Abstract
This study investigated the radioactivity of groundwater and bottom silt from wells in southern Sinai, Egypt. Eight well sites were chosen (Abu Redis, Abu Zenima, and Al-Tor) and composite samples of water and silt were created from each. Southern Sinai well water (Egypt) was safe for drinking based on tested elements (226Ra < 300 Bq/L, 232Th < 100 Bq/L). However, some bottom silt samples showed elevated 226Ra, 232Th, and 222Rn-, potentially posing health risks through inhalation or ingestion. Further investigation is needed on these specific silt samples due to potential internal and external radiation exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Radioactive materials can naturally occur in groundwater and soil, and depending on the levels, they may pose health risks. This study investigates the radioactivity of groundwater and bottom silt from wells in southern Sinai, Egypt. The researchers specifically chose well sites in Abu Redis, Abu Zenima, and Al-Tor to assess potential radioactivity levels in these areas. By analyzing the water and silt samples, the study aimed to determine if the radioactivity fell within safe limits for drinking water and habitation.
The safe disposal of radioactive waste remains a significant global challenge. While deep geological repositories are often considered the most secure option, concerns persist regarding potential long-term leakage and contamination of surrounding environments. investigated natural radioactivity levels in groundwater from a Saharan aquifer in Algeria. It highlights the importance of such studies in desert regions where water resources are limited (Sellaoui et al., 2016). Desert regions, frequently chosen for waste burial due to their low population density and seemingly stable geology, present unique concerns (Ewing, 2016). This study investigates the potential for radioactive contamination of groundwater and silt in desert wells situated near radioactive waste disposal sites. Here, we explore the possibility of radioactive materials migrating from buried waste and reaching nearby water sources. The focus is on desert environments, where factors like low precipitation and high evaporation rates can influence the movement of contaminants. Several studies have documented the dangers of radioactive contamination in groundwater. (Ai et al., 2020; Zhang et al., 2024) Research by Kim et al. (2016) (Ai et al., 2020) highlights the risks associated with radionuclide migration from nuclear facilities, emphasizing the importance of monitoring groundwater quality near such sites. Similarly, a study by Wang et al. (2018) (Zhang et al., 2024) investigates the long-term behavior of radioactive isotopes in groundwater systems, underlining the potential for persistent contamination. Desert environments pose additional challenges. Silt accumulation in wells can act as a trap for contaminants, potentially concentrating radioactive materials over time. The work of Zhang et al. (2019) (Zhang et al., 2019) explores the interaction of radionuclides with desert soil components, highlighting the potential for long-term retention within the desert ecosystem. This study aims to contribute to a growing body of research on the potential consequences of radioactive waste disposal in desert regions. By analyzing groundwater and silt samples from wells near a designated waste burial site, we hope to gain valuable insights into the risks of radioactive contamination in these arid environments.
Due to minimal weathering and erosion, desert soils tend to concentrate naturally occurring radioactive elements like Radium (226Ra) and Thorium (232Th) present in the underlying rocks. This can lead to higher levels of radioactivity in groundwater and surface water compared to other regions. Non-desert areas, In areas with more active weathering and erosion, these radioactive elements are dispersed and diluted, resulting in lower background radioactivity levels in water bodies. Human activities like mining, uranium exploration, and nuclear waste disposal can significantly contribute to radioactivity in desert water bodies. These activities can introduce additional radioactive contaminants into the environment. While human activities can also affect radioactivity in non-desert water bodies, the impact is often less pronounced due to the higher background levels and the greater dilution effect of water resources [29.30]. The horizontal distribution of groundwater can vary significantly depending on several factors, but here are some key characteristics. Topography, Higher elevations: Groundwater tends to flow from areas of high elevation (recharge zones) towards lower elevations (discharge zones). This creates a topography-driven horizontal flow pattern. Valleys, Groundwater often accumulates in valleys, where it's closer to the surface. Permeability, The permeability of the rock and soil layers plays a crucial role. Highly permeable materials like sand and gravel allow for easier groundwater movement, leading to a more even distribution. Less permeable materials like clay can create barriers and pockets of stagnant water. Aquifers: Groundwater primarily flows through saturated rock and soil formations called aquifers. The type of aquifer (confined vs. unconfined) and its geological structure influence the horizontal distribution. Confined aquifers have impermeable layers above and below, restricting horizontal flow compared to unconfined aquifers [31.32].
Desert communities, often isolated and reliant on limited water resources, face a unique environmental challenge – the potential contamination of their vital groundwater supplies (Bhandari et al., 2018). While the image of a shimmering oasis might evoke serenity, the reality is that desert wells can become unwitting repositories of harmful pollutants (Offstein et al., 2017). One such threat comes from the insidious presence of radioactive materials. This study delves into the concerning issue of radioactivity in desert well water and the role of silt in its potential spread (Wang et al., 2018). By exploring the presence of radioactivity in desert well water and the role of silt, this study aims to contribute to a growing body of research focused on protecting these vital resources and ensuring the safety of desert communities. The disposal of radioactive waste remains a significant environmental concern. While deep geological repositories are often considered the most secure option, the potential for radioactive materials to migrate into surrounding groundwater and soil raises serious health and ecological risks. This is particularly worrisome in arid regions where desert communities rely heavily on well water for drinking and irrigation (IAEA, 2021). This study investigates the potential for radioactive contamination of groundwater and silt in desert wells located near the burial sites of radioactive waste. Here, we explore the concerning possibility of radioactive contamination in desert well water and surrounding sediments. Several studies have highlighted this risk (Epa, 2020). By examining the potential for radioactive contamination of desert well water and silt, this study aims to contribute to a growing body of research focused on the safe and sustainable management of radioactive waste disposal, particularly in sensitive desert environments.
2 Materials and Methods
2.1 Sample Collection and Preparation
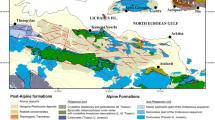
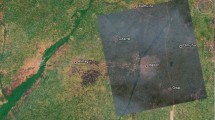
Eight groundwater and corresponding bottom silt samples were collected from wells located in southern Sinai, Egypt (Abu Redis, Abu Zenima, and Al-Tor). Abu Redis: This appears to be a smaller town located on the western coast of the Gulf of Suez, north of the much larger town of El Tor. Abu Zenima: This appears to be a larger town on the eastern coast of the Gulf of Suez, directly opposite El Tor. It's important to note that the scale of the map might not allow for precise pinpointing of the well locations within these towns Fig. 1A. To create representative composite samples, five subsamples were obtained from each well and thoroughly homogenized for both water and silt fractions. Each sample was weighed and assigned a unique identifier (W for water, S for silt). Water Samples: Five liters were collected from each well. In the laboratory, water and silt samples were transferred into Marinelli packed sealed 300 cm3 containers and left for 25 days to reach equilibrium. Silt Samples: Samples were air-dried in natural sunlight to remove surface moisture and then oven-dried at 110 °C for 24 h. Subsequently, they were sieved through a 200-µm mesh to achieve a uniform particle size ≤ 200 μm. This aging period allowed for secular equilibrium between 226 and 228Ra, a prerequisite for accurate radon concentration measurements. CR-39 plastic track detectors (2 cm × 2 cm, Track Analysis Systems Ltd.) were positioned inside each container lid before sealing. These standard radon detectors capture tracks formed when alpha particles from radon gas interact with the detector material. The number of tracks per unit area directly correlates to the radon gas concentration within the water and silt samples.
