Abstract
Heavy metal(loid)s pose a concern that has drawn significant attention on a worldwide scale due to their persistence, toxicity, and bioaccumulation. For instance, research estimates that globally, the environment has been exposed to around 800,000 t of lead (Pb) and 30,000 t of chromium (Cr) over the course of the last 50 years. In response to this global problem, several techniques such as electrokinetic extraction, surface capping, encapsulation, soil flushing, soil washing, and chemical immobilization have been applied for the remediation of metal-contaminated soils. However, many of these have been classified as expensive, non-ecofriendly and time-consuming. Research has shown that proteobacteria is one of the most abundant phyla in metal-contaminated soils. Proteobacteria are well known for their pathogenic potential, but little is known about their potential for cleaning up contaminated soil. This study assessed the remediation potentials of the proteobacteria consortium on leachate-contaminated soil. About 18 bacterial species that were isolated from the leachate-contaminated soil were reintroduced to the soil. The metals with the highest levels of bioreduction activity in the microcosm modified with proteobacteria isolates were As (61%), Cu (64%), Zn (53%), Mn (47%), and Cr (61%). The shortest bioreduction time detected during remediation by proteobacteria isolates is 69.3 days, which is related to Cu reduction at a rate of 0.01 day−1. It is inferred that the selective blending of proteobacteria isolates promotes the bioreduction of metals in contaminated systems by manipulating microbial diversity. Therefore, it is possible to exploit a potential synergy toward metal reduction through microbial interaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Population expansion, improved living standards, urbanization, and industrialization are the main causes of the rise in waste generation. Municipal solid waste (MSW) disposal practices frequently involve dumping or landfilling the waste in public spaces. Municipal solid waste disposal is a pressing issue worldwide, as improper management practices can lead to various environmental and health concerns (Sohoo et al., 2022). Pollution emanating from MSW landfills account for one of the main threats to the environment since landfill leachate contains potentially toxic elements, including metallic pollutants (Moustafa et al., 2022). Heavy metals and metalloids, also known as heavy metal(loid)s are significant agricultural soil pollutants due to their toxicity, prevalence, non-biodegradability, and bioavailability for crop uptake (Ayangbenro & Babalola, 2020; Hou et al., 2020). Globally, the generation of MSW is steadily rising. The worldwide solid waste production amounted to over 2.1 billion t annually in 2017, and by 2050, it is anticipated to increase to 3.4 billion t (Abu-Qdais et al., 2023; Kaza et al., 2018). Gokgoz et al. (2023) reported that in 2021, the Orange County Landfill in Florida, USA generated about 34 million t of waste, while 0.2 million t was recorded for the Botetourt County Landfill in Virginia, USA. Canada produces about 49,616 t of MSW each day, as against the USA that generates approximately 624,700 t per day (Nanda & Berruti, 2021). The European Union produces approximately 392 million t of MSW annually (Shah et al., 2023). Landfilling has emerged as one of the most effective ways to dispose of waste in developing nations like Malaysia, Indonesia, Thailand, and many others. For instance, in 2021, Malaysia’s population was about 32.7 million. This potentially generated a staggering quantity of solid garbage, predicted to be about 38,427 metric t per day (1.17 kg/capita/day), and with 82.5% of the waste being disposed of in landfills (MIDA, 2021).
Landfill leachate is the fluid that emerges from the trash owing to the increased moisture content of waste due to water percolation or degradation (Jayanthi et al., 2016). Leachate is a highly concentrated effluent. It has a significant impact on landfill design and operation. Leachate has a high concentration of organic substances like alkenes, aromatic hydrocarbons, acids, esters, alcohols, hydroxybenzene, and amides, as well as ammonia nitrogen, and a significant number of heavy metal(loid)s (Cheng et al., 2018; Emenike et al., 2016; Mishra et al., 2023). The raw leachate can laterally infiltrate into the soil compartments of landfills that are not well-engineered with liners and leachate collection systems, thereby contaminating the soil. Leachate can spread into surface and groundwater if sufficient prevention is not taken. The transported heavy metal(loid)s have the potential to bioaccumulate in living things and can enter the food chain. Heavy metal(loid)s from contaminated sources, including water or air, can enter the bodies of humans through the food chain, inhalation, and/or ingestion (Oves et al., 2016).
Heavy metal(loid)s from leachate can be harmful to any environmental media even in low quantities. Many studies have shown that heavy metal(loid)s have negative effects on living things (Wuana & Okiemen, Wuana and Okieimen, 2011) . The health of humans is negatively impacted by potentially toxic metals and metalloids. These potentially toxic elements can build up in the body after exposure. Toxic metal bioaccumulation has a variety of harmful consequences on various body tissues and organs. Metal toxicity symptoms can be sudden or build up over time. It has the potential to impair cellular processes such as apoptosis, differentiation, development, proliferation, and damage repair (Alameri et al., 2020). In addition to the common health complications associated with metal toxicity, such as cancer, kidney damage, and effects on the central nervous system, exposure to metal may also result in epigenetic changes that may affect the control of gene expression (Balali-Mood et al., 2021). For instance, the study by Forsyth et al., (2018) reported that some pregnant women in a rural hamlet in Bangladesh exhibited increased levels of Pb in their blood. In that study, it was discovered that more than 30% of the women whose blood were examined had Pb levels that were more than 5 µg/dL. They discovered that food storage cans were the main source of Pb exposure to these women. Nearly 18% of the examined food storage cans contained soldering made of Pb. This possibly caused the poisoning in the women. Another study conducted by Ying et al., (2018) in China found that children receiving treatment at clinics that treat Pb poisoning had Pb levels ranging from 5 to 126 µg/dL in their blood. The presence of heavy metal(loid)s in the soil can have a negative effect on the growth of earthworms (Eisenia fetida) (Žaltauskaitė et al., 2020). Toxicity owing to heavy metal(loid)s contamination also pose serious threats to flora and wildlife. For example, vegetation that is being cultivated on Pb-contaminated soil can transfer the Pb content to crops (Jyothi, 2021).
It is challenging to remove heavy metal(loid)s from any environmental media owing to the mobility, persistency, and their deterioration capacity. Herein, lies the need for the present studies and related work. Consequently, to achieve the metal remediation of polluted soil, it is necessary to consider the circumstances that may immobilize and/or convert metal ions within a specific environmental media, such as soil. The development of diverse ways for restoring the natural ecosystem affected by contamination, to its original circumstance is required when using microorganisms to remove/bioreduce metal ions from the contaminated soil.
Microorganisms such as proteobacteria display distinctive behavior in metal-induced and hazardous environments via their ability to be resistant to metal ions (Karelová et al., 2011). Proteobacteria, also now referred to as Pseudomonadota represents a large group of prokaryotic microorganisms that are a major phylum of bacteria (Oren & Garrity, 2021). The group includes Vibrio, Escherichia, Salmonella, and a variety of other pathogenic organisms (Berman, 2012). Proteobacteria have been found to have immense metabolic and degradative abilities, in addition to their great habitat adaptability (Gong et al., 2023; Liu et al., 2022). Studies have shown that ureolytic (urease-producing) microorganisms such as proteobacteria possess the potential to ameliorate soils contaminated with a vast spectrum of heavy metal(loid)s (Gong et al., 2023; Hu et al., 2021). Consequently, the current study examined the impact of altering microbial diversity on the cleanup of soil contaminated with leachate metals. It further investigated the relative metal reducibility potentials of native proteo- and non-proteobacteria found in an unhygienic landfill that had been contaminated by extremely heterogeneous leachate.
With respect to impact and significance, the present study adds to the existing knowledge which affirms that the selective use of a blend of surviving microbes from contaminated soil can positively influence heavy metal(loid)s remediation. Furthermore, the present study has also identified a new direction in the use of bacteria species different from the popular option that prioritizes the blend of gram-positive consortia over the gram-negative species. Additionally, this study will cause a shift from the prior understanding that has classified proteobacteria as a microbial signature of disease. Hence, it will draw scientific attention to the ecological restoration potentials of this phylum of bacteria. Furthermore, the study could shed light on the difference in the general metabolism and impact of proteobacteria in soil than in humans.
2 Materials and Method
2.1 Study Site and Collection of Samples
The present study entails the treatment of leachate-impacted soil collected from Taman Beringin Landfill (TBL). Taman Beringin Landfill is a closed, non-sanitary landfill that is situated in the North Jinjang district in Kuala Lumpur, Malaysia. Some leachate samples were collected from pipes that were connected to the landfill cells of TBL, and also from seepages that were adjoining it. There were two categories of soil samples: contaminated soil and uncontaminated soil. The contaminated soil was taken from TBL (3° 13.78′ N, 101° 39.72′ E) while the uncontaminated soil was collected from a garden (3° 7′ 24.15″ N, 101° 39′ 16.79″ E) at the University of Malaya in Malaysia. The contaminated soil was contaminated with leachate due to seepage emanating from the waste cells of TBL. Specifically, the leachate-contaminated soil was removed from the surface (0–30 cm) during sample collection, in accordance with the ASTME—1197 standard guideline for performing terrestrial soil-core microcosm test (American Society for Testing and Materials (ASTM), 1987; Sprocati et al., 2012). A one-piece auger was used for the soil sample collection from selected sampling points located on TBL. The soil samples were transferred into a soil bag and thereafter, taken to the laboratory so that the resident microbes could be isolated from it. Conversely, heavy metal(loid)s analyses were performed on a portion of soil from the uncontaminated soil that had been air-dried. At the laboratory, the samples were stored at 4 °C and − 20 °C for further studies.
2.2 Characterization of Leachate and Soil
The raw leachate samples were characterized using American Public Health Association (APHA) (1998). The soil sample from TBL was also characterized using the ASTME-1197 procedure (American Society for Testing and Materials (ASTM), 1987; Sprocati et al., 2012). Total nitrogen, potassium, and phosphorus contents were determined using the ASTM E978-87, ASTM E96-94, and ASTM D5198-92 methods. The samples’ level of acidity was also assessed (American Society for Testing and Materials (ASTM), 1987). With the exception of mercury (Hg) that the USEPA 3052 method (US Environmental Protection Agency (USEPA), 1996) was utilized for, the study used the USEPA 3050B method to analyze the total concentrations of all the other elements in the soil (US Environmental Protection Agency (USEPA), 1996). All evaluations were properly triple-checked (including different trials). The samples were also evaluated for other physicochemical properties, including pH, color, odor, dissolved oxygen, total organic carbon (TOC), salinity, conductivity, suspended particles, and total dissolved solids (TDS). The Hach Sension 7 was used to detect salinity, conductivity, and TDS, while the HANNA HI8424 portable pH meter and the DO 6 + dissolved oxygen meter were used to monitor pH and dissolved oxygen levels, respectively.
