Abstract
Vernal pools are small, shallow and ephemeral freshwater wetlands, often located in forests. Despite harboring high levels of biodiversity and being biochemical hotspots, they have been severely degraded by human activities. Restoration of vernal pools has become a priority for resource managers, and monitoring of restoration projects is needed to track the effectiveness of restoration techniques. In this study, we analyzed the ecological functioning of natural and restored vernal pools in the forest of Chinon (France) four years after a restoration project. Specific aims were to: (1) compare organic-matter decomposition rates between natural and restored pools, and (2) evaluate the main drivers of organic-matter decomposition in these two types of pool. In five natural and nine restored vernal pools, a standardized organic-matter decomposition assay (cotton strip) was deployed for up to 57 days. Each pool was characterised in terms of vegetation type, water and soil quality. We found that decomposition rates did not differ between natural and restored pools. The natural pools, however, had the greater stores of total organic carbon (TOC) content, and Sphagnum moss cover. Across both pool types, decomposition rates were positively associated with canopy openness and vascular plant cover, suggesting a possible ‘priming effect’ of primary producers on microbial decomposers. Sphagnum moss cover and some soil variables, such as TOC, were associated with slowed decomposition, highlighting the potential of Sphagnum spp. at influencing microbial activity. We suggest that future studies couple the cotton strip assay with biotic indices to assess the overall functioning of these wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide, approximately 60% of inland wetlands were lost due to human activities since the start of the twentieth century (Davidson 2014). Among these wetlands, vernal pools have been particularly impacted due to their ephemeral nature, an ecosystem trait that facilitates their destruction during annual low-water phases. Across the globe, projects for the conservation and restoration of vernal pool have been initiated but with uncertain results. Restoration success is most commonly evaluated using indices based on ecosystem structure (Matthews et al. 2009), whereas functional indicators are still relatively rarely used, representing less than 10% of the restoration monitoring programs between 2004 and 2013 (Kollmann et al. 2016). This is in contrast to the way in which ecologists perceive vernal pools—as ecosystems with structure and function—and the focus on ecosystem structure in many ways neglects the valuable ecosystem services that vernal pools provide (e.g. nutrient transformation, carbon sequestration).
Organic-matter (OM) decomposition can be used as a proxy of ecosystem biogeochemical functioning (Neher et al. 2003; Fennessy et al. 2008; Young et al. 2008) because this process integrates the biological activity of mostly invertebrates and microorganisms and the environmental variables that constrain it. Decomposition rates covary with the chemical quality of the OM, and since plant species vary widely in their quality, the composition of vegetation communities can influence overall OM decomposition rates in an ecosystem (Webster and Benfield 1986; Bot and Benites 2005; Kayranli et al. 2010). Sphagnum spp. are known as ecosystem engineer species, because they can reduce the pH of their environment and increase moisture and anoxia (Rydin et al. 2006). These species also produce phenolic compounds as secondary metabolites which can reduce hydrolase enzyme activity (Freeman et al. 2004; Rydin et al. 2006; Chiapusio et al. 2013). Combined with their common low degradability, they could make decomposition rates decrease (van Breemen 1995). In addition to leaf-litter quality, OM decomposition is also influenced by temperature (Tiegs et al. 2019), nutrient (Colas et al. 2019) and oxygen availability (Webster and Benfield 1986; Graça et al. 2015), high nutrient content (Woodward et al. 2012), type and size of decomposer populations including invertebrates and microorganisms (Webster and Benfield 1986; Graça et al. 2015), and water current and wave action (Pabst et al. 2008). Several methods can be used to quantify OM decomposition rates with the most common being litter bag assays, which allow researchers to measure OM mass loss through time (Bärlocher 2005), and cotton strip (CS) assays (Tiegs et al. 2007, 2013), that are based on the loss of tensile loss of cotton fabric, a process that reflects the microbial catabolism of cellulose. Cotton-strips are composed of 95% cellulose, which is the most abundant organic polymer on earth and the main constituent of plant litter. Thus, CS are in many ways an ecologically relevant form of organic matter that can be used in decomposition experiments (Tiegs et al. 2019). They do not necessarially reflect the decomposition rate of the locally availably OM, but rather, provide a highly standardized and reproducible means of comparing the decomposition potential of ecosystems (InfoClimat 2017; Météo-France 2017). Additional advantages include their ease of use, their cost effectiveness, and the fact that the incubation periods required are generally less than those of leaf litter (Tiegs et al. 2007, 2013; Slocum et al. 2009). Originally used in soil studies (Trettin et al. 1996; Mendelssohn et al. 1999), this method is increasingly used in stream ecology (Tiegs et al. 2019; Burdon et al. 2020) and more recently in freshwater ponds, and to assess above-ground decomposition rates (Tiegs et al. 2007; Vizza et al. 2017).
Vernal pools are small, temporary freshwater wetlands, often found in forests. In the temperate and boreal climates of Europe and North America, their hydroperiods generally vary from three to eleven months, even more than one year in some cases (Colburn 2004), and their wet-and-dry cycle is subject to multi-annual patterns. Vernal pools may be filled in different proportions by snowmelt, groundwater, direct precipitation, surface runoff, endorheic streamflow or floodwater from temporary connectivity to close surface-water bodies (Colburn 2004). These ecosystems are known for their high level of biodiversity at local and landscape scales, adding aquatic habitats to the upland matrix, and providing feeding, breeding and resting places for many species (Calhoun et al. 2017). However, very few studies have focused on the ecological functioning of vernal pools, especially on OM decomposition. Capps et al. (2014) showed that inundation increased above-ground decomposition rates in vernal pools, supporting similar studies on other types of freshwater wetlands (Neckles and Neill 1994; Kelley and Jack 2002; Battle and Golladay 2007). A microcosm study demonstrated how decomposition rates were influenced by the inundation period and by the presence of Molinia grasses and Sphagnum spp. (Hervé et al. 2018). In addition, Holgerson and Raymond (2016) highlighted their important biogeochemical activity by quantifying CO2 and CH4 emissions.
