Abstract
This study employs multivariate analysis to evaluate the effectiveness of common indices in assessing surface water quality. The research involves collecting and analyzing 60 samples from the Bomadi and Gbotebo rivers and their associated tributaries. The samples are subjected to comprehensive testing to determine their hydrogeochemical composition, suitability for irrigation, and pollution levels. The study finds that most parameters conform to the standards set by the World Health Organization (WHO) and Standard Organization of Nigeria (SON), although occasional instances of elevated cadmium and iron levels are observed. The evaluation of various indices offers insights into the overall quality of the water and its suitability for different uses. The values of the potential salinity (PSi), sodium absorption ratio (SARi), and residual sodium carbonate (RSCi) indices indicate excellent water quality and health conditions. However, other indices like sodium soluble percentage (SSPi), magnesium hazard ratio (MHRi), Kelly ratio (KRi), and percentage sodium (%Na) suggest only marginal suitability for irrigation. Pollution levels, gauged by the pollution index of the surface water (PISw) index, remain consistently low. Assessing health risks stemming from trace elements (TEs) in the water reveals minimal carcinogenic threats through oral route exposure. Correlation analysis establishes a meaningful connection between water evolution indices and the broader water quality and health risks, although this correspondence is not mirrored among ratio indices—except for K/Cl. Within the spectrum of 22 indices, the study identifies four that notably contribute to water classification. Utilizing principal component analysis (PCA), the research successfully highlights the primary sources of variability among the indices, accounting for an impressive 92.392% of the total variance. In conclusion, this study showed the effectiveness of commonly employed indices in evaluating surface water quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water, an invaluable natural resource, holds a paramount position on our planet, serving critical roles for all life forms and functioning as a cornerstone of social and economic dynamics within society (Madhav et al., 2021a). The chemistry of water, largely influenced by human activities and to a lesser extent by geological formations, can also be impacted by natural factors such as precipitation patterns, geological characteristics of watersheds and aquifers, climatic conditions, and diverse interactions between rocks and water in aquifers (Elangovan et al., 2018; Madhav et al., 2021a, b).
Regrettably, the quality of natural water has seen a decline due to numerous human interventions. The nature of these interventions varies according to regional human activities. For instance, in Nigeria’s coastal regions, activities like dredging (both motorized and artisanal), agricultural practices along riverbanks and creeks, water transportation, oil and gas exploration, urbanization, industrialization, and the discharge of effluents and solid waste materials, including abattoir waste, into surface water bodies, have collectively led to pollution of these resources (Agedah et al., 2015; Aghoghovwia et al., 2018a, b; Ben-Eledo et al., 2017; Izah & Angaye, 2016; Izah et al., 2021a, 2022a, 2023; Ogamba et al., 2015a, b, c, 2021; Seiyaboh & Izah, 2017a, b). Furthermore, groundwater has not been spared, particularly in areas with high water tables.
Waste, a prominent pollution source, has gained significant attention. In Nigeria’s Bayelsa State, solid municipal waste is released untreated into surface water bodies. Effluents from food processing industries like cassava and oil processing also find their way into these waters. Moreover, treated effluents from oil and gas operations are discharged into surface water resources. These waste products often contain toxic or hazardous substances, causing adverse effects on both humans and aquatic organisms. Notably, heavy metals are frequently identified as potential hazards in waterborne wastes. Consequently, these wastes can disrupt water chemistry, altering factors such as pH, conductivity, salinity, total dissolved solids, nitrate, sulfate, phosphate, bicarbonate, and the presence of potassium, magnesium, sodium, and calcium.
Understanding the evolving hydrogeochemical and geochemical characteristics is pivotal for effective water resource management. Water quality is intricately tied to physicochemical parameters, influenced by geological attributes and human behavior unique to each location (Madhav et al., 2018). Feldspar and carbonate, prevalent minerals within the Earth’s crust, are important for maintaining natural water chemistry. Rapid dissolution of carbonates like calcite and dolomite significantly impacts water chemistry (Kim et al., 2004; Madhav et al., 2021b).
Manipulation of water quality through domestic and industrial effluent control and industrial and agricultural practices exemplifies human influence (Madhav et al., 2021b). Similar to groundwater, surface water serves diverse functions, including drinking, domestic uses like cooking and washing, agricultural needs, and involvement in biogeochemical processes (Huang et al., 2013; Madhav et al., 2021b).
Numerous studies have explored hydrogeochemical features and pollution levels in water bodies due to human activities like waste deposition and urban runoff (Ben-Eledo et al., 2017; Izonfuo & Bariweni, 2001; Ogamba et al., 2015a, b, c; Seiyaboh et al., 2017). Water suitability is assessed through hydrogeochemical facies, with common indices including sodium absorption ratio (SARi), residual sodium carbonate (RSCi), sodium soluble percentage (SSPi), magnesium hazard ratio (MHRi), Kelly ratio (KRi), percentage sodium (%Na), permeability index (PIi), potential salinity (PSi) (Verma & Singh, 2021; Verma et al., 2020; Madhav et al., 2018, 2021a, b), sodium-magnesium ratio, sodium-chloride ratio, bicarbonate-to-(sum of bicarbonate and sulfate) ratio, potassium–chloride ratio, and sulfate–chloride ratio (Madhav et al., 2021a, b). Other indices used in assessing the hydrogeochemical quality of water include overall water quality (OWQ) and pollution index (Verma & Singh, 2021; Verma et al., 2020; Madhav et al., 2018, 2021a, b). These ratios and indices are usually calculated based on the ions (calcium, sodium, magnesium, sodium, sulfate, nitrate, chloride) found in water and other physicochemical parameters such as pH, total dissolved solids, and conductivity.
High levels of trace elements (TEs) in surface water pose health concerns (Izah et al., 2016, 2017). A risk-hazard model evaluates TEs’ risk and toxicity in ecological studies, involving air, water, and soil (Izah et al., 2021b; Ogamba et al., 2021; Uzoekwe et al., 2021). Indices assess non-carcinogenic hazards, including daily intake, hazard quotient, total target hazard quotient, and hazard index.
Multivariate analyses like correlation matrices, factor analyses, and cluster studies reveal pollution sources and anthropogenic contributions (Lu et al., 2011). Polluted water can cause health issues through exposure modes like ingestion and skin contact. Multivariate analysis has been applied to assess water quality (Madhav et al., 2018; 2021a, b; Ogamba et al., 2021), highlighting human activities and natural factors as primary pollution causes. However, applying multivariate analyses to commonly used water assessment indices is underrepresented in the literature.
Hence, this research aims to understand hydrogeological and geochemical processes by (i) assessing the concentration of hydrogeochemical and trace elements; (ii) applying correlational and multivariate analyses in the study of the hydrogeochemical and trace elements variables in water; (iii) determining the suitability of the water for drinking, domestic, and agricultural purposes using water quality, health risk indices, and ratios; and (iv) applying Pearson correlation and MA in the study to determine the suitability of the water sources for drinking, domestic, and agricultural purposes using established water quality, health risk indices and ratios of water from the Bomadi and Gbotebo rivers, and their environs in Bayelsa State, Nigeria. The findings of this study will be useful to water quality scientists and environmental scientists who use models or indices to study the pollution level of water resources.
2 Materials and Methods
2.1 Study Area
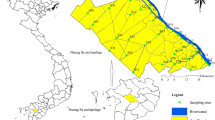
The research was conducted within the vicinity of Bomadi and Gbotebo rivers in the Ekeremor Local Government Area of Bayelsa State (Fig. 1). These rivers are part of the Focardos River’s tributary system, originating in Delta State and flowing into Bayelsa State. The local population benefits economically from the resources these rivers offer.
2.2 Sample Collection
In the context of this study, a total of 12 sampling stations were established. Five water samples were collected at each of these stations, resulting in a combined total of 60 water samples (calculated by multiplying 5 samples per station by 12 stations). For the purpose of analyzing TE, the water samples were gathered from the midsection of the river using 1-L plastic containers. These containers were thoroughly rinsed with HNO3 before collection. On the other hand, for other types of analysis, water samples were taken using 1-L containers rinsed with distilled water. The field analysis involves assessing various in situ characteristics of the water, including temperature, conductivity, total dissolved solids, salinity, and pH. To facilitate proper identification, the surface water samples were assigned labels ranging from SaP1 to SaP12, corresponding to the different sampling points. In preparation for laboratory analysis, the samples intended for such purposes were carefully stored in a cooler surrounded by ice to maintain their integrity. Additionally, the samples intended for analysis of TEs were preserved by adding 1 mL of HNO3 acid for every 500 mL of water samples. It is worth noting that all samples were analyzed within a 96-h timeframe following their collection.
2.3 Laboratory Analysis
2.3.1 In Situ and Anion Analysis of the Water Samples
The in situ parameters, including pH, conductivity, salinity, temperature, and total dissolved solids, were assessed using a multimeter known as the OAKTON PCTSTestrtm 5 multiparameter device. These analyses were conducted according to the manufacturer’s instructions. A 3-point calibration was followed using solutions with pH values of 7.00, 4.00, and 10.01 to determine pH. Meanwhile, the meter was calibrated for conductivity measurement using 3-point standard calibration standards: 84 µS/cm, 1413 µS/cm, and 12.88 mS/cm. The multimeter also facilitated the conversion of conductivity measurements into total dissolved solids and salinity measurements through a specific conversion factor. The range of measurements provided by the multimeter involves the following intervals: pH (− 2.00 to 16.00), conductivity (0.00 µS/cm to 20.00 mS/cm), total dissolved solids (0.10 ppm to 10.00 ppt), salinity (0.00 to 10.00 ppt), and temperature (0.00 to 50.00 °C).
2.3.2 Sulfate, Nitrate, Carbonate, Chloride Determination
Nitrate, phosphate, and sulfate concentrations in the water were evaluated using UV spectrophotometric screening (APHA 4500-NO3-B), ascorbic acid technique (APHA 4500-PE), and turbidimetric method (APHA 4500-SO42-E), respectively, as previously described by Izah (2019).
2.3.3 Trace Elements and Macronutrients Analysis of the Water Samples
The macronutrients and TEs in the water samples were analyzed using a flame atomic absorption spectrophotometer (GBC 908 PBMT model, GBC Scientific-Australia). This involved aspirating the water directly into the flame atomic absorption spectrophotometer system. The concentration of each component was determined by extrapolating from the absorbance-concentration plot and was then recorded in milligrams per liter (mg/L).
