Abstract
A quantitative investigation of the effect of external mass transfer on the overall rate of biodegradation of Congo Red dye in a recirculating packed bed bioreactor (RPBB) was performed. The isolated Bacillus subtilis MN372379 species was immobilized on the packing material made of low-density polyethylene (LDPE) foam cubes in the RPBB. The overall dye removal rates were measured at varied recirculating flow conditions and an empirical correlation for the external mass transfer coefficient was determined. The observed rate of removal of Congo Red dye in the RPBB was found to be increasing with the recirculation rate. An empirical correlation to predict the non-dimensional Colburn factor was obtained as JD = 1.34 Re−0.3. The dimensionless correlation for mass transfer coefficient would enable the design and scale-up of an RPBB with minimal impact of external mass transfer resistance on the overall rate of dye bio-degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A large amount of color-containing industrial effluents are released into the environment due to the widespread use of azo dyes in food, textiles, cosmetics, leather, plastics, paper printing, pharmaceutical, photography, and pigment production. Azo dyes are the most common (60–70% of all dyes) synthetic commercial dyes. It makes them one of the significant environmental contaminants (Srinivasan et al., 2011). Azo dyes are aromatic compounds containing one or more (R–N = N–R) groups. Such hazardous colored compounds in the industrial effluent raise severe health and environmental issues (Mata et al., 2015; Sessa & Palmquist, 2008). The traditional methods for decolorizing textile dyes include membrane filtration, chemical precipitation, coagulation, electrochemical degradation, and adsorption (Phugare et al., 2011). The methods mentioned above successfully remove color, but they use a lot of energy and chemicals. Therefore, they are expensive and generate a lot of secondary pollutants. An efficient and cost-effective treatment technology is required for environment-neutral manufacturing in the color-related industry (Chaturvedi et al., 2021a). Various microorganisms, including bacteria, fungi, and yeasts, have been discovered to decolorize azo dyes. Among them, a thorough investigation of the bacterial breakdown of azo dyes in free cell cultures has already been published (Gopinath et al., 2009; Ip et al., 2010; Jadhav et al., 2011; Junghanns et al., 2012; Manu & Chaudhari, 2002; Niebisch et al., 2010). When the free cells are employed at an industrial scale, it witnesses several operational issues, such as shear forces, cell toxicity, cell stability under an agitated environment, and biomass-effluent separation.
In recent years, researchers have focused on developing immobilized bacterial cells for the degradation of azo dyes. It offers many advantages such as high stability, regeneration, reuse, simpler separation, accelerated response rates, enhanced cell metabolism, limited cell wash-out, and efficient control. Many immobilized cell systems have been developed and widely used for the complete degradation of textile dyes, including Basic Blue 41 (Elizalde-González et al., 2009), Reactive Blue 4 (Binupriya et al., 2010), Remazol Brilliant Blue R (Osma et al., 2010), Reactive red 45 (Bilal & Asgher, 2015), Malachite Green (Yang et al., 2011), Anthraquinone (Champagne & Ramsay, 2010), Direct Blue 1 (Karagoz et al., 2011), and Congo Red (Chaturvedi et al., 2021b). Several researchers have utilized continuous immobilized bioreactors such as air-lift, packed bed, trickling bed, fluidized bed, membrane-based, and rotating biological contactors to decolorize textile dyes (Wang et al., 2023). The packed-bed reactors are generally preferred among the other bioreactors for continuous and scalable wastewater treatment applications (Chaturvedi et al., 2021c; Dizge & Tansel, 2010; Zilouei et al., 2006). The dyeing effluents have also been treated using a packed bed bioreactor (PBBR) with a single or multi-column configuration (Córdoba et al., 2008; Fiol et al., 2006; Sahu et al., 2009).
The internal and external mass transfer resistances play a critical role in the overall reaction kinetics of the immobilized bioreactors, and hence, are essential in the design and scale-up of bioreactors. Many researchers have investigated the effects of mass transfer on the overall reaction rate in the immobilized packed bed reactor systems and concluded that the reaction kinetics were restricted by internal and external mass transfer resistances (Banerjee & Ghoshal, 2016; Geed et al., 2018; Parawira et al., 2006). Mudliar and coworkers (Mudliar et al., 2008) constructed a steady-state model for evaluating internal pore diffusion and exterior liquid film diffusion effects in an immobilized biofilm system in a continuous mode. However, there are few published studies on the external mass transfer aspects during the degradation of azo dyes in bioreactors operating in the recirculation mode. The current study investigated the effect of external mass transfer on the biodegradation rate of Congo Red dye in a recirculating packed bed bioreactor (RPBB). The RPBB employed isolated Bacillus subtilis MN372379 (Chaturvedi et al., 2021b) species immobilized on the packing material of low-density polyethylene (LDPE) foam cubes. The effect of recirculation was accounted for in the calculations of the “observed” reaction rate constant in the RPBB. The input flow rate was varied to determine the empirical correlation for external mass transfer coefficient and the surface reaction rate constant.