Despite their importance for biodiversity and materials cycling, vernal pools are threatened ecosystems and they are often overlooked because of their small size, as shown by Burne and Griffin (2005) for vernal pools in the USA. They are vulnerable to land use changes, and impacted by runoff from forest plantations, urbanization or agriculture, and to climate change, all of which lead to modifications of their hydrology (Williams 2005; Calhoun et al. 2017). As with other wetlands, the evaluation of restoration success in vernal pools rarely uses functional indicators and research has yet to be conducted that compares OM decomposition rates between natural and restored vernal pools.
This study was carried out in natural and newly restored (i.e. four years prior to the study) vernal pools located in a temperate forest in Chinon (France). Our objectives were to (1) compare the above-ground OM decomposition rates between natural restored vernal pools, and (2) identify environmental variables that explain variation in OM decomposition rates in these two pool types. The study was based on the following hypotheses: (i) four years after the restoration project, the OM decomposition rates in the natural vernal pools are slower than in restored vernal pools due to the presence of Sphagnum spp., (ii) Sphagnum spp. and vascular plant cover is greater in natural pools, and some soil characteristics, such as OM content, differ between the two types of pools and (iii) Sphagnum spp. are a main driver of OM decomposition. The overarching goal of our study was to evaluate the effects of vernal pool restoration, and help identify ecological indicators that are sensitive to this increasingly common means of resource management.
Methods
Study area
The study was carried out in the spring–summer of 2016, in the Chinon forest, in France (47°12′53.3″N, 0°19′45.3″E). This area has a temperate oceanic climate, with mean annual temperature of 11.8 °C and mean annual precipitations of 695 mm (1981–2010) (Météo-France). Temperature and precipitation data from a meteorological station situated at 50 km North-East from the forest were recorded (InfoClimat) (Online Resource 1). Principal soils in the study site are haplic albeluvisols [according to WRB 2006 classification from IUSS Working Group WRB (2006)], acidic and hydromorphic (Boutin 1989, 1996). Their thickness varies in mean from 0.4 to 3.0 m. They are mainly formed by Quaternary aeolian deposits (sands and loam) covering Eocene clays with siliceous conglomerates (Alcaydé and Rasplus 1971; Alcaydé 1975).
The state-owned part of the forest spreads over 5141 ha (Office national des forêts and Jammes 2003) and is currently managed for forestry with stands dominated by Quercus petraea Liebl. (1784), Pinus sylvestris L. (1753) or Pinus pinaster Aiton (1789), and mixed stands of these species. Vernal pools on the study site were generally dry for 2–6 months in summer/autumn. They are mainly filled by precipitation during winter and spring and some are connected to perched water tables. The water was nutrient-poor (water conductivity ranged from 45 to 110 µS.cm−1 in spring/summer 2016) and acidic, with pH ranging from 4 to 5.5.
Restoration of vernal pools in the Chinon forest
Pollen analyses suggest that the formation of the Chinon depressions, which evolved into vernal pools, date back to 26,000 BP; although their origin is still uncertain, the hypothesis of a natural periglacial formation prevails (Couderc 1980). Before the 1970s, many wetlands, including vernal pools, were present in the forest of the study site but most of them were severely degraded or destroyed by industrialization of forest plantations. The vernal pools that remain are typically vegetated with different species of Sphagnum (subgenus cuspidata, subgenus subsecunda, S. palustre) that form dense carpets and hummocks. Typical vegetation of inundated oligotrophic pools can also be found.
We surveyed the study site in 2011 and 2012 and identified degraded pools, mostly filled, drained and planted, as well as undisturbed or lightly disturbed pools, here called “natural vernal pools”. In September 2012, a restoration project was initiated that had the goal of restoring 32 pools. The restoration works consisted of digging, cutting trees, scraping of purple-moor grass [Molinia caerulea (L.) Moench] and blocking drain ditches. Depending on the topography, the size of the original pools and their riparian zones, pools of different sizes and depths (on average 200 m2 to 1000 m2, and 80 cm to 2 m deep) were restored (Online resource 2). Pool depth was also a function of the depth of the impermeable clay layer underneath the pools, which was not disturbed in order to better restore nutrient-poor, acidic vernal-pool waters.
Experimental design
Since the objectives of this study were to measure OM decomposition under inundated conditions and to investigate the effects of restoration, we selected the pools that we believe had similar hydroperiods and morphology (mean max depth of 61 cm). Among these pools, we choose pools with and without Sphagnum spp., and under different forest stands (Pinus spp. and Quercus spp.). As a result, 14 out of 50 pools present in the forest met the conditions and were chosen for the study: five natural and nine restored vernal pools.