Throughout the analysis process, specific operational conditions outlined in Supplementary Materials 1–2 and rigorous quality control methods were adhered to. These quality control measures encompassed actions such as aspirating reagent blanks, repeating sample collection and analysis, and conducting spike recovery tests. The percentage of recovery was calculated using Eq. 1. Spike recovery procedures were carried out in parallel using certified reference standards in conjunction with the actual test samples. The concentration of trace elements and macronutrients was subsequently computed to ascertain the analyte’s retrievable amount. The recovery rates achieved ranged from 92.0 to 98.3%. Following the analysis of every set of ten samples, a quality standard check was performed to ensure consistency and reliability. To determine the limits of detection (LOD) and limits of quantitation (LOQ), the standard deviation of the absorbance response and the slope value of the calibration curve were employed. These values and the percent spike recoveries of the test metals are detailed in Supplementary Materials 2 and 3. Calculations for recovery rate, LOD, and LOQ were performed using Eqs. 1, 2, and 3, respectively.
where MLSS is the metal level in the spiked sample, MLU.SS is the metal level in the unspiked sample, Std dev is the standard deviation, SCC is the slope calibration curve, and 3.3 and 10 are constant factors.
Note that when the concentration of the metals in the sample is below the equipment detection level, it is denoted as BEDL.
2.4 Water Quality Indices
2.4.1 Overall Water Quality or Water Pollution Index
In this research, 12 parameters were carefully selected to compose the comprehensive water quality index (OWQ). These parameters encompass conductivity, pH, total dissolved solids, calcium, magnesium, sodium, potassium, nitrate, sulfate, bicarbonate, chloride, and iron. The OWQ is a significant metric for assessing water quality suitability for human consumption, as indicated by studies conducted by Sahu and Sikdar (2008) and Guettaf et al. (2017).
To establish the OWQ, a five-stage methodology, previously employed by Verma and Singh (2021), was adopted in this study. The initial stage involved assigning relative weights (RWi) to the parameters on a scale ranging from 1 to 5, as established by Rao (2012) and further detailed in the work of Verma and Singh (2021). Subsequently, the second stage of the process entailed the computation of weight parameters (WPi) using Eq. 4, which is expounded upon in Table 1.
The third stage involves computing each parameter’s concentration status (CSi) using Eq. 5. The fourth stage involves determining the overall water quality parameter through Eq. 6. Subsequently, the fifth stage involves the evaluation of the pollution index of the surface water (PISw) for each sample. This is achieved through the application of Eq. 7, using the 12 parameters that were selected. The specific relative weights, as well as the standards from the World Health Organization (WHO) and the Nigerian Standard Organization (SON) water guidelines, are presented in Table 1.
where RWi is the related weight of the selected parameters.
where CWi is the concentration of each selected parameter in the surface water and SLi is the standard limit (WHO and SON, Nigeria) of the water.
where WPi is the weighted parameters and CSi is the concentration station of the parameters.
where OWQ is the overall water quality parameter.
The criteria for classification for PISw according to Rao, 2012, 2018) and Verma and Singh (2021) include < 1.0 (no pollution); PISw < 1.0 (no pollution); 1.0 ≥ PISw ≤ 1.5 (low pollution); 1.5 ≥ PISw ≤ 2.0 (slight/moderate pollution); 2.0 ≥ PISw ≤ 2.5 (heavy pollution); and PISw > 2.5 (very heavy pollution).
2.4.2 Natural Evolution of Water and Water Type
The analysis of water suitability for natural and domestic uses, particularly in the context of irrigation, involves the examination of ion levels such as calcium, sodium, magnesium, sulfate, nitrate, chloride, and bicarbonate in water (Madhav et al., 2018, 2021a, b; Verma & Singh, 2021; Verma et al., 2020). These ions are crucial in determining the type of water and its intended purpose. These waterborne ions serve as the basis for calculating significant indices and ratios, including SARi, RSCi, SSPi, MHRi, KRi, %Na, PIi, and PSi (Madhav et al., 2018, 2021a, b; Verma & Singh, 2021; Verma et al., 2020). Furthermore, additional ratios are often computed, such as the sodium-magnesium ratio, sodium-chloride ratio, bicarbonate-to-(sum of bicarbonate and sulfate) ratio, potassium–chloride ratio, and sulfate–chloride ratio (Madhav et al., 2021a, b).
The SARi, RSCi, SSPi, MHRi, KRi, %Na, PIi, and PSi were calculated following the method previously applied by Verma and Singh (2021) and Madhav et al., (2018, 2021a, b). The concentration of all ions is expressed in meq/L.
The criteria for classifying SARi based on irrigation purposes include 0 ≥ SARi ≤ 10 (excellent), 10 ≥ SARi ≤ 18 (good), 18 ≥ SARi ≤ 26 (fair), and SARi > 26 (poor) (Verma & Singh, 2021; Verma et al., 2020; Madhav et al., 2021a, b; Guettaf et al., 2017).
The criteria for classifying RSCi by Richards (1954) as applied by Verma and Singh (2021) and Madhav et al., (2018, 2021a, b) include RSCi < 1.25 (Safe), 1.25 ≥ RSCi ≤ 2.50 (marginal), and RSCi > 2.5 (unsuitable).
The criteria for classifying %Nai by Wilcox (1955) as applied by Verma and Singh (2021), Madhav et al., (2018, 2021a, b), and Guettaf et al. (2017) include 0 ≥ %Nai ≤ 20 (excellent), 20 ≥ %Nai ≤ 40 (good), 40 ≥ %Nai ≤ 60 (permissible), and 60 ≥ %Nai ≤ 80 (doubtful) (Verma & Singh, 2021; Verma et al., 2020; Madhav et al., 2021a, b; Guettaf et al., 2017).
Sodium-magnesium ratio, sodium-chloride ratio, bicarbonate -(sum of bicarbonate and sulfate) ratio, potassium–chloride ratio, and sulfate–chloride ratio were calculated following the method previously applied by Madhav et al., (2021a, b).
2.4.3 Health Risk Assessment of Trace Metals in Water Samples
The health risk was calculated through two pathways (dermal and ingestion route). The average daily intake for the two routes (dermal and oral) was calculated following the method previously discribed by Ogamba et al. (2021). The meaning of the abbreviations used in this section and the corresponding values are presented in Supplementary Material 3.
Average Daily Dose (ADD)
Hazard Quotient (HQ) and Hazard Index (HI)
Hazard index and hazard quotients were calculated following the method previously described by Mohammadi et al. (2019), Rezaei et al. (2019), and Ogamba et al. (2021).
where the RFD (µg/kg/day) for ingestion and dermal are 0.50 and 0.025 (cadmium), 40 and 8 (copper), 700 and 140 (iron) (Ogamba, 2021), and 300 and 60 (zinc), respectively (Mohammadi et al., 2019).
Carcinogenic Hazard
Carcinogenic hazards of TEs are often used to determine the probable risk of developing cancer over a life time due to exposure to the carcinogenic metals. Carcinogenic hazards of TEs are the product of the ADD and carcinogen slope factor (CSF) (Aigberua et al., 2021)
The CSF of cadmium is 6.10 (Aigberua et al., 2021).
The carcinogenic hazard was compared based on the criteria previously presented by the authors (Adeyemi & Ojekunle, 2021; Aigberua et al., 2021; Joel et al., 2018) (Supplementary Material 4).
2.5 Statistical Analysis
The data analysis involves the utilization of both Minitab 17 and SPSS version 20. Initially, descriptive statistics were conducted on the raw data, and values were presented as mean ± standard deviation (n = 5) and range, i.e., minimum–maximum in parentheses. Additionally, a one-way analysis of variance was employed to highlight any significant differences among water samples taken from the various sampling stations. The Tukey Honestly Significant Difference Test (THSD) statistics were employed to elucidate the origins of these dissimilarities where they exist. The connections between measurement variables were carried out using both Pearson correlation and multivariate analyses (PCA and cluster analysis (CA) utilizing the Euclidean distance and Ward linkage). These methods served to illustrate the interrelationships and associations within the hydrogeochemical and geochemical characteristics of the water. Furthermore, multivariate analysis and Pearson correlation were employed to elucidate the links and relationships driving the variations observed in the water’s hydrogeochemical and geochemical attributes.
3 Results and Discussion
3.1 Hydrogeochemical and Geochemical Characteristics of the Surface Water
3.1.1 Temperature
Table 2 displays the distribution of the in situ characteristics found in the surface water of the Bomadi and Gbotebo rivers and their surrounding areas within Bayelsa State, Nigeria. The water temperature fell within the range of 26.50–27.20 °C. Notably, the temperature data exhibited a statistically significant variation (p < 0.05) across the different sampling points. Further examination using the Tukey-HSD test revealed that the concentration in SaP3 accounted for the observed statistical discrepancy. This variation might possibly be attributed to the sun’s intensity before the water was collected from the mentioned sampling station. This notion is supported by findings from Ogamba et al. (2015a) and Agedah et al. (2015), who indicated that the timing of sampling could indeed impact the temperature of surface water.
Temperature holds a significant role in maintaining the health of aquatic ecosystems and influencing the rate of chemical reactions. The magnitude of temperature variation is contingent upon prevailing weather conditions. The temperature data obtained in this study falls within the guidelines established by the World Health Organization (WHO) at 40 °C (Soleimani et al., 2022), as well as aligns with the ambient temperature of the specific region, as indicated by Richard et al. (2019). Furthermore, the recorded temperature aligns with the range of values (25.00–32.00 °C) documented in various studies conducted within Bayelsa State (Ebuete & Bariweni, 2019; Eremasi et al., 2015; Izonfuo & Bariweni, 2001).
3.1.2 pH
The pH levels of the water exhibited a range from 5.45 to 7.35, displaying statistically significant differences (p < 0.05) across the various sampling points. Analyzing the THSD test statistics, it is evident that SaP8 and SaP9, SaP2 and SaP11, SaP3 and SaP5, and SaP1 and SaP5 did not exhibit any noteworthy dissimilarity (p > 0.05). It is worth noting that the instability of water pH could potentially account for the observed variations. Variations in pH values could be attributed to factors like the timing of sampling and prevailing anthropogenic activities, including direct disposal of household waste into the water, human activities such as boating, and surface runoff following precipitation.
An elevated pH in water suggests robust photosynthetic activity by phytoplankton and algae, leading to the removal of dissolved carbon dioxide and a shift in acidity toward an alkaline level (Hennemann and Petrucio, 2011; Jayakumar et al., 2013; Nazneen et al., 2019). Overall, only the mean values of SaP9 and SaP10, accounting for 16.67% of the sampling stations, showed a pH lower than the permissible limits established by the World Health Organization (WHO) and the Nigerian Standard Organization (SON) (6.50–8.50).
The recorded values are consistent with previously reported concentrations (ranging from 5.53 to 7.70) in various surface waters within Bayelsa State (Izonfuo & Bariweni, 2001; Seiyaboh et al., 2013; Daka et al., 2014; Agedah et al., 2015; Eremasi et al., 2015; Ogamba et al., 2015a, b, c; Ben-Eledo et al., 2017; Aghoghovwia et al., 2018a; Aigberua & Tarawou, 2019; Ebuete & Bariweni, 2019). The consistency in pH values across these bodies of water suggests a resemblance in the human activities occurring in the region’s waterways.