2 Materials and Methods
2.1 Dyes and Chemicals
The analytical grade Congo Red dye (λmax: 496.5 nm), nutrient agar (consisting of, in g/L, beef extract 1.5, the peptic digest of animal tissue 5.0, yeast extract 1.5, agar–agar 15.0, sodium chloride 5.0), glucose, and nutrient broth (consisting of, in g/L, sodium chloride 5.0, peptone 5.0, yeast extract 1.5, meat extract 1.5) were purchased from Merck, Germany. All of the other chemicals utilized in this investigation (for mineral salt media and trace elements) were of analytical grade.
2.2 Packing Material and the Adsorption Study
The LDPE foam was obtained from a local store, and had an average density of around 930 kg/m3. The foam sheets were cut and used in the form of 1 cm3 cubes. Foam pieces were carefully cleaned with distilled water, then three times with 100% ethanol, and finally with distilled water. They were pressed and dried at 60 °C in an oven overnight. The RPBB was then packed with these dried foam pieces as packing material. The adsorption of Congo Red dye was first studied on the LDPE foam in the batch mode, and it was observed that the LDPE foam showed minimal adsorption for the Congo Red dye and became saturated with dye within 1–2 h. Therefore, the continuous decolorization of Congo Red dye in the RPBB was attributed primarily to biodegradation.
2.3 Preparation of the Simulated Dye-Bearing Wastewater
For the preparation of simulated dye-bearing wastewater (SDW) of a given concentration used in this study, the Congo Red dye was added to the MSM (mineral salts medium) in the required amount. The MSM composition (g/L) used in this study is as follows: K2HPO4 (1.6); KH2PO4 (0.4); MgSO4.7H2O (0.2); NaCl (0.1); and CaCl2 (0.02), with 1.0 mL/L of the trace element stock solution and 1 mL of FeSO4.6H2O stock solution at 5 g/L. The trace element stock solution included the following components (in g/L): MnSO4.H2O (1.8); ZnSO4 (0.2); CuSO4 (0.1); Na2MoO4 (0.25) and H3BO3 (2) (Geed et al., 2017).
2.4 Bioreactor Configuration
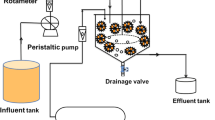
For the biodegradation of SDW, a cylindrical bioreactor made of borosilicate glass with a total volume of about 1500 mL and a working volume of 1200 mL (outer diameter = 6 cm, height = 53 cm) was built. Since biodegradation is a slow process, it was not possible to get complete degradation of dye in a single pass through a packed bed bioreactor. Therefore, the biodegradation process was run in the recirculating mode. A total of 2000 mL of SDW with 200 mg/L dye concentrations was used at varied flow rates in every biodegradation experiment in the RPBB. The sample collection port was provided at the top, and the influent feeding and drainage ports were provided at the bottom of the RPBB. The samples were collected, centrifuged at 8000 rpm for 10 min, and filtered through 0.22-µm filters before color analysis. The absorbance of the treated dyeing water was monitored using a UV–Vis spectrophotometer (Model No.: SL-159, Elico, India) at 497 nm to assess the extent of decolorization. The bioreactor was filled with the washed and dried LDPE foam cubes (packing medium) up to a height of 43 cm. Stainless steel sieves were used to prepare the support for the fixed bed of packing material at the top and bottom of the bioreactor. The temperature of the inlet reservoir was maintained at ~ 35 0C using a water bath. A schematic of the RPBB used in this study is shown in Fig. 1.
2.5 Bacterial Immobilization and Acclimatization in the Congo Red Dye Environment in the RPBB
In the RPBB, the isolated Bacillus subtilis MN372379 species (Chaturvedi et al., 2021b) were immobilized on the LDPE packing medium. The details of the molecular characterization and phylogenetic tree of Bacillus subtilisMN372379 species have been elaborated on in the previous study (Chaturvedi et al., 2021b). The bacterial immobilization procedure took a total of 20 days to complete. Subsequently, the bacterial mass was acclimatized to the SDW (made of Congo Red dye) environment in the RPBB. The goal of the acclimatization procedure was to get the bacterial population adapted to the anaerobic environment of SDW. The concentration of dye in the RPBB inlet stream was gradually raised from 50 to 500 mg/L. At each concentration, the RPBB was operated until the full decolorization of SDW was achieved followed by a successive cycle of decolorization of SDW at the same dye loading. The second cycle of decolorization at every inlet dye concentration exhibited a relatively higher dye-breakdown rate than the prior cycle implying that the bio-mass had been adapted to the SDW environment in the RPBB.
3 Mass Transfer Study: Theoretical Method
In the RPBB, the bacterial species are immobilized onto porous LDPE foam cubes. According to the film hypothesis of bio-transport, the surface of the packing medium (in contact with a moving fluid) develops a fictional external liquid film engulfing the attached biomass/enzyme (Kathiravan et al., 2010; Mudliar et al., 2008; Cheng et al., 2011). Therefore, the substrate (Congo Red dye) must be transported from bulk liquid to the biofilm through the surrounding liquid film via molecular diffusion. After the substrate is diffused to the surface of biomass attached to the LDPE foam, the biochemical reactions occur at the biofilm. While the presence of a surrounding liquid film offers a mass transfer barrier to the transport of a substrate, it enhances the toxicity tolerance of the microorganism present in the biofilm.