2.3 Isolation and Identification of Bacteria
The method of serial dilution was utilized to isolate the bacteria. Dilutions (0.1 mL) were disseminated over newly made nutritional agar under aseptic conditions (Kauppi et al., 2011). The duplicates and inoculation media plates underwent a 24-h incubation period at 37 °C. Developed colonies were subsequently sub-cultured to ensure the purity of materials prior to identification. To prevent the loss of vigor and viability that is common to most organisms in the stationary phase, the cells were regrown every 16–24 h. Each target cell was injected with inoculation fluid (IF) using Protocols A (IF-A catalog number 72401) and B (IF-B catalog number 72403) at a turbidity range of 95–98%. A 3-mm-diameter region of cell growth was scooped up from the agar plate’s surface using a cotton-tipped inoculators swab (catalog no. 3321), which was then dipped into the chosen IF. To maintain uniform suspension, it was required to smash any cell clumps against the tube wall.
Thereafter, the pre-grown isolates on agar medium (suspended in IF) was then transferred to the GEN III Microplate and allowed to incubate. Subsequently, the isolated microorganisms were then characterized using the Biolog GEN III Microplate methodology (Bochner, 1989a, 1989b). A wide variety of gram-negative and gram-positive bacteria can be profiled and identified using the 94 biochemical tests on the GEN III Microplate test panel, which offers a standardized micro technique. It consists of 23 chemical sensitivity tests and 71 assays for carbon source utilization. A tetrazolium dye that changes color as a result of substrate metabolism is present in the GEN III Microplate. A phenotypic fingerprint of the microbe is provided by the test panel in particular, which can be used to identify it at the species level. The Microbial Identification Systems software by “Biolog” (OmniLog Data Collection) then uses the phenotypic pattern on the GEN III Microplate to identify the bacteria species.
2.4 Bacteria Consortium Formulation for Bioremediation of Soil Contaminated with Heavy Metal(loid)s
The microbial consortia employed in this study are made up of 18 strains that were obtained utilizing the aforementioned technique. Each strain was reactivated on nutrient agar for 18 h at 33 °C, and then it was inoculated into the nutrient broth and cultivated to stationary phase in a shaker at 150 revolutions per minute. Before being applied to the polluted soil, each strain was pooled in equal portions (50 mL of each microbial strain) until it had grown to 1.5 ABS at 600 nm (Emenike et al., 2016).
2.5 Heavy Metal(loid)s Sensitivity Test
For the heavy metal(loid)s sensitivity test, agar diffusion was used to evaluate the metal tolerance for each bacterial isolate. Each sterile plate was seeded with the standard suspension of each organism (5 × 105 CFU/ml), and nutrient agar (20 ml) was added. Before creating 4 diameter wells (6 mm) on the seeded plates, pre-diffusion was permitted. These concentrations: 5, 10, 15, and 20 ppm of each metal were created. About 70 µl of the metals were poured into the appropriate wells. Therefore, each plate had four concentrations of a particular metal and was let to stand for 1 h to allow pre-diffusion to occur. After that, plates were incubated for 24 h at 37 °C. Based on the observed growth pattern, the minimum inhibitory concentrations (MIC) of the heavy metal(loid)s on the microorganisms were calculated. The inhibition zone diameter (IZD) was calculated from the diameters of the matching clear zones, which represented the metal concentrations that exhibited no discernible increase. Three replicates were employed. A linearity plot was used to assess the relationship between resistance and IZD after a one-way ANOVA was used to compare metal resistance across isolated microorganisms.
2.6 Experimental Setup for Bioremediation
A portion of the soil from TBL that had been excavated was used for the bioremediation study in a lab setting. Three treatments made up the experiment: Treatment A, which contained proteobacteria, Treatment B, which contained non-proteobacteria, and Treatment C, also referred to as the “Control” (no amendment). Except for the control setup, the experiment was carried out with 2 kg of leachate-contaminated soil (in polybags) amended with 10% v/w (200 ml to 2 kg of soil) of microbial inoculum. The experiment was carried out in triplicates for all treatments, and each treatment included approximately (3 × 109 CFU/g) of inoculum. There was no stirring, as the experiments were static. It is significant to note that the soil used in the control microcosm was not pre-sterilized because the experiment’s goal is to assess the actual performance differences between treatments while taking into account the inherent potentials of local microbes in the soil at unaltered concentrations and diversity. About 100 mL of distilled water was added every 2 days to the soil to maintain its moisture level between 60 and 65%. For 100 days, the analysis of the amount of metals in the soil, including soil pH and soil redox potential across the treatments were conducted every 20th day (Emenike et al., 2016).
2.7 Metal Analysis
An inductively coupled plasma optical emission spectrometer (ICP-OES) was used to analyze the soil’s metal content every 20 days for all treatments in accordance with USEPA 3050B regulations. Before the acid digestion process, 0.5 g of soil sample from each analysis was put in a beaker. This involved the use of 2 mL of nitric acid 65%, 2 mL of deionized water, and 4 mL of diluted nitric acid solution, as well as 2 mL of nitric acid 65% and 2 mL of hydrochloric acid 37%. After that, a hot block was used to heat the soil and acid mixture to 85 °C for 30 min. The soil sample was heated, then placed in a desiccator for chilling, and the sample volume was then prepared to 50 mL by adding the deionized water. The digested sample was aspirated into the ICP-OES for As, Cd, and Pb analysis, while for Hg, the sample was analyzed with cold vapour atomic absorption spectrophotometer (CVAAS) FIMS-400. The concentration of metals in dry weight basis was calculated after the instrumental reading was acquired.
2.8 Rate Constant of Metal Bioreduction
The daily rate of metal ion bioreduction was calculated using first-order kinetic model:
- k:
-
first-order rate constant for metal uptake per day
- t:
-
time in days
- C:
-
concentration of residual metal in the soil (mg kg−1)
- C0:
-
initial concentration of metal in the soil (mg kg−1)
and half-life of metal removal was calculated using,
k = first-order rate constant for metal bioreduction per day
2.9 Quality Assurance and Quality Control
The obtained data’s accuracy, consistency, and dependability were confirmed through quality assurance and quality control. All lab equipment and glassware were cleaned with 5% (v/v) nitric acid before being rinsed with deionized water. Before analysis, equipment and glassware were oven-dried at 65 °C for 24 h to reduce the likelihood of interferences and cross-contamination. All experiments and evaluations were conducted in triplicates.
2.10 Statistical Analysis
To examine the data statistically, SPSS 21.0 was used for ANOVA, and the LSD post-hoc test was conducted with a 0.05 significance level.
3 Results and Discussion
3.1 Study Site
The specific sampling points on TBL are shown in Fig. 1, and the general conditions of TBL are presented in Table 1.
Table 2 provides a description of the leachate from the TBL. According to the results of the analysis, several of the metals had concentrations that were higher than the standards established by Ministry of Health Malaysia (MHM) (Ministry of Health Malaysia (MHM), 2016) and the Malaysian Department of Environment (Department of Environment (DOE), 2009). The landfill has not been in operation since 2005, yet the concentration of metals in the leachate kept rising. Perhaps, this is due to the type of trash placed and the decomposition and dissociation processes that take place in the landfill. The environment in the landfill has a huge influence on how quickly MSW decomposes. The moisture content and acidity level have been found to regularly have an impact on the pace of trash decomposition. The MSW has an average moisture content of roughly 55%.
Biochemical changes and physiological chemical processes, such as dilution, volatilization, adsorption, precipitation, and dissolution, can affect the quality of leachate (Podlasek, 2023). Leachate’s composition may vary depending on the age of the landfill, making it difficult to characterize (Lindamulla et al., 2022). Large variations in leachate quality have been shown to exist for several landfills and even at various areas inside the same landfill (Podlasek, 2023). The raw leachate of TBL had a brownish color upon visual inspection, which may be related to the waste content and suspended materials present. The pH level is a crucial factor in the effluent discharge and wastewater quality. The pH range is frequently used to symbolize how aggressive the leachate and biochemical conditions are, within the solid waste (Fazzino et al., 2023). The pH of the landfill leachate at TBL is slightly alkaline (7.57 ± 0.8) (Table 2) in the neutrality range. This is consistent with the results of Salleh and Hamid (2013) who noted a shift in pH from 8.49 to 6.96 at Air Hitam Dump in Malaysia. This was linked to excessive alkalinity in samples from a typical old landfill. Mature landfills have a pH that is somewhat alkaline and are full of tough organic material that are non-biodegradable (Fazzino et al., 2023). Usually, the leachate found in landfills in Malaysia shows elevated concentrations for BOD5 and COD (Department of Environment (DOE), 20099) but the leachate from TBL showed 127 ± 45 mg/L BOD5 and 482 ± 103 mg/L COD (Table 2) typical of a mature landfill. This is possibly due to its non-operational status. The BOD5 values of mature landfills are typically low, as a leachate’s organic components may be considered highly biodegradable if the BOD5 value is high (Lindamulla et al., 2022).
According to the leachate analysis from Table 2, the metal with the highest concentration was iron (Fe), which had a concentration of 134.6 ± 16 mg/L. On the other hand, zinc (Zn) had a concentration of 24.3 ± 3 mg/L. Among other metals, Cr, Al, and Mn had readings of 6.2 ± 1.4 mg/L, 5.47 ± 1.2 mg/L, and 3.1 ± 0.32 mg/L, respectively.
The soil samples taken from TBL were characterized (Table 3). The metal content included Al (49,600 mg/kg), Fe (42,900 mg/kg), Mn (281 mg/kg), As (103 mg/kg), Cu (59 mg/kg), Zn (49 mg/kg), Cr (46 mg/kg), Ni (21 mg/kg), and Pb (18 mg/kg) (Table 3). Heavy metal(loid)s including Cd, Zn, Cu, Cr, Pb, and Ni are typically found in soil or plants. The allowable level of Cd in plants, as approved by the World Health Organization World Health Organization (WHO) (1996) is 0.02 mg/kg. The WHO recommends tolerable limits of 0.6, 10, 1.6, 2, and 10 mg/kg for Zn, Cu, Cr, Pb, and Ni, respectively.The findings of the current study show that the levels of heavy metal(loid)s in the soil were higher than the limits recommended by World Health Organization (WHO) (1996). It is a well-known fact that the metal concentration of soil removed from unlined landfills (non-sanitary) frequently reflects the sorts of garbage that were dumped there. It is customary in Malaysia for residential and industrial hazardous waste to be dumped in landfills. This may be the probable reason for the elevated levels of potentially toxic elements found in this study. Different forms of a given metal are distributed differently depending on the chemistry of that metal (Hou et al., 2020). There is always a possibility that heavy metal(loid)s in the soil could contaminate the groundwater, and subsequently, surface water as well (Sohoo et al., 2022). The possibility that heavy metal(loid)s could linger in landfills for a long time is another issue that provides cause for concern (Ayangbenro & Babalola, 2020). Heavy metal(loid)s may linger in landfills for around 150 years if they are leached at a rate of 400 mm/year, which is another issue that is cause for concern (Adelopo et al., 2018). In addition to being among the most poisonous and carcinogenic contaminants, metals pose a major risk to the environment and human health due to their non-degradable nature. Consequently, the purpose of the study was to use proteobacteria to treat soil that had been contaminated by leachate.
