Abstract
Large quantities of phosphogypsum originate from accumulated Tunisian industrial waste each year. Thus, mining effluents that do not comply with Tunisian discharge standards affect the hydrographic network and cause not only soil contamination of trace metal elements but are also considered a source of toxicity in the food chain and ultimately in the human body organs. In a bid to determine the contamination status and assess heavy metal pollution and the health risk of agricultural soils in a typical peri-urban area in southwestern Tunisia—M’Dhilla Basin, sex samples subdivided into 17 sub-samples from three different depths (0–60 cm), (0–80 cm), and (0–200 cm) were collected with a stainless steel auger. This study is based on physic-chemical analysis using standard laboratory procedures, X-ray fluorescence technique, and multivariate statistical analysis. Results showed that trace metal element concentration ranged as follows: Sr > Zn > Cr > Cd > Pb. A significant contamination was discovered in the study area that indicated a high Cr and Zn topsoil contamination and a homogenous pattern in Cd and Pb (p < 0.05). Assessment of the trace metal element pollution was determined with calculations of the contamination factor and the pollution load index. Our finding proved that the study area was highly polluted with Cd, Sr, and Zn. Accordingly, high concentrations of trace metal elements cause serious environmental problems to the atmosphere, soil, and water. Carcinogenic health risks worsen and affect most of the inhabitants in the mining basin of M’Dhilla.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Trace metal elements (TME) in soils have been considered important tracers to monitor the impact of human activities (Manta et al., 2002). Due to their toxicity and environmental persistence, TME can accumulate in soils increasing potential risks to the environment and population (Sey & Belford, 2019). In agricultural soils, these toxic cations can pose health risks to humans attributed to the consumption of crops and chronic exposure to soil particles (Boim et al., 2016). Previous researchers have shown that mining activity is an important source of TME in agricultural soils near mining areas worldwide (Khelifi et al., 2020; Pen-Mouratov et al., 2008). In Tunisia, many investigations are being carried out on the environmental aspect of TME as a potential source of contamination once they surpass the permissible limits of the international guideline (Hamed et al., 2014; Khelifi et al., 2020; Mekki & Sayadi, 2017; Sleimi et al., 2014, 2021).
The anthropogenic sources in urban soils include vehicle exhaust emissions, waste treatment, wastewater, and industrial activities (Besser & Hamed, 2021; Dridi et al., 2020; Hachani et al., 2022; Khoualdia et al., 2018; Missaoui et al., 2022). Indeed, mining activities were considered within the major anthropogenic sources of TME pollution. The study of this contamination in urban soils will help develop strategies to protect the urban environment and human health from long-term TME accumulation. The physicochemical properties of TME which cause soil contamination are as follows: (i) TME are not destroyed and often accumulate naturally in the soil instead of being depleted (Maas et al., 2010); (ii) they cause a wide range of health risks worsened by differences in their oxidative status and associated bioavailability (Walker et al., 2003) and (iii) multiple contamination sources (Nriagu & Pacyna, 1988). In Tunisia, the concentration of TME in the soils near the phosphate mining areas was significantly higher than that of the unused area (Hentati et al., 2015). This industry has developed since the beginning of the year 1950 and is emitting a high quantity of phosphogypsum (PG) inevitably sterile at the same time as the acid. PG is produced during the wet process of phosphoric acid synthesis by a chemical reaction of rock phosphate with sulphuric acid as described in Eq. 1.
Due to the residual phosphoric, sulphuric, and hydrofluoric acids contained within the porous PG, it is considered an acidic by-product (pH < 3). Tunisian PG is predominated by fine particle size ranged between 45 and 250 µm in diameter (Tayibi et al., 2009). It is reported to contain very high Cd, Hg, and Zn concentrations (Rutherford et al., 1995; Choura et al., 2009).
The manufacturing of 01 ton of phosphate P2O5 generated the production of 5 tons of PG (Pérez-López et al., 2010). However, more than 10 million tons per year of PG were produced. Thus, Rutherford et al. (1995) identified PG as a potential hazardous waste because of its radium-226 content and the large volumes produced. The presence of TME in PG may pose a potential hazard to human health (Al-Masri & Al-Bich, 2002). Its composition shows generally high total contents of Cr, Zn, Cd, Sr, and As (Garbaya et al., 2021; Hammas et al., 2016; Tayibi et al., 2009). In this context, the present paper aims to (i) evaluate the TME pollution using multivariate statistical analysis and the health risk assessment of agricultural soils in a typical peri-urban area in southwestern Tunisia—M’Dhilla Basin and (ii) give a simple overview of the environmental management of PG.
2 The Study Area
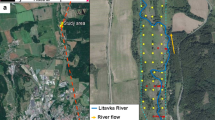
The study area is located in the region of M’Dhilla, Gafsa, southwest of Tunisia. The selected sampling points are located 4 km north of M’Dhilla. It encompasses both the discharge from the laundry located in M’Dhilla and the discharge from the Tunisian Chemical Group (GCT) (Fig. 1). The study area is characterized by gypsum soil of low resistance that is locally present along El Melah Wadi to the north of the study area. These soils are shallow (60–90 cm) with a coarse to medium texture; halomorphic soils (or saline soils) are with coarse sand that becomes more clayey at depth (Fig. 2).
The sampling point is located at 477,712.74 m E latitude, 3,798,008.92 m N longitude, in the vicinity of “M’Dhilla discharge.” A control sample, from an uncontaminated site, was used to compare the results. These samples were collected from a pit of 200 cm deep localized at 481,418.70 m E latitude, and 3,798,571.24 m N at 2 km from CTG. Soil samples and water samples were collected in February 2022 and April 2022. Seventeen samples from different depths (0 to 200 cm) were collected with a stainless steel auger. Two pits were created using a drilling machine to get a 2-m depth, which are sample D and control sample T, from an uncontaminated site, localized at 481,418.70 m E latitude and 3,798,571.24 m N at 2 km from CTG. Samples are subdivided into sub-samples and are classified in Table 1.
3 Methodology
3.1 Sample Collection
Two pits of 2 m each were dug; six samples “T1 to T6” were collected from a control site that was not contaminated. Samples were counted and transported in plastic bags to the laboratory. All samples were air-dried, homogenized, sieved, and ground in two particle size (sieve opening) ranges: course > 1 mm and fine < 80 μm. Some of the samples were subject to physic-chemical analyses. The other section was used for the X-ray fluorescence analyses using the Niton FXL 950, Thermo Scientific. The measure of pH was determined using a pH meter. The EC and TDS were determined using a laboratory multi-parameter analyzer “Consort C5020.” The percentage of CaO3 was determined by Bernard’s calcimeter method. The total nitrogen concentration was determined by the KJELDAHL method.
3.2 X-Ray Fluorescence Spectrometry (FXL)
The TME concentrations (Cd, Sr