Organic-matter decomposition rates
The experiment was carried out under in situ conditions, in the Chinon forest. OM decomposition was measured using a cotton strip (CS) assay and following the protocols detailed in Tiegs et al. (2013, 2019). Since an appropriate incubation time for CS had yet been determined for vernal pools, we used three exposure times to calibrate the measurements: at Time1, CS were incubated between 22 and 24 days, at Time2, they were incubated between 39 and 42 days, and at Time3 they were incubated between 51 and 57 days. The longest period of incubation was determined by the level of the water table of the pool; when the water depth was less than 5 cm, the CS were removed. For each incubation time in each vernal pool, four CS replicates were installed at a position a few centimetres above the sediment surface, at the deepest part of the pool near its geographic center. At the end of each incubation time, the CS were removed, immediately hand-washed with 70% ethanol to stop microbial activity and placed in aluminium envelopes. In the lab, they were dried at 40 °C for at least 30 h and placed in a desiccator before measuring the tensile strength remaining with a tensiometer (Mark-10 brand, Model #MG100, Copiague, NY, USA) at the Oakland University Aquatic Ecology Lab (Tiegs et al. 2013). Reference strips, i.e. strips not incubated in the field but washed with ethanol and dried, were used to determine the initial tensile strength. Then, we calculated the percentage of tensile strength loss (%TSL, Eq. 1) and the percentage of tensile strength loss per day (%TSLd), dividing the %TSL by the incubation time expressed in days. These coefficients represent the decomposition rate of the cotton strips.
Tensile Strength reference strips was the mean tensile strength of twenty reference strips and Tensile Strength treatment strips the remaining tensile strength in CS after incubation.
Calibration procedure for CS decomposition
In some instances the CS strips that were incubated in the ponds had slightly greater tensile-strength relative to the reference strips, an outcome we addributed to the strips being insufficiently decomposed, and to slight variation among individual cotton strips. In these instances, the value of the measured tensile-strength was replaced by a “NA” (not available) value. “NA” values were not used in statistical analyses. All pools used in analyses had a minimum of two TSL values for each sampling date.
Environmental variables
We assessed which environmental variables influence decomposition rates by comparing natural and restored pools. These variables related to vegetation, water, and soil (Table 1). Water variables included electrical conductivity, concentration of dissolved organic carbon, pH and RedOx potential, water temperature and water level. Vegetation variables included tree canopy openness above the pool, the species composition of the surrounding forest with the cover of Quercus spp., and the cover of vascular plants and Sphagnum spp. in the pool. The tree canopy openness was estimated by measuring the light intensity (in lux) at the pool surface in comparison with fully open area close to the forest, i.e. where the light intensity received by a light sensor was not intercepted by tree canopy (Eq. 2). High percentages indicated low canopy cover above the considered pools.
We estimated the tree canopy cover in a belt of 10 m around each pool, from the annual maximum water level of the pool 0–10 m in the direction of the surrounding forest. The percentages of relative cover of Quercus spp., Pinus spp. and other species were visually estimated in the belt. We retained only the percentage of Quercus spp. cover for the analyses, knowing that the percentage of other species was between 0 and 23% (estimated in the field). The percentage of tree cover was used because it reflects the type and quality of the OM entering each pool, light availability and consequently OM dynamics. Soil variables included soil granulometric composition (clay, silt, and sand), OM content, pH, total nitrogen (TN), total organic carbon (TOC) and available phophorus. The samples were taken on the upper layers of the pool centre, at 0–5 cm and 5–10 cm depth (Table 1, Online resource 3).
Statistical analyses
Calibration procedure for CS decomposition
Since no CS incubation time was defined for vernal pools in the literature, we used three different incubation times. The most reliable time was that with the fewest NA values (see Calibration procedure for CS decomposition). We first counted the number of NA values found at each time and for each pool. Then, we used a generalized linear model (Poisson family, package stats version 3.3.1), followed by a multi-comparison post-hoc test using a Tukey test (package multcomp for R), to assess if the number of NA values were different among incubation times. As the number of NA values differ among times, the incubation time with the fewest NA (all pools included) was chosen (see the sections methods and results for the calibration procedure for more details).
OM decomposition rates, restoration status and environmental variables
To assess the difference in term of %TSLd between restored and natural pools, a mixed-effects model was used with the pool identity treated as random effect and CS replicates were nested within pools (lme4 package for R). Assumptions of normality were confirmend by visually inspecting QQplots. Wilcoxon rank sum tests were performed (package stats version 3.3.1) to compare environmental variables between natural and restored pools (Table 1). To assess which environmental variables best explain variability of %TSLd, we first calculated Pearson’s r coefficients for each of the environmental variables (Table 1, function "rcorr" from Hmisc package on R software) after these values were centred around the mean (i.e. subtracting the variable means to each individual value) and scaled to their standard deviation (i.e. dividing each centred individual value by the mean standard deviation), with the function “scale” on R. Among the variables significantly correlated with each other (α = 0.05), we considered those with a strong correlation (|r|≥ 0.7) (Dormann et al. 2013); a correlogram was built to visualize the correlation matrix (functions "cor" and “corrplot” from corrplot package for R). From this list, ten variables were chosen that were not correlated with each other. A global mixed linear model was created on the basis of the centred and scaled data, with the %TSLd as response variable, all chosen variables as fixed factors and the pool identity as random factor in a nested design. The linear model assumptions were checked graphically and with a Shapiro test for normal data distribution. All of the model combinations were performed and the best models were selected on the basis of the AIC values using the function “dredge” from the R package MuMIn. The selected models had a delta AIC inferior to 2.