2.2 Natural Radioactivity Analysis
2.2.1 Radioactivity Analysis
Natural radioactivity levels in the samples were quantified using a high-purity germanium detector (HPGe) manufactured by Ortec USA, with 70% counting efficiency (Fig. 2 and 3). Data acquisition and analysis were performed with Ortec's MAESTRO-32 software. To ensure measurement accuracy, both energy and efficiency calibrations were conducted using a commercially available multi-nuclide standard source. Density, 1.0 g/cm3 is a typical density for water at standard temperature and pressure. It's unlikely that the isotope product has the same density as water. Volume and container, the isotope product has a volume of 1.300 mL (milliliters), which is equivalent to 1.300 cm3 (cubic centimeters) only if dealing with water (Measurement of Radionuclides in Food & the Environment, 2007). For other substances, the volume in mL won't necessarily translate directly to cm3. Container size: The isotope product is housed in a 250-mL cylindrical container. If the isotope product itself has a volume of 1.300 mL, it would likely occupy a significant portion of the container's volume (more than half). This standard source encompassed a diverse range of radionuclides (109Cd, 241Am, 123Te, 57Co, 113Sn, 51Cr, 85Sr, 88Y, 137Cs, and 60Co), facilitating full-energy peak efficiency calibration across a broad energy spectrum (80 keV to 2500 keV). Activity concentrations of 226Ra were determined by analyzing the gamma-ray emissions of its progeny, 214Pb and 214Bi, at their characteristic energies of 351.9 keV and 609.3 keV, respectively. Similarly, activity concentrations of 232Th were ascertained in the silt sample (Naturally Occurring Radioactive Materials) scale residue samples by measuring the gamma-ray lines emitted by its decay products, 228Ac and 208Tl, at 911.1 keV and 583.1 keV, respectively (Beckhoff et al., 2008). Finally, the activity concentration of naturally occurring 40 K was directly measured using its distinctive gamma-ray peak at 1460 keV.
2.2.2 Physical Properties
Appearance: Generally colorless and odorless, though it might have a slight turbidity due to suspended particles. Temperature: the cooler than surface water due to the insulating effect of the ground. However, in some cases, geothermal activity can elevate groundwater temperature. Electrical conductivity (EC): This high was due to dissolved salts, especially in arid environments where evaporation concentrates minerals. pH: range from slightly acidic to slightly alkaline depending on the minerals present.
2.2.3 Chemical Properties
Major dissolved ions: include calcium (Ca2 +), magnesium (Mg2 +), sodium (Na +), potassium (K +), chloride (Cl-), sulfate (SO42⁻), and bicarbonate (HCO3⁻). The specific dominant ions will depend on the local geology. Total dissolved solids (TDS): This refers to the total amount of dissolved minerals in the water. It is high in desert well water due to evaporation. Trace elements: the well water contains trace elements like iron (Fe), manganese (Mn), arsenic (As), and fluoride (F⁻) depending on the rock formations it interacts with. Organic matter: The level of organic matter is low due to limited vegetation and biological activity in desert environments.
2.2.4 Relationship to Radioactive Elements
Desert groundwater contain natural radioactive elements like Radium (226Ra) and Thorium (232Th). This occurs because minimal weathering and erosion in desert environments lead to a concentration of these elements in the soil, which can then leach into the groundwater. Impact on Properties: Radionuclides can affect the water's physical and chemical properties. For example, they can increase electrical conductivity and influence pH. Health Risks: Radionuclides in drinking water can pose health risks with prolonged exposure, potentially causing issues with kidneys, bones, and even increasing cancer risk.
2.3 Radon Concentration Measurement in Water and Silt Samples
CR-39 plastic track detectors are a widely employed technique for quantifying the rate of radon exhalation from materials. However, these detectors require calibration to convert the number of detected alpha particles into a radon gas concentration (IAEA (International Atomic Energy Agency) 2018). The measurement process involves placing samples within a standardized cylindrical container with a diameter of 3.5 cm and a length of 10 cm. Dosimeters are prepared by attaching two CR-39 detectors (each 250 μm thick and 2 cm2 in area) to the underside of the container lid before sealing. Following this preparation, the samples undergo a minimum of 25 days of storage to allow for radon gas accumulation. The sealed cup technique serves as the chosen method for determining the radon concentration emanating from the silt samples (Abu-Jarad et al., 2019). This technique relies on enclosing the sample within an airtight container, enabling radon to accumulate within the headspace over a designated period. The CR-39 detectors capture alpha particles emitted by the accumulated radon gas. After an exposure time of 45 days, the detectors are etched using a 6.25 N sodium hydroxide (NaOH) solution at 60°C for 6 h. This established sealed cup technique offers a simple and reliable method for quantifying radon concentration within silt samples (Fig. 4) (Knoll, 2010).
2.4 Activity Concentration Calculations
To isolate the actual radioactivity levels in the samples, background radiation was subtracted from each measurement (IAEA (International Atomic Energy Agency) 2018). The activity concentration for each identified gamma-ray line was determined by considering the sample mass, gamma decay branching ratios, and counting time. All samples were measured under identical conditions to ensure consistent detector efficiency and counting time, as recommended by Knoll (2010). Equation 1 depicts the formula used to calculate the activity concentration (A) in Becquerel per kilogram (Bq/kg) for each sample:
where: (CPS)net: Net counts per second, (I): Intensity of the specific gamma-ray line for the targeted radionuclide, (Eff): Measured efficiency for the detected gamma-ray line and (M): Mass of the sample in kilograms (Measurement of Radionuclides in Food & the Environment, 2007). A correction factor was applied to account for the potential interference of 232Th, specifically its daughter nuclide 228Ac (emitting a gamma ray at 1459.2 keV), on the measurement of 40K (emitting a gamma ray at 1460.8 keV) (Measurement of radioactivity in food & the environment - Guidance on sampling, measurement & uncertainty estimation., 2016). Equation 2 illustrates the formula used for this correction:
where: (Ath) Activity concentration of 232Th (Bq/kg), (AK) Activity concentration of 40K (Bq/kg).
2.5 Minimum Detectable Activity Concentration (AMDC)
This section explores the calculation of the minimum detectable activity concentration (AMDC) for radionuclides identified in the water and silt samples. AMDC represents the lowest level of confidence. Equation 3 depicts the formula used to calculate AMDC (Measurement of Radionuclides in Food & the Environment, 2007):
where: FC: Statistical coverage factor (typically set at 1.64 for a 95% confidence level), σΝΒ: Standard deviation of the background signal within the region of interest and ε: Detector efficiency (Knoll, 2010). The study determined the following minimum detectable activity concentrations: 0.6 Bq/kg for 226Ra, 0.3 Bq/kg for 232Th, and 6.2 Bq/kg for 40K (Measurement of radioactivity in food & the environment - Guidance on sampling, measurement & uncertainty estimation., 2016). These values indicate that the instrument possesses the capability to detect these radionuclides at activity levels as low as 0.6 Bq/kg for 226Ra, 0.3 Bq/kg for 232Th, and 6.2 Bq/kg for 40K, with a 95% confidence level. The average minimum detectable activity concentration across all investigated radionuclides was estimated to be 1.4 Bq/kg. In simpler terms, this signifies that the instrument can, on average, reliably detect radionuclide concentrations as low as 1.4 Bq/kg with a 95% confidence level.