3.1.3 Salinity
The salinity levels of the water fell within the range of 0.01–0.07 ppt. There were statistically significant deviations (p < 0.05) observed across the various sampling stations. However, upon analyzing the THSD test statistics, it was revealed that SaP9, SaP11, and SaP12 were the sources of these deviations. These statistical deviations indicate that human-induced factors present at these specific sampling stations are causing alterations in the concentration of non-carbonate salts within the water. This alteration suggests the presence of certain factors that elevated water conductivity during the time of sampling. A notable example is SaP12, which exhibited a mean salinity of 0.07 ppt, and its conductivity was considerably higher than the readings recorded at other sampling points (as detailed in Table 2).
Salinity represents the cumulative content of non-carbonate salts, predominantly chloride and sodium ions, dissolved within water. Marine waters may also contain potassium, magnesium, or sulfate ions. Salinity significantly influences the diversity of organisms populating aquatic ecosystems, ranging from fish, reptiles, and mammals to amphibians, plankton, microbes, macrophytes, and plants.
The salinity distribution exhibited notable stratification within the study area, as evidenced by prior research (Nazneen et al., 2019). The observed salinity values share similarities with concentrations reported earlier (ranging from 0.00 to 2.58 ppt) in the Nun River and other surface water bodies in Bayelsa State (Agedah et al., 2015; Aghoghovwia et al., 2018a; Ben-Eledo et al., 2017; Ogamba et al., 2015a, b, c). Notably, the salinity values in this study and previous research on freshwater resources in Bayelsa State remain below the World Health Organization (WHO) limits of 100–200 ppt, as reported by Iroha et al. (2020).
3.1.4 Conductivity
The conductivity of the water ranged from 29.44 to 175.80 µS/cm. Statistically, there were discernible differences (p < 0.05) in the concentration of conductivity across the various sampling locations. The THSD test statistics indicated no significant variance between SaP1 and SaP5 nor between SaP2 and SaP3. A more substantial and statistically significant variation was evident in SaP12, which suggests an elevated level of human activity preceding the sampling. Importantly, the values remained consistently below the limits established by the SON and the WHO, which set the threshold at 1000 µS/cm, as noted by Soleimani et al. (2022) and SON (2007).
The relatively low conductivity values reflect the water’s freshness and provide initial insights into its salt content. These recorded values at the sampling points exhibit similarities with concentrations reported in surface water resources within Bayelsa State, Nigeria. Except for the control point (SaP12), these values fall within the ranges of 47.70–89.33 µS/cm (Izonfuo & Bariweni, 2001) and 31.60–78.80 µS/cm (Ogamba et al., 2015a, b). Importantly, they remain lower than ranges reported by other authors, such as 56.08–64.95 µS/cm (Agedah et al., 2015), 54.00–63.00 µS/cm (Daka et al., 2014), 57.30–78.80 µS/cm (Ogamba et al., 2015c), and 59.70–71.65 µS/cm (Aghoghovwia et al., 2018a).
The recorded conductivity values across all sampling points were also notably lower than those (ranging from 265.00 to 1096.75 µS/cm) found in Epie Creek, as Ben-Eledo et al. (2017) reported. The variations in conductivity observed in this study compared to prior research indicate the influence of human activities in diverse water bodies. A similar trend was reported by Ben-Eledo et al. (2017) in Epie Creek, where elevated conductivity was attributed to waste deposition from various sources, including markets, abattoirs, households, and runoff after rainfall.
Numerous human activities occurring directly in the water or near the sampling locations could alter essential characteristics like conductivity. These activities include artisanal sand mining, fishing, ridge farming using herbicides, boating, waste disposal, sawmills, mechanic workshops, crude oil pipelines, welding, and slaughterhouses.
The notable increase in SaP12’s conductivity implies that a substantial concentration of ions contributes to the heightened conductivity in the water before sampling. These ions could originate from waste disposal into the water or other materials washed into the water through runoff following precipitation prior to sampling.
3.1.5 Total Dissolved Solid
The total dissolved solid (TDS) concentrations in the water spanned a range of 21.40–125.70 mg/L (as detailed in Table 2). Notably, there were statistically significant differences (p < 0.05) in TDS concentrations among the various sampling points. The THSD analysis revealed no considerable variation between SaP2 and SaP3 and between SaP5 and SaP11. The observed disparities in TDS concentration across the rivers could be attributed to escalated human activities within these aquatic environments. A sudden elevation in TDS content in SaP12 indicates the impact of human activities that lead to heightened ion levels. These activities include the disposal of waste materials containing elevated amounts of nitrate, phosphate, sulfate, ammonia, and more, into the water (Angaye et al., 2015).
Essentially, TDS values provide insights into inorganic salt levels and other dissolved substances within the water. Importantly, the recorded TDS values stand well below the permissible limits established by the SON and the WHO, which set a 500-mg/L threshold. These lower values may stem from reduced water flow rates and minimal human activities. Conversely, higher values could be linked to agricultural practices, organic matter, anthropogenic actions, surface runoff, etc.
The concentrations recorded in this study exhibit similarities with previous research within Bayelsa State, Nigeria, particularly the value range of 10.33–548.50 mg/L in rivers and creeks (Epie, Taylor, Kolo, Ikoli creeks, and Nun river) (Agedah et al., 2015; Aghoghovwia et al., 2018a; Aigberua & Tarawou, 2019; Ben-Eledo et al., 2017; Daka et al., 2014; Ebuete & Bariweni, 2019; Izonfuo & Bariweni, 2001; Ogamba et al., 2015a, b, c).
Moving on to the cationic and anionic characteristics of the water from the Bomadi and Gbotebo rivers and their surroundings in Bayelsa State, Nigeria, these are detailed in Table 3. The concentration of anions covered a range of 0.00–4.00 mg/L for sulfate, 0.20–0.90 mg/L for nitrate, 1.00–7.00 mg/L for bicarbonate, and 10.00–2.00 mg/L for chloride. Similarly, statistical differences (p < 0.05) were noted in the concentration of nitrate, sulfate, and chloride, whereas bicarbonate displayed no statistical deviation across the sampling points. The observed variations indicate human activities contributing to water ionic composition changes.
The recorded anion concentrations in this study remain lower than the guideline limits set by the WHO and SON. The WHO and SON stipulated allowable levels of 250 mg/L for sulfate and 50 mg/L for nitrate (SON, 2007; Soleimani et al., 2022). Additionally, the WHO set limits of 500 mg/L for bicarbonate and 200 mg/L for chloride, while the SON does not provide specific limits for bicarbonate and chloride.
Furthermore, the concentration of these ions aligns with previous studies conducted in Bayelsa State, Nigeria. Concentration ranges of 1.79–4.25 mg/L for bicarbonate, 0.50–61.75 mg/L for chloride, 0.41–24.74 mg/L for sulfate, and 0.01–4.12 mg/L for nitrate have been documented in various water bodies, including Taylor Creek and Nun River (Aghoghovwia et al., 2018a; Aigberua & Tarawou, 2019; Ben-Eledo et al., 2017; Daka et al., 2014; Ebuete & Bariweni, 2019; Izonfuo & Bariweni, 2001; Ogamba et al., 2015a, b, c; Seiyaboh et al., 2013).
Anions play crucial roles in various chemical and biological processes, impacting functions such as acid–base balance, neuronal transmission, cell membrane permeability, and hormone production (Kawashima et al., 2013). Iodide and bicarbonate play important roles in producing hormones, although iodide is particularly important in this process (Vargas-Zúñiga & Sessler, 2017). The other anions also have distinctive chemical and biological activities, and for a very long time, people have wished for the development of detection methods that are both efficient and accurate for the other anions (Kawashima et al., 2013). These anions’ presence suggests their beneficial role in supporting aquatic organisms within the studied surface water. Furthermore, the study suggests no significant pollution from human activities in the study area.
Table 4 shows the TE characteristics of the surface water of the Bomadi and Gbotebo rivers and their environs in Bayelsa State, Nigeria. The study showed that elements such as cobalt, nickel, and lead were not detected in the water, suggesting that pollution due to their presence was not present in the studied water. The concentrations of the detected elements were in the range of 1.323–2.813 mg/L, BEDL–0.045 mg/L, BEDL–0.177 mg/L, and BDEL–0.008 mg/L for iron, copper, zinc, and cadmium, respectively.
There were statistical deviations (p < 0.05) in the mean concentrations of iron and zinc, while no statistical deviation (p > 0.05) was observed in the mean values of copper and cadmium. The study revealed the presence of iron and zinc in all sampling points, whereas copper and cadmium were detected in only a subset of the sampling points. Specifically, copper and cadmium were detected in four of the 12 sampling points (SaP4, SaP6, SaP10, and SaP12). The detection of cadmium at these points could be attributed to the level of human activity in those locations. The concentrations of the detected elements exceeded the permissible values set by the SON, with iron exceeding the limit of 0.30 mg/L and cadmium exceeding both the SON and WHO limits of 0.003 mg/L. However, the concentrations remained below the SON and WHO limits of 1.00 mg/L and 2.00 mg/L for copper and the SON limit of 3.00 mg/L for zinc. Based on these values, iron and cadmium are the dominant ions in the water. The elevated concentration of iron may be attributed to the geological composition of the area, as reported by Izah et al. (2016), a trend noted across various environmental matrices and food products from the region (Izah & Aigberua, 2017).
Iron is a vital element essential for the human body. Various authors have extensively reported its significance in hemoglobin, transferrin, ferritin, and iron-containing enzyme formation (Sevcikova et al., 2011), along with its role in oxygen transport, DNA synthesis, the electron transport chain, and the regulation of cell growth and differentiation (Abbaspour et al., 2014; Izah et al., 2016). Moreover, elevated iron concentrations can lead to siderosis, a benign pneumoconiosis (Muhammad et al., 2014). Conversely, cadmium, a non-essential element, lacks direct biological functions, and its high concentrations can result in bone demineralization and liver and kidney diseases (Muhammad et al., 2014).