3.2 Isolated Bacteria Species
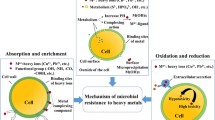
Using the conventional isolation technique, 18 isolates were successfully removed from the soil taken from TBL. It is worth mentioning that the identified bacteria isolates used in the present study are the complete set of culturable species from the soil sample. To further investigate their ability for removing certain metals, the isolates were divided into proteobacterial and non-proteobacterial groups (Table 4). Microorganisms have the propensity to evolve a variety of survival strategies in environments contaminated by heavy metal(loid)s. Microorganisms have created inventive metal resistance and detoxifying systems in response to metals in the environment. Microorganisms adopt a variety of strategies to avoid becoming poisonous to metals, including biotransformation, extrusion, the utilization of enzymes, the manufacture of exopolysaccharide, and the synthesis of metallothionein (Igiri et al., 2018). The existence of anionic structures that enable germs to bind to metal cations, also results in the presence of a negative charge on the surface of microorganisms (Huang et al., 2023a).
NB, no bacteria addition; *Treatment C also referred to as Control.
Microbes can survive in situations where there is metal contamination owing to one or more of their resistance mechanisms. Also, it is anticipated that the combination of bacteria will increase the metabolic potential for heavy metal(loid)s (Emenike et al., 2016). Complex pollutants such as heavy metal(loid)s may put a single species under stress, obstruct its metabolism, and render them incapable of surviving in a toxic environment (Brisson et al., 2012). However, microbial consortia, or groups of bacteria, have a higher propensity for resistance and multifunctionality because different species cooperate to effectively utilize a variety of substrates. Certain species within microbial consortia can promote or inhibit the growth of other species, change interactions among them, and even affect the function of the whole community (Li et al., 2023).
3.3 Microbial Resistance to Selected Heavy Metal(loid)s
3.3.1 Arsenic
Out of the 18 isolates, the proteobacteria species including Pseudomonas alcaligenes, Pseudomonas mendocina, Aeromonas caviae, and Stenotrophomonas acidaminiphilia showed the highest resistance to exposure to As. The studies by Banerjee et al., (2011), Ghosh et al., (2015), and Mishra et al., (2022) support the findings of the present study.
3.3.2 Copper
When exposed to Cu, B. aryabhattai was completely inhibited, whereas B. diminuta demonstrated inhibition at 5 ppm. The other 16 isolates all exhibited complete resistance to Cu at concentrations of 20 ppm and higher. These results agree with the research by Jayanthi et al., (2016).
3.3.3 Zinc
All isolates, with the exception of B. kochii, B. aryabhattai, J. hoylei, B. diminuta, and D. tsuruhatensis demonstrated positive growth beyond 20 ppm of Zn, according to the results of the heavy metal(loid) sensitivity test. While B. diminuta and J. hoylei showed growth inhibition in response to exposure to more than 5 ppm of Zn, B. aryabhattai showed growth inhibition in response to more than 10 ppm. Even at low levels of Zn (<5 ppm), the growth of D. tsuruhatensis and B. kochii was hindered.
3.3.4 Manganese
The exposure to Mn revealed that all isolates were highly resistant up to 20 ppm, except for C. gleum that showed inhibition even at 5 ppm concentration. The microbe, S. marcescens, particularly showed great resistance to Mn. This finding is in consonance with the studies by Barboza et al., (2017) and Huang et al., (2023b).
3.3.5 Chromium
The three species that showed great resistance to exposure to Cr above 20 ppm include B. cereus, S. marcescens, and J. hoylei. This is probably due to the high level of resistance by the bacteria species to harmful metals. Similar recommendations for the bioremediation of hazardous metals have been made by earlier studies (Campos et al., 2013; Ramírez et al., 2019). The other isolated bacteria species exhibited growth inhibition when exposed to Cr at varied concentrations below 20 ppm.
3.4 Removal Efficiency for Selected Heavy Metal(loid)s Across Treatments
3.4.1 Arsenic
Arsenic is a semi metallic, odorless, tasteless metal with prominently toxic or human carcinogenic properties (Singh et al., 2007). Both organic and inorganic forms of As are prevalent in the soil, and the inorganic forms are quite poisonous (Shrivastava et al., 2015). Arsenic is known to occur mostly as As5+,(arsenate) in surface soil, whereas As3+ (arsenite) predominates in waterlogged soil. The relative concentrations of As5+ and As3+ have a significant impact on the mobility, toxicity, and environmental behavior of As (Gonzalez Henao & Ghneim-Herrera, 2021). The ingestion or inhalation of As can cause detrimental effect to humans. It can cause skin cancer, lung disease, and the impairment of the liver and bladder (Bhat et al., 2023).
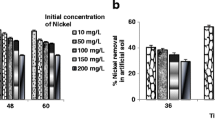
Figure 2 shows the concentration of extractable As in the soil throughout the current study, spanning various time intervals and employing various combinations of microbial consortia. The soil amended with Treatment A (Proteo-consortia) demonstrated a larger As decrease than the Treatments B and C following 100 days of remediation with various combinations of microbial consortia. The initial As concentration was measured to be 103 mg/kg. The highest removal for As (61%) was recorded for the soil amended with Treatment A in comparison to Treatments B and C (Fig. 2). At 20-day intervals throughout the investigation, a rapid As reduction in Treatment A was seen during metal analyses. The findings show that proteo groups have the ability to bioreduce As in contaminated soil. Bacterial resistance to heavy metal(loid)s is a crucial element to consider when studying remediation. This is because potentially toxic metals and metalloids directly affect the survival and development of bacteria employed to recover contaminated locations (Kang et al., 2016). Consequently, the selection and optimization of the microorganisms that boosted the reduction of As can be linked to the higher As reduction by Treatment A (proteobacteria). This agrees with Pepi et al., (2007) and Yamamura et al., (2007) who reported that Aeromonas sp. and Pseudomonas sp. are highly resistant bacteria to As. These two types of bacteria belong to the proteo-group and may have caused a larger removal of As from contaminated soil.
3.4.2 Copper
Copper has an excellent thermal and electrical conductivity, in addition to being soft, malleable, and ductile (Jha et al., 2015). Copper can damage the kidneys and liver, as well as, irritate the intestines and stomach when ingested in excessive doses (Jyothi, 2021). The consumption of drinking water from Cu-treated pipes may cause contamination of the water (Wuana & Okieimen, 2011). Copper forms strong complexes to the organic molecules in the soil. Consequently, only a tiny fraction of Cu will be found in solution as ionic copper, Cu (II) (Filipović et al., 2023).
The remediation experiments for Cu across the different treatments are shown in Fig. 3. Treatments A, B, and C (Control) had mean residual Cu concentrations of 21.33 mg/kg, 23.67 mg/kg, and 37 mg/kg, respectively. Therefore, the most efficient elimination of Cu (64%) was observed with Treatment A, followed by Treatment B and the Control at 60% and 37%, respectively. As a result, a significant difference (p < 0.001) was observed between the modified treatments (A–B) and the Control (Treatment C). The way that microorganisms react to metal-pollution might vary depending on the type of metals present and the range of concentrations, even among different species (different treatments). The significant reduction of Cu that is observed with the modified treatments demonstrates the efficiency of the microorganisms.
This result correlates with the hypothesis suggested by Nieto et al., (1989) and later confirmed by Fakhar et al., (2022) that some microorganisms have a tendency to be more sensitive to particular metals but can have great tolerance for other metals. The type of metabolites produced by the species in the amended treatments during the experiment as opposed to the Control is possibly related to the reduction in Cu content. For example, Bacillus species, Rhodococcus species, Brevundimonas species, and Stenotrophmonas species have all been linked to the removal of Cu from contaminated systems in the past (Choudhary and Sar, 2009; Emenike et al., 2017). This is likely the cause of the treatments’ higher reduction levels.
3.4.3 Zinc
Zinc is commonly found in soil as a result of industrial activities such as waste incineration, mining, and steel processing (Luo et al., 2023). Zinc may be present in trace amounts in some foods and beverages, and these concentrations may rise if the products are kept in metal tanks (Wuana & Okieimen, 2011). Zinc deficiency in women can result in birth abnormalities because it is a trace element that is crucial for human health (Wuana & Okieimen, 2011). However, the Zn concentrations in drinking water may increase to dangerous levels owing to industrial sources and toxic waste sites (Sankhla et al., 2019; Wuana & Okieimen, 2011).
The microcosm proteobacteria group showed substantial Zn bioreduction (Fig. 4). Initial Zn levels in the polluted soil were 49 mg/kg, but the experiment showed that Treatments A and B experienced roughly 53% and 50% Zn bioreduction, respectively. In this study, a significant difference between the treatments (A, B) and the Control group was found, due to the almost twofold smaller variation in Zn concentration between additions with Treatments A and B as opposed to Treatment C (Control). Microorganisms have a great deal of untapped potential for remediating soil. The results show the potential effects of these bacteria on the in situ microbial population (Fauziah et al., 2017; Tatiana et al., 2011).
3.4.4 Manganese
Manganese is a chemically active element that is pinkish gray in color (Nieder et al., 2018). It is a hard, extremely brittle metal that oxidizes readily (Nieder et al., 2018). Pure manganese is highly reactive. When it is in its powdery form, it can burn if exposed to oxygen (Williams et al., 2012). Manganese reacts with water and can dissolve in dilute acids (Williams et al., 2012). Inhaling huge doses of Mn through dust or fumes can irritate the lungs and result in pneumonia (Dobson et al., 2004).
In the present study, the Treatment A microcosm showed the largest Mn reduction (Fig. 5). The percentage of extractable metal reduction for Treatment A was 47%, and the residual Mn content was 149.33 mg/kg. When compared to the Control trial, the bioreduction of Mn caused by the inclusion of proteobacteria in Treatment A showed a fourfold higher degree of bioreduction. It is evident that adding inoculum had a huge impact on removing Mn from the contaminated soil. This was most likely caused by the organisms in Treatment A being able to start metabolic processes that improved the breakdown or uptake of Mn, leading to increased Mn removal by the treatment. Hence, the order of reduction for all treatment was Treatment A (47%) > B (30%) > Control (11%). Significant differences between Treatment A, Treatment B, and Control were recorded (p < 0.001). The variations in Mn reduction among the treatments can be possibly linked to the bacterial strains in each treatment. The combination of Burkholderia sp., Ochrobactrum sp., and Pseudomonas sp. may have played a major role in higher Mn reduction in Treatment A. This is probably because Burkholderia sp. has multiple natural resistance-associated macrophage protein (NRAMP) isoforms (Kehres & Maguire, 2003) which enabled Mn uptake in bacteria using the transporter, NRAMP (MntH). The transporter comprises of both high and low-affinity Mn uptake systems, and the utilized transporter depends on the concentration of Mn in the environment.
Similarly, it has been observed that a strain of Ochrobactrum sp. lowers the concentration of extractable Mn in contaminated environments (Lebuhn et al., 2006; Ozdemir et al., 2003). Also, it has been determined that Pseudomonas sp. is an effective Mn remediation agent (Santelli et al., 2014). As a result, Treatment A’s ideal reduction of Mn in comparison to other treatments may have been caused by the synergistic form of interaction among the three microorganisms.