Statistical analyses were performed with R software version 3.3.1 (Rstudio version 0.99.903), and the figures with ggplot2 and corrplot packages for R, Microsoft Office Excel 2013 and Inkscape version 0.92.
Results
Calibration procedure for CS decomposition
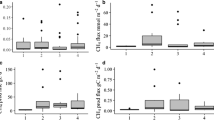
At Time1 (22–25 days of incubation) and Time2 (39–42 days), there were respectively 39% and 38% of the NA values, and only 13% at Time3 (51–57 days). The generalized linear model analysis confirmed that the percentage of NA values were significantly lower at Time3 than at Time1 and Time2 (p-values < 0.05, Fig. 1). For the analyses, we selected the pools with two NA values at the most (Fig. 2). Only one pool (VP31) never met this condition and was excluded from the data set. To have the most reliable results, the %TSL after incubation should be close to 50% (Hill et al. 1988; Slocum et al. 2009). The pools were closer to this value at Time3 (Fig. 2, the dotted line shows the 50% TSL), therefore, we decided to analyse only the % TSLd measured at Time3.
Boxplot of NA values in the tensile strength loss of the cotton strips over time, all hydrological and vegetation treatments included: Time1 was between 22 and 24 days of incubation, Time2 between 39 and 42 days, and Time3 between 51 and 57 days. Diamonds indicate mean values, bold horizontal lines indicate median values, whiskers extend from the 1st or 3rd quartile to upper or lower extreme data, and circles show outlying points. Capital letters indicate significant differences between the incubation times
Percentage of Tensile Strength Loss (%TSL) of the cotton strips incubated in natural (NAT) and restored (REST) vernal pools over time. Only the vernal pools with at the most two NA values (not available values) for the %TSL were included. Incubation times: Time1 (22–24 days), Time2 (39–42 days), and Time3 (51–57 days). The dotted line represents 50% TSL. Diamonds indicate mean values, bold horizontal lines indicate median values, whiskers extend from the 1st or 3rd quartile to upper or lower extreme data, and circles show outlying points. Box are arranged in the same order as the labelling below the plot and each colour correspond to a vernal pool
Organic-matter decomposition rates and influence of environmental variables
Differences between natural and restored vernal pools
After 51 to 57 days of incubation (Time3), the rate of OM decomposition (%TSLd) was not significantly different between natural and restored pools (F-value: 0.128, p-value: 0.72). The median value was 0.57% TSLd and the mean value was 0.72% TSLd, with a maximum value of 1.43% (%TSLd) and a minimum value of 0.19%.
Several environmental variables differed between natural and restored pools (Table 2, Online Resource 3). For the water variables, only electrical conductivity was diffent between pool types and it was greater in natural pools (76 ± 12 µS.cm−1 versus 62 ± 4 µS.cm−1). Tree species composition of the forest, measured as the cover of Quercus spp., and tree-canopy openness, did not differ between natural and restored pools. The cover of Sphagnum spp. and vascular plants in the pools were greater in the natural pools (respectively 53% ± 34% and 50% ± 34% versus 4.8% ± 6% and 28% ± 19% in the restored pools). For the soil variables, TOC (0–5 cm and 5–10 cm), TN (0–5 cm) and OM content (5–10 cm) were greater in the natural pools. Furthermore, in both natural and restored pools, granulometric analyses showed that mean clay contents were 37% (some pools achieved 70%), mean sand contents were 30 to 45% and mean silt contents were 17 to 35%. Both pool types showed low pH values (Online Resource 3).
Correlations between environmental variables
Before assessing the variables that explained the variability of the %TSLd and which differenciated natural from restored vernal pools, we evaluated correlation between them. Several variables were strongly correlated, with |r coefficient|≥ 0.7 such as water temperatures and water levels, soil TOC from 0-5 cm and soil TOC from 5–10 cm, and cover of vascular plants and tree canopy openness (Fig. 3, Table 3). Variables that were either uncorrelated or weakly correlated (i.e. significant correlation with a p-value < 0.05 and Pearson’s r coefficient <|0.70|) were selected to be used in the Wilcoxon rank sum tests and the global mixed linear model. Among all variables, only one variable by correlated variable group was chosen to be use in the models. Among the water variables, water levels, pH, redox potential and dissolved organic carbon were selected; among the vegetation variables, the tree canopy openness and the cover of Quercus spp. were selected; and among the soil variables, the TOC of the layer 0–5 cm, the pH of the layers 0–5 cm and 5–10 cm, and the TN of the layer 5–10 cm were selected.
Correlogram of the environmental variables. Significant correlations are shown with a filled circle (α = 0.05). The size of the circles and the colour intensity are proportional to the r coefficients: high coefficient are bigger than the low coefficients (absolute values); positive correlations are drawn in black whereas the negative correlations are in grey
Environmental variables influencing CS decomposition rates
To create the linear models explaining the %TSLd, the ten variables presented in the result section "Correlation between environmental variables" were used. The model selection procedures identified six models which best explained the variability of %TSLd (Table 4) among all of the model combinations performed. The tree canopy openness (VEG_canopy) and the content of soil total organic carbon in the layer 0–5 cm (SOIL_TOC) had a significant influence on %TSLd (α = 0.05) in all the six models. Water levels (WATER_levels) had also a significant effect on %TSLd but it appeared only in one model (model 6). The tree canopy openness and the water levels were positively linked to the %TSLd with mean estimates calculated from the six best models of + 0.72 and + 0.51, respectively. The soil TOC was negatively linked to the %TSLd, with a mean estimate of − 0.62. The other environmental variables that did not appear as significant parameters in the six best models did not significantly influence the %TSLd.