2.6 External Gamma Radiation Exposure Assessment
This section evaluates the potential external gamma radiation exposure arising from the water and silt (Naturally Occurring Radioactive Materials) samples. Equation 4 serves as the basis for calculating the absorbed dose rate in air, specifically one meter above the ground surface, attributable to gamma radiation emitted by 226Ra, 232Th, and 40K within the samples (Radiation Protection & Safety in Ionizing Radiation Applications, 2014a). The following equation (Eq. 4) estimates the absorbed dose rate (National Council on Radiation Protection and Measurements (NCRP) 2005):
where: (ARa) Activity concentration of 226Ra (Bq/kg), (ATh) Activity concentration of 232Th (Bq/kg) and (AK) Activity concentration of 40K (Bq/kg). This equation allows for an estimation of the potential radiation dose an individual might receive when standing in close proximity to the water and silt samples. The recommended limit for absorbed dose from external gamma radiation exposure is 0.059 mGy h⁻1 (National Council on Radiation Protection and Measurements (NCRP) 2005). This signifies that the combined absorbed dose rate from 226Ra, 232Th, and 40K must not exceed this established limit. It's important to recognize that the absorbed dose rate represents just one factor contributing to an individual's total radiation dose. Additional considerations include the duration of exposure near the samples and the presence of any shielding materials that could attenuate the radiation exposure (UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) 2008).
2.7 Annual Effective Dose Equivalent (AEDE) from Gamma Radiation
This section explores the calculation of annual effective dose equivalent (AEDE) arising from external gamma radiation exposure for adults. Equation 5 serves as the basis for this calculation (The, 2007 Recommendations of the ICRP 2007):
where: (Fγ) Conversion factor for gamma rays (0.7 Sv Gy⁻1), (Dabs) Absorbed dose rate (mGy h⁻1), (Texp) Annual exposure time (hours) (National Council on Radiation Protection and Measurements (NCRP) 2005). The equation incorporates a typical occupancy factor of 0.7. This factor represents the estimated fraction of a year an individual spends in the exposed location. Occupational settings necessitate a different approach. Equation 6 is employed to calculate the annual effective dose (AED) in such environments:
The factor of 0.2 in Eq. 6 accounts for the increased exposure duration typically encountered in occupational settings (National Council on Radiation Protection and Measurements (NCRP) 2005). It's important to note that these equations provide estimations, and the actual AEDE may deviate based on several factors. These factors include the presence of shielding materials and the individual's distance from the radiation source. The calculated annual effective dose from gamma rays should be considered in conjunction with the global average background radiation level of 0.460 mSv y⁻1 (National Council on Radiation Protection and Measurements (NCRP) 2005).
2.8 Annual Gonadal Dose Equivalent (AGDE)
This section delves into the estimation of the annual gonadal dose equivalent (AGDE), which represents the average yearly radiation dose absorbed by the reproductive organs of an exposed population. Equation 7 serves as the basis for this calculation, utilizing the activity concentrations of 226Ra, 232Th, and 40K measured in the water and silt samples (UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) 2008). Equation 7:
where: (ARa) Activity concentration of 226Ra (Bq/kg), (ATh) Activity concentration of 232Th (Bq/kg) and (AK) Activity concentration of 40K (Bq/kg). It's crucial to note that the AGDE calculation presented here presupposes the availability of the annual effective dose equivalent (AEDE) for adult exposure, obtainable from either Eq. 5 or Eq. 6 (refer to Sect. 2.7) (The, 2007 Recommendations of the ICRP 2007). The varying conversion factors (3.09, 4.18, and 0.314) within Eq. 7 account for the differential sensitivities of various tissues to radiation. Compared to other organs, the gonads exhibit a heightened susceptibility to radiation, which is reflected in these factors. It's important to recognize that Eq. 7 provides an estimated AGDE, and the actual value may deviate due to several factors (National Council on Radiation Protection and Measurements (NCRP) 2008). These factors encompass the age and sex distribution of the exposed population, as well as the presence of any shielding materials that could attenuate the radiation exposure.
2.9 Radiological Risk Assessment of Water and Silt Samples
Water and silt samples accumulating in wells can harbor significant quantities of naturally occurring radioactive materials such as 226Ra, 232Th, and 40K. These radionuclides emit gamma rays, which pose a potential health risk to humans. To quantify the radiological risks associated with these samples, a suite of parameters is employed: Radium Equivalent (Raeq): This parameter provides a combined metric for gamma-ray exposure from 226Ra, 232Th, and 40K within the sample. External Hazard Index (Hex): This index specifically evaluates the external gamma-ray exposure hazard stemming from the water and silt sample. Internal Hazard Index (Hin): This index signifies the potential for internal exposure if the radionuclides are inhaled or ingested (Radiation Protection & Safety in Ionizing Radiation Applications, 2014b). Gamma Index (Iγ): This parameter offers an overall measure of gamma-ray exposure arising from the sample. Activity Utilization Index (AUI): This index reflects the efficiency with which the sample's radionuclides produce gamma rays. Established global averages exist for these parameters (Raeq: 370 Bq/kg, Hex: 0.4, Hin: 0.2, Iγ: 0.6, and AUI: 0.5) (National Council on Radiation Protection and Measurements (NCRP) 2009). Water and silt samples exceeding these average values warrant further investigation due to a potentially elevated health risk. It's noteworthy that 98.5% of the radiological effects attributable to the 238U decay series can be ascribed to 226Ra. This underscores the importance of 226Ra as the primary radionuclide of concern when assessing the radiological hazards associated with water and silt samples. Finally, it's crucial to recognize that the radiological risks posed by water and silt samples depend on both the specific radionuclide composition and the exposure scenario. For example, samples with high concentrations of 226Ra and 232Th pose a greater health threat compared to those with lower activities of these radionuclides. This section explores the application of several parameters to quantify the radiological risks associated with water and silt sample. These parameters provide insights into the potential for gamma-ray exposure and internal contamination (UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) 2008).
2.9.1 Radium Equivalent (Raeq)
Radium equivalent (Raeq) serves as a metric for the combined gamma-ray exposure arising from the presence of 226Ra, 232Th, and 40K within a material. Equation 8 facilitates the calculation of Raeq (Basic Safety and Standards: The IAEA Basic Safety Standards (https:, , www.iaea.org, resources, safety-standards) offer recommendations for radiation protection, including dose rate limits for various exposure scenarios. xxxx):
where: (ARa) Activity concentration of 226Ra (Bq/kg), (ATh) Activity concentration of 232Th (Bq/kg) and (AK) Activity concentration of 40K (Bq/kg). The conversion factors (1.43 and 0.077) account for the differences in gamma-ray energies emitted by 232Th and 40K compared to 226Ra. Raeq is a valuable tool for assessing the radiological hazards of water and silt sample because it incorporates the contributions of all three prominent radionuclides. A recommended limit of 370 Bq/kg has been established for Raeq (National Council on Radiation Protection and Measurements (NCRP) 2005). water and silt sample exceeding this limit warrant further investigation due to a potentially elevated health risk.