3.2 Pearson Correlation and Multivariate Analysis of the Hydrogeochemical and Geochemical Characteristics of the Water
Table 5 presents the Pearson correlations among the hydrogeochemical characteristics of the surface water from the Bomadi and Gbotebo rivers and their surroundings in Bayelsa State, Nigeria. The analysis reveals several correlations among the variables. Temperature exhibited a positive correlation with pH (r = 0.384) at a significance level of p = 0.01, as well as with sodium (r = 0.262) at p = 0.05. Salinity exhibited a significant positive association (p = 0.01) with both conductivity (r = 0.962) and total dissolved solids (r = 0.958). Conductivity displayed a positive link with pH (r = 0.278) at p = 0.05 and a strong connection with total dissolved solids (r = 0.999) at p = 0.01. pH demonstrated a positive correlation with total dissolved solids (r = 0.279), sulfate (r = 0.320), and bicarbonate (r = 0.323) at p = 0.05 and with nitrate (r = 0.435), chloride (r = 0.494), potassium (r = 0.672), and sodium (r = 0.653) at p = 0.01. Sulfate exhibited positive links (p = 0.01) with nitrate (r = 0.625), bicarbonate (r = 0.710), chloride (r = 0.700), potassium (r = 0.503), and sodium (r = 0.477). Similarly, nitrate displayed a positive correlation (p = 0.01) with bicarbonate (r = 0.673), chloride (r = 0.800), potassium (r = 0.692), and sodium (r = 0.643). Bicarbonate indicated positive relationships (p = 0.01) with chloride (r = 0.715), potassium (r = 0.469), and sodium (r = 0.365), alongside a negative correlation with calcium (r = 0.421). Chlorine demonstrated positive associations (p = 0.01) with potassium (r = 0.755) and sodium (r = 0.739). Potassium exhibited a positive correlation (p = 0.01) with sodium (r = 0.955). Calcium showcased a positive link (p = 0.01) with magnesium (r = 0.499). Furthermore, copper displayed a positive relationship (p = 0.01) with zinc (r = 0.331) and cadmium (r = 0.939), while zinc exhibited a positive correspondence (p = 0.01) with cadmium (r = 0.425).
These correlations provide insights into the interrelatedness and mutual dependence of the parameters, suggesting shared sources and similar behavior during transport (Aigberua et al., 2021; Izah et al., 2021b). Some variables display both positive and negative associations, signifying complex interdependencies (Singh et al., 2015). Singh et al. (2015) noted that correlated parameters often hold greater utility. The significance of these correlational findings is further reflected in the PCA and varimax rotation of the principal components.
Supplementary Materials 5 and 6 display the outcomes of hierarchical cluster analyses utilizing squared Euclidean distance and Ward linkage. These analyses were applied to the sampling points and hydrogeochemical characteristics derived from the water of the Bomadi and Gbotebo rivers and their surroundings in Bayelsa State, Nigeria. The results yielded two main clusters in both cases.
Regarding the sampling points, the clusters were as follows: Cluster 1 was represented by SaP12, while the remaining sampling points (SaP1–SaP11) formed Cluster 2. Within Cluster 2, further sub-clusters emerged, namely, Sub-cluster B encompassing SaP1, SaP2, SaP3, SaP5, and SaP11, and Sub-cluster A divided into Sub-cluster I with SaP4, SaP6, SaP7, and SaP10, and Sub-cluster II consisting of SaP8 and SaP9 (depicted in Supplementary Material 5).
Similarly, the hydrogeochemical characteristics led to the formation of two primary clusters: Cluster 1 comprised conductivity and total dissolved solids, while Cluster 2 encompassed the remaining parameters. In Cluster 2, additional subdivisions were evident: Sub-cluster A encompassed temperature and chloride, and Sub-cluster B further broke down into Sub-cluster I with pH; Sub-cluster II comprising bicarbonate, sodium, calcium, magnesium, sulfate, and iron; and Sub-cluster III involving copper, cadmium, salinity, zinc, nitrate, and potassium (as illustrated in Supplementary Material 6).
The proximity of clusters indicates statistical associations, whereas clusters with greater distance demonstrate lower degrees of association (Izah et al., 2022b; Ogamba et al., 2021). The diversity observed in sampling activities underscores variations in anthropogenic influences on water characteristics. Moreover, the distinctions in hydrogeochemical features reveal differences in the information conveyed by the various parameters. For example, conductivity provides preliminary insights into the overall ion content within the water.
In line with Singh et al. (2015), correlational analysis serves to establish spatial relationships among sampling points within a study area, offering an effective means of classifying water sampling stations through clustering. This approach is economically feasible and retains valuable statistical insights. Therefore, by analogy, Supplementary Material 6 allows the analysis of four distinct sampling stations based on distances or sampling points, while Supplementary Material 7 accommodates the analysis of seven parameters without significant loss of essential water quality information within the area.
Table 6 shows the PCA analysis of the hydrogeochemical and geochemical analysis of surface water of the Bomadi and Gbotebo rivers and their environs in Bayelsa State, Nigeria. There is a total variance of 84.137% for the five principal components (PCs), thus having an eigenvalue > 1. Furthermore, the values for each PC include the loadings, eigenvalue, and variance. PC-1 explains 33.053% of the total variance and has significant (p ≥ 0.50) loadings of pH (r = 0.649), sulfate (r = 0.746), nitrate (r = 0.840), bicarbonate (r = 0.733), chloride (r = 0.923), potassium (r = 0.860), sodium (r = 0.848), and iron (r = 0.895). PC-2 explains 19.32% of the total variance and has statistically significant (p ≥ 0.50) loadings with salinity (r = 0.781), conductivity (r = 0.799), total dissolved solid (r = 0.799), and copper (r = 0.566). PC-3 explains which correlates with zinc (r = 0.885), chromium (r = 0.812), iron (r = 0.781), lead (r = 0.929), and copper (r = 0.854), while PC-2 contributed 24.8% to the variance, which correlates with manganese (r = 0.854) and cobalt (r = 0.837).
Table 7 presents the varimax-rotated component matrix of the hydrogeochemical analysis conducted on the surface water of the Bomadi and Gbotebo rivers and their surroundings in Bayelsa State, Nigeria. Five factors were deemed sufficient to account for 84.17% of the variance within the component matrix.
Factor 1, explaining 31.716% of the total variance, sheds light on the relationships involving pH, total dissolved solids, sulfate, nitrate, bicarbonate, chloride, potassium, sodium, and iron. This cluster of elements reflects influences from both natural processes and human activities. A notable positive correlation between sodium and chloride suggests the occurrence of halite weathering (Madhav et al., 2021b; Patel et al., 2016). Moreover, the strong statistical linkage between nitrate, bicarbonate, chloride, potassium, and sodium points toward an anthropogenic origin of these ions in the surface water, often stemming from waste disposal, agricultural runoff, and industrial drainage.
Factor 2, accounting for 18.392% of the total variance, highlights the interplay of conductivity, salinity, and total dissolved solids. These elements showcase a blend of natural influences and human activities. Conductivity demonstrates a robust correlation with both total dissolved solids and salinity. Essentially, the conductivity and total dissolved solid readings provide insights into the ionic load and presence of contaminants in the water. The relatively low values suggest a certain level of water cleanliness.
Factor 3, explaining 15.98% of the variance, draws attention to copper and zinc. While these elements typically share common sources, this study suggests their presence might be linked to human activities.
Factor 4, accounting for 9.83% of the variance, revolves around calcium and magnesium. Their correlation hints at potential carbonate weathering in the area (Madhav et al., 2021a, b). This could elucidate the weak negative correlation observed between sulfate and calcium and the limited positive correlation with magnesium.
Factor 5, explaining 8.216% of the variance, emphasizes temperature as a pivotal water quality parameter. It plays a significant role in shaping the composition and viability of aquatic life within the water body. Additionally, temperature exerts influence over the speed of chemical and biological reactions. The temperature variations are attributed to the region’s prevailing climatic conditions.
3.3 Suitability of the Water
3.3.1 Natural Evolution of the Surface Water and Water Type
Supplementary Material 7, in the context of this study, displays the descriptive statistics about the natural evolution of surface water and its classification based on water type extracted from the sampling points within the Bomadi and Gbotebo rivers and their environs in Bayelsa State, Nigeria. The SARi ranges from 0.28 to 0.71 across all sampling points. Employing the classification criteria, the water demonstrates a SARi value of ≤ 10 (excellent), signaling its suitability for irrigation purposes (Guettaf et al., 2017; Madhav et al., 2021a, b; Verma & Singh, 2021; Verma et al., 2020). This implies that soils with low sodium levels are conducive to diverse soil environments for irrigation.
The %Nai spans a range of 30.91 to 70.26. This range indicates water quality levels from good (20 ≥ %Nai ≤ 40) to doubtful (60 ≥ %Nai ≤ 80) (Guettaf et al., 2017; Madhav et al., 2021a, b; Verma & Singh, 2021; Verma et al., 2020). Nonetheless, the doubtful classification is only evident in the maximum values of SaP5 and SaP11. Further examination reveals that SaP1, SaP3, SaP4, and SaP7 are permissible for minimum, maximum, and mean values. In contrast, SaP2 and SaP6 display good minimum, maximum, and mean values. SaP5 demonstrates doubtful readings for mean and minimum, while SaP9 and SaP10 exhibit good values for mean and minimum, and SaP12 displays permissible values for maximum. The overall interpretation suggests that the water is generally suitable for use, with exceptions observed in SaP5 and SaP11 instances.
The RSCi exhibits negative values for mean, minimum, and maximum, except for SaP5, which shows values of 0.02, 0.00, and 0.05, respectively, along with a maximum value of 0.05 for SaP11 (RSCi 1.25—considered safe) (Madhav et al., 2021a, b; Verma & Singh, 2021; Verma et al., 2020). These findings collectively suggest excellent water quality. Notably, bicarbonate and hydrogen bicarbonate influence calcium and magnesium precipitation in water, contributing to increased sodium ions (Ahamad et al., 2018). The RSCi outcomes here imply favorable conditions for irrigation purposes.
SSPi ranges from 28.21 to 65.67%. The values indicate that SaP1, SaP2, SaP3, SaP4, SaP6, SaP7, SaP8, SaP9, SaP10, and SaP12 share a value of 50. SaP11’s maximum and SaP5’s mean, minimum, and maximum values exceed 50. According to Kadyampakeni et al. (2018) and Verma and Singh (2021), SSPi values greater than 50 suggest sodium iron precipitation in soil, leading to soil quality deterioration. Verma and Singh (2021) emphasize SSPi’s significance as a determinant of water quality, particularly for irrigation purposes. Drawing from the criteria presented by Wilcox (1955) and Verma and Singh (2021), SSPi ranges of < 20, 20–40, 40–80, and > 80 indicate excellent, good, marginal, and poor water types for irrigation, respectively. Results indicate that most water types in the study range between good and marginal quality for irrigation, signaling potential pollution at some sampling points.
Moving beyond Nai, SSPi, and RSCi, other indices like KRi, PIi, Psi, and MHRi are commonly employed to assess water quality for irrigation (Ahamad et al., 2018). The PIi encompasses a range from 67.23 to 217.44%, classifying the water from marginally fit to suitable for irrigation. While SaP2, SaP6, SaP8, and SaP11’s minimum values fall below 75% but exceed 25%, the remaining minimums and the means and maximums are above 75%, indicating irrigation suitability. Overall, the majority of water samples align with group 1, a pattern consistent with groundwater findings in Varanasi, India (Ahamad et al., 2018), and the Pungar subbasin in Tamilnadu, India (Srinivasamoorthy et al., 2014).