Discussion
OM decomposition rates: natural vs. restored vernal pools
Cotton-strips were used in this study as a proxy for natural OM. Whereas they do not possess the same composition and the same characteristics than natural litter, their decomposition follows the same patterns (Tiegs et al. 2007). We found no significant difference in OM decomposition rates between the natural and restored vernal pools in our study sites. Such similar values of % TSL between pool types means that the cellulolytic activity that we measured was similar in both pool types, but not necessarily that the global microbial activities involved in the decomposition processes were similar (Tiegs et al. 2007).
To our knowledge, our study is the first that used CS assay to measure OM decomposition rates in vernal pools. For this reason, we could not compare our results to similar studies. However, in a study using leaf litter Gingerich and Anderson (2011), studied seasonal and permanently flooded wetlands in West Virginia (floodplain, headwater and impoundment wetlands) but did not find differences in term of OM decomposition rates between natural and created wetlands. They used a standard litter (Typha latifolia L.), with the litter bag method during the experiment, instead of the in-situ litter, and they explained that it could be part of the reason why they did not observe differences. They measured a decomposition potential which reflected all the factors involved in this process but without considering the importance of litter quality. Like Gingerich and Anderson (2011), the decomposition rates of cotton-strips, which are not in-situ litter and not as complex as natural litter in term of composition, gave the decomposition potential in our studied vernal pools.
Which environmental variables differed between natural and restored vernal pools?
In the Chinon forest, most of the restored vernal pools were dug four years before this study, modifying the soil structure. Vegetation and tree clear-cutting were also performed. Natural pools were formed about 26,000 BP (Couderc 1980), leading to developed soils with OM and carbon accumulation. Considering that the upper soil strata of restored pools were formed for only four years, it is not surprising that soil parameters, especially the TOC, differed between natural and restored vernal pools. This result is consistent with previous studies such as Ballantine & Schneider (2009) and Marton et al. (2014).
Vegetation cover (i.e. vascular plants and Sphagnum spp.) was greater in natural than restored pools. The presence of Sphagnum spp. in the aquatic-terrestrial transition zone and within the pool is characteristic of the natural pools, which show more advanced evolution stage than the restored ones. However, in some of the restored pools, these bryophytes have begun to colonise the banks and the centre of very shallow pools. Regarding canopy openness and Quercus spp. cover, we observed slight differences on the field, but they were not significantly different.
Vernal pools studied were selected based on similar hydroperiod and morphology and no differences in water levels and temperatures were observed. Other water parameters were similar, except water electrical conductivity which is linked to soil composition, reflecting the similar water source (precipitation and surface runoffs).
Environmental variables influencing organic-matter decomposition rates
Hydrology and temperature have often been shown to be important drivers for OM decomposition, notably in temporary wetlands where flooding increases the decomposition rates compared to unflooded areas (Kelley and Jack 2002; Battle and Golladay 2007; Inkley et al. 2008; Capps et al. 2014). In our study, the chosen vernal pools had similar hydroperiods and our analyses revealed that the tree canopy openness and the soil TOC were the strongest drivers of the CS decomposition.
The amount of light reaching the water surface could be crucial for OM dynamics, whether it be growth of algae which stimulate fungal growth or production of fungal spores which is enhanced by light (Rier et al. 2007; Lagrue et al. 2011). In the vernal pools of our study site, there was a significant and positive correlation between tree canopy openness and CS decomposition. Moreover, tree canopy openness was positively correlated with the cover of vascular plants in the pool, which can grow better. These results could lead to several hypothesis which would need extra-measurements in future studies. First, CS decomposition could be enhanced by an increase of microorganism growth due to the light. An estimation of microorganism population should be estimated to strengthen this hypothesis. Moreover, plant roots exudates could enhance microbial activity through a priming effect (Guenet et al. 2014) therefore a high cover of vascular plants in the pool could increase microbial activity and thus, CS decomposition. This hypothesis would need the measurements of labile organic-matter content and microorganism populations.
Decomposition rates were also negatively influenced by soil TOC, which was in turn correlated with Sphagnum moss cover. Sphagnum spp. are known to influence their environment, especially by producing phenolic compounds which could lead to decrease OM decomposition rates (Freeman et al. 2004; Rydin et al. 2006; Chiapusio et al. 2013). High contents of TOC and OM in soils could be the consequence of low decomposition rates induced by the presence of Sphagnum spp.
The use of cotton strips as indicators of OM decomposition rates in vernal pools
Cotton strips were used in this study because they are standard materials with a high repeatability, which allow site comparisons, within a relatively short time of incubation (Tiegs et al. 2007). Moreover, it had been shown in an Italian floodplain—which included river, pond and terrestrial habitats—that the decomposition rates of CS followed the same patterns as natural litter mass loss (Tiegs et al. 2007). Even though CS have been regularly used as a proxy for OM in decomposition experiments, this method does not measured the same process as litter decomposition; the decomposition of plant litter is made by a large range of decomposers, such as invertebrates, whereas the decomposition of CS expresses the potential cellulolytic activity in the ecosystems (Tiegs et al. 2007).