2.9.2 External and Internal Hazard Indices (Hex, Hin)
External hazard index (Hex) and internal hazard index (Hin) are additional parameters employed to evaluate the radiological risks associated with water and silt sample. External Hazard Index (Hex): This index specifically assesses the external exposure hazard stemming from gamma-ray emissions. Equation 9 calculates Hex (National Council on Radiation Protection and Measurements (NCRP) 2009; Radiation Protection & Safety in Ionizing Radiation Applications, 2014b;):
where: (Raeq) Radium equivalent (Bq/kg), Internal Hazard Index (Hin): This index focuses on the potential for internal exposure to radon, a recognized carcinogen. Equation 10 provides the calculation for Hin (Radiation Protection & Safety in Ionizing Radiation Applications, 2014b):
where: (ARa) Activity concentration of 226Ra (Bq/kg), (ATh) Activity concentration of 232Th (Bq/kg) and (AK) Activity concentration of 40K (Bq/kg). For both Hex and Hin, values below 1 are generally considered insignificant concerning radiological hazard (National Council on Radiation Protection and Measurements (NCRP) 2009).
2.9.3 Gamma Index (Iγ)
The gamma index (Iγ) serves as a comprehensive metric for quantifying the gamma-ray exposure arising from water and silt sample. Equation 11 facilitates the calculation of Iγ (Radiation Protection & Safety in Ionizing Radiation Applications, 2014b):
where: (ARa) Activity concentration of 226Ra (Bq/kg), (Ath) Activity concentration of 232Th (Bq/kg) and (AK) Activity concentration of 40K (Bq/kg). The European Commission has established guidelines that categorize the potential radiological hazard of water and silt samples based on Iγ values (Landman et al., 2011): Negligible Radiological Hazard: Iγ values below 0.5. Low Radiological Hazard: Iγ values between 0.5 and 1. It's crucial to recognize that Iγ is just one piece of the puzzle when assessing radiological risk. Other factors, such as the physical form (e.g., dust, solid) of the water and silt sample and the specific exposure scenario (ingestion, inhalation, etc.), significantly influence the overall hazard (National Council on Radiation Protection and Measurements (NCRP) 2009).
2.9.4 Activity Utilization Index (AUI)
The activity utilization index (AUI) serves as a metric for evaluating the efficiency with which radionuclides within water and silt sample emit gamma rays relative to their activity concentration. In simpler terms, AUI reflects how effectively the radioactive materials in the water and silt sample translate their inherent radioactivity into gamma radiation. Equation 12 facilitates the calculation of AUI (National Council on Radiation Protection and Measurements (NCRP) 2009; Radiation Protection & Safety in Ionizing Radiation Applications, 2014b;):
where: (CfRa) Conversion factor for 226Ra (0.462) (nGy h⁻1 per Bq/kg), (CfTh) Conversion factor for 232Th (0.604) (nGy h⁻1 per Bq/kg) and (CfK) Conversion factor for 40K (0.041) (nGy h⁻1 per Bq/kg). The AUI is expressed in units of nGy h⁻1 per Bq/kg (nanogray per hour per becquerel per kilogram). Regulatory limits have been established for the activity concentrations (Bq/kg) of individual radionuclides (226Ra, 232Th, 40K) in water and silt samples, with a recommended maximum of 50 Bq/kg for each. Consequently, an ideal AUI for water sample would be below 50 nGy h⁻1 per Bq/kg (UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) 2008).
2.9.5 Radon Gas Concentration Measurement
This section details the methodology employed to measure radon gas concentrations emanating from water and silt samples.
2.9.6 Track Detection and Density Calculation
A CR-39 detector, a passive solid-state device, was utilized to record tracks left behind by alpha particles emitted during radon decay within the samples. The number of these tracks is directly proportional to the concentration of radon gas present. Equation 13 facilitates the calculation of track density (ρ) for each sample:
where: (ρ) Track density (tracks/cm2·d), (N) Total number of tracks counted, (A) Area of the detector (cm2) and (T) Exposure time (days). Radon Gas Concentration and Effective Radium Content. The radon gas concentration (CRn) is determined using Eq. 14 (Landman et al., 2011):
where: (CRn) Radon gas concentration (Bq/m3), (ρo) Path density of the radioactive source (tracks/cm2·d), (ρ) Path density of the sample (tracks/cm2·d), (No) Activity concentration of the radioactive source of radium (Bq/kg) and (To) Exposure time to a radioactive element (days). Equation 15 allows the calculation of the effective radium content (ARE) within a sample (Landman et al., 2011):
where: (ARE) Effective radium (Bq/kg), (ρ) Path density (tracks/cm2·d), (k) Calibration factor of the CR-39 detector (tracks/cm2·d per Bq/kg), (Te) Effective exposure time (days), (M) Mass of the sample (kg), (A) Cross-sectional area of the container holding the samples (cm2) and (h) Distance between the detector and the top of the container (cm).
2.9.7 Radon Exhalation Rate and Annual Effective Dose
The radon exhalation rate (Ex) is calculated using Eq. 16 (Radiation Protection & Safety in Ionizing Radiation Applications, 2014b):
where: (Ex) Radon exhalation rate (Bq/kg·h), (C) Radon concentration (Bq/m3), (α) Radon decay constant (h⁻1), (T) Exposure time (h), (λ) Decay constant for the radioactive source of radium (h⁻1), (V) Chamber volume (m3) and (A) Measured sample mass (kg). Finally, Eq. 17 estimates the annual effective dose (HE) for the public exposed to the radon gas (UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) 2008):
where: (HE) Annual effective dose (mSv·y⁻1), (C) Radon concentration (Bq/m3), (F) Indoor occupancy factor (0.4), (T) Total time in a year (hours) (8760 h·y⁻1) and (D) Dose conversion factor ((mSv·y⁻1)/(Bq·m⁻3)⁻1) (9 × 10⁻⁶ (mSv·y⁻1)/(Bq·m⁻3)⁻1) (Landman et al., 2011).
3 Results
3.1 Radionuclide Activity Concentrations (A Ra, Ath, Ak), Ra Equivalent (Raeq), and AUI in Water and Silt Samples: Comparison with IAEA Limits
World Health Organization (WHO): Radon-226: 100 Bq/L, Thorium-238 (not directly regulated, but often considered as part of total alpha activity): 20 Bq/L and Potassium-40: 10 mBq/L (milliBecquerel per Liter). International Atomic Energy Agency (IAEA): Radon-226: 300 Bq/L, Thorium-238 (similar to WHO, considered as part of total alpha activity): 100 Bq/L and Potassium-40: 10 mBq/L. European Union (EU): Radon-226: 100 Bq/L, Thorium-238: 100 Bq/L and Potassium-40: 10 mBq/L. United States Environmental Protection Agency (EPA): (It's important to note that the US EPA regulates drinking water, and radon is a gas that primarily enters through air exposure. However, these values can provide a reference point). Radon-226: 150 pCi/L (converted to Bq/L: 150 pCi/L * 0.037 Bq/pCi = 5.55 Bq/L), Thorium-238: 5 pCi/L (converted to Bq/L: 5 pCi/L * 0.037 Bq/pCi = 0.185 Bq/L), Potassium-40: 30 pCi/L (converted to Bq/L: 30 pCi/L * 0.037 Bq/pCi = 1.11 Bq/L).