The KRi spans a range of 0.39 to 1.91, suggesting suitability to marginal unsuitability as previously classified by Ahamad et al. (2018). All values fall under the “suitable” category (KRi < 1) except for SaP5, which ranges from 1 to 2. This indicates that nearly all the water from the different sampling points is suitable for irrigation processes. This trend is in the range previously reported in groundwater from Varanasi, India (Ahamad et al., 2018).
The MHRi ranged from 50.82 to 76.90%. The water samples were > 50 (an indication of harmful irrigation) based on the classification previously presented by Ahamad et al. (2018). The authors have variously reported that water with > 50 MHRi is unsafe for plants because it can lead to a decline in crop productivity (Rao et al., 2013; Singaraja et al., 2014).
GRiI and GRiII are acceptable for classifying water in three fields: evaporation, precipitation, and rock dominance (Verma & Singh, 2021). The GRiI, which depicts cations, showed precipitation dominance, while the GRiII, which depicts anions, also showed precipitation dominance. This is because the values are 10. The GRiI and GRiII provided detailed information about water chemistry (particularly anions and cations) and the factors influencing the ions in the water (Verma & Singh, 2021).
The techniques for assessing the ion exchange process in water and its movement in the host rock and unsaturated zone have been developed (Schoeller, 1977; Verma & Singh, 2021). The indices include chloroalkaline indices (CAI I and CAI II). The CAI I showed positive values < 1.0, an indication of reverse ion exchange rather than the forward ion exchange processes in discharging alkali elements (Na+ + K+), while the CAI II showed negative values, an indication of forward ion exchange, whereas positive values designate the occurrence of reverse ion exchange (Schoeller, 1977; Verma & Singh, 2021). The CAI II showed the role of forward ion exchange processes in the determination of the chemistry of water at all the sampling points.
The sodium-magnesium ratio ranged from 0.54 to 2.54. This suggests that carbonate weathering is the main cause of calcium in the surface water in the study area. In carbonate weathering, precipitation dominance is dominant. However, in instances where the ratio of weathering is above 2 (SaP5 and the maximum of SaP11), silicate weathering contributes to the weathering processes in a minute amount (Karunanidhi et al., 2020; Madhav et al., 2021a).
The bicarbonate-to-(sum of bicarbonate and sulfate) ratio ranged from 0.28 to 1.00. The values are close to the 0.66–0.97 recorded in groundwater in Bhadohi, India (Madhav et al., 2021a).
The sodium-chloride ratios were < 1 at all the sampling points. According to Madhav et al. (2021a), silicate weathering contributes to the sodium in the water; however, since the value is < 1, it indicates that carbonate is the main iron contributing to the ions in the water. The low levels in the water suggest the absence of human activities such as textile industries.
The potassium–chloride ratio ranged from 0.04 to 0.08, while the sulfate–chloride was 0.00–0.14. These ratios reflect the amount of sulfate and potassium in the water and the human activities contributing to these ions in the study area. The breakdown of organic matter causes the addition of sulfate.
Potential salinity (PSi) ranged from 0.28 to 0.61. The PSI values were < 3, indicating that the water can be used for irrigation. According to Doneen (1964), Verma and Singh (2021) found that water with a PSI of < 3 Moreover > 3 indicated that the water was suitable and unsuitable for irrigation, respectively.
Supplementary Material 8 shows the bivariate plots showing the relationship between some parameters and ratios. These ratios showed the potential sources of important ions and exchange processes regulating the chemistry of the water chemistry. Some of these important bivariate plots include (a) total cation vs. HCO−3; (b) (HCO−3 + SO2−4) vs. HCO−3; (c) (Ca2+ + Mg2+)—(HCO−3—SO2−4) vs. (Na+ + K+)- Cl (Madhav et al., 2021a); (d) Na+ vs. Cl−; (e) HCO−3 vs. (Na+ + K+) (Madhav et al., 2021b); (f) (Ca2+ + Mg2+) and (HCO−3 + SO2−4); (g) Ca2+ and SO2−4; (h) Na+ and (Ca2+ + Mg2+); (i) Ca2+ and Mg2+; (j) Na+ and Cl−; and (k) (NO−3 + Cl−)/HCO−3 and TDS, which have been used by several authors in the study of water chemistry (Barzegar et al., 2016a, b; Barzegar et al., 2016a, b; Rao et al., 2018; Khan & Umar, 2019; Marghade et al., 2019).
The total cation vs. HCO−3 plot (Supplementary Material 8a) suggests that weathering is the main cause of iron in the surface water in the study area. The plot of (HCO−3 + SO2−4) vs. HCO−3 (Supplementary Material 8b) indicates that carbonate is the main weathering process (Husain et al., 2020; Raju, 2012). The plot of (Ca2+ + Mg2+)—(HCO−3—SO2−4) vs. (Na+ + K+)—Cl (Supplementary Material 8c) provides information about the ion processes. From the plot, the cations are occurring slightly because the plot is nearly linear. According to Mthembu et al. (2020), when cation processes are not occurring, all the data points will be close to the origin. The plot of Na+ vs. Cl− (Supplementary Material 8d) indicates carbonates as the predominant source of ions in the water. While plotting HCO−3 vs. (Na+ + K+) (Supplementary Material 9e), we saw that carbonates are the major source of weathering in the water.
The (Ca2+ + Mg2+) vs. (HCO−3 + SO2−4) plot (Supplementary Material 8f) showed that in the surface water sample, precipitation (silicate weathering) and mineral dissolution (carbonate/sulfate) are dominant in the area. Previous studies on water chemistry have revealed a similar trend but with a different percentage distribution (Barzegar et al., 2016b; Verma & Singh, 2021). The study also showed that ion exchange processes primarily rule the water ion (Verma & Singh, 2021). In Supplementary Material 8 g, the plot of Ca2+ vs. SO2−4 showed anhydrite and sulfur dissolution processes (Verma & Singh, 2021). The plot of Na2+ and (Ca2+ + Mg2+) recommends that Ca2+ and Mg2+ ions are the offshoots of the reverse ion exchange process, and Na+ ions are derivatives of the forward ion exchange process (Supplementary Material 8 h) (Verma & Singh, 2021). The plot of Mg2+ versus Ca2+ (Supplementary Material 8i) showed the occurrence of CaCO3 precipitation. The plot of Na+ and Cl− (Supplementary Material 8j) showed the existence of silicate weathering and forward ion exchange processes (Barzegar et al., 2016b; Rao et al., 2018; Verma & Singh, 2021). The plot of (NO−3 + Cl−)/HCO−3 and TDS (Supplementary Material 8 k) showed the effect of human activities on water quality.
In Supplementary Material 8b, c, d, e, i, j, and k, the positive relationship suggests the effect of human activities, particularly agrochemicals, on the water (Rao et al., 2018), while the negative relationships, as shown in Supplementary Material 8a, f, and g, indicate that the activities come from natural causes.
3.3.2 Evolution of the Surface Water Pollution
Within the context of the Bomadi and Gbotebo rivers and their environs in Bayelsa State, Nigeria, Supplementary Material 9 presents the descriptive statistics encompassing the comprehensive water quality evaluation across various parameters at each sampling point. The table illustrates the water quality status for each parameter and provides the cumulative outcome represented as the PISw for each respective sampling point.
The PISw values exhibit a range from PISw < 1.0 (indicating no pollution) to 1.0 ≥ PISw ≤ 1.5 (reflecting low pollution), by the criteria set forth by Rao (2012) and Verma and Singh (2021). Several sampling points, including SaP1, SaP3, SaP4, SaP6, SaP7, SaP9, SaP10, and SaP12, consistently indicate no pollution across all parameters. For maximum values, SaP8 and SaP11 equally display no pollution, while mean and minimum values reiterate this status. Moreover, SaP2 and SaP5’s mean, minimum, and maximum values all underscore an absence of pollution.
Moving further, parameters such as pH, conductivity, total dissolved solids, sulfate, nitrate, bicarbonate, chloride, potassium, calcium, sodium, and magnesium consistently maintain values below 0.1. This signifies that these parameters contribute minimally or insignificantly to the overall pollution levels in the region’s surface water. In contrast, iron exhibits values exceeding 0.1 across all sampling points for mean, minimum, and maximum. This occurrence is attributed to the geological characteristics of the area, a notion reinforced by various authors who have highlighted the association of elevated iron concentrations in the Niger Delta region with its geological attributes (Izah et al., 2016, 2017).
3.3.3 Health Risk Assessment of Trace Elements in Surface Water of Bomadi and Gbotebo Rivers and Their Environs in Bayelsa State, Nigeria
There are two distinct categories of health risks to consider: carcinogenic risks and non-carcinogenic risks. These categories measure the extent to which exposure to harmful substances through oral and dermal routes leads to adverse health effects. To evaluate these risks, a health risk assessment was conducted. This assessment involved determining the non-carcinogenic risks using indices such as ADD, hazard quotients (HQ), and hazard index (HI) and assessing carcinogenic hazards. These assessments were performed for two main age groups: adults and children.
Supplementary Materials 10 and 11 provide information on the average daily dose (ADD) of TEs from the Bomadi and Gbotebo rivers and their surroundings in Bayelsa State, Nigeria. The data consider both the consumption and dermal contact of surface water by adults and children. The calculated ADD values for the detected elements (iron, copper, zinc, and cadmium) were all below 1.0 for both age groups and exposure routes.
Supplementary Materials 12 and 13 detail the hazard quotients (HQs) of trace metals through ingestion and dermal exposure in adults and children. These values were also below 1 across all sampling points, indicating a lower risk for adults than children.
Furthermore, Supplementary Materials 14 and 15 present the hazard index (HI) and carcinogenic hazard for both age groups. The HI values, which are below 1, signify that there is no non-carcinogenic hazard due to exposure to the water from the Bomadi and Gbotebo rivers and their surroundings. A HI value greater than 1 would suggest a potential health hazard. The carcinogenic hazards, primarily observed in SaP4, SaP6, SaP10, and SaP12, were measured in the range of 10−6 for adults. According to Mohammadi et al. (2019), their daily consumption determines the toxicity of heavy metals to humans. The ADD values in our study showed similarity to earlier works on drinking water in Iran by Mohammadi et al. (2019) and Rezaei et al. (2019).
The fact that both HQ and HI values remained below 1 indicates that the levels of non-carcinogenic health impacts are within acceptable limits. Therefore, the trace elements analyzed in the Bomadi and Gbotebo rivers and their surroundings are unlikely to have a cumulative detrimental non-carcinogenic health effect. Considering various exposure scenarios, the primary mode of exposure to these water sources is ingestion. These findings align with prior studies (Mohammadi et al., 2019; Rezaei et al., 2019) conducted on Iran’s drinking water supply.
Carcinogenic hazards suggest the potential for cancer development due to exposure to these water resources. The observed carcinogenic hazards were 10−5 for SaP4, SaP6, SaP10, and SaP12 for dermal route and 10,3 for SaP4, SaP6, SaP10, and SaP12 for ingestion route. Referring to the classification in Supplementary Material 4 (Adeyemi & Ojekunle, 2021; Aigberua et al., 2021; Joel et al., 2018), the carcinogenic hazard in sampling points that cadmium was detected depicts moderate hazards for ingestion.