3.4.5 Chromium
Chromium is a hard, steel-gray, and lustrous transition metal (Briffa et al., 2020). Most soils contain Cr in its trivalent form, Cr3+, but it is harmful to humans, animals, and plants in its hexavalent state, Cr6+ (Karthik et al., 2014). Hexavalent Cr contamination might result through welding, burning coal or oil, steel manufacturing, or fertilizer production (Monalisa & Kumar, 2013). Large amounts of wastewater contaminated with Cr have been dumped into water bodies as a result of the widespread usage of Cr in industrial applications (Monalisa & Kumar, 2013). Consequently, an exposure to excessive levels of Cr can harm the liver and kidneys, lead to skin ulcers, and have an impact on the central nervous system (Briffa et al., 2020).
Figure 6 shows the reduction of Cr across the treatments in the current study. It revealed that the Treatment A microcosm had a significant influence. It is observed that Cr decreased from 46 to 18 mg/kg (61%) in Treatment A. Therefore, it can be inferred that the inclusion of Treatment A improved Cr removal from the polluted soil. The residual Cr concentrations for Treatments B and C were 26.67 mg/kg and 25 mg/kg, respectively (Fig. 6). Similar results was found in Emenike et al., (2017), where it was shown that the addition of microbial consortia of various groups had a higher percentage of removal of Cr than natural bioremediation. A substantial difference between Treatment A versus the Control was confirmed by statistical analysis (p < 0.001). The difference between Treatment B and the Control was only 1%, which was the smallest reduction. This possibly happened because of Treatment B’s microorganisms mineralizing due to an unidentified but distinctive metabolic reaction to the Cr-induced environment. Most prevalent species isolated from soil polluted with Cr belonged to the proteo group (Flavio et al., 2005). This could be the probable cause for the isolate’s Cr-affinity, which ultimately led to the bioreduction of extractable Cr from the treated microcosm. The greater elimination of Cr observed in Treatment A may be related to the involvement of the Pseudomonas sp. (Hassan et al., 1999). Conversely, as suggested by Ajay Kumar et al., (2009), the variations in the degree of reduction across various treatments could be attributed to the variations in the affinity and electronegativity of the metal ions during the exposure to microbes. In addition, Sprocati et al., (2012) found that strain selection, bacterial concentration, and inoculum heterogeneity are crucial for efficient bioremediation.
3.4.6 Removal Rate Constant of Heavy Metal(loid)s Across Treatments
Generally, the treatments augmented with microorganisms showed more potential for the removal of heavy metal(loid)s than the Control experiment. Table 5 displays the rate and half-life of the elimination of potentially toxic elements. Evaluation of the various treatments for various metals showed that Treatment A for Cu showed the highest removal rate. The rate of removal was 0.010 day−1. Also, when compared to other treatments, Treatment A exhibited the highest removal rates for As, Zn, Mn, and Cr, with removal rates of 0.0095 day−1, 0.0076 day−1, 0.0063 day−1, and 0.0094 day−1, respectively (Table 5). It is worth mentioning that the grouping of treatments, particularly those containing only proteobacteria has not been explored before for bioremediation. Furthermore, since some microorganisms as separate cultures had been reported to remove metals successfully in prior trials, the substantial decrease of metal by this treatment may be related to the blending of microbes. A function of the bioremoval rate constant is the half-life. According to the data on the half-lives of the metals and metalloids, the shortest half-life for Cu was reported by Treatment A during the bioremediation of soil from TBL (Table 5). It took approximately 69.3 days to reduce the Cu concentration in the system to half. The shortest half-life was reported for As, Zn, Fe, Mn, and Cr when the polluted soil was supplemented with Treatment A; these values were 72.96 days, 91.2 days, 110.02 days, and 73.73 days, respectively (Table 5). The concentration of heavy metal(loid)s removed by each treatment determines how different they are from one another in terms of their half-life values. The Control (Treatment C) had the longest half-life value. This was similarly reported by Emenike et al., (2016) and Auta et al., (2018).
Besides the investigation of heavy metal(loid)s, other parameters that were assessed include soil pH and soil redox potential. The soil redox potential was assessed across the treatments during the bioremediation process. The variation of the redox potential across the different treatments reflects the solubility of the metals in the soil. It was observed that Treatment A had a higher redox potential value at the end of the 100 days (294.96 mV), in comparison to Treatment B and the Control that had 275.06 mV and 228.63 mV, respectively. The increase in soil redox potential as the days progressed from day 0 to day 100 for all treatments indicates that metal transformation was possibly occurring. It is anticipated that as leachate-contaminated soil goes through bioremediation, the amount of metals that can be extracted will decrease, thereby increasing the redox potential. This is supported by findings from Rinklebe et al., (2016), Chuang et al., (2020), and Cavazzoli et al., (2023). Contrarily, Chen et al., (2021) reported a significant reduction in the soil redox potential of a Cr-contaminated soil upon the introduction of microbial strains.
With respect to soil pH, a pH of 8.2 was recorded for the soil at the onset of the bioremediation studies for Treatments A, B, and the Control experiment (Fig. 7). This initial soil pH was suitable for microbial growth. With reference to the changes in the soil pH during the 100-day remediation study, the soil pH varied from slightly acidic at day 20 (6.93, 6.83, and 7.32 for Treatments A, B, and Control, respectively) to neutral pH (8.21, 7.91, and 8.05 for Treatments A, B, and Control, respectively) at day 100 (Fig. 7). The pH changes in the soil are likely caused by the immobilization of metals following bacterial inoculum introduction. The studies by Wang et al., (2021) and Ji et al., (2022) support the bioreduction of hazardous metals at neutral soil pH as reported in the present study. For instance, the addition of biochars containing Bacillus cereus, Bacillus velezensis, and Bacillus amyloliquefaciens raised the pH of the soil and improved the bioreduction of Cd, Pb, and As in the study by Ji et al., (2022). As a soil’s pH increases from slightly acidic to neutral, functional groups such as phenolic, hydroxyl, and carbonyl are more likely to deprotonate. This possibly enhances the negatively charged surfaces of soil colloids and positively influences the bioremoval of the heavy metal(loid)s from the soil (Wang et al., 2021). Conversely, Syed et al., (2021) reported a decrease in the pH of the inoculated metal-contaminated media at various intervals during their bioremediation study.
With respect to the possible involvement of other microbes such as fungi in the bioremoval of heavy metal(loid)s in the soil, it is important to note that the Control experiment in the present study was not sterilized. This implies that the bioreduction of metals that was observed with the Control experiment, recorded as 11%, 26%, 37%, 42%, and 46% for the percentage removal of Mn, Zn, Cu, Cr, and As, respectively, occurred as a result of natural remediation. Natural remediation could have been possible due to inherent fungi that may have been present in the unamended soil (Control experiment).
Furthermore, with regards to the possible effects of other microbes or organisms on the survival of the bioaugmented bacteria; it is important to note that low inocula can lead to limited survival of bioaugmented bacteria. Since colonization by bioaugmented bacteria is a significant factor in the success of bioaugmentation, the use of high inocula was ensured during the study. Specifically, each treatment included approximately 3 × 109 CFU/g of inoculum. The bacterial count was also considered during the 100-day remediation period, and Fig. 8 displays the bacterial count over time with various treatments for the TBL’s soil remediation. It is crucial to comprehend the distribution of bacteria in a bioremediation setup because a bacterial count reflects the survival of microbes in a metal-contaminated soil. The count was conducted every 20 days. Day 0 saw an average bacterial count of 1.4 × 108 CFU/g for all treatments, as opposed to the Control’s 2.5 × 107 CFU/g. Throughout the 100 days, there was a shifting tendency in the bacterial count. Similar trends in bacterial count was seen by Lin et al., (2010) for bioremediation investigations of heavy metal(loid)s with inoculation of various groups of microorganisms. At day 20, all treatments showed an increase in the number of microorganisms (Fig. 8). This was anticipated due to the inoculum being introduced at the start of the experiment. An increase in bacterial count was observed at both day 60 and day 80 for both Treatments A and B (Fig. 8). The availability of nutrients in the soil is a probable cause of the increase in bacterial count. The bacterial count may have increased also as a result of the occurrence of conditions that are favorable for cell duplication. A significant reduction in the bacterial count was observed across board at the end of the experiment (day 100) (Fig. 8). This trend may have occurred due to cell stress associated with continued metal toxicity. It is important to note that the remediation process has the tendency to limit the availability of nutrients at this stage.
The findings from the majority of the data in the current study allude to the interplay of the significant role of the proteobacteria species in Treatment A, toward the bioreduction of heavy metal(loid)s. Some of the proteobacteria species include Ochrobactrum intermedium, Burkholderia vietnamiensis, Aeromonas caviae, Serratia marcescens, and Pseudomonas spp. Therefore, these microbes have been briefly expatiated on, hereafter.
Ochrobactrum intermedium is a species of bacteria that has been found to be capable of degrading potentially toxic elements, oil, and hydrocarbons in contaminated soil. This bacterium belongs to the class of proteobacteria and is known for its versatile metabolic capabilities. Researchers have identified Ochrobactrum intermedium as a potential candidate for soil remediation due to its ability to produce certain enzymes that facilitate the breakdown of complex compounds (Raklami et al., 2022; Sharma & Shukla, 2021). For instance, Khan et al., (2022) reported that Ochrobactrum intermedium was able to remove 93% of reactive black 5 dye from textile waste-contaminated soil. Also, the study by Sharma and Shukla (2021) showed that Ochrobactrum intermedium can resist up to 2400 mgL−1 and 850 mgL−1 of Pb and Ni, respectively. Furthermore, studies have reported the presence of ATPase (metal-specific transporter) and extracellular polymeric substances (EPS) in the genome of Ochrobactrum intermedium (Khan et al., 2022; Raklami et al., 2022). While the ATPase is important for the import of metals, the EPS enables metal ion binding, which enhances the adsorption and exchange of metal ions (Sharma & Shukla, 2021). Raklami et al., (2022) reported that Ochrobactrum intermedium can cause the detoxification of Cr by reducing the highly toxic form of Cr ion (Cr (VI)) to a less toxic state (Cr (III)).
Burkholderia vietnamiensis is another bacterium that is commonly isolated for the microbial remediation of polluted soil. The studies by Gan et al., (2014) demonstrated the ability of Burkholderia vietnamiensis to degrade crystal violet dye from textile-waste contaminated media. This finding was corroborated in the study by Zhou et al., (2014) where it was concluded that Burkholderia vietnamiensis has the potential to bioadsorb copper ions and simultaneously, degrade crystal violet dye. Burkholderia vietnamiensis can also reduce the toxicity of uranium and nickel; as well as influence the degradation of trichloroethylene (Van Nostrand et al., 2007). Strains of Burkholderia can produce indoleacetic acid (IAA), a substance that can solubilize inorganic phosphates to aid in the uptake of metals from soil (Jiang et al., 2008; Wang et al., 2022a). Additionally, Burkholderia strains can synthesize exopolysaccharides and siderophores (low molecular weight molecules that can scavenge iron), which facilitate the removal of heavy metals from the soil (Ferreira et al., 2010; Wang et al., 2022a). In fact, Burkholderia vietnamiensis has been identified as the strain with the highest siderophore-producing capacity among the genus Burkholderia (Wang et al., 2022a).