Table 1 (Fig. 5) indicates: Radium-226 (Ra): The IAEA limit for 226Ra in drinking water is 300 Bq/L. All samples in the table are in Bq/L, and none exceed the 300 Bq/L limit. This is good news; the 226Ra levels in these samples fall within the IAEA's recommended safe range for drinking water. Thorium (232Th): The statement "there is no permissible limit" for 232Th isn't entirely accurate. While the IAEA doesn't have a specific limit for 232Th itself, it's often regulated through total alpha activity. This combined activity includes alpha particles emitted by 232Th and other alpha-emitting isotopes. The table doesn't show total alpha activity, so it's impossible to determine if the samples exceed any regulations related to 232Th. Potassium-40 (K): The IAEA limit for 40K is 10 Bq/L, as mentioned correctly. All samples in the table exceed this limit. However, it's important to note that 40K is a naturally occurring radioactive isotope present in all living things, including humans. The 10 Bq/L limit is very low because 40K is not considered a significant health risk at these levels. Overall Safety: Based on the data provided in the table, we cannot definitively say that exposure to all the water samples is unsafe. Here's why: 226Ra levels are within the IAEA's recommended limit for drinking water. The table doesn't show total alpha activity, so we can't assess 232Th compliance. While 40K exceeds the IAEA limit, it's a naturally occurring element and the limit is very low due to its minimal health risk at these levels (Sellaoui et al., 2016).
Table 1 (Fig. 6) shows the activity concentrations of Radium-226 (ARa), Thorium-232 (Ath), Potassium-40 (Ak), and Radium Equivalent (Raeq) in eight silt samples (S1-S8). The activity concentrations are measured in Becquerel per kilogram (Bq/Kg). The table also shows the recommended limits for these elements in drinking water set by the International Atomic Energy Agency (IAEA). Analysis of the Data: (226ARa): The IAEA limit for ARa in drinking water is 300 Bq/Kg. All of the silt samples in the table have ARa levels below this limit. This indicates that the ARa levels in these samples are within the safe range for drinking water. (232Ath): The IAEA limit for Ath in drinking water is 100 Bq/Kg. All of the silt samples in the table have Ath levels below this limit. This indicates that the Ath levels in these samples are also within the safe range for drinking water. Potassium-40 (Ak): The IAEA limit for Ak in drinking water is 1000 Bq/Kg. All of the silt samples in the table have Ak levels below this limit. This indicates that the Ak levels in these samples are also within the safe range for drinking water. Radium Equivalent (Raeq): The Raeq is a calculated value that represents the combined radiological impact of ARa, Ath, and Ak. The IAEA limit for Raeq is 370 Bq/Kg (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000). All of the silt samples in the table have Raeq levels below this limit. This indicates that the Raeq levels in these samples are also within the safe range for drinking water. Additional Notes: It is important to note that the IAEA limits are for drinking water and may not be applicable to other types of water, such as groundwater or wastewater (Guidelines for drinking-water quality, 2011). The activity concentrations of radioactive elements in water can vary depending on a number of factors, such as the source of the water, the geology of the area, and the presence of human activities.
AUI is a metric that reflects how efficiently radioactive materials in a sample emit gamma rays relative to their total activity concentration (Bq/kg). Higher AUI indicates a greater tendency of the radioactive materials to emit detectable gamma rays (in nGy h^-1). The data reveals the presence of three radionuclides: 226Ra, 238Th and 40K. AUI values for eight water samples (W1-W8) and eight silt samples (S1-S8) along with the activity concentration of 226Ra, 238Th, and 40K for each sample. Water samples (W1-W8): AUI ranges from 0.09 nGy h^-1 per Bq/kg to 0.48 nGy h^-1 per Bq/kg. Silt samples (S1-S8): AUI ranges from 0.21 nGy h^-1 per Bq/kg to 1.38 nGy h^-1 per Bq/kg. Silt samples generally have higher AUI values compared to water samples. This might be due to the tendency of Ra and Th to bind to soil particles, leading to higher concentrations in silt. AUI variations within each sample type (water and silt) are likely due to differences in the total activity concentration and the relative abundance of 226Ra, 238Th, and 40K among the samples. Samples with higher concentrations of Ra or Th, which generally have higher gamma emission probabilities than 40K, would be expected to have higher AUI values (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000). Correlations between AUI, Radionuclides, and Sample Type: By looking at the data in the table, you can see if there are any correlations between: AUI and Radionuclide Activity Concentration: Samples with higher activity concentrations of Ra or Th (shown in the table) might tend to have higher AUI values. Sample Type (Water vs. Silt) and AUI: As discussed earlier, silt samples generally have higher AUI due to potential enrichment of Ra and Th. The AUI data, along with the information about the specific radionuclides (226Ra, 238Th, 40K) in the water and silt samples, provides valuable insights into their gamma ray emission characteristics. While AUI doesn't directly tell you the exact contribution of each radionuclide, the presence of Ra, Th, and 40K helps explain some of the observed trends.
The figure does mention a margin of error of ± 5%. This likely applies to all the data points in the figure.
3.2 Interpreting the Radiation Exposure Levels relative to Global Limits
Table 2 (Figs. 7, 8) indicate this analysis evaluates gamma ray intensity (Iγ) and potential radiation hazards in eight silt samples (S1-S8) and eight water samples (W1-W8). Gamma Ray Intensity (Iγ): Iγ measurements varied across the samples, with the highest values observed in S8, S1, and W8 (1.82 mSv/h, 1.27 mSv/h, and 1.27 mSv/h, respectively). However, it's important to note that Iγ alone doesn't provide a complete picture of radiation risk. External Dose Estimates (Deff (AEDE)): The data provides "Deff (AEDE) outdoor" values (mSv/y) for both silt and water samples. Deff represents the dose conversion factor for external exposure, and "outdoor" indicates external exposure from the samples while outdoors. Silt samples ranged from 0.02 mSv/y to 0.14 mSv/y, and water samples ranged from 0.01 mSv/y to 0.10 mSv/y. Importantly, all samples were below the international permissible limit of 1 mSv/y for external exposure. Internal Dose and Hazard Indices: The analysis included the Internal Hazard Index (Hin) and External Hazard Index (Hex) for the silt samples (S1-S8). Hin is a preliminary indicator of potential risk from inhaling or ingesting radioactive materials, with higher values suggesting a greater potential for internal dose. Hex reflects the potential hazard from external gamma radiation exposure while near the samples. Hin: Values ranged from 0.06 mSv/y to 0.63 mSv/y. Notably, samples S1 (0.36 mSv/y) and S8 (0.63 mSv/y) exceeded 0.1 mSv/y, suggesting a potentially higher risk for internal dose if radioactive materials are inhaled or ingested. Hex: Values ranged from 0.05 mSv/y to 0.49 mSv/y. While there are no universally accepted regulatory limits for Hex, some countries use it as a screening tool. Similar to Hin, samples S1 (0.31 mSv/y) and S8 (0.49 mSv/y) had Hex exceeding 0.1 mSv/y, indicating a potentially higher external hazard from gamma radiation exposure. Overall Considerations: Although all samples were below the international permissible limit for external exposure (1 mSv/y), Hin values for S1 and S8 suggest a potential for higher internal dose if radioactive materials are ingested or inhaled. Hex values for S1 and S8 indicate a potentially higher external hazard from gamma radiation exposure for these specific samples.