3.1 Concentration Profile and Observed Biodegradation Rate in the RPBB
For a steady-state plug flow with no axial dispersion through immobilized LDPE foam in a packed bed bioreactor (PBBR), the following material balance equation is used to calculate the overall rate of biodegradation of dye:
where \(Q\) is the feed flow rate (L/h), \(W\) represents the dry cell mass (biocatalyst) in the immobilized foam cubes (g), \(h\) represents the bioreactor column height (cm), W/h is the dry cell mass in the unit length of the bioreactor, \(dC/dz\) represents the Congo Red dye concentration gradient along the length of the bioreactor (mg/L/cm), \({k}_{p}\)(L/g/h) represents is the “observed” (overall) biodegradation rate constant, and \(C\)(mg/L) is the substrate concentration. In reality, W will change with time because the growth and decay of the active biomass is a continuous process. The dry cell mass was measured by taking an immobilized LDPE foam cube from the reactor and drying it at 60 °C for 12 h. This was followed by taking a mass difference of LDPE foam cube before and after immobilization. Considering the complexity of the biochemical systems and experimental limitations, the value of W was determined at the steady-state and assumed to be constant throughout the process.
The following equation is derived by integrating Eq. 1 from inlet (at z = 0) to outlet (at z = h):
where \({C}_{in}\) and \({C}_{out}\) stands for the inlet and outlet dye concentrations (mg/L) from the column, respectively. Finally, the concentration of dye at the outlet of the PBB is given by the following expression:
3.1.1 Modification in the Material Balance Equation to Account for Recirculation in a RPBB
Equations 1, 2, and 3 will be valid only for a single pass PBB. Few research papers published in the literature applied Eq. (2) for a RPBB that is incorrect (Banerjee & Ghoshal, 2016; Swain et al., 2021a). In a RPBB, the outlet stream is recycled back to the inlet, the \({C}_{in}\) keeps changing with time. Therefore, the Eqs. (1) and (2) will hold good only for a single residence time (τ) from any time t to (t + τ). When a mass balance of dye is applied on the inlet reservoir, which was assumed to be a perfectly mixed tank in this study, it yields the following equation:
Further substituting the Eq. (3) into Eq. (4), the following equation is obtained:
Finally, the variations of dye concentration in the reservoir with time are obtained by integrating Eq. (5) along the height of the RPBB as:
where C0 is the initial concentration of dye for the RPBB and C(t) is the dye concentration after every τ residence time. The observed dye degradation rate constant, \({k}_{p}\) can be determined from the slope of the straight line plotted for ln (C/CO) against time according to Eq. (6). At varied circulating flow rates, different values of \({k}_{p}\) can be obtained for the same RPBB.
The approach mentioned in Section 3.1 is quite general, and the derived governing equations can be used either as is or with some modifications to predict the transient concentration of a reactant in a reactor running in the recirculating mode. There are several such applications, e.g., the operation of an artificial kidney (Kim et al., 2011), recirculating MBBR (Swain et al., 2021b), and membrane reactors or filtrations (Parasyris et al., 2020), that can adopt such an approach in their mathematical formulations and modelling.
3.2 Evaluation of External Mass Transfer in the RPBB
In the RPBB, the molecular diffusion of dye through the thin laminar film formed on the biomass immobilized on the LDPE foam might be significantly slow (Sonwani et al., 2020). This extrinsic film diffusion can considerably reduce the observed reaction rate. The rate of substrate diffusion from bulk fluid to the external surface of biomass layer can be described as follows (Parawira et al., 2006; Tepe & Dursun, 2008):
where \({r}_{m}\) is the mass transfer rate (mg/g/h),\({A}_{m}\) is the surface area per unit mass of LDPE foam available for mass transfer (cm2/g), \({k}_{m}\) is the external mass transfer rate constant (L/cm2/h), \(C\) and \({C}_{s}\) are the dye concentration in bulk liquid and at the surface of the LDPE foam (mg/L). \({A}_{m}\) of the porous foam can be determined from Eq. (8) (Kafshgari et al., 2013).
where \({d}_{p}\) represents the equivalent spherical diameter of the foam cube (cm),\({\rho }_{p}\) represents the density of the LDPE (g/cm3) and \(\varepsilon\) is the bed porosity. The porosity (i.e., pore volume fraction) of the LDPE foam was determined from the values of \({\rho }_{p}\) and the masses of the wet and dried foam cubes (Kafshgari et al., 2013).