The genus Aeromonas consist of gram-negative bacteria that are widely distributed in aquatic systems, as well as in the terrestrial environments. Aeromonas spp. have great genetic flexibility and significant capacity to thrive in diverse habitats (Canellas & Laport, 2022). When compared to other bacterial groups, research on the biotechnological potentialities of Aeromonas spp. is significantly sparse because attention is usually paid to their harmful potential (Canellas & Laport, 2022). Mobile elements such as transposons, bacteriophages, plasmids, and chromosomal genomes (with integrons) mediate heavy metal(loid)s resistance in Aeromonas species (Tataje-Lavanda et al., 2019). These metal resistance genes include those for Cr, As, Zn, Hg, Cd, Co, Ni, and others (Uhrynowski et al., 2017). Therefore, Aeromonas species possess the ability to convert and eliminate heavy metal(loid)s and other contaminants from contaminated environments (Huang et al., 2019). For instance, Aeromonas species have been shown to breakdown hydrocarbons and bioreduce Ni, Cu, and Zn (Qurbani et al., 2022), remove nitrates from wastewater (Rajta et al., 2020), and decolorize trimalachite green dye (Du et al., 2018).
The genus Pseudomonas is one of the most diverse bacterial genera that play a critical role in natural microbial communities. According to Hesse et al., (2018), Pseudomonas spp. are thin, rod-shaped, mostly unicellular organisms with a straight or curved long axis. They are non-spore-forming, gram-negative organisms (Fakhar et al., 2022). Pseudomonas species are frequently found in soil, aquatic environments, humans, and animals (Hesse et al., 2018). They are common and can grow on harmful metal-contaminated soil. For instance, Pseudomonas spp. can methylate mercury (Hg2+) and transform it to its gaseous form, methyl mercury (Igiri et al., 2018). Similar conversions of As to gaseous arsines, Pb to dimethyl lead, and selenium (Se) to its volatile form, dimethyl selenide are also possible with Pseudomonas spp. (Fakhar et al., 2022). Pseudomonas spp. can tolerate polluted settings, owing to a number of well-established processes. During biosorption, polysaccharides on the cell surface of Pseudomonas spp. form a flaccid bond with heavy metal(loid) ions (Fakhar et al., 2022). Pseudomonas spp. have defense mechanisms that rely greatly on extracellular polysaccharides secreted around their cells (Fakhar et al., 2022). Furthermore, rhamnolipids which is a type of biosurfactant made by Pseudomonas can form complexes with heavy metal(loid)s including Zn, Pb, and Cd. Consequently, this causes them to speciate (Meliani, 2015).
Serratia marcescens is a gram-negative rod-shaped member of the Enterobacteriaceae family. It has been isolated from a variety of environmental and nosocomial sources (Díaz et al., 2022). Since Serratia marcescens has been identified as the cause of a variety of symptoms in hospitalized patients, its significance as an opportunistic human pathogen has come to light (Díaz et al., 2022). Some of these virulence characteristics, especially those of secondary relevance, may be one of the reasons why the bacterial species is able to compete in a variety of hosts, including soil, water, insects, and plant surfaces (Abreo & Altier, 2019). Published findings have shown Serratia marcescens to be highly resistant to certain toxic metals (Marrero et al., 2009; Díaz et al., 2022) . Serratia species have been suggested as effective biosorbents for Cd and Cr (Sun et al., 2023) and as a bacterium that may reduce molybdenum (Mo) (Shukor et al., 2008). The Serratia marcescens strains 16, C-1, and C4 that were discovered in a Ni deposit in Cuba were able to remove Ni and Co ions from monometallic systems (Marrero et al., 2009). The biosorption of Co (II), Zn (II), Cu (II), and Ni (II) via Serratia marcescens was also reported in the study by Díaz et al. (2022). In the present study, Serratia marcescens is a member of the proteobacteria treatment group (Treatment A) that exhibited the highest bioreduction of As, Cu, Zn, Mn, and Cr in leachate-contaminated soil.
Overall, it is important to realize that the bioaugmentation strategy aimed to rearrange the collection of microbes isolated from the contaminated area to find the dominant microorganisms that can remove pollutants and boost the pace at which heavy metal(loid)s are removed. The proper strains of microorganisms or their consortia are necessary for a successful bioaugmentation technique. The ability of microorganisms to grow quickly, be easily cultivated, tolerate contaminants well, even at high concentrations, and thrive in a variety of environmental conditions should all be considered when choosing the right culture (Agnieszka and Zofia, 2010; Gong et al., 2023). Consortia are frequently more effective than single strains because the intermediates of one strain’s catabolic route may be further degraded by other bacteria with suitable catabolic pathways (Heinaru et al., 2005).
4 Conclusions
An environment contaminated with potentially toxic metals and metalloids poses a serious challenge due to their harmful effects across the food chain. The formation of large amounts of leachate is influenced by landfilling. Leachate from improperly managed landfills can seriously contaminate surface and groundwater sources by flowing laterally into the soil. Some microorganisms have the capacity to thrive in metal-laden environments and convert those metals into less dangerous forms. Yang et al., (2023) listed proteobacteria as a major microbial strain that have the innate ability to tolerate potentially toxic elements. Yang et al., (2023) and Gong et al., (2023) further suggested that there could be a close correlation between the presence of proteobacteria in a soil’s microbial community and the removal of metals. Both studies recommended this notion for future study. The proposition by Yang et al., (2023) and Gong et al., (2023) were investigated and corroborated in this study.
The current study found that in comparison to the other treatments, the proteobacteria group considerably increased the rate at which As, Cu, Zn, Mn, and Cr were bioreduced, making them potential remediation agents for the bioreduction of heavy metal(loid)s in contaminated environments. Specifically, in the present study, the highest percentage removal was recorded as 61%, 64%, 53%, 47%, and 61% for As, Cu, Zn, Mn, and Cr, respectively. Therefore, this study reports that the proteobacteria isolates demonstrated better metal removal from the leachate-impacted soil. Following the findings of the current study, future strategies and applications will include multiple investigations centered on identifying and exploring the target genes for metal-remediation using proteobacteria. In view of the proposed upscaling, some factors will be considered and further optimized. These factors include oxygen supply, possible interferences from environmental conditions such as sunlight, rain, temperature, microbial competitions, soil texture and moisture, concentration, toxicity, and bioavailability of target contaminants, as well as the presence of other co-contaminants. In some instances, there may be the possibility of suppression of introduced bacterial strains by indigenous microorganisms which may lead to failure of the field-scale remediation of metal-contaminated soils. This may occur despite the application of a microbial inoculum that was successful at the laboratory stage. In order to mitigate this possibility, further standardization of the growth parameters of the performing isolates will be conducted prior to large-scale studies.
With respect to contributions of the present study, to the best of the authors’ knowledge, this is the first time that the proteobacteria group have been categorically prioritized as bioremediation agents. Furthermore, the microbial formulation protocol described in the current study can be optimized by industry for land reclamation. The studies by Fakhar et al., (2022), Cao et al., (2022), and Wang et al., (2022b) reported an increase in the abundance of proteobacteria after the bioreduction of heavy metal(loid)s from contaminated media. These studies further alluded that the proteobacteria group may have played a significant role in the bioremediation of metal-contaminated soils. It is apparent that the work by Cao et al., (2022), Fakhar et al., (2022), and Wang et al., (2022b) support the findings of the current study, with regards to the conclusion that proteobacteria possibly possess remarkable potential for the bioreduction of heavy metal(loid)s in contaminated soils.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
Abreo, E., & Altier, N. (2019). Pangenome of Serratia marcescens strains from nosocomial and environmental origins reveals different populations and the links between them. Scientific Reports, 9(1), 46. https://doi.org/10.1038/s41598-018-37118-0
Abu-Qdais, H. A., Shatnawi, N., & Al-Shahrabi, R. (2023). Modeling the impact of fees and circular economy options on the financial sustainability of the solid waste management system in Jordan. Resources, 12(3), 32. https://doi.org/10.3390/resources12030032
Adelopo, A. O., Haris, P. I., Alo, B. I., Huddersman, K., & Jenkins, R. O. (2018). Multivariate analysis of the effects of age, particle size and landfill depth on heavy metals pollution content of closed and active landfill precursors. Waste Management, 78, 227–237. https://doi.org/10.1016/j.wasman.2018.05.040
Agnieszka, M., & Zofia, P. S. (2010). Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiology Research, 165, 363–375. https://doi.org/10.1016/j.micres.2009.08.001
Ajay Kumar, A. V., Naif, A. D., & Hilal, N. (2009). Study of various parameters in the biosorption of heavy metals on activated sludge. World Applied Science Journal, 5, 32–40.
Alameri, A. A., Mahdi, I. J., Ameer, F. S. A., Khaleel, A. K., & Harbi, S. T. (2023). Study into how heavy metal exposure increases the chance of developing lung cancer. Journal of Global Scientific Research, 8(1), 2851–2860. https://doi.org/10.5281/zenodo.7541417
American Public Health Association (APHA). (1998). In L. Clescert, A. Greenberg, & A. Eaton (Eds.), Standard methods for the examination of water and wastewater (20th ed.). Washington. https://www.standardmethods.org/pb-assets/downloads/SM%20SOP-2023-2.0-1676654450900.pdf. Accessed 5 Apr 2023
American Society for Testing and Materials (ASTM). (1987). Standard guide for conducting a terrestrial soil-core microcosm test. Annual Book of ASTM, 1104, 743–755.
Auta, H. S., Emenike, C. U., Jayanthi, B., & Fauziah, S. H. (2018). Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Marine Pollution Bulletin, 127, 15–21. https://doi.org/10.1016/j.marpolbul.2017.11.036
Ayangbenro, A. S., & Babalola, O. O. (2020). Genomic analysis of Bacillus cereus NWUAB01 and its heavy metal removal from polluted soil. Scientific Reports, 10(1), 19660. https://doi.org/10.1038/s41598-020-75170-x
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M. R., & Sadeghi, M. (2021). Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Frontiers in Pharmacology, 227. https://doi.org/10.3389/fphar.2021.643972
Banerjee, S., Datta, S., Chattyopadhyay, D., & Sarkar, P. (2011). Arsenic accumulating and transforming bacteria isolated from contaminated soil for potential use in bioremediation. Journal of Environmental Science and Health, Part A, 46(14), 1736–1747. https://doi.org/10.1080/10934529.2011.623995
Barboza, N. R., Morais, M. M., Queiroz, P. S., Amorim, S. S., Guerra-Sá, R., & Leão, V. A. (2017). High manganese tolerance and biooxidation ability of Serratia marcescens isolated from manganese mine water in Minas Gerais Brazil. Frontiers in Microbiology, 8, 1946. https://doi.org/10.3389/fmicb.2017.01946
Berman, J. J. (2012). Gamma Proteobacteria. Taxonomic Guide to Infectious Diseases, 1, 37–47.
Bhat, A., Hara, T. O., Tian, F., & Singh, B. (2023). Review of analytical techniques for arsenic detection and determination in drinking water. Environmental Science: Advances, 2, 171–195. https://doi.org/10.1039/D2VA00218C
Bochner, B. (1989). Breathprints at the microbial level. ASM American Society for Microbiology News, 55(10), 536–539.