Based on the Table 2, all the samples (water W1-W8 and silt S1-S8) appear to have Annual Gonadal Dose Equivalent (AGDE) values exceeding the international limit of 1 mSv/y set by the International Atomic Energy Agency (IAEA) for public exposure. Here's a breakdown of what this means and what to consider: The table shows AGDE values for each sample, all exceeding the IAEA's public exposure limit of 1 mSv/y. This indicates a potential concern for radiation exposure if people were to be continuously exposed to these samples. Nature of AGDE: AGDE only considers external exposure from gamma rays emitted by the samples. It doesn't account for internal exposure from inhaling or ingesting radioactive materials. Public Health Significance: The data suggests potential health risks due to radiation exposure from these samples. However, the extent of the risk depends on several factors. Overall, The AGDE values in the table indicate a potential concern for radiation exposure from the water and silt samples.
The figure does mention a margin of error of ± 5%. This likely applies to all the data points in the figure.
The figure does mention a margin of error of ± 5%. This likely applies to all the data points in the figure.
3.3 Radon Concentration and Dose Comparison with IAEA Limits
Table 3 (Fig. 9) presents data on 222Rn concentration in various environmental samples and estimated human exposure levels for Eight locations (W1-W8 for water sources, S1-S8 for silt samples). It also includes calculated doses from inhalation and compares the values to limits set by the International Atomic Energy Agency (IAEA). The table allows for comparison of measured 222Rn concentrations in silt, air, and vegetables with the IAEA limits. It also provides estimates of potential radiation exposure through inhalation for each location. Comparison with IAEA Limits: Silt: The IAEA doesn't have a specific regulatory limit for Radon concentration in silt. However, the listed range (10–2000 Bq/m3) provides a general idea of typical background levels. Air: The IAEA recommends a maximum indoor Radon concentration of 300 Bq/m3. Several locations in the table (both water and silt samples) exceed this limit when considering estimated indoor Radon levels (Rnindoor). Vegetables: The IAEA doesn't have a regulatory limit for Radon in vegetables. The listed range (0.1–10 Bq/kg) provides context for the measured values.
The figure does mention a margin of error of ± 5%. This likely applies to all the data points in the figure.
4 Discussion
This study investigated the radionuclide activity concentrations and potential radiation exposure risks associated with water and silt samples collected from eight locations. The analysis focused on Radium (226Ra), Thorium (232Th), Potassium (40 K), and Radon (222Rn).
4.1 Radionuclide Activity Concentrations in Water and Silt
The activity concentrations of 226Ra in all water samples were within the recommended limit (300 Bq/L) set by the International Atomic Energy Agency (IAEA) for drinking water. This indicates no immediate threat from 226Rain terms of drinking water safety. Data on total alpha activity, which can be used to assess compliance with 232Th regulations, was missing. Further analysis is needed to determine 232Th compliance. 40 K activity concentrations exceeded the IAEA limit (10 Bq/L) in all water samples. However, 40 K is a naturally occurring, low-risk element at environmental levels. The low limit reflects this characteristic.
4.2 Radionuclide Activity Concentrations in Silt
The activity concentrations of 226Ra, 232Th and 40 K in all silt samples fell within the recommended limits set by the IAEA for drinking water. It's important to note that these limits apply to drinking water and may not be directly applicable to silt.
4.3 External Dose Estimates and Gamma Radiation Exposure
The calculated external dose equivalent (Deff) from gamma rays for both water and silt samples were below the international permissible limit of 1 mSv/y for external exposure. This suggests no significant external radiation risk from gamma rays under the conditions assessed in this study.
4.4 Internal Dose Estimates and Potential Health Risks
While the AGDE values suggested a potential concern for radiation exposure, they only consider external exposure from gamma rays and may not reflect the full picture of radiation risk. The Internal Hazard Index (Hin) for two silt samples (S1 and S8) exceeded 0.1 mSv/y, indicating a potentially higher risk of internal dose if radioactive materials are inhaled or ingested. Further investigation into the potential for inhalation or ingestion of these materials is warranted.
4.5 Radon Concentration and Potential Inhalation Risks
The estimated indoor Radon levels based on Radon concentration in silt samples exceeded the IAEA's recommended limit of 300 Bq/m3 for several locations. This suggests a potential health risk from Radon inhalation in these areas. However, it's important to note that this is an estimated value, and actual indoor Radon measurements are necessary for a more accurate assessment.
5 Radionuclide Transport and Interaction Between Silt and Water
5.1 Factors Affecting How Radioactive Elements Move between Silt and Water
The influence how radioactive elements move between silt and water. Several factors influence how radioactive elements move between silt and water: Physical characteristics: Particle size, surface area, and mineralogy of silt particles all play a role. Smaller particles with a larger surface area tend to adsorb (cling) to radioactive elements more effectively than larger particles. The mineral composition of the silt also affects how strongly it binds to radioactive elements. Chemical properties: The element's charge, ionic form, and complexation (association with other molecules) influence its mobility in water and its affinity for binding to silt particles. Water chemistry: Factors like pH, salinity, and the presence of other dissolved ions can affect the solubility and adsorption of radioactive elements. These factors can cause radionuclides to partition between the water column (where they are dissolved or suspended) and the bottom sediment (silt). Understanding these interactions is important for predicting the fate and transport of radioactive contaminants in aquatic environments.
5.2 Causes of High Radioactivity at 2 Sites
The study identified two sites with elevated radioactivity levels. Here are some explanations: Natural sources: Certain geological formations contain naturally radioactive elements like uranium or thorium. The weathering and erosion of these rocks can release radioactive materials into the surrounding water and soil. Anthropogenic sources: Human activities like mining, nuclear waste disposal, or industrial processes can introduce radioactive contaminants into the environment.
5.3 Actual Indoor Radon Measurements
The study estimated indoor Radon levels based on silt concentrations. Actual measurements are essential for an accurate assessment of potential inhalation risks. Investigation into human exposure pathways: The study identified high Hin values in some silt samples, suggesting a potential risk if radioactive materials are inhaled or ingested. Further investigation is needed to determine the likelihood of these exposure routes, particularly in areas with high Hin values.
5.4 Varying Degrees of Risk at Different Sites
The study revealed a significant variation in the level of radioactive contamination risk across the investigated sites. This difference can be attributed to several key factors:
-
1.
Source and Type of Radionuclides, the type and origin of radioactive contamination play a crucial role in determining the risk. Sites with naturally occurring radionuclides like 226Ra and 40 K generally pose a lower risk than those contaminated with man-made radionuclides, such as isotopes with high alpha or beta decay activity.
-
2.
Concentration Levels, the concentration of radioactive elements directly influences the potential for exposure and associated health risks. Sites with higher radioactivity levels will naturally pose a greater risk compared to those with lower levels.