We designed this experiment to calibrate the incubation time in vernal pools under a temperate climate and we found that CS needed at least 51–57 days of incubation to get close to 50% TSL (Fig. 3, Time3), the amount of decay that is believed to maximize the sensitivity of the assay. Comparing our values with the river ecosystems studied in Tiegs et al. (2013), we observed relatively slow decomposition rates. Indeed, Tiegs et al. (2013) incubated cotton strips in streams in the U.S.A. (Midwestern rivers with little riparian vegetation and receiving water drainage from row-crop agricultural field, and Michigan rivers with low human impacts) and in New Zealand (several soil uses in the river watersheds) for respectively 27 and 14 days. They found on average 2.3% TSLd for Midwestern streams, 1.8% for Michigan streams and 1.5% for New Zealand streams. In the vernal pools, after 51 to 57 days of incubation, we found 40.7% TSL on average for natural and restored pools, i.e. 0.7% TSLd.
In each pool, the cotton strips were placed close to each other, at a distance of about 10 cm. However, the data range was high (Fig. 3), suggesting that the cotton strips were sensitive to the micro-environmental conditions within the pools. This was also observed by Vysna et al. (2014) in their study of CS decomposition in Australian rivers. Future monitorings should use more within-pool replicates, and they should be dispersed randomly around the pool.
Conclusion
Like other small wetlands, vernal pools are strongly affected by human activities, especially because of their small size and their seasonality, making their detection more difficult during the dry period (see references in Holgerson and Raymond (2016)). This study was designed to assess the functioning of natural and restored vernal pools in the Chinon forest (France) in terms of OM decomposition, and more specifically, in terms of potential cellulolytic activity. After 51–57 days, no difference were found between OM decomposition rates in natural and restored vernal pools. Even though this result suggests that decomposition was restored, cellulolytic decomposition is only one dimension of the overall process of OM decomposition. The main drivers of cellulolytic activity measured with the CS assay was TOC and tree-canopy openness. They were also part of the environmental variables which differentiated natural from restored pools, with OM content, vascular plants and Sphagnum covers. These differences were mainly linked to the impacts of restoration work.
Decomposition indices, which are rarely used for ecological monitoring of wetlands, could be considered as a "global indicator" because they reflect several aspects of the systems characteristics such as biological activity, OM sources and foodwebs (Young et al. 2008). Future studies should use more CS replicates in order to better characterize the intra-pool variability. Moreover, questions of the re-establishment of biota were not addressed here. We therefore suggest to integrate other biotic indices with indicator species and biodiversity assessments, as well as measurements of specific microbial activities to assess the overall functioning of those systems. Lastly, the data presented here are a useful baseline for tracking the recovery of these restored vernal pools.
Abbreviations
- CS:
-
Cotton strips
- OM:
-
Organic-matter
- TN:
-
Total nitrogen
- TOC:
-
Total organic carbon
- %TSL, %TSLd:
-
Percentage of tensile strength loss, Percentage of tensile strength loss per day
References
Alcaydé G (1975) Carte géologique de la France à 1/50000, feuille Chinon (486). Bureau de recherches géologiques et minières, Orléans
Alcaydé G, Rasplus L (1971) Carte géologique détaillée de la France (1/50000), feuille Langeais (487). Bureau de recherches géologiques et minières, Orléans
Ballantine K, Schneider R (2009) Fifty-five years of soil development in restored freshwater depressional wetlands. Ecol Appl 19(6):1467–1480. https://doi.org/10.1890/07-0588.1
Bärlocher F (2005) Leaf mass loss estimated by litter bag technique. In: Graca MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition. Springer, Netherlands, pp 37–42
Battle JM, Golladay SW (2007) How hydrology, habitat type, and litter quality affect leaf breakdown in wetlands on the gulf coastal plain of Georgia. Wetlands 27(2):251–260. https://doi.org/10.1672/0277-5212(2007)27[251:hhhtal]2.0.co;2
Bot A, Benites J (2005) The importance of soil organic matter: key to Drought-resistant Soil and Sustained Food Production. FAO soils bulletin, Food and Agriculture Organization of the United Nations, Rome
Boutin D (1989) Carte des sols du département d’Indre-et-Loire (1/50000)–1823: Langeais. Chambre d’agriculture de l’Indre-et-Loire, France
Boutin D (1996) Carte des sols du départment de l’Indre-et-Loire (1/50000)–1723: Chinon. Chambre d’agriculture de l’Indre-et-Loire, France
Burdon FJ, Bai Y, Reyes M, Tamminen M, Staudacher P, Mangold S, Singer H, Räsänen K, Joss A, Tiegs SD, Jokela J, Eggen RIL, Stamm C (2020) Stream microbial communities and ecosystem functioning show complex responses to multiple stressors in wastewater. Glob Change Biol 00:1–20. https://doi.org/10.1111/gcb.15302
