for language editing of the manuscript.
Conflict of interest
All the authors have contributed equally to this work and they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kumar, M., Chand, R., Dubey, R.S. et al. Effect of Tricyclazole on morphology, virulence and enzymatic alterations in pathogenic fungi Bipolaris sorokiniana for management of spot blotch disease in barley. World J Microbiol Biotechnol 31, 23–35 (2015). https://doi.org/10.1007/s11274-014-1756-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1756-3