3.2.1 Relation Between the Mass Transfer Coefficient, Biochemical Surface Reaction, and Overall Dye Removal Rate Constants
Suppose a first-order reaction rate is assumed for the biodegradation of dyes at the surface of the bacterial layer immobilized at the LDPE foam cubes. In that case, the biochemical reaction rate can be described as follows:
where \(r\) is the substrate (i.e., dye) removal rate (mg/g/h), and \(k\) is the biochemical surface reaction rate constant (L/cm2h). The substrate removal rate becomes equal to the rate of external mass transfer at the steady-state. Hence, the surface concentration of the substrate can be determined using Eqs. (7) and (9) as:
The observed dye removal rate constant (\({k}_{p}\)) can be calculated from Eqs. (1), (9), and (10) as follows:
The rearrangement of Eq. (11) results in the following equation that relates the observed dye removal rate in the RPBB to the mass transfer and biochemical reaction rates:
3.2.2 Determination of the Correlation for External Mass Transfer Coefficient in the RPBB
The dimensionless Colburn factor, \({J}_{D}\) is used to link the external mass transfer coefficient to operational factors, e.g., reactor diameter, fluid flow rate, and fluid characteristics (Fogler, 2006).
where \(Re\) is Reynolds number, \(\rho\) is the feed density, K and n are empirical parameters, \({D}_{f}\) is the diffusivity, \(\mu\) is the feed viscosity, and G (g/cm2 h) is the superficial mass velocity defined as \(G=\frac{Q\rho }{A}\).The value of n is found in the range from 0.1 to 1.0 in the literature (19). Equation (13) is rearranged to express the external mass transfer rate constant as follows:
where
Finally, the following equation for \({k}_{p}\) is obtainedafter substituting Eq. (14) in Eq. (12):
In this study, the experimentally observed values of \(1/{k}_{p}\) is plotted against \(1/{G}^{n}\) for different values of K and \(n\). Next, the values of \({A}_{m}\) are determined from the plot and compared with the calculated \({A}_{m}\) (Eq. (8)) to identify the correct values of \(K\) and \(n\) for the bioreactor. It enables the determination of a dimensionless correlation for the external mass transfer coefficient and the magnitude of biochemical surface reaction rate constant in the given RPBB.
4 Results and Discussion
Although the bacterial population uses dyes as the carbon source, the literature shows that the high concentration of dyes inhibits their metabolic activities (Kathiravan et al., 2014). The microbial strains are immobilized in this study’s LDPE foam cube matrix to reduce the substrate inhibitory effect. This is because the microorganisms immobilized on a porous support medium are exposed to a relatively lower concentration of dyes due to the mass transfer barrier than the freely suspended biomass. It improves their tolerance against any shock of dye loading in the inlet stream and enhances their dye-degrading efficiency. The biodegradation kinetics of immobilized bacterial cells in such cases are affected by various factors driving the mass transfer diffusion rate from bulk to the biofilm.
4.1 The Effect of Inlet Mass Flow Rate on the Overall Rate of Dye Removal IN the RPBB
The influence of variation in the recirculation flow rate on the overall “observed” rate of dye-degradation in the RPBB was investigated by changing the feed flow rate between 25 and 100 ml/h while maintaining the dye loading constant 200 mg/L. Figure 2 shows the overall observed dye removal rate constant (kp) against the volumetric flow rates of the recirculating stream. The kp was determined from Eq. (6) according to the method discussed in Section 2.2. The low values of kp at a low flow rate indicated high mass transfer resistance, which is attributed to the formation of a thick laminar layer around the surface of the LDPE foam under a low Reynolds flow condition (Kureel et al., 2018). A study by Kathiravan et al. (Kathiravan et al., 2014) has shown that the low residence time at high flow rates affects the diffusion of solutes into the pores of the LDPE foam. As the feed flow rate was increased, the thickness of the laminar layer around the LDPE foam was reduced. It led to the high concentration gradient of Congo Red dye within the boundary layer and, therefore, reduced external mass transfer resistance. It eventually increased the value of the overall observed dye removal rate constant in the RPBB.
4.2 Determination of the Correlation for External Mass Transfer Coefficient
The mass transfer rate of the substrate into a porous immobilizing matrix plays a critical role in influencing the efficacy of a packed bed bioreactor system (Yeo & Yuniarto, 2019; Tepe & Dursun, 2008). The external mass transfer and intra-particle diffusion are two types of diffusional limitations. The external diffusion resistance is caused by forming a laminar boundary layer between the immobilizing matrix and the surrounding fluid. In contrast, the intraparticle diffusion resistance is caused by the concentration difference of the substrate within the matrix. Out of these two, the external mass transfer restriction is significant in governing the biodegradation kinetics in a packed bed bioreactor system. Further, the external mass transfer resistance depends on the feed flow rate, substance concentration, diffusivity, nature of the support material, and other hydrodynamic conditions in a bioreactor.
This study determined the external mass transfer coefficient correlation according to Eq. (14), which involved two empirical parameters, n and N. The parameter N depends on another empirical constant, K (Eq. (14a)). First, a range of random values for n and K are chosen based on the literature data. kp was determined (from Eq. (6)) by running the biodegradation experiments at varied volumetric flow rates of dyeing water in the RPBB. Next, the experimentally determined 1/kp were plotted against 1/Gn for various values of n in Fig. 3, and the corresponding data are summarized in Table 1. The straight-line nature of the plots in Fig. 3 was consistent with Eq. (15).