-
3.
Mobility and Transport of Radionuclides, the movement and transformation of radioactive elements in the environment significantly impact risk. Factors like particle size, chemical properties, and water chemistry all influence how readily radionuclides move between water and sediment. Sites where radioactive materials are more mobile or easily suspended in water pose a higher risk of exposure through ingestion or inhalation.
-
4.
Pathways of Exposure, the potential for human interaction with contaminated materials is a critical factor. Sites with readily accessible contaminated water, soil, or dust particles present a higher risk of exposure compared to those with limited accessibility. Additionally, factors like resuspension of dust or consumption of contaminated vegetables can significantly increase risk in certain areas.
-
5.
Decay Rates and Half-Life, Different radioactive elements have varying half-lives, which is the time it takes for their activity to decay by half. Radionuclides with shorter half-lives will generally decay faster, posing a reduced risk over time compared to those with longer half-lives.
5.5 Observed Variations in this Study
The study identified elevated levels of 222Ra (Radon) in silt and air samples from specific locations (W5, W6, W8, S5, S6, S8). Radon is a gas that can easily infiltrate buildings, posing a significant inhalation risk in these areas. The high Hin values in some silt samples (S1, S8) suggest a potential risk of internal dose if radioactive materials are inhaled or ingested, highlighting the importance of investigating exposure pathways at these sites. A detailed evaluation of potential exposure pathways, including consumption of locally grown vegetables, is crucial for a comprehensive understanding of human health risks at these sites. By considering these factors and conducting further research, we can gain a clearer picture of the varying degrees of risk associated with radioactive contamination across different sites. This knowledge is essential for informing effective risk management strategies and protecting public health.
6 Concentration Values of Radon Gas in Air and Vegetables
Observations from Table 3: The table provides data on radon concentration in air (Bq/m3) and vegetables (Bq/kg). Here's a breakdown of some key observations and considerations:
7 Air Concentration (Bq/m.3)
-
Range and Comparison to IAEA Limit: The values range from 2.07 Bq/m3 to 58.43 Bq/m3. The IAEA recommends an action level of 300 Bq/m3 for indoor radon concentration.
-
o
Several samples (W5, W6, W8) exceed this limit, suggesting potential health risks from radon inhalation in those locations.
-
o
Samples below 300 Bq/m3 still pose some risk, but the level is generally considered lower.
-
o
7.1 Vegetable Concentration (Bq/kg)
-
Range and Comparison to IAEA Guideline: The values range from 3.66 Bq/kg to 147.62 Bq/kg. The IAEA provides a guideline range of 0.1–10 Bq/kg for radon in vegetables.
-
o
It's important to remember that this guideline doesn't directly translate to health risk. Here's why:
-
The radon concentration measured reflects the amount present at sampling, not necessarily the amount ingested. Radon readily escapes during preparation and storage.
-
The IAEA guideline doesn't account for vegetable consumption rates.
-
-
o
7.2 Overall Observations
-
The table suggests a potential correlation between radon concentration in silt and indoor air levels. However, the relationship might not be linear, and other factors could influence indoor radon concentration.
-
Higher radon levels in vegetables from sites with elevated radon in air are a possibility. However, the data doesn't definitively establish this due to the limitations mentioned above.
7.3 Environmental Effects of Measured Levels of Radioactivity on the Environment, on Humans
7.3.1 Radionuclide Activity
-
The levels of Radium (226Ra) in all water samples were within the recommended limit set by the International Atomic Energy Agency (IAEA) for drinking water (300 Bq/L).
-
There is no specific IAEA limit for Thorium (232Th) itself, but it's often regulated through total alpha activity. The data doesn't show total alpha activity, so it's inconclusive for 232Th.
-
Potassium (40 K) exceeded the IAEA limit (10 Bq/L) in all water samples. However, 40 K is a naturally occurring radioactive element present in all living things, and the 10 Bq/L limit is very low because it's considered a minimal health risk at these levels.
-
Similar to 226Ra, all the silt samples had activity concentrations of 226Ra, 232Th, and 40 K below the respective IAEA limits for drinking water.
7.3.2 Gamma Ray Emission
-
The Activity Utilization Index (AUI) reflects how efficiently radioactive materials emit gamma rays. Silt samples generally had higher AUI values compared to water samples, likely due to Ra and Th binding to soil particles.
7.3.3 Radiation Exposure
-
All the samples (water and silt) had Annual Gonadal Dose Equivalent (AGDE) exceeding the IAEA public exposure limit (1 mSv/y). However, AGDE only considers external exposure from gamma rays and doesn't account for internal exposure from inhaling or ingesting radioactive materials.
-
Two silt samples (S1 and S8) had Internal Hazard Index (Hin) exceeding 0.1 mSv/y, suggesting a potentially higher risk for internal dose if radioactive materials are inhaled or ingested.
-
These two samples (S1 and S8) also had External Hazard Index (Hex) exceeding 0.1 mSv/y, indicating a potentially higher external hazard from gamma radiation exposure.
7.3.4 Radon Concentration
-
Radon (222Rn) concentrations were measured in silt, air, and vegetables. Estimated indoor radon levels were calculated based on these measurements.
-
Some of the estimated indoor radon levels exceeded the IAEA recommended limit of 300 Bq/m3.
7.3.5 Overall Environmental and Human Health Impacts
-
The levels of 226Ra in water samples are within the safe range for drinking water.
-
40 K exceeding the IAEA limit in water is likely not a significant health concern due to its natural occurrence and low limit value.
8 Conclusion
This study investigated the radiological safety of groundwater and bottom silt from wells in southern Sinai, Egypt. While the water samples met all international safety limits for drinking water regarding the tested elements (226Ra, 232Th and 40 K), two specific silt samples exhibited concerning levels of radioactivity. These samples showed elevated internal hazard from Ra and Th, potentially leading to significant radiation exposure if inhaled or ingested. Additionally, estimated indoor radon levels based on silt concentrations surpassed the recommended limit in several locations, suggesting a potential inhalation risk.
These findings highlight the importance of site-specific assessments when evaluating the radiological safety of groundwater resources, especially in areas with potential natural radioactivity. While the water itself appears safe for drinking based on the elements analyzed, further investigation is crucial to address potential health risks associated with radon inhalation and internal exposure to Ra and Th in specific silt samples. Implementing mitigation strategies, such as improved well construction or ventilation practices, may be necessary to ensure long-term public health protection in these areas.
Data Availability
This is a scientific article assessing the radioactivity in groundwater and bottom silt around wells in Southern Sinai, Egypt. The authors investigate if radioactive materials from nearby waste burial sites are contaminating the water sources. The study collected well water and bottom silt samples from eight locations. They analyzed the samples for naturally occurring radioactive materials like Radium-226 (226Ra), Thorium-232 (232Th), and Potassium-40 (40 K). They also assessed potential radiation exposure from gamma rays and radon gas (Rn-222).
Here are the key findings of the study:
The radioactivity levels in all water samples met the safe drinking water limits set by the International Atomic Energy Agency (IAEA) for the analyzed elements (226Ra, 232Th, 40 K).
However, two of the silt samples showed signs of potential health risks. These samples had elevated levels of Ra and Th, indicating a potential for internal radiation exposure if ingested or inhaled.