Bochner, B. (1989). Sleuthing out bacterial identities. Nature, 339(6220), 157–158. https://doi.org/10.1038/339157a0
Brenner, K., You, L., & Arnold, F. H. (2008). Engineering microbial consortia: A new frontier in synthetic biology. Trends in Biotechnology, 26, 483–489. https://doi.org/10.1016/j.tibtech.2008.05.004
Briffa, J., Sinagra, E., & Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon, 6(9). https://www.cell.com/heliyon/pdf/S2405-8440(20)31534-6.pdf. Accessed 11 Apr 2023
Brisson, V. L., West, K. A., Lee, P. K., Tringe, S. G., Brodie, E. L., & Alvarez-Cohen, L. (2012). Metagenomic analysis of a stable trichloroethene-degrading microbial community. The ISME Journal, 6(9), 1702–1714. https://doi.org/10.1038/ismej.2012.15
Campos, V., Moraga, R., Fernández, Í., Yáñez, F., Valenzuela, A., & Mondaca, M. A. (2013). Reduction of hexavalent chromium by Serratia marcecens immobilized on active carbon and their potential use in bioremediation. Gayana, 77(1), 60. https://doi.org/10.4067/S0717-65382013000100008
Canellas, A. L. B., & Laport, M. S. (2022). The biotechnological potential of Aeromonas: A bird’s eye view. Critical Reviews in Microbiology, 1–13. https://doi.org/10.1080/1040841X.2022.2083940
Cao, X., Cui, X., Xie, M., Zhao, R., Xu, L., Ni, S., & Cui, Z. (2022). Amendments and bioaugmentation enhanced phytoremediation and micro-ecology for PAHs and heavy metals co-contaminated soils. Journal of Hazardous Materials, 426, 128096. https://doi.org/10.1016/j.jhazmat.2021.128096
Cavazzoli, S., Squartini, A., Sinkkonen, A., Romantschuk, M., Rantalainen, A. L., Selonen, V., & Roslund, M. I. (2023). Nutritional additives dominance in driving the bacterial communities’ succession and bioremediation of hydrocarbon and heavy metal contaminated soil microcosms. Microbiological Research, 270, 127343. https://doi.org/10.1016/j.micres.2023.127343
Chen, Y., Wu, H., Sun, P., Liu, J., Qiao, S., Zhang, D., & Zhang, Z. (2021). Remediation of chromium-contaminated soil based on Bacillus cereus WHX-1 immobilized on biochar: Cr (VI) transformation and functional microbial enrichment. Frontiers in Microbiology, 12, 641913. https://doi.org/10.3389/fmicb.2021.641913
Cheng, W., Quan, X., Huang, X., Cheng, C., Yang, L., & Cheng, Z. (2018). Enhancement of micro-filtration performance for biologically-treated leachate from municipal solid waste by ozonation in a micro bubble reactor. Separation and Purification Technology, 207, 535–542. https://doi.org/10.1016/j.seppur.2018.07.005
Choudhary, S., & Sar, P. (2009). Characterization of a metal resistant Pseudomonas sp. isolated from uranium mine for its potential in heavy metal (Ni2+, Co2+, Cu2+, and Cd2+) sequestration. Bioresource Technology, 100(9), 2482–2492. https://doi.org/10.1016/j.biortech.2008.12.015
Chuang, S., Wang, B., Chen, K., Jia, W., Qiao, W., Ling, W., & Jiang, J. (2020). Microbial catabolism of lindane in distinct layers of acidic paddy soils combinedly affected by different water managements and bioremediation strategies. Science of the Total Environment, 746, 140992. https://doi.org/10.1016/j.scitotenv.2020.140992
Department of Environment (DOE). (2009). Environmental quality - pollution control from solid waste transfer stations and landfills, Regulations 2009 – P.U. (A) 433/2009. In Contaminated land management and control guidelines No 1: Malaysian recommended site screening levels for contaminated land. Malaysia: Ministry of Natural Resources and Environment. http://58.82.155.201/AEC/pdf/laws2/1en/2my/7.EN-MY-07.pdf. Accessed 5 Apr 2023
Díaz, A., Marrero, J., Cabrera, G., Coto, O., & Gómez, J. M. (2022). Biosorption of nickel, cobalt, zinc and copper ions by Serratia marcescens strain 16 in mono and multimetallic systems. Biodegradation, 33(1), 33–43. https://doi.org/10.1007/s10532-021-09964-9
Dobson, A. W., Erikson, K. M., & Aschner, M. (2004). Manganese neurotoxicity. Annals of the New York Academy of Sciences, 1012(1), 115–128. https://doi.org/10.1196/annals.1306.009
Du, L. N., Pan, K. K., Li, G., Yang, Y. Y., & Xu, F. C. (2018). Efficient degradation of malachite green by Aeromonas sp. strain DH-6. Applied Environmental Biotechnology, 3, 2–8.
Emenike, C. U., Agamuthu, P., & Fauziah, S. H. (2016). Blending Bacillus sp., Lysinibacillus sp. and Rhodococcus sp. for optimal reduction of heavy metals in leachate contaminated soil. Environmental Earth Sciences, 75(1), 26. https://doi.org/10.1007/s12665-015-4805-9
Emenike, C. U., Liew, W., Fahmi, M. G., Jalil, K. N., Pariathamby, A., & Hamid, F. S. (2017). Optimal removal of heavy metals from leachate contaminated soil using bioaugmentation process. CLEAN–Soil, Air, Water, 45(2). https://doi.org/10.1002/clen.201500802
Environmental Quality Act (EQA). (1974) (Act 127). https://www.env.go.jp/en/recycle/asian_net/Country_Information/Law_N_Regulation/Malaysia/Malaysia_mal13278.pdf. Accessed 20 Sept 2023.
Fakhar, A., Gul, B., Gurmani, A. R., Khan, S. M., Ali, S., Sultan, T., & Rizwan, M. (2022). Heavy metal remediation and resistance mechanism of Aeromonas, Bacillus, and Pseudomonas: A review. Critical Reviews in Environmental Science and Technology, 52(11), 1868–1914. https://doi.org/10.1080/10643389.2020.1863112
Fauziah, S. H., Jayanthi, B., Emenike, C. U., & Agamuthu, P. (2017). Remediation of heavy metal contaminated soil using potential microbes isolated from a closed disposal site. International Journal of Bioscience, Biochemistry and Bioinformatics., 7(4), 230–237.
Fazzino, F., Pedullà, A., & Calabrò, P. S. (2023). Boosting the circularity of waste management: Pretreated mature landfill leachate enhances the anaerobic digestion of market waste. Biofuel Research Journal, 10(1), 1764–1773. https://doi.org/10.18331/BRJ2023.10.1.2
Ferreira, A. S., Leitao, J. H., Silva, I. N., Pinheiro, P. F., Sousa, S. A., Ramos, C. G., & Moreira, L. M. (2010). Distribution of cepacian biosynthesis genes among environmental and clinical Burkholderia strains and role of cepacian exopolysaccharide in resistance to stress conditions. Applied and Environmental Microbiology, 76(2), 441–450. https://doi.org/10.1128/AEM.01828-09
Filipović, L., Defterdarović, J., Chen, R., Krevh, V., Gerke, H. H., Baumgartl, T., & Filipović, V. (2023). Leached copper correlation with dissolved organic carbon in sloped vineyard soil. Water, 15(4), 800. https://doi.org/10.3390/w15040800
Flavio, C. A., Okeke, B. C., Bento, F. M., & Frankenberger, W. T. (2005). Diversity of chromium-resistant bacteria isolated from soils contaminated with dichromate. Applied Soil Ecology, 29(2), 193–202. https://doi.org/10.1016/j.apsoil.2004.10.006
Forsyth, J. E., Islam, M. S., Parvez, S. M., Raqib, R., Rahman, M. S., Muehe, E. M., & Luby, S. P. (2018). Prevalence of elevated blood lead levels among pregnant women and sources of lead exposure in rural Bangladesh: A case control study. Environmental Research, 166, 1–9. https://doi.org/10.1016/j.envres.2018.04.019
Gan, L., Cheng, Y., Palanisami, T., Chen, Z., Megharaj, M., & Naidu, R. (2014). Pathways of reductive degradation of crystal violet in wastewater using free-strain Burkholderia vietnamiensis C09V. Environmental Science and Pollution Research, 21, 10339–10348. https://doi.org/10.1007/s11356-014-3037-y
Ghosh, P., Rathinasabapathi, B., Teplitski, M., & Ma, L. Q. (2015). Bacterial ability in AsIII oxidation and AsV reduction: Relation to arsenic tolerance, P uptake, and siderophore production. Chemosphere, 138, 995–1000. https://doi.org/10.1016/j.chemosphere.2014.12.046
Gokgoz, M., Zhang, W., Manage, N., Mbengue, M., Bolyard, S., & Chen, J. (2023). Survey on the current leachate treatments of public municipal solid waste landfills and the potential impact of per-and polyfluorinatedalkyl substances in the Eastern and Northwestern United States. Journal of the Air & Waste Management Association, 73(8), 638–648. https://doi.org/10.1080/10962247.2023.2235313
Gong, Y., Yang, S., Chen, S., Zhao, S., Ai, Y., Huang, D., & Cheng, H. (2023). Soil microbial responses to simultaneous contamination of antimony and arsenic in the surrounding area of an abandoned antimony smelter in Southwest China. Environment International, 174, 107897. https://doi.org/10.1016/j.envint.2023.107897
Gonzalez Henao, S., & Ghneim-Herrera, T. (2021). Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Frontiers in Environmental Science, 9, 15. https://doi.org/10.3389/fenvs.2021.604216
Hassan, M. N., Awang, M., Chong, T. L., Zakaria, Z., Lay, L. B., Yusoff, N., & Sino, H. (1999). The application of a life cycle inventory (LCI) model for solid waste disposal systems in Malaysia. The International Journal of Life Cycle Assessment, 4(4), 188–190. https://doi.org/10.1007/BF02979493
Heinaru, E., Merimaa, M., Viggor, S., Lehiste, M., Leito, I., Truu, J., & Heinaru, A. (2005). Biodegradation efficiency of functionally important populations selected for bioaugmentation in phenol-and oil-polluted area. FEMS Microbiology Ecology, 51(3), 363–373. https://doi.org/10.1016/j.femsec.2004.09.009
Hesse, C., Schulz, F., Bull, C. T., Shaffer, B. T., Yan, Q., Shapiro, N., & Loper, J. E. (2018). Genome-based evolutionary history of Pseudomonas spp. Environmental Microbiology, 20(6), 2142–2159. https://doi.org/10.1111/1462-2920.14130
Hou, D., O’Connor, D., Igalavithana, A. D., Alessi, D. S., Luo, J., Tsang, D. C., & Ok, Y. S. (2020). Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nature Reviews Earth & Environment, 1(7), 366–381. https://doi.org/10.1038/s43017-020-0061-y
Hu, X., Liu, X., Qiao, L., Zhang, S., Su, K., Qiu, Z., & Yu, C. (2021). Study on the spatial distribution of ureolytic microorganisms in farmland soil around tailings with different heavy metal pollution. Science of the Total Environment, 775, 144946. https://doi.org/10.1016/j.scitotenv.2021.144946
Huang, X. N., Min, D., Liu, D. F., Cheng, L., Qian, C., Li, W. W., & Yu, H. Q. (2019). Formation mechanism of organo-chromium (III) complexes from bioreduction of chromium (VI) by Aeromonas hydrophila. Environment International, 129, 86–94. https://doi.org/10.1016/j.envint.2019.05.016
Huang, Y., Huangfu, X., Ma, C., & Liu, Z. (2023a). Sequestration and oxidation of heavy metals mediated by Mn (II) oxidizing microorganisms in the aquatic environment. Chemosphere, 138594. https://doi.org/10.1016/j.chemosphere.2023.138594
Huang, X., Nong, X., Liang, K., Chen, P., Zhao, Y., Jiang, D., & Xiong, J. (2023b). Efficient Mn (II) removal mechanism by Serratia marcescens QZB-1 at high manganese concentration. Frontiers in Microbiology, 14, 1150849. https://doi.org/10.3389/fmicb.2023.1150849
Igiri, B. E., Okoduwa, S. I., Idoko, G. O., Akabuogu, E. P., Adeyi, A. O., & Ejiogu, I. K. (2018). Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. Journal of Toxicology, 2568038. https://doi.org/10.1155/2018/2568038
Jayanthi, B., Emenike, C. U., Agamuthu, P., Simarani, K., Mohamad, S., & Fauziah, S. H. (2016). Selected microbial diversity of contaminated landfill soil of Peninsular Malaysia and the behavior towards heavy metal exposure. CATENA, 147, 25–31. https://doi.org/10.1016/j.catena.2016.06.033
Jha, K., Balakumar, D., & Paluchamy, R. (2015). Experimental analysis of microstructure and mechanical properties of copper and brass based alloys. International Journal of Automotive & Mechanical Engineering, 11. https://doi.org/10.15282/ijame.11.2015.14.0195
Ji, X., Wan, J., Wang, X., Peng, C., Wang, G., Liang, W., & Zhang, W. (2022). Mixed bacteria-loaded biochar for the immobilization of arsenic, lead, and cadmium in a polluted soil system: Effects and mechanisms. Science of the Total Environment, 811, 152112. https://doi.org/10.1016/j.scitotenv.2021.152112
Jiang, C. Y., Sheng, X. F., Qian, M., & Wang, Q. Y. (2008). Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere, 72(2), 157–164. https://doi.org/10.1016/j.chemosphere.2008.02.006
Jyothi, N.R. (2021). Heavy metal sources and their effects on human health. In M. K. Nazal, H. Zhao (Eds), Heavy metals – Their environmental impact and mitigating measures. IntechOpen. https://doi.org/10.5772/intechopen.95370
Kang, C. H., Kwon, Y. J., & So, J. S. (2016). Bioremediation of heavy metals by using bacterial mixtures. Ecological Engineering, 89, 64–69. https://doi.org/10.1016/j.ecoleng.2016.01.023
Karelová, E., Harichová, J., Stojnev, T., Pangallo, D., & Ferianc, P. (2011). The isolation of heavy-metal resistant culturable bacteria and resistance determinants from a heavy-metal-contaminated site. Biologia, 66(1), 18–26. https://doi.org/10.2478/s11756-010-0145-0
Karthik, K., Sharavanan, P. S., & Arivalagan, V. (2014). Effects of hexavalent chromium exposures and control measures through phytoremediation. International Journal of Reciprocal Symmetry and Theoretical Physics, 1(2), 111–115.