These findings fall within the commonly reported USEPA limit of 10−6 to 10−4 for oral and dermal exposure, as observed in various literature sources (Izah et al., 2021b, b; Ogamba et al., 2021). Consequently, the potential carcinogenic risk associated with cadmium exposure is considered moderate. This study’s results are consistent with previous investigations on drinking water in Iran by Mohammadi et al. (2019) and Rezaei et al. (2019).
3.4 Application of Correlational and Multivariate Analyses to Suitability of the Water Tools or Indices
3.4.1 Pearson Correlation
Determining water suitability encompasses several factors, including overall water quality, the natural evolution of surface water, water type, and health risk indices. This comprehensive assessment evaluates the quality and potential risks associated with the water. Table 8 illustrates the Pearson correlation among various indices used to assess hydrogeochemical and geochemical quality of surface water from the Bomadi and Gbotebo rivers and their vicinity in Bayelsa State, Nigeria. The Pearson correlation quantifies the relationships between these indices.
Positive correlations were observed for several pairs of indices. For instance, PISw exhibited a strong positive correlation (p = 0.01) with SARi, SSPi, MHRi, KRi, %Nai, PIi, PSi, Gibbs Ratio I, Gibbs Ratio II, and CAI II. Similarly, indices like SARi, SSPi, MHRi, KRi, %Nai, Pli, PSi, RSCi, Gibbs Ratio I, Gibbs Ratio II, and CAI II displayed positive correlations.
On the other hand, MHRi showed positive correlations with Gibbs Ratio I, Gibbs Ratio II, and CAI I at p = 0.01, as well as with Gibbs Ratio II at p = 0.05. Furthermore, certain indices exhibited both positive and negative correlations with each other. For instance, HI Adult (ingestion) was positively correlated with HI Children (ingestion), HI Adult (dermal), HI Children (dermal), and K/Cl at p = 0.01.
Positive correlations between certain indices indicate that they share similar influencing factors. This implies that a change in one parameter could potentially lead to corresponding changes in the other parameters with which it is correlated. On the other hand, negative correlations suggest that changes in one variable may not necessarily result in corresponding changes in the associated variables, indicating variations in the dynamics of these indices. Additionally, weak associations between certain indices suggest multiple contributing factors could influence their distribution.
3.4.2 Multivariate Analysis
Figures 2 and 3 depict the results of a hierarchical cluster analysis employing Euclidean distance and Ward linkage. This analysis focused on TEs and hydrogeochemical parameters, utilizing the sampling points and indices to assess water suitability within the vicinity of the Bomadi and Gbotebo rivers in Bayelsa State, Nigeria.
The outcome of the analysis revealed the formation of two primary clusters: one centered around SaP5 and another encompassing the remaining sampling points (SaP1-SaP4, SaP6–SaP12). Within the second cluster, several sub-clusters emerged, reflecting a more intricate pattern (Fig. 2). As a result of this clustering, the total number of sampling points was condensed to 7, while the indices were streamlined into four major classes based on the calculated distances and similarities (Fig. 3). This process has the potential to streamline the multitude of indices typically employed in water quality assessments, facilitating more efficient analyses in the future.
Interestingly, this analysis also demonstrated the absence of significant spatial differences among certain sampling stations (Fig. 2) and the similarity between specific indices (Fig. 3). The clustering approach effectively coherently grouped stations and indices, enhancing the credibility of the findings. In Fig. 2, the indices grouped share similar characteristics, as evidenced by their proximity, and the range of correlational coefficients is detailed in Table 8. This approach confirms that the technique effectively categorizes surface water conditions across the region and similarly groups common indices for assessing trace elements and hydrogeochemical properties. Consequently, this method offers a practical means to categorize water quality across the region, and it also simplifies the assessment process by potentially reducing the number of required sampling points. For future spatial sampling in the study location, only seven strategically selected locations might suffice, as opposed to the initial 12, without compromising the reliability of the results. By decreasing the number of sampling points in a monitoring network, costs can be minimized without compromising the value of the findings, a strategy supported by studies conducted by Singh and Singh (2010) and Singh et al. (2015). Furthermore, the four indices that constitute the grouped clusters could serve as representative water quality indicators, offering a more efficient alternative to employing the full set of 22 indices. This streamlined approach could enhance the practicality and effectiveness of future water quality assessments.
Table 9 shows the PCA of indices used to assess surface water’s hydrogeochemical and geochemical quality from the Bomadi and Gbotebo rivers and their environs in Bayelsa State, Nigeria. PCA was performed on the combined hydrogeochemical and trace element indices, and PCs with eigenvalues > 1 were retained. Results from the analysis, including the loadings, eigenvalues, and variance, were explained by each PC. The extracted four PCs explain 92.392% of the total variance, as shown in Table 4. PC-1 explains 43.693% of the variance and has statistical loading with PISw (r = 0.585), SARi (r = 0.925), SSPi (0.907), KRi (r = 0.939), %Nai (r = 0.939), Pli (0.901), PSi (r = 0.690), RSCi (r = 0.845), Gibbs Ratio I (r = 0.892), Gibbs Ratio II (r = 0.876), Na/Mg (r = 0.869), and negative loading with Na/Cl (0.579). PC-2 explains 18.595% of the variance and has statistical loadings with HI Adult (ingestion) (r = 0.818), HI Children (ingestion) (r = 0.818), HI Adult (dermal) (r = 0.790), HI Children (dermal) (r = 0.790), and K/Cl (r = 0.649). PC-3 explains 14.452% of the variance and has statistical loadings with PISw (r = 0.562), PSi (r = 0.551), and SO4/Cl (r = 0.781). PC-4 explains 10.593% of the variance and has statistical loading with CAI I (r = 0.703).
Table 10 shows the principal component (varimax with Kaise normalization) analysis of indices used to assess surface water’s hydrogeochemical and geochemical quality from the Bomadi and Gbotebo rivers and their environs in Bayelsa State, Nigeria. The total variance is 92.392%. Factor 1 explains 37.084% of the variance and has statistical loading with SARi (r = 0.965), SSPi (r = 0.987), KRi (r = 0.965), %Nai (r = 0.988), Pli (r = 0.927), RSCi (r = 0.931), Gibbs Ratio I (r = 0.836), and Na/Mg (r = 0.975). Factor 2 explains 18.587% of the variance and has statistical loading with HI Adult (ingestion) (r = 0.982), HI Children (ingestion) (r = 0.982), HI Adult (dermal) (r = 0.975), and HI Children (dermal) (r = 0.975). Factor 3 explains 16.76% of the variance and has statistical loadings of PISw (r = 0.573), PSi (r = 0.682), CAI I (r = 0.909), and SO4/CL (r = 0.908). Factor 4 explains 12.851% of the variance and has a statistical loading with Na/Cl (r = 0.834).
After the rotation, there were no major changes in the cumulative values for each component. Similar trends have been reported in the literature by Izah et al. (2022c) and Keshavarzi et al. (2021). The PC-1 showed that most of the indices that have to do with the evolution of natural water are influenced by certain factors. At the same time, the PC-2 also shows that the indices resulting from TEs analysis have probably caused health effects due to water consumption. This suggests that the factors that affect the hydrogeochemical characteristics of the water tend to have a major influence on the overall water quality. The resultant TEs in water tend to appear in the overall health risk assessment. PCA is used to assess several parameters to ascertain the sources responsible for the variations in the indices used to assess surface water’s hydrogeochemical and geochemical quality, explaining 92.392% of the total variability.
4 Conclusion
Over the past 30 years, environmental pollution has substantially risen, attributed primarily to urbanization, industrialization, and population growth. This has prompted environmental health experts to renew their focus on assessing the quality of public and environmental health by applying various indices. In this study, an evaluation was conducted on commonly utilized indices for assessing surface water quality, employing correlational analysis and MA techniques.
Key findings from the study encompass a comprehensive analysis of various water attributes, with certain physical and chemical factors, including temperature, salinity, pH, and conductivity, being present, while others such as chromium, nickel, cobalt, and lead were notably absent. Interestingly, iron, zinc, cadmium, and copper were detected in specific locations, displaying significant statistical variations in their concentrations. In general, most of the hydrogeochemical characteristics observed adhered to the permissible levels set by the WHO and the SON; however, cadmium and iron concentrations exceeded acceptable thresholds at specific sampling points.
The study further employed statistical tools, including Pearson’s correlation and MA using CA and PCA, to discern the influence of both human activities and natural processes on water characteristics within the study area. Water suitability assessment revealed diverse conditions, ranging from excellent to marginal quality, based on parameters such as Psi, SARi, RSCi, SSPi, KRi, MHRi, and %Nai.
Furthermore, a meticulous categorization of water, based on factors such as evaporation, precipitation, and rock dominance, indicated precipitation dominance in both cation-based GRiI and anion-based GRiII. Additionally, assessments of water quality involved ion exchange processes within the host rock and unsaturated zone techniques, with CAI I indicating reverse ion exchange for discharging alkali elements (Na+ and K+), while CAI II showed negative values, signifying forward ion exchange. Positive CAI II values implied reverse ion exchange occurrences.
The sodium-magnesium ratio highlighted that calcium presence in water primarily results from carbonate weathering, with sporadic contributions from silicate weathering. Sodium-chloride ratios suggested sodium origins linked to silicate weathering processes.
Pollution indices (PISw) indicated generally insignificant to low levels of pollution in surface water, with parameters such as pH, conductivity, total dissolved solids, sulfate, nitrate, bicarbonate, chloride, potassium, calcium, sodium, and magnesium contributing minimally or insignificantly. An exception was iron, which exhibited higher levels attributed to natural effects.
Furthermore, health risk assessments related to TEs in the water revealed no non-carcinogenic hazards resulting from exposure through contact or ingestion. Additionally, carcinogenic hazard for cadmium revealed moderate risk. The application of Pearson correlations and MA techniques emphasized significant correlations among various parameters, suggesting shared influencing factors. Additionally, CA and PCA results highlighted the potential for reducing the number of indices in a single study while still obtaining equivalent information.
Therefore, the utilization of MA and Pearson correlation in the examination of water quality, along with the commonly employed indices for assessing environmental and health quality, indicated that it is possible to reduce the number of indices and sampling points without compromising the quality of the results.
Data Availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
References
Abbaspour, N., Hurrell, R., & Kelishadi, R. (2014). Review on iron and its importance for human health. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences, 19(2), 164.
Adeyemi, A. A., & Ojekunle, Z. O. (2021). Concentrations and health risk assessment of industrial heavy metals pollution in groundwater in Ogun state. Nigeria. Scientific African, 11, e00666.