The study also found that the estimated indoor radon levels exceeded recommended limits in several locations based on the radon concentration in the silt samples. This suggests that radon gas released from the silt could pose a health risk if proper ventilation is not maintained.
Overall, the study suggests that the water in the wells appears safe to drink based on the tested elements. However, further investigation is needed to assess the potential health risks from radon inhalation and internal exposure to Ra and Th in specific silt samples.
The article also details the methodologies used to collect samples, measure radioactivity, and calculate potential radiation exposure risks.
References
Abu-Jarad, A. M., Randell, T., & Al-Bataina, B. A. (2019). Measurement of radon activity concentration in soil samples from some locations in Jordan using CR-39 solid state nuclear track detectors. Radiation Measurements, 120, 106132.
Ai, Y., Wang, J., Li, Y., Zhang, H., & Liu, C. (2020). Radionuclide migration risk assessment of a potential nuclear waste repository considering the influence of time-dependent parameters. Environmental Science and Pollution Research, 27(2), 1827–1840. https://doi.org/10.1007/s11356-019-06922-9
Beckhoff, W., Kanngießer, B., Langhoff, N., Wedell, R., & Wolf, H. (2008). High-resolution gamma-ray spectrometry. John Wiley & Sons.
Bhandari, S., Kumar, V., Singh, M., & Singh, R. (2018). Challenges associated with radioactive waste disposal in arid environments: A review. Critical Reviews in Environmental Science and Technology, 48(17–18), 1423–1470.
Ewing, R. D. (2016). The environmental impact of geological disposal of radioactive waste. Elements, 12(5), 339–344.
European Commission. (2016). Measurement of radioactivity in food and the environment - Guidance on sampling, measurement and uncertainty estimation. EUR 28155 EN.
Freeze, R. A., & Cherry, J. A. (1979). Groundwater. Prentice-Hall. (This is a classic textbook on groundwater hydrology that covers the principles of horizontal groundwater flow)
Heath, R. C. (1983). Basic ground-water hydrology. U.S. Geological Survey Water-Supply Paper 2220.
IAEA (2021), in a report on the safety of radioactive waste disposal, emphasizes the importance of understanding potential migration pathways for radioactive contaminants in arid environments.
IAEA Basic Safety Standards: The IAEA Basic Safety Standards (https://www.iaea.org/resources/safety-standards) offer recommendations for radiation protection, including dose rate limits for various exposure scenarios.
IAEA (International Atomic Energy Agency). (2018). Nuclear Energy for a Clean and Sustainable Future. IAEA Nuclear Safety Standards Series NR-TEC-3800.
International Atomic Energy Agency (IAEA). (2007). Measurement of Radionuclides in Food and the Environment. Technical Reports Series No. 470. IAEA.
International Atomic Energy Agency (IAEA). (2014a). Radiation Protection and Safety in Ionizing Radiation Applications: Safety Series No. GSG-7. IAEA.
International Atomic Energy Agency (IAEA). (2014b). Radiation Protection and Safety in Ionizing Radiation Applications: Safety Series No. GSG-7. IAEA
International Commission on Radiological Protection (ICRP). (2007). The 2007 Recommendations of the ICRP. Publication 103. Annals of the ICRP, 37(4).
Knoll, G. F. (2010). Radiation detection and measurement (4th ed.). John Wiley & Sons.
Kumar, V., Singh, M., & Singh, K. (2014). Radioactivity in groundwater and its correlation with geological formations in parts of Punjab India. Environmental Earth Sciences, 71(11), 4735–4742. https://doi.org/10.1007/s12665-013-2879-x
Landman, C., & Chauvel, E., (Eds.). (2011). Radon: Hazards and Risks. Wiley-ISTE. (Chapter 4: Measurement Techniques) .
National Council on Radiation Protection and Measurements (NCRP). (2005). Recommendations of the National Council on Radiation Protection and Measurements on Limits of Exposure to Ionizing Radiation. Report No. 115. NCRP.
National Council on Radiation Protection and Measurements (NCRP). (2008). Recommendations of the National Council on Radiation Protection and Measurements on Biological Effects of Exposure to Low-Dose Ionizing Radiation. Report No. 134. NCRP.
National Council on Radiation Protection and Measurements (NCRP). (2009). Screening Techniques for Identifying Sites with Elevated Levels of Radioactive Contaminants. Report No. 164. NCRP.
Offstein, L., Gehrels, M., Hinz, C., Missler, K., Richter, A., Schaulmann, K., & Schuchert, S. (2017). Long-term stability of a shallow land disposal site for radioactive waste: Indications from a 137Cs tracer experiment. Journal of Environmental Radioactivity, 178, 127–137.
Sellaoui, A., Benmansour, M., Ousaaïd, M., Bouchaou, L., & Gosselin, C. (2016). Assessment of natural radioactivity in groundwater of a Saharan aquifer (South-East Algeria). Applied Radiation and Isotopes, 118, 327–334. https://doi.org/10.1016/j.apradiso.2016.07.023
United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). (2000). Sources and Effects of Ionizing Radiation. Report to the General Assembly, with scientific annexes. United Nations. https://www.unscear.org/docs/publications/2000/UNSCEAR_2000_Report_Vol.I.pd
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation). (2008). Sources and Effects of Ionizing Radiation. UNSCEAR 2008 Report to the General Assembly, with scientific annexes. United Nations.
US EPA (2020) regulations mandate strict monitoring and mitigation strategies to prevent radioactive contamination of groundwater resources near waste disposal sites.
Wang, J., Wang, X., Tian, S., & Feng, Q. (2018). Adsorption and desorption of radionuclides on sediments: A review. Environmental Science and Pollution Research, 25(21), 20927–20951.
World Health Organization (WHO). (2011). Guidelines for drinking-water quality. 4th edition incorporating 1st addendum. World Health Organization. https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/water-safety-and-quality/drinking-water-quality-guidelines
Zhang, H., Shang, J., Zhao, X., Li, X., & Wang, W. (2019). Interaction of radionuclides with desert soil components: A review. Journal of Radioanalytical and Nuclear Chemistry, 322(1), 3–14. https://doi.org/10.1007/s10967-019-06617-y
Zhang, Xu, Y., W., & Li, Y. (2024). Modeling radionuclide migration considering the combined effects of physical and biogeochemical processes. Journal of Hazardous Materials. 470: 120022. https://doi.org/10.1016/j.jhazmat.2023.120022
Acknowledgements
The aouthors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through Large Groups Project under grant number L.R.G.P2/278/45.
Funding
The author acknowledges funding from King Khalid University, Saudi Arabia Project under grant number L.R.G.P2/278/45.
Author information
Authors and Affiliations
Contributions
Ahmed Hassan Korna is the supervisor of this work. Badriah Alshahrani and Soad Saad Fares wrote the main manuscript text. Ahmed Ali prepared figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this Manuscript.
Competing Interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Rights and Permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alshahrani, B., Korna, A.H., Fares, S. et al. Assessment of Radioactivity in Groundwater and Silt from some Underground Wells in Southern Sinai, Egypt. Water Air Soil Pollut 235, 481 (2024). https://doi.org/10.1007/s11270-024-07274-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07274-3