Burne MR, Griffin CR (2005) Protecting vernal pools: a model from Massachusetts, USA. Wetlands Ecol Manage 13(3):367–375. https://doi.org/10.1007/s11273-004-7528-3
Calhoun AJK, Mushet DM, Bell KP, Boix D, Fitzsimons JA, Isselin-Nondedeu F (2017) Temporary wetlands: challenges and solutions to conserving a ‘disappearing’ ecosystem. Biol Conserv 221:3–11. https://doi.org/10.1016/j.biocon.2016.11.024
Capps KA, Rancatti R, Tomczyk N, Parr TB, Calhoun AJK, Hunter MJ (2014) Biogeochemical hotspots in Forested Landscapes: the role of vernal pools in denitrification and organic matter processing. Ecosystems 17(8):1455–1468. https://doi.org/10.1007/s10021-014-9807-z
Chiapusio G, Jassey VEJ, Hussain MI, Binet P (2013) Evidences of Bryophyte Allelochemical Interactions: the case of Sphagnum. In: Cheema ZA, Farooq M, Wahid A (eds) Allelopathy: Current Trends and Future Applications. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 39–54
Colas F, Woodward G, Burdon FJ, Guérold F, Chauvet E, Cornut J, Cébron A, Clivot H, Danger M, Danner MC, Pagnout C, Tiegs SD (2019) Towards a simple global-standard bioassay for a key ecosystem process: organic-matter decomposition using cotton strips. Ecol Indicators 106:105466. https://doi.org/10.1016/j.ecolind.2019.105466
Colburn EA (2004) Vernal pools: natural history and conservation. McDonald & Woodward Pub. Co., Saline
Couderc J-M (1980) Les mardelles de Touraine et leurs groupements végétaux. In: Géhu JM (ed) La végétation des sols tourbeux, Lille–1978. Colloques phytosociologiques, VII, Strauß & Cramer GmbH, pp 35–60
Davidson NC (2014) How much wetland has the world lost? long-term and recent trends in global wetland area. Mar Freshw Res 65(10):934–941. https://doi.org/10.1071/mf14173
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36(1):27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Fennessy MS, Rokosch A, Mack JJ (2008) Patterns of plant decomposition and nutrient cycling in natural and created wetlands. Wetlands 28(2):300–310. https://doi.org/10.1672/06-97.1
Freeman C, Ostle NJ, Fenner N, Kang H (2004) A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol Biochem 36(10):1663–1667. https://doi.org/10.1016/j.soilbio.2004.07.012
Gingerich RT, Anderson JT (2011) Litter decomposition in created and reference wetlands in West Virginia. USA 19(5):449–458
Graça MAS, Ferreira V, Canhoto C, Encalada AC, Guerrero-Bolaño F, Wantzen KM, Boyero L (2015) A conceptual model of litter breakdown in low order streams. Int Rev ges Hydrobiol Hydrogr 100:1–12
Guenet B, Danger M, Harrault L, Allard B, Jauset-Alcala M, Bardoux G, Benest D, Abbadie L, Lacroix G (2014) Fast mineralization of land-born C in inland waters: first experimental evidences of aquatic priming effect. Hydrobiologia 721:35–44
Hervé P, Tiegs S, Grellier S, Matthias Wantzen K, Isselin-Nondedeu F (2018) Combined Effects of Vegetation and Drought on Organic-Matter Decomposition in Vernal Pool Soils. Wetlands. https://doi.org/10.1007/s13157-018-1091-9
Hill MO, Latter PM, Bancroft G (1988) Standardization of rotting rates by a linearizing transformation. In: Harrison AF, Latter PM, Walton DWH (eds) Cotton strip assay: an index of decomposition in soils. NERC/ITE, 21–24 (ITE Symposium, 24), Grange-over-Sands,
Holgerson MA, Raymond PA (2016) Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nature Geosci 9(3):222–226. https://doi.org/10.1038/ngeo2654
InfoClimat (2017) Climatologie de l'année 2016 à Tours–Parçay-Meslay. https://www.infoclimat.fr/climatologie/annee/2016/tours-parcay-meslay/valeurs/07240.html. Accessed 21/08/2017
Inkley MD, Wissinger SA, Baros BL (2008) Effects of drying regime on microbial colonization and shredder preference in seasonal woodland wetlands. Freshw Biol 53(3):435–445. https://doi.org/10.1111/j.1365-2427.2007.01908.x
IUSS Working Group WRB (2006) World reference base for soil resources 2006. FAO, Rome
Kayranli B, Scholz M, Mustafa A, Hedmark A (2010) Carbon storage and fluxes within Freshwater Wetlands: a critical review. Wetlands 30(1):111–124. https://doi.org/10.1007/s13157-009-0003-4
Kelley RH, Jack JD (2002) Leaf litter decomposition in an ephemeral karst lake (Chaney Lake, Kentucky, USA). Hydrobiologia 482(1–3):41–47. https://doi.org/10.1023/a:1021209906661
Kollmann J, Meyer ST, Bateman R, Conradi T, Gossner MM, Mendonça MdSJ, Fernandes GW, Hermann J-M, Koch C, Müller SC, Oki Y, Overbeck GE, Paterno GB, Rosenfield MF, Toma TSP, Weisser WW (2016) Integrating ecosystem functions into restoration ecology—recent advances and future directions. Restor Ecol 24(6):722–730. https://doi.org/10.1111/rec.12422
Lagrue C, Kominoski JS, Danger M, Baudoin J-M, Lamothe S, Lambrigot D, Lecerf A (2011) Experimental shading alters leaf litter breakdown in streams of contrasting riparian canopy cover. Freshw Biol 56(10):2059–2069. https://doi.org/10.1111/j.1365-2427.2011.02637.x
Marton JM, Fennessy MS, Craft CB (2014) Functional differences between natural and restored wetlands in the Glaciated Interior Plains. J Environ Qual 43(1):409–417. https://doi.org/10.2134/jeq2013.04.0118