For the random values of n and K, the value of N was determined from Eq. (14a), as has been summarized in Table 2 for a few cases. The necessary physical parameters used in the computation were as follows: \(\rho\) = 1.205 g/cm3, \({d}_{p}\) = 1 cm, \(\varepsilon\) = 0.32, \({A}_{c}\) = 28.286 cm2, and \(\mu\) = 0.0085 g/cm.s, \({D}_{f}\) = 7.88 × 10−5 cm2/s (Sonwani et al., 2020). It enables the determination of Am from Fig. 3 and Eq. (15) for the given value of N. The same process was repeated for a series of random values of n and K, and the corresponding values of Am were obtained. The calculated values of Am have been summarized in Table 2 for some cases of n and K. It is apparent from Table 2 that the calculated value (3.22 cm2/g) of \({A}_{m}\) for \(n\) = 0.7 and \(K\) = 1.34 was very close to experimentally determined value (3.48 cm2/g) of \({A}_{m}\). Therefore, the true values for \(n\) and \(K\) were selected as 0.7and1.34, respectively. The corresponding surface reaction rate constant (k) for the biodegradation in the biofilm is 2.5 mL/cm2 h. Finally, the correlation for external mass transfer coefficient (\({k}_{m}\)) was obtained (from Eq. (14)) using the true values of the empirical parameters n and N as:
4.3 Determination of the Colburn Factor for External Mass Transfer Coefficient
The Colburn factor provides a dimensionless correlation for the external mass transfer coefficient. After obtaining the true values of the empirical parameters n and k, the following Colburn factor was obtained for the biodegradation of Congo Red dye using immobilized Bacillus subtilis MN372379in the RPBB used in this study (using Eq. (13)).
The above dimensionless correlation for the Colburn factor can be used in designing the scaled-up RPBB with optimized external mass transfer resistance.
4.4 The Effect of Recirculation Flow Rate on the External Mass Transfer
It is apparent from Eq. (16) that the mass transfer coefficient, \({k}_{m}\) increases with increasing the recirculation flow rate. The values of \({k}_{m}\) for various volumetric flow rates in the range of 25–100 ml/h were calculated and summarized in Table 3. The mass transfer coefficients were found to be 0.13 to 0.35 ml/cm2 h for this flow rate range.
Figure 4 displays the external mass transfer coefficient (along with the overall dye degradation rate constant, kp) as the recirculation flow rate function. The increase in kp with the recirculation flow rate is attributed to the increased mass transfer coefficient at a high recirculation rate. When the recirculation flow rate was doubled, the overall biodegradation rate constant was increased by ~ 36%. Generally, at a high feed flow rate through a packed bed reactor, the overall kinetic becomes reaction limited and independent of the mass transfer resistance. Therefore, a high recirculation flow rate would lower the external mass transfer barrier. However, an extremely high recirculation flow may destabilize the biofilm adhered to the LDPE packing; hence, the recirculation flow rate needs optimization to achieve maximum overall dye degradation while ensuring a stable biochemical operation in the RPBB. As the scope of this work was related to the mass transfer aspect of a PBBR, the detection of metabolites post-biodegradation of dye was not included in this work. However, it is necessary to detect the generated metabolites for an understanding of the mechanism pathways of dye degradation. Further, the identification of these metabolites gives insight into their hazardous nature.
5 Conclusion
The presented study aimed at investigating the effect of external mass transfer resistance on the overall dye removal rate in a RPBB which contained isolated microorganisms immobilized onto LDPE packing medium. The measured dye removal rates at varied feed flow conditions and the diffusion and reaction kinetics were used to develop an empirical correlation for the external mass transfer coefficient in the RPBB. The Chilton–Colburn analogy related the flow characteristics with the mass transfer parameters. The order of the biochemical reaction at the bacterial surface was taken as one. The observed rate of removal of Congo Red dye in the RPBB was obtained and shown to be increasing with the recycle rate. A correlation for the non-dimensional Colburn factor which can be used to predict the mass