Agedah, E. C., Ineyougha, E. R., Izah, S. C., & Orutugu, L. A. (2015). Enumeration of total heterotrophic bacteria and some physico-chemical characteristics of surface water used for drinking sources in Wilberforce Island. Nigeria. Journal of Environmental Treatment Techniques, 3(1), 28–34.
Aghoghovwia, O. A., Umoru, O. D., & Izah, S. C. (2018a). Physicochemical characteristics of nun river at Gbarantoru and Tombia Axis in Bayelsa State. Nigeria. Bioscience Methods, 9(1), 1–11.
Aghoghovwia, O. A., Izah, S. C., & Miri, F. A. (2018b). Environmental risk assessment of heavy metals in sediment of Nun River around Gbarantoru and Tombia Towns, Bayelsa State. Nigeria. Biological Evidence, 8(3), 21–31.
Ahamad, A., Madhav, S., Singh, P., Pandey, J., & Khan, A. H. (2018). Assessment of groundwater quality with special emphasis on nitrate contamination in parts of Varanasi City, Uttar Pradesh, India. Applied Water Science, 8, 1–13.
Aigberua, A., & Tarawou, T. (2019). Water quality index (WQI) assessment along inland fresh waters of Taylor Creek in Bayelsa State. Nigeria. Journal of Environmental Treatment Techniques, 7(3), 260–269.
Aigberua, A. O., Izah, S. C., & Richard, G. (2021). Hazard analysis of trace metals in muscle of Sarotherodon melanotheron and Chrysichthys nigrodigitatus from Okulu River, Rivers State, Nigeria. Journal of Environmental Health and Sustainable Development, 6(3), 1340–1356.
Angaye, T. C. N., Zige, T. C., & Izah, S. C. (2015). Microbial load and heavy metals properties of leachates from solid wastes dumpsites in the Niger Delta, Nigeria. Journal of Environmental Treatment Techniques, 3(3), 148–153.
Barzegar, R., Asghari Moghaddam, A., Najib, M., Kazemian, N., & Adamowski, J. (2016a). Characterization of hydrogeologic properties of the Tabriz plain multilayer aquifer system, NW Iran. Arabian Journal of Geosciences, 9, 1–17.
Barzegar, R., Asghari Moghaddam, A., & Tziritis, E. (2016b). Assessing the hydrogeochemistry and water quality of the Aji-Chay River, northwest of Iran. Environmental Earth Sciences, 75, 1–15.
Ben-Eledo, V. N., Kigigha, L. T., Izah, S. C., & Eledo, B. O. (2017). Water quality assessment of Epie creek in Yenagoa metropolis, Bayelsa state. Nigeria. Archives of Current Research International, 8(2), 1–24.
Daka, E. R., Amakiri-Whyte, B., & Inyang, I. R. (2014). Surface and groundwater quality in some oil field communities in the Niger Delta: Implications for domestic use and building construction. Research Journal of Environmental and Earth Sciences, 6(2), 78–84.
Doneen, L. D. (1964). Notes on water quality in agriculture. University of California.
Ebuete, A. W., & Bariweni, P. A. (2019). Water quality index of Kolo Creek, Bayelsa State, Nigeria. Journal of Applied Sciences and Environmental Management, 23(11), 1923–1927.
Elangovan, N. S., Lavanya, V., & Arunthathi, S. (2018). Assessment of groundwater contamination in a suburban area of Chennai, Tamil Nadu, India. Environment, Development and Sustainability, 20, 2609–2621.
Eremasi, Y. B., Alagoa, K. J., & Daworiye, P. (2015). Water quality evaluation and heavy metals concentration of Kolo Creek, Imiringi, Bayelsa State. International Journal of Current Research in Biosciences and Plant Biology, 2(2), 61–66.
Guettaf, M., Maoui, A., & Ihdene, Z. (2017). Assessment of water quality: A case study of the Seybouse River (North East of Algeria). Applied Water Science, 7(1), 295–307.
Hennemann, M. C., & Petrucio, M. M. (2011). Spatial and temporal dynamic of trophic relevant parameters in a subtropical coastal lagoon in Brazil. Environmental Monitoring and Assessment, 181, 347–361.
Huang, G., Sun, J., Zhang, Y., Chen, Z., & Liu, F. (2013). Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. Science of the Total Environment, 463, 209–221.
Husain, M. S., Umar, R., & Ahmad, S. (2020). A comparative study of springs and groundwater chemistry of Beas and Parbati valley, Kullu District, Himachal Pradesh, India. HydroResearch, 3, 32–47.
Iroha, I. R., Ude, I. U., Okoronkwo, C., Kenneth, O., Okafor, C. O. O., & Akuma, S. O. (2020). Comparative assessment of physicochemical characteristics, metal levels and anion contents of water from different aquatic environments in Ebonyi State. Biomed J. Sci. & Tech Res, 29(5), 22834–22865.
Izah, S. C., & Aigberua, A. O. (2017). Comparative assessment of selected heavy metals in some common edible vegetables sold in Yenagoa metropolis. Nigeria. Journal of Biotechnology Research, 3(8), 66–71.
Izah, S. C., & Angaye, T. C. (2016). Heavy metal concentration in fishes from surface water in Nigeria: Potential sources of pollutants and mitigation measures. Sky Journal of Biochemistry Research, 5(4), 31–47.
Izah, S. C., Chakrabarty, N., & Srivastav, A. L. (2016). A review on heavy metal concentration in potable water sources in Nigeria: Human health effects and mitigating measures. Exposure and Health, 8, 285–304.
Izah, S. C., Richard, G., & Sawyer, W. E. (2021a). Distribution of fungi density and diversity in a surface water of Epie Creek in Yenagoa Metropolis, Nigeria. Archive of Epidemiology and Public Health, 3, 1–5.
Izah, S. C., Uzoekwe, S., & Aigberua, A. (2021b). Source, geochemical spreading and risks of trace metals in particulate matter 2.5 within a gas flaring area in Bayelsa State Nigeria. Advances in Environmental Technology, 7(2), 101–118.
Izah, S. C., Richard, G., Aigberua, A. O., & Ekakitie, O. (2021c). Variations in reference values utilized for the evaluation of complex pollution indices of potentially toxic elements: A critical review. Environmental Challenges, 5, 100322.
Izah, S. C., Aigberua, A. O., & Srivastav, A. L. (2022a). Factors influencing the alteration of microbial and heavy metal characteristics of river systems in the Niger Delta region of Nigeria. In S. Madhav, S. Kanhaiya, A. L. Srivastav, V. B. Singh, & P. Singh (Eds.), Ecological Significance of River Ecosystems (pp. 51–78). Elsevier.
Izah, S. C., Aigberua, A. O., & Richard, G. (2022b). Concentration, source, and health risk of trace metals in some liquid herbal medicine sold in Nigeria. Biological Trace Element Research, 200, 3009–3302.
Izah, S. C., Aigberua, A. O., & Ogwu, M. C. (2022c). Trace element composition of Gallus gallus domesticus eggs and health risks associated with their consumption in Port Harcourt, Nigeria. Journal of Food Safety and Hygiene, 8(3), 202–222.
Izah, S. C., Bassey, S. E., & Ohimain, E. I. (2017). Assessment of heavy metal in cassava mill effluent contaminated soil in a rural community in the Niger Delta region of Nigeria. EC Pharmacology and Toxicology, 4(5), 186–201.
Izah, S. C., Iyiola, A. O., & Richard, G. (2023). Impacts of pollution on the hydrogeochemical and microbial community of aquatic ecosystems in Bayelsa State, Southern Nigeria. In: Madhav S, Singh VB, Kumar M, Singh S (Editors). Hydrogeochemistry of Aquatic Ecosystems, (pp. 283 – 305). John Wiley & Sons Ltd. https://doi.org/10.1002/9781119870562.ch13.
Izah, S. C. (2019). Cassava mill effluents: Environmental risk assessment of heavy metals, treatment and potentials for the production of Saccharomyces cerevisiae biomass (Doctoral dissertation, Ph. D dissertation submitted to School of Post Graduate Studies, Niger Delta University, Wilberforce Island, Bayelsa state, Nigeria. 290pp).
Izonfuo, L. W. A., & Bariweni, A. P. (2001). The effect of urban runoff water and human activities on some physico-chemical parameters of the Epie Creek in the Niger Delta. Journal of Applied Sciences and Environmental Management, 5(1), 47–55.
Jayakumar, R., Steger, K., Chandra, T. S., & Seshadri, S. (2013). An assessment of temporal variations in physicochemical and microbiological properties of barmouths and lagoons in Chennai (Southeast coast of India). Marine Pollution Bulletin, 70(1–2), 44–53.
Joel, E. S., Maxwell, O., Adewoyin, O. O., Ehi-Eromosele, C. O., Embong, Z., & Oyawoye, F. (2018). Assessment of natural radioactivity in various commercial tiles used for building purposes in Nigeria. MethodsX, 5, 8–19.
Kadyampakeni, D., Appoh, R., Barron, J., & Boakye-Acheampong, E. (2018). Analysis of water quality of selected irrigation water sources in northern Ghana. Water Science and Technology: Water Supply, 18(4), 1308–1317.
Karunanidhi, D., Aravinthasamy, P., Deepali, M., Subramani, T., & Roy, P. D. (2020). The effects of geochemical processes on groundwater chemistry and the health risks associated with fluoride intake in a semi-arid region of South India. RSC Advances, 10(8), 4840–4859.
Kawashima, T., Agou, T., & Yoshino, J. (2013). Chemical sensors: Main group compounds for anion detection. Comprehensive Inorganic Chemistry II (Second Edition) Reedijk, J., & Poeppelmeier, K. (Eds). Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Volume 1 (pp. 1053–1068). Elsevier.
Kenoyer, G. J., & Bowser, C. J. (1992). Groundwater chemical evolution in a sandy silicate aquifer in northern Wisconsin: 1. Patterns and rates of change. Water Resources Research, 28(2), 579–589.
Keshavarzi, A., Kumar, V., Ertunç, G., & Brevik, E. C. (2021). Ecological risk assessment and source apportionment of heavy metals contamination: An appraisal based on the Tellus soil survey. Environmental Geochemistry and Health, 43(5), 2121–2142.
Khan, I., & Umar, R. (2019). Environmental risk assessment of coal fly ash on soil and groundwater quality, Aligarh, India. Groundwater for Sustainable Development, 8, 346–357.
Kim, K., Rajmohan, N., Kim, H. J., Hwang, G. S., & Cho, M. J. (2004). Assessment of groundwater chemistry in a coastal region (Kunsan, Korea) having complex contaminant sources: A stoichiometric approach. Environmental Geology, 46, 763–774.
Li, P., Karunanidhi, D., Subramani, T., & Srinivasamoorthy, K. (2021). Sources and consequences of groundwater contamination. Archives of Environmental Contamination and Toxicology, 80, 1–10.