Kauppi, S., Sinkkonen, A., & Romantschuk, M. (2011). Enhancing bioremediation of diesel-fuel-contaminated soil in a boreal climate: Comparison of biostimulation and bioaugmentation. International Biodeterioration & Biodegradation, 65(2), 359–368. https://doi.org/10.1016/j.ibiod.2010.10.011
Kaza, S., Yao, L., Bhada-Tata, P., & Van Woerden, F. (2018). What a waste 2.0: A global snapshot of solid waste management to 2050. World Bank Publications. https://openknowledge.worldbank.org/server/api/core/bitstreams/92a50475-3878-5984-829e-0a09a6a9badc/content. Accessed 24 Aug 2023
Kehres, D. G., & Maguire, M. E. (2003). Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiology Reviews, 27(2–3), 263–290. https://doi.org/10.1016/S0168-6445(03)00052-4
Khan, S., Zeyad, M. T., & Malik, A. (2022). Genotoxicity assessment of textile waste contaminated soil and characterization of textile dye degradation by a novel indigenous bacterium Ochrobactrum intermedium BS39. Chemosphere, 299, 134082. https://doi.org/10.1016/j.chemosphere.2022.134082
Lebuhn, M., Bathe, S., Achouak, W., Hartmann, A., Heulin, T., & Schloter, M. (2006). Comparative sequence analysis of the internal transcribed spacer 1 of Ochrobactrum species. Systematic and Applied Microbiology, 29(4), 265–275. https://doi.org/10.1016/j.syapm.2005.11.003
Li, S. N., Zhang, C., Li, F., Ren, N. Q., & Ho, S. H. (2023). Recent advances of algae-bacteria consortia in aquatic remediation. Critical Reviews in Environmental Science and Technology, 53(3), 315–339. https://doi.org/10.1080/10643389.2022.2052704
Lin, T. C., Pan, P. T., & Cheng, S. S. (2010). Ex situ bioremediation of oil-contaminated soil. Journal of Hazardous Materials, 176, 27–34. https://doi.org/10.1016/j.jhazmat.2009.10.080
Lindamulla, L., Nanayakkara, N., Othman, M., Jinadasa, S., Herath, G., & Jegatheesan, V. (2022). Municipal solid waste landfill leachate characteristics and their treatment options in tropical countries. Current Pollution Reports, 8(3), 273–287. https://doi.org/10.1007/s40726-022-00222-x
Liu, B., Yao, J., Chen, Z., Ma, B., Li, H., Wancheng, P., & Duran, R. (2022). Biogeography, assembly processes and species coexistence patterns of microbial communities in metalloids-laden soils around mining and smelting sites. Journal of Hazardous Materials, 425, 127945. https://doi.org/10.1016/j.jhazmat.2021.127945
Luo, X., Wu, C., Lin, Y., Li, W., Deng, M., Tan, J., & Xue, S. (2023). Soil heavy metal pollution from Pb/Zn smelting regions in China and the remediation potential of biomineralization. Journal of Environmental Sciences, 125, 662–677. https://doi.org/10.1016/j.jes.2022.01.029
Malaysian Investment Development Authority (MIDA). (2021). Waste to energy for a sustainable future. Malaysia’s Stellar Investment Performance -Jan to Sept 2021. https://www.mida.gov.my/waste-to-energy-for-a-sustainable-future/. Accessed 5 Apr 2023
Marrero, J., Diaz, A., Valle, A., Cantero, D., Gómez, J. M., & Coto, O. (2009). Nickel and cobalt removal capacities of native metal-resistant bacteria. Advanced Materials Research, 71, 617–620. https://doi.org/10.4028/www.scientific.net/AMR.71-73.617
Meliani, A. (2015). Bioremediation strategies employed by Pseudomonas species. Bacterial Metabolites in Sustainable Agroecosystem, 351–383. https://doi.org/10.1007/978-3-319-24654-3_14
Min, D., Wu, J., Cheng, L., Liu, D. F., Lau, T. C., & Yu, H. Q. (2021). Dependence of arsenic resistance and reduction capacity of Aeromonas hydrophila on carbon substrate. Journal of Hazardous Materials, 403, 123611. https://doi.org/10.1016/j.jhazmat.2020.123611
Ministry of Health Malaysia (MHM). (2016). Drinking water quality standard. Engineering Services Division. https://environment.com.my/wp-content/uploads/2016/05/Drinking-Water-MOH.pdf. Accessed 5 Apr 2023
Mishra, S., Kumar, S., & Verma, S. K. (2022). Arsenic resistance mechanisms in Pseudomonas mendocina SMSKVR-3 strain isolated from Khetri Copper Mines, Rajasthan. India. Current Microbiology, 79(2), 69. https://doi.org/10.1007/s00284-021-02749-6
Mishra, S., Singh, V., Ormeci, B., Hussain, A., Cheng, L., & Venkiteshwaran, K. (2023). Anaerobic–aerobic treatment of wastewater and leachate: A review of process integration, system design, performance and associated energy revenue. Journal of Environmental Management, 327, 116898. https://doi.org/10.1016/j.jenvman.2022.116898
Monalisa, M., & Kumar, P. H. (2013). Effect of ionic and chelate assisted hexavalent chromium on mung bean seedlings (Vigna radiata L. wilczek. var k-851) during seedling growth. Journal of Stress Physiology & Biochemistry, 9(2), 232–241.
Moustafa, A., Hamzeh, M., Baroudi, M., Ouddane, B., & Net, S. (2022). 55 xenobiotic organic compounds in Tripoli landfill-Lebanon leachate and their fluxes to the Abou Ali River and Mediterranean Sea. Environmental Monitoring and Assessment, 194(12), 856. https://doi.org/10.1007/s10661-022-10522-w
Nanda, S., & Berruti, F. (2021). Municipal solid waste management and landfilling technologies: A review. Environmental Chemistry Letters, 19, 1433–1456. https://doi.org/10.1007/s10311-020-01100-y
Nieder, R., Benbi, D. K., Reichl, F. X., Nieder, R., Benbi, D. K., & Reichl, F. X. (2018). Microelements and their role in human health. In Soil components and human health (pp. 317–374). Dordrecht: Springer. https://doi.org/10.1007/978-94-024-1222-2_7
Nieto, J. J., Fernandez-Castillo, R., Marquez, M. C., Ventosa, A., Quesada, E., & Ruiz-Berraquero, F. (1989). Survey of metal tolerance in moderately halophilic eubacteria. Applied and Environmental Microbiology, 55(9), 2385–2390. https://doi.org/10.1128/aem.55.9.2385-2390.1989
Oren, A., & Garrity, G. M. (2021). Valid publication of the names of forty-two phyla of prokaryotes. International Journal of Systematic and Evolutionary Microbiology, 71(10), 005056. https://doi.org/10.1099/ijsem.0.005056
Oves, M., Saghir Khan, M., Huda Qari, A., Nadeen Felemban, M., & Almeelbi, T. (2016). Heavy metals: Biological importance and detoxification strategies. Journal of Bioremediation and Biodegradation, 7(2), 1–15. https://doi.org/10.4172/2155-6199.1000334
Ozdemir, G., Ozturk, T., Ceyhan, N., Isler, R., & Cosar, T. (2003). Heavy metal biosorption by biomass of Ochrobactrum anthropi producing exopolysaccharide in activated sludge. Bioresource Technology, 90(1), 71–74. https://doi.org/10.1016/S0960-8524(03)00088-9
Pepi, M., Volterrani, M., Renzi, M., Marvasi, M., Gasperini, S., Franchi, E., & Focardi, S. E. (2007). Arsenic-resistant bacteria isolated from contaminated sediments of the Orbetello Lagoon, Italy, and their characterization. Journal of Applied Microbiology, 103(6), 2299–2308. https://doi.org/10.1111/j.1365-2672.2007.03471.x
Podlasek, A. (2023). Modeling leachate generation: Practical scenarios for municipal solid waste landfills in Poland. Environmental Science and Pollution Research, 30(5), 13256–13269. https://doi.org/10.1007/s11356-022-23092-8
Qurbani, K., Khdir, K., Sidiq, A., Hamzah, H., Hussein, S., Hamad, Z., & Azizi, Z. (2022). Aeromonas sobria as a potential candidate for bioremediation of heavy metal from contaminated environments. Scientific Reports, 12(1), 21235. https://doi.org/10.1038/s41598-022-25781-3
Rajta, A., Bhatia, R., Setia, H., & Pathania, P. (2020). Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. Journal of Applied Microbiology, 128(5), 1261–1278. https://doi.org/10.1111/jam.14476
Raklami, A., Meddich, A., Oufdou, K., & Baslam, M. (2022). Plants—Microorganisms-based bioremediation for heavy metal cleanup: Recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. International Journal of Molecular Sciences, 23(9), 5031. https://doi.org/10.3390/ijms23095031
Ramírez, V., Baez, A., López, P., Bustillos, R., Villalobos, M. Á., Carreño, R., & Munive, J. A. (2019). Chromium hyper-tolerant Bacillus sp MH778713 assists phytoremediation of heavy metals by mesquite trees (Prosopis laevigata). Frontiers in microbiology, 10, 1833. https://doi.org/10.3389/fmicb.2019.01833
Rinklebe, J., Shaheen, S. M., & Frohne, T. (2016). Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere, 142, 41–47. https://doi.org/10.1016/j.chemosphere.2015.03.067
Salleh, N. F. D. M., & Hamid, K. H. K. (2013). Leachate characterization from a closed landfill in Air Hitam, Puchong. Malaysia. Malaysian Journal of Analytical Sciences, 17(1), 24–29.