transfer coefficient of Congo Red dye in the RPBB was obtained as \({J}_{D}=1.34 {Re}^{-0.3}\). The use of this empirical correlation is recommended to quantify external diffusion effects for the biodegradation of dyewater in an anaerobic immobilized packed bed bioreactor. The reaction rate constant for the biodegradation of Congo Red dye was also determined to be as 2.5 ml/cm2. h. The determination of mass transfer coefficient will help assess and minimize the impact of external mass transfer barrier on the biodegradation of Congo Red dye in the RPBB. Further, it would also assist in the design and scale-up of the RPBB for a continuous treatment of dye-bearing wastewater.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Banerjee, A., & Ghoshal, A. K. (2016). Biodegradation of phenol by calcium-alginate immobilized @@ Bacillus cereus in a packed bed reactor and determination of the mass transfer correlation. Journal of Environmental Chemical Engineering, 4(2), 1523–1529. https://doi.org/10.1016/j.jece.2016.02.012
Bilal, M., & Asgher, M. (2015). Sandal reactive dyes decolorization and cytotoxicity reduction using manganese peroxidase immobilized onto polyvinyl alcohol-alginate beads. Chemistry Central Journal, 9, 47. https://doi.org/10.1186/s13065-015-0125-0
Binupriya, A. R., Sathishkumar, M., Ku, C. S., & Yun, S.-I. (2010). Sequestration of Reactive Blue 4 by free and immobilized Bacillus subtilis cells and its extracellular polysaccharides. Colloids and Surfaces b: Biointerfaces, 76(1), 179–185. https://doi.org/10.1016/j.colsurfb.2009.10.031
Champagne, P.-P., & Ramsay, J. A. (2010). Dye decolorization and detoxification by laccase immobilized on porous glass beads. Bioresource Technology, 101(7), 2230–2235. https://doi.org/10.1016/j.biortech.2009.11.066
Chaturvedi, A., Rai, B. N., Singh, R. S., & Jaiswal, R. P. (2021a). A comprehensive review on the integration of advanced oxidation processes with biodegradation for the treatment of textile wastewater containing azo dyes. Reviews in Chemical Engineering, 38(6). https://doi.org/10.1515/revce-2020-0010
Chaturvedi, A., Rai, B. N., Singh, R. S., & Jaiswal, R. P. (2021b). A computational approach to incorporate metabolite inhibition in the growth kinetics of indigenous bacterial strain Bacillus subtilis MN372379 in the treatment of wastewater containing Congo Red dye. Applied Biochemistry and Biotechnology, 193(7), 2128–2144. https://doi.org/10.1007/s12010-021-03538-4
Chaturvedi, A., Rai, B. N., Singh, R. S., & Jaiswal, R. P. (2021c). Comparative toxicity assessment using plant and luminescent bacterial assays after anaerobic treatments of dyeing wastewater in a recirculating fixed bed bioreactor. Journal of Environmental Chemical Engineering, 9(4), 105466. https://doi.org/10.1016/j.jece.2021.105466
Cheng, C. W., Abdulla, R., Sridhar, R. R., & Ravindra, P. (2011). Determination of external mass transfer model for hydrolysis of jatropha oil using immobilized lipase in recirculated packed-bed reactor. Advances in Chemical Engineering and Science, 1, 289–298. https://doi.org/10.4236/aces.2011.14040
Córdoba, A., Vargas, P., & Dussan, J. (2008). Chromate reduction by Arthrobacter CR47 in biofilm packed bed reactors. Journal of Hazardous Materials, 151(1), 274–279. https://doi.org/10.1016/j.jhazmat.2007.10.072
Dizge, N., & Tansel, B. (2010). External mass transfer analysis for simultaneous removal of carbohydrate and protein by immobilized activated sludge culture in a packed bed batch bioreactor. Journal of Hazardous Materials, 184(1), 671–677. https://doi.org/10.1016/j.jhazmat.2010.08.090
Elizalde-González, M. P., Fuentes-Ramírez, L. E., & Guevara-Villa, M. R. G. (2009). Degradation of immobilized azo dyes by Klebsiella sp. UAP-b5 isolated from maize bioadsorbent. Journal of Hazardous Materials, 161(2), 769–774. https://doi.org/10.1016/j.jhazmat.2008.04.023
Fiol, N., Escudero, C., Poch, J., & Villaescusa, I. (2006). Preliminary studies on Cr(VI) removal from aqueous solution using grape stalk wastes encapsulated in calcium alginate beads in a packed bed up-flow column. Reactive and Functional Polymers, 66(8), 795–807. https://doi.org/10.1016/j.reactfunctpolym.2005.11.006
Fogler, H. S. (2006). External diffusion effect on heterogeneous reactions. In Elements of Chemical Reaction Engineering (4th ed. pp. 757–810). Prentice Hall International.