Matthews J, Spyreas G, Endress A (2009) Trajectories of vegetation-based indicators used to assess wetland restoration progress. Ecol Appl 19(8):2093–2107
Mendelssohn IA, Sorrell BK, Brix H, Schierup HH, Lorenzen B, Maltby E (1999) Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquat Bot 64(3–4):381–398. https://doi.org/10.1016/s0304-3770(99)00065-0
Météo-France (2017) Météo et climat, Données climatiques de la station de Tours. https://www.meteofrance.com/climat/france/tours/37179001/releves. Accessed 06 May 2017
Neckles HA, Neill C (1994) Hydrologic control of litter decomposition in seasonally flooded prairie marshes. Hydrobiologia 286(3):155–165. https://doi.org/10.1007/bf00006247
Neher DA, Barbercheck ME, El-Allaf SM, Anas O (2003) Effects of disturbance and ecosystem on decomposition. Appl Soil Ecol 23(2):165–179. https://doi.org/10.1016/s0929-1393(03)00043-x
Office national des forêts, Jammes D (2003) Document d'objectifs Natura 2000 "Complexe forestier de Chinon landes du Ruchard". https://www.centre.developpement-durable.gouv.fr/complexe-forestier-de-chinon-landes-du-ruchard-a1948.html?id_rubrique=817. Accessed 25 08 2017
Pabst S, Scheifhacken N, Hesselschwerdt J, Wantzen KM (2008) Leaf litter degradation in the wave impact zone of a pre-alpine lake. Hydrobiologia 613(1):117–131. https://doi.org/10.1007/s10750-008-9477-y
Rier ST, Kuehn KA, Francoeur SN (2007) Algal regulation of extracellular enzyme activity in stream microbial communities associated with inert substrata and detritus. J N Am Benthol Soc 26(3):439–449. https://doi.org/10.1899/06-080.1
Rydin H, Gunnarsson U, Sundberg S (2006) The role of Sphagnum in peatland development and persistence. In: Wieder RK, Vitt DH (eds) Boreal peatland ecosystems. Springer-Verlag, Berlin Heidelberg, Ecological Studies, pp 47–67
Slocum MG, Roberts J, Mendelssohn IA (2009) Artist canvas as a new standard for the cotton-strip assay. J Soil Sci Plant Nutr 172:71–74. https://doi.org/10.1002/jpln.200800179
Tiegs SD, Langhans SD, Tockner K, Gessner MO (2007) Cotton strips as a leaf surrogate to measure decomposition in river floodplain habitats. J N Am Benthol Soc 26(1):70–77. https://doi.org/10.1899/0887-3593(2007)26[70:csaals]2.0.co;2
Tiegs SD, Clapcott JE, Griffiths NA, Boulton AJ (2013) A standardized cotton-strip assay for measuring organic-matter decomposition in streams. Ecol Indic 32:131–139. https://doi.org/10.1016/j.ecolind.2013.03.013
Tiegs SD, Costello DM, Isken MW et al (2019) Global patterns and drivers of ecosystem functioning in rivers and riparian zones. Sci Adv. https://doi.org/10.1126/sciadv.aav0486
Trettin CC, Jurgensen MF, Grigal DF, Gale MR, Jeglum JR (1996) Northern forested wetlands ecology and management. Taylor & Francis, Routledge, New York
van Breemen N (1995) How Sphagnum bogs down other plants. Trends Ecol Evol 10(7):270–275. https://doi.org/10.1016/0169-5347(95)90007-1
Vizza C, Zwart JA, Jones SE, Tiegs SD, Lamberti GA (2017) Landscape patterns shape wetland pond ecosystem function from glacial headwaters to ocean. Limno Oceano 62(S1):S207–S221. https://doi.org/10.1002/lno.10575
Vysna V, Dyer F, Maher W, Norris R (2014) Cotton-strip decomposition rate as a river condition indicator–Diel temperature range and deployment season and length also matter. Ecol Indic 45:508–521. https://doi.org/10.1016/j.ecolind.2014.05.011
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. Annual Rev Ecol Syst 17:567–594. https://doi.org/10.1146/annurev.es.17.110186.003031
Williams DD (2005) The biology of temporary waters. Oxford University Press, New York
Woodward G, Gessner MO, Giller PS, Gulis V, Hladyz S, Lecerf A, Malmqvist B, McKie BG, Tiegs SD, Cariss H, Dobson M, Elosegi A, Ferreira V, Graça MAS, Fleituch T, Lacoursière JO, Nistorescu M, Pozo J, Risnoveanu G, Schindler M, Vadineanu A, Vought LBM, Chauvet E (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336(6087):1438. https://doi.org/10.1126/science.1219534
Young RG, Matthaei CD, Townsend CR (2008) Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. J N Am Bentho Soc 27(3):605–625. https://doi.org/10.1899/07-121.1
Acknowledgements
We thank Hugo Basquin and Théo Destang-Quelen who helped with the installation and the monitoring of the experiment, Diana Ethaiya and Megan Maul for determining cotton-strip tensile strength, and the Laboratoire de Touraine and Laboratoire Inovalys (Nantes) for water and soil analyses. We are grateful to the staff of the Office National des Forêts for their support, and to Bertrand Guenet and the GRET-PERG researchers for their advice. This study was part of the GEREZOH project and was supported by the Région Centre-Val de Loire.
Funding
This study was part of the GEREZOH project and was supported by the Région Centre-Val de Loire (France).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hervé, P., Grellier, S., Tiegs, S.D. et al. Monitoring organic-matter decomposition and environmental drivers in restored vernal pools. Wetlands Ecol Manage 28, 937–952 (2020). https://doi.org/10.1007/s11273-020-09759-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-020-09759-4