Sankhla, M. S., Kumar, R., & Prasad, L. (2019). Zinc impurity in drinking water and its toxic effect on human health. Indian Congress of Forensic Medicine & Toxicology, 17(1), 84–87. https://doi.org/10.5958/0974-4487.2019.00015.4
Santelli, C. M., Chaput, D. L., & Hansel, C. M. (2014). Microbial communities promoting Mn (II) oxidation in Ashumet Pond, a historically polluted freshwater pond undergoing remediation. Geomicrobiology Journal, 31(7), 605–616. https://doi.org/10.1080/01490451.2013.875605
Shah, H. H., Amin, M., & Pepe, F. (2023). Maximizing resource efficiency: opportunities for energy recovery from municipal solid waste in Europe. Journal of Material Cycles and Waste Management, 1–17. https://doi.org/10.1007/s10163-023-01733-5
Sharma, B., & Shukla, P. (2021). A comparative analysis of heavy metal bioaccumulation and functional gene annotation towards multiple metal resistant potential by Ochrobactrum intermedium BPS-20 and Ochrobactrum ciceri BPS-26. Bioresource Technology, 320, 124330. https://doi.org/10.1016/j.biortech.2020.124330
Shrivastava, A., Ghosh, D., Dash, A., & Bose, S. (2015). Arsenic contamination in soil and sediment in India: Sources, effects, and remediation. Current Pollution Reports, 1, 35–46. https://doi.org/10.1007/s40726-015-0004-2
Shukor, M. Y., Habib, S. H. M., Rahman, M. F. A., Jirangon, H., Abdullah, M. P. A., Shamaan, N. A., & Syed, M. A. (2008). Hexavalent molybdenum reduction to molybdenum blue by S. marcescens strain Dr. Y6. Applied Biochemistry and Biotechnology, 149, 33–43. https://doi.org/10.1007/s12010-008-8137-z
Singh, N., Kumar, D., & Sahu, A. P. (2007). Arsenic in the environment: Effects on human health and possible prevention. Journal of Environmental Biology, 28(2), 359–365.
Sohoo, I., Ritzkowski, M., Guo, J., Sohoo, K., & Kuchta, K. (2022). Municipal solid waste management through sustainable landfilling: In view of the situation in Karachi, Pakistan. International Journal of Environmental Research and Public Health, 19(2), 773. https://doi.org/10.3390/ijerph19020773
Sprocati, A. R., Alisi, C., Tasso, F., Marconi, P., Sciullo, A., Pinto, V., & Cremisini, C. (2012). Effectiveness of a microbial formula, as a bioaugmentation agent, tailored for bioremediation of diesel oil and heavy metal co-contaminated soil. Process Biochemistry, 47(11), 1649–1655. https://doi.org/10.1016/j.procbio.2011.10.001
Sun, X., Feng, H., Luo, J., Lin, L., Zhang, H., Duan, Y., & Zhu, Z. (2023). A novel N-arachidonoyl-l-alanine-catabolizing strain of Serratia marcescens for the bioremediation of Cd and Cr co-contamination. Environmental Research, 222, 115376. https://doi.org/10.1016/j.envres.2023.115376
Syed, A., Zeyad, M. T., Shahid, M., Elgorban, A. M., Alkhulaifi, M. M., & Ansari, I. A. (2021). Heavy metals induced modulations in growth, physiology, cellular viability, and biofilm formation of an identified bacterial isolate. ACS Omega, 6(38), 25076–25088. https://doi.org/10.1021/acsomega.1c04396
Tataje-Lavanda, L., Ormeño-Vásquez, P., Altamirano-Díaz, R., Espinoza-Salazar, L., Zimic, M., & Tantaleán, J. C. (2019). Draft genome sequence of heavy metal-resistant Aeromonas veronii CTe-01, isolated from a Peruvian wastewater treatment plant. Microbiology Resource Announcements, 8(49), 10–1128. https://doi.org/10.1128/mra.01147-19
Tatiana, V. A., Mosher, J. J., Palumbo, A. V., Yang, Z. K., Podar, M., Brown, S. D., & Brandt, C. C. (2011). Mercury and other heavy metals influence bacterial community structure in contaminated Tennessee streams. Applied and Environmental Microbiology, 77(1), 302–311. https://doi.org/10.1128/AEM.01715-10
Uhrynowski, W., Decewicz, P., Dziewit, L., Radlinska, M., Krawczyk, P. S., Lipinski, L., & Drewniak, L. (2017). Analysis of the genome and mobilome of a dissimilatory arsenate reducing Aeromonas sp. O23A reveals multiple mechanisms for heavy metal resistance and metabolism. Frontiers in Microbiology, 8, 936. https://doi.org/10.3389/fmicb.2017.00936
US Environmental Protection Agency (USEPA). (1996). Soil screening guidance: Technical background document. USEPA Rep. 540/R-95/128. US Gov. Print. Office, Washington, DC. https://archive.epa.gov/region9/superfund/web/pdf/ssg_nonrad_technical-2.pdf. Accessed 5 Apr 2023
Van Nostrand, J. D., Khijniak, T. J., Neely, B., Sattar, M. A., Sowder, A. G., Mills, G., & Morris, P. J. (2007). Reduction of nickel and uranium toxicity and enhanced trichloroethylene degradation to Burkholderia vietnamiensis PR1301 with hydroxyapatite amendment. Environmental Science & Technology, 41(6), 1877–1882. https://doi.org/10.1021/es0616581
Wang, L., Chen, H., Wu, J., Huang, L., Brookes, P. C., Rodrigues, J. L. M., ... & Liu, X. (2021). Effects of magnetic biocharmicrobe composite on Cd remediation and microbial responses in paddy soil. Journal of Hazardous Materials, 414, 125494. https://doi.org/10.1016/j.jhazmat.2021.125494
Wang, Y., Huang, W., Ali, S. W., Li, Y., Yu, F., & Deng, H. (2022). Isolation, identification, and characterization of an efficient siderophore producing bacterium from heavy metal contaminated soil. Current Microbiology, 79(8), 227. https://doi.org/10.1007/s00284-022-02922-5
Wang, F., Wang, H., Zhao, Z., Dong, W., Wu, Z., Zhang, S., & Wu, X. (2022). Simultaneous elimination of black-odor and stabilization of heavy metals in contaminated sediment using calcium peroxide/hydroxyapatite: Microbial responses and ecotoxicological effects. Journal of Hazardous Materials, 429, 128298. https://doi.org/10.1016/j.jhazmat.2022.128298
Williams, M., Todd, G. D., Roney, N., Crawford, J., Coles, C., McClure, P. R., & Citra, M. (2012). Toxicological profile for manganese. Agency for Toxic Substances and Disease Registry (US), Atlanta (GA). https://www.ncbi.nlm.nih.gov/books/NBK158872/. Accessed 5 Apr 2023
World Health Organization (WHO). (1996). Permissible limits of heavy metals in soil and plants. Geneva. https://www.omicsonline.org/articles-images/2161-0525-5-334-t011.html. Accessed 5 Apr 2023
Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. International Scholarly Research Notices, 2011. https://doi.org/10.5402/2011/402647
Yamamura, S., Yamashita, M., Fujimoto, N., Kuroda, M., Kashiwa, M., Sei, K., Fujita, M., & Ike, M. (2007). Bacillus selenatarsenatis sp. nov., a selenate- and arsenate reducing bacterium isolated from the effluent drain of a glass-manufacturing plant. International Journal of Systematic and Evolutionary Microbiology, 57, 1060–1064. https://doi.org/10.1099/ijs.0.64667-0
Yang, R., Ma, G., Liu, C., Wang, C., Kang, X., Wu, M., & Zhang, B. (2023). Effects of different heavy metal pollution levels on microbial community structure and risk assessment in Zn-Pb mining soils. Environmental Science and Pollution Research, 1–13. https://doi.org/10.1007/s11356-023-26074-6
Ying, X. L., Gao, Z. Y., Yan, J., Zhang, M., Wang, J., Xu, J., & Yan, C. H. (2018). Sources, symptoms and characteristics of childhood lead poisoning: Experience from a lead specialty clinic in China. Clinical Toxicology, 56(6), 397–403. https://doi.org/10.1080/15563650.2017.1391392
Žaltauskaitė, J., Kniuipytė, I., & Kugelytė, R. (2020). Lead impact on the earthworm Eisenia fetida and earthworm recovery after exposure. Water, Air, & Soil Pollution, 231, 1–8. https://doi.org/10.1007/s11270-020-4428-y
Zhou, F., Cheng, Y., Gan, L., Chen, Z., Megharaj, M., & Naidu, R. (2014). Burkholderia vietnamiensis C09V as the functional biomaterial used to remove crystal violet and Cu (II). Ecotoxicology and Environmental Safety, 105, 1–6. https://doi.org/10.1016/j.ecoenv.2014.03.028
Acknowledgements
We appreciate the management of Taman Beringin Landfills, Jabatan Pengurusan Sisa Pepejal Negara (JPSPN), and Dewan Bandaraya Kuala Lumpur (DBKL) for their support during the research activities.
Funding
This study received funding support from Dalhousie University (GR-39364), University of Malaya (RP011A-14SUS), and Inti International University (INTI-FHLS-06–03-2021).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emenike, C.U., Agamuthu, P., Fauziah, S.H. et al. Enhanced Bioremediation of Metal-Contaminated Soil by Consortia of Proteobacteria. Water Air Soil Pollut 234, 731 (2023). https://doi.org/10.1007/s11270-023-06729-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06729-3