Geed, S. R., Kureel, M. K., Giri, B. S., Singh, R. S., & Rai, B. N. (2017). Performance evaluation of Malathion biodegradation in batch and continuous packed bed bioreactor (PBBR). Bioresource Technology, 227, 56–65. https://doi.org/10.1016/j.biortech.2016.12.020
Geed, S. R., Kureel, M. K., Prasad, S., Singh, R. S., & Rai, B. N. (2018). Novel study on biodegradation of malathion and investigation of mass transfer correlation using alginate beads immobilized Bacillus sp. S4 in bioreactor. Journal of Environmental Chemical Engineering, 6(2), 3444–3450. https://doi.org/10.1016/j.jece.2018.05.025
Gopinath, K. P., Murugesan, S., Abraham, J., & Muthukumar, K. (2009). Bacillus sp. mutant for improved biodegradation of Congo red: Random mutagenesis approach. Bioresource Technology, 100(24), 6295–6300. https://doi.org/10.1016/j.biortech.2009.07.043
Ip, A. W. M., Barford, J. P., & McKay, G. (2010). Biodegradation of Reactive Black 5 and bioregeneration in upflow fixed bed bioreactors packed with different adsorbents. Journal of Chemical Technology & Biotechnology, 85(5), 658–667. https://doi.org/10.1002/jctb.2349
Jadhav, S. B., Phugare, S. S., Patil, P. S., & Jadhav, J. P. (2011). Biochemical degradation pathway of textile dye Remazol red and subsequent toxicological evaluation by cytotoxicity, genotoxicity and oxidative stress studies. International Biodeterioration & Biodegradation, 65(6), 733–743. https://doi.org/10.1016/j.ibiod.2011.04.003
Junghanns, C., Neumann, J. F., & Schlosser, D. (2012). Application of the aquatic fungus Phoma sp. (DSM22425) in bioreactors for the treatment of textile dye model effluents. Journal of Chemical Technology & Biotechnology, 87(9), 1276–1283. https://doi.org/10.1002/jctb.3797
Kafshgari, M. H., Mansouri, M., Khorram, M., & Kashani, S. R. (2013). Kinetic modeling: A predictive tool for the adsorption of zinc ions onto calcium alginate beads. International Journal of Industrial Chemistry, 4(1), 5. https://doi.org/10.1186/2228-5547-4-5
Karagoz, B., Bayramoglu, G., Altintas, B., Bicak, N., & Yakup Arica, M. (2011). Amine functional monodisperse microbeads via precipitation polymerization of N-vinyl formamide: Immobilized laccase for benzidine based dyes degradation. Bioresource Technology, 102(13), 6783–6790. https://doi.org/10.1016/j.biortech.2011.03.050
Kathiravan, M. N., Karthiga Rani, R., Karthick, R., & Muthukumar, K. (2010). Mass transfer studies on the reduction of Cr(VI) using calcium alginate immobilized Bacillus sp. in packed bed reactor. Bioresource Technology, 101(3), 853–858. https://doi.org/10.1016/j.biortech.2009.08.088
Kathiravan, M. N., Praveen, S. A., Gim, G. H., Han, G. H., & Kim, S. W. (2014). Biodegradation of Methyl Orange by alginate-immobilized Aeromonas sp. in a packed bed reactor: external mass transfer modeling. Bioprocess and Biosystems Engineering, 37(11), 2149–2162. https://doi.org/10.1007/s00449-014-1192-7
Kim, J. C., Garzotto, F., Nalesso, F., Cruz, D., Kim, J. H., Kang, E., Kim, H. C., & Ronco, C. (2011). A wearable artificial kidney: Technical requirements and potential solutions. Expert Review of Medical Devices, 8(5), 567–579. https://doi.org/10.1586/erd.11.33
Kureel, M. K., Geed, S. R., Rai, B. N., & Singh, R. S. (2018). Novel investigation of the performance of continuous packed bed bioreactor (CPBBR) by isolated Bacillus sp. M4 and proteomic study. Bioresource Technology, 266, 335–342. https://doi.org/10.1016/j.biortech.2018.06.064
Manu, B., & Chaudhari, S. (2002). Anaerobic decolorisation of simulated textile wastewater containing azo dyes. Bioresource Technology, 82(3), 225–231. https://doi.org/10.1016/S0960-8524(01)00190-0
Mata, A., Pinheiro, H., & Lourenço, N. (2015). Effect of sequencing batch cycle strategy on the treatment of a simulated textile wastewater with aerobic granular sludge. Biochemical Engineering Journal, 104, 106. https://doi.org/10.1016/j.bej.2015.04.005
Mudliar, S., Banerjee, S., Vaidya, A., & Devotta, S. (2008). Steady state model for evaluation of external and internal mass transfer effects in an immobilized biofilm. Bioresource Technology, 99, 3468–3474. https://doi.org/10.1016/j.biortech.2007.08.001
Niebisch, C. H., Malinowski, A. K., Schadeck, R., Mitchell, D. A., Kava-Cordeiro, V., & Paba, J. (2010). Decolorization and biodegradation of reactive blue 220 textile dye by Lentinus crinitus extracellular extract. Journal of Hazardous Materials, 180(1), 316–322. https://doi.org/10.1016/j.jhazmat.2010.04.033
Osma, J. F., Toca-Herrera, J. L., & Rodríguez-Couto, S. (2010). Transformation pathway of Remazol Brilliant Blue R by immobilised laccase. Bioresource Technology, 101(22), 8509–8514. https://doi.org/10.1016/j.biortech.2010.06.074
Parasyris, A., Discacciati, M., & Das, D. B. (2020). Mathematical and numerical modelling of a circular cross-flow filtration module. Applied Mathematical Modelling, 80, 84–98. https://doi.org/10.1016/j.apm.2019.11.016