Lu, X., Zhou, Z., & Wang, J. (2011). Analysis on interactions among atmosphere, surface and ground water using EARTH model in the unsaturated zone. Procedia Environmental Sciences, 8, 134–139.
Madhav, S., Ahamad, A., Kumar, A., Kushawaha, J., Singh, P., & Mishra, P. K. (2018). Geochemical assessment of groundwater quality for its suitability for drinking and irrigation purpose in rural areas of Sant Ravidas Nagar (Bhadohi), Uttar Pradesh. Geology, Ecology, and Landscapes, 2(2), 127–136.
Madhav, S., Raju, N. J., Ahamad, A., Singh, A. K., Ram, P., & Gossel, W. (2021a). Hydrogeochemical assessment of groundwater quality and associated potential human health risk in Bhadohi environs. India. Environmental Earth Sciences, 80(17), 585.
Madhav, S., Raju, N. J., & Ahamad, A. (2021b). A study of hydrogeochemical processes using integrated geochemical and multivariate statistical methods and health risk assessment of groundwater in Trans-Varuna region, Uttar Pradesh. Environment, Development and Sustainability, 23, 7480–7508.
Marghade, D., Malpe, D. B., & Subba Rao, N. (2021). Applications of geochemical and multivariate statistical approaches for the evaluation of groundwater quality and human health risks in a semi-arid region of eastern Maharashtra, India. Environmental Geochemistry and Health, 43, 683–703.
Mohammadi, A. A., Zarei, A., Majidi, S., Ghaderpoury, A., Hashempour, Y., Saghi, M. H., Alinejad, A., Yousefi, M., Hosseingholizadeh, N., & Ghaderpoori, M. (2019). Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad. Iran. Methodsx, 6, 1642–1651.
Mthembu, P. P., Elumalai, V., Brindha, K., & Li, P. (2020). Hydrogeochemical processes and trace metal contamination in groundwater: Impact on human health in the Maputaland coastal aquifer, South Africa. Exposure and Health, 12, 403–426.
Muhammad, I., Ashiru, S., Ibrahim, I. D., Salawu, K., Muhammad, D. T., & Muhammad, N. A. (2014). Determination of some heavy metals in wastewater and sediment of artisanal gold local mining site of Abare Area in Nigeria. Journal of Environmental Treatment Techniques, 1(3), 174–182.
Nazneen, S., Raju, N. J., Madhav, S., & Ahamad, A. (2019). Spatial and temporal dynamics of dissolved nutrients and factors affecting water quality of Chilika lagoon. Arabian Journal of Geosciences, 12, 1–23.
Ogamba, E. N., Izah, S. C., & Oribu, T. (2015a). Water quality and proximate analysis of Eichhornia crassipes from River Nun, Amassoma Axis. Nigeria. Research Journal of Phytomedicine, 1(1), 43–48.
Ogamba, E. N., Seiyaboh, E. I., Izah, S. C., Ogbugo, I., & Demedongha, F. K. (2015b). Water quality, phytochemistry and proximate constituents of Eichhornia crassipes from Kolo creek, Niger Delta, Nigeria. International Journal of Applied Research and Technology, 4(9), 77–84.
Ogamba, E. N., Izah, S. C., & Toikumo, B. P. (2015c). Water quality and levels of lead and mercury in Eichhornia crassipes from a tidal creek receiving abattoir effluent, in the Niger Delta. Nigeria. Continental Journal of Environmental Science, 9(1), 13–25.
Ogamba, E. N., Charles, E. E., & Izah, S. C. (2021). Distributions, pollution evaluation and health risk of selected heavy metal in surface water of Taylor creek, Bayelsa State. Nigeria. Toxicology and Environmental Health Sciences, 13(2), 109–121.
Patel, P., Raju, N. J., Reddy, B. S. R., Suresh, U., Gossel, W., & Wycisk, P. (2016). Geochemical processes and multivariate statistical analysis for the assessment of groundwater quality in the Swarnamukhi River basin, Andhra Pradesh, India. Environmental Earth Sciences, 75, 1–24.
Raju, N. J. (2012). Evaluation of hydrogeochemical processes in the Pleistocene aquifers of Middle Ganga Plain, Uttar Pradesh, India. Environmental Earth Sciences, 65, 1291–1308.
Rao, S. N. (2012). PIG: A numerical index for dissemination of groundwater contamination zones. Hydrological Processes, 26(22), 3344–3350.
Rao, G. T., Rao, V. V. S. G., Rao, Y. S., & Ramesh, G. (2013). Study of hydrogeochemical processes of the groundwater in Ghatprabha river sub-basin, Bagalkot District, Karnataka, India. Arabian Journal of Geosciences, 6, 2447–2459.
Rao, N. S., Sunitha, B., Rambabu, R., Rao, P. N., Rao, P. S., Spandana, B. D., Sravanthi, M., & Marghade, D. (2018). Quality and degree of pollution of groundwater, using PIG from a rural part of Telangana State, India. Applied Water Science, 8, 1–13.
Rezaei, H., Zarei, A., Kamarehie, B., Jafari, A., Fakhri, Y., Bidarpoor, F., Karami, M. A., Farhang, M., Ghaderpoori, M., Sadeghi, H., & Shalyari, N. (2019). Levels, distributions and health risk assessment of lead, cadmium and arsenic found in drinking groundwater of Dehgolan’s villages, Iran. Toxicology and Environmental Health Sciences, 11(1), 54–62.
Richard, G., Nwagbara, M. O., & Weli, V. E. (2019). Public health implications of seasonality in noxious gases from dumpsites in some Niger Delta States, Nigeria. International Journal of Research Studies in Bioscience, 7(5), 1–14.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. Scientific Publishers.
Sahu, P., & Sikdar, P. K. (2008). Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environmental Geology, 55, 823–835.
Sasu, D. D. (2022). Life expectancy at birth in Nigeria 2021, by gender. https://www.statista.com/statistics/1122851/life-expectancy-in-nigeria-by-gender/#:~:text=In%202021%2C%20life%20expectancy%20at,well%20as%20in%20the%20world. Accessed February 20, 2022.
Schoeller, H. (1977). Geochemistry of groundwater. Groundwater studies, an international guide for research and practice, UNESCO, Paris, 1–18.
Seiyaboh, E. I., & Izah, S. C. (2017a). Review of impact of anthropogenic activities in surface water resources in the Niger Delta region of Nigeria: A case of Bayelsa state. International Journal of Ecotoxicology and Ecobiology, 2(2), 61–73.
Seiyaboh, E. I., & Izah, S. C. (2017b). Bacteriological assessment of a tidal creek receiving slaughterhouse wastes in Bayelsa state, Nigeria. Journal of Advances in Biology and Biotechnology, 14(1), 1–7.
Seiyaboh, E. I., Inyang, I. R., & Gijo, A. H. (2013). Environmental impact of Tombia bridge construction across Nun river in central Niger delta, Nigeria. The International Journal of Engineering and Science, 2(11), 32–41.
Seiyaboh, E. I., Izah, S. C., & Oweibi, S. (2017). Physico-chemical characteristics of sediment from Sagbama Creek. Nigeria. Biotechnological Research, 3(1), 25–28.
Sevcikova, M., Modra, H., Slaninova, A., & Svobodova, Z. (2011). Metals as a cause of oxidative stress in fish: A review. Veterinarni Medicina, 56(11), 537–546.
Singaraja, C., Chidambaram, S., Anandhan, P., Prasanna, M. V., Thivya, C., Thilagavathi, R., & Sarathidasan, J. (2014). Determination of the utility of groundwater with respect to the geochemical parameters: A case study from Tuticorin District of Tamil Nadu (India). Environment, Development and Sustainability, 16, 689–721.
Singh, S., & Singh, K. N. (2010). Physio-chemical analysis of sewage discharged into Varunariver at Varanasi. Current World Environment, 5(1), 201–203.
Singh, P., Chaturvedi, R. K., Mishra, A., Kumari, L., Singh, R., Pal, D. B., Giri, D. D., Singh, N. L., Tiwary, D., & Mishra, P. K. (2015). Assessment of ground and surface water quality along the river Varuna, Varanasi, India. Environmental Monitoring and Assessment, 187, 1–10.
Soleimani, H., Nasri, O., Ghoochani, M., Azhdarpoor, A., Dehghani, M., Radfard, M., Darvishmotevalli, M., Oskoei, V., Heydari, M., & Heydari, M. (2022). Groundwater quality evaluation and risk assessment of nitrate using Monte Carlo simulation and sensitivity analysis in rural areas of Divandarreh County, Kurdistan province. Iran. International Journal of Environmental Analytical Chemistry, 102(10), 2213–2231.
Srinivasamoorthy, K., Gopinath, M., Chidambaram, S., Vasanthavigar, M., & Sarma, V. S. (2014). Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu. India. Journal of King Saud University-Science, 26(1), 37–52.
Standard organization of Nigeria (SON) (2007). Nigerian standard for drinking water quality. Nigerian industrial standard, Nigeria
Uzoekwe, S. A., Izah, S. C., & Aigberua, A. O. (2021). Environmental and human health risk of heavy metals in atmospheric particulate matter (PM 10) around gas flaring vicinity in Bayelsa State. Nigeria. Toxicology and Environmental Health Sciences, 13(4), 323–335.
Vargas-Zúñiga, G. I., & Sessler, J.L. (2017). Anion and ion pair recognition under interfacial aqueous conditions. Comprehensive Supramolecular Chemistry II. Atwood, J. L. (Ed). Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. (pp. 161–189). Elsevier
Verma, A., & Singh, N. B. (2021). Evaluation of groundwater quality using pollution index of groundwater (PIG) and non-carcinogenic health risk assessment in part of the Gangetic Basin. Acta Geochimica, 40, 419–440.
Verma, A., Yadav, B. K., & Singh, N. B. (2020). Data on the assessment of Groundwater Quality in Gomti-Ganga alluvial plain of Northern India. Data in Brief, 30, 105660.
Wilcox, L. V. (1955). Classification and use of irrigation water (Circular 969). USA.
World Health Organization (WHO) (2011). Guideline for drinking water quality. 4th Edition.
Acknowledgements
This paper constitutes a segment of the Ph.D. dissertation by the lead author, guided by Authors 2 through 5 at the Niger Delta University, Wilberforce Island, Nigeria.
Funding
The study was part of the PhD dissertation of the SCI.
Author information
Authors and Affiliations
Contributions
SCI conceived the idea; participated in the field data/sample collection, laboratory, and statistical analyses; managed the literature search; and wrote the initial draft. GR participated in the field data/sample collection. Authors HOS, GR, WES, and ORU edited the final work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This paper does not contain any human participants or animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Izah, S.C., Stanley, H.O., Richard, G. et al. Surface Water Quality: A Statistical Perspective on the Efficacy of Environmental and Human Health Assessment Tools. Water Air Soil Pollut 235, 205 (2024). https://doi.org/10.1007/s11270-024-06965-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-06965-1