Parawira, W., Murto, M., Zvauya, R., & Mattiasson, B. (2006). Comparative performance of a UASB reactor and an anaerobic packed-bed reactor when treating potato waste leachate. Renewable Energy, 31(6), 893–903. https://doi.org/10.1016/j.renene.2005.05.013
Phugare, S. S., Kalyani, D. C., Patil, Av., & Jadhav, J. P. (2011). Textile dye degradation by bacterial consortium and subsequent toxicological analysis of dye and dye metabolites using cytotoxicity, genotoxicity and oxidative stress studies. Journal of Hazardous Materials, 186(1), 713–723. https://doi.org/10.1016/j.jhazmat.2010.11.049
Sahu, A. K., Conneely, T., Nüsslein, K. R., & Ergas, S. J. (2009). Biological perchlorate reduction in packed bed reactors using elemental sulfur. Environmental Science & Technology, 43(12), 4466–4471. https://doi.org/10.1021/es900563f
Sessa, D. J., & Palmquist, D. E. (2008). Effect of heat on the adsorption capacity of an activated carbon for decolorizing/deodorizing yellow zein. Bioresource Technology, 99(14), 6360–6364. https://doi.org/10.1016/j.biortech.2007.11.076
Sonwani, R. K., Giri, B. S., Jaiswal, R. P., Singh, R. S., & Rai, B. N. (2020). Performance evaluation of a continuous packed bed bioreactor: Bio-kinetics and external mass transfer study. Ecotoxicology and Environmental Safety, 201, 110860. https://doi.org/10.1016/j.ecoenv.2020.110860
Srinivasan, R., Kathiravan, M. N., & Gopinath, K. P. (2011). Degradation of Tectilon Yellow 2G by hybrid technique: Combination of sonolysis and biodegradation using mutant Pseudomonas putida. Bioresource Technology, 102(3), 2242–2247. https://doi.org/10.1016/j.biortech.2010.10.022
Swain, G., Sonwani, R. K., Giri, B. S., Singh, R. S., Jaiswal, R. P., & Rai, B. N. (2021a). A study of external mass transfer effect on biodegradation of phenol using low-density polyethylene immobilized Bacillus flexus GS1 IIT (BHU) in a packed bed bioreactor. Water and Environment Journal, 35(1), 285–294. https://doi.org/10.1111/wej.12626
Swain, G., Sonwani, R. K., Singh, R. S., Jaiswal, R. P., & Rai, B. N. (2021b). A comparative study of 4-chlorophenol biodegradation in a packed bed and moving bed bioreactor: Performance evaluation and toxicity analysis. Environmental Technology & Innovation, 24, 101821. https://doi.org/10.1016/j.eti.2021.101820
Tepe, O., & Dursun, A. Y. (2008). Combined effects of external mass transfer and biodegradation rates on removal of phenol by immobilized Ralstonia eutropha in a packed bed reactor. Journal of Hazardous Materials, 151(1), 9–16. https://doi.org/10.1016/j.jhazmat.2007.05.049
Wang, Y., Zhu, T., Wong, Y. J., Zhang, K., & Chang, M. (2023). Treatment performance of multistage active biological process (MSABP) reactor for saline sauerkraut wastewater: acclimatization, optimization and improvement. Bioprocess and Biosystems Engineering, 23. https://doi.org/10.1007/s00449-023-02877-2
Yang, Y., Hu, H., Wang, G., Li, Z., Wang, B., Jia, X., & Zhao, Y. (2011). Removal of malachite green from aqueous solution by immobilized Pseudomonas sp. DY1 with Aspergillus oryzae. International Biodeterioration & Biodegradation, 65(3), 429–434. https://doi.org/10.1016/j.ibiod.2011.01.007
Yeo, W. S., Yuniarto, A. (2019). The external mass transfer model for the hydrolysis of palm olein using immobilized lipase in a scaled-up recirculated packed-bed reactor. after anaerobic treatments of dyeing wastewater in a recirculating fixed bed bioreactor. Journal of Environmental Chemical Engineering, 7(4), 103185. https://doi.org/10.1016/j.jece.2019.103185
Zilouei, H., Guieysse, B., & Mattiasson, B. (2006). Biological degradation of chlorophenols in packed-bed bioreactors using mixed bacterial consortia. Process Biochemistry, 41(5), 1083–1089. https://doi.org/10.1016/j.procbio.2005.11.019
Acknowledgements
Author wants to acknowledge Prof. Ram Sharan Singh and Prof. Birendra Nath Rai for their helpful suggestions, and the department of chemical engineering and technology, IIT (BHU) for providing necessary laboratory facility to conduct the research activities.
Author information
Authors and Affiliations
Contributions
Conceptualization: Anuj Chaturvedi, Ravi Prakash Jaiswal;
Methodology: Ravi Prakash Jaiswal, Anuj Chaturvedi.
Formal analysis and investigation: Anuj Chaturvedi;
Writing—original draft preparation: Anuj Chaturvedi;
Writing—review and editing: Anuj Chaturvedi, Ravi Prakash Jaiswal;
Funding acquisition: Ravi Prakash Jaiswal;
Resources: Ravi Prakash Jaiswal; Ram Sharan Singh.
Supervision: Ravi Prakash Jaiswal;
Corresponding author
Ethics declarations
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The participant has consented to the submission of the case report to the journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaturvedi, A., Jaiswal, R.P. Investigation of External Mass Transfer during Biodegradation of Congo Red Dye in a Recirculating Packed Bed Bioreactor. Water Air Soil Pollut 234, 360 (2023). https://doi.org/10.1007/s11270-023-06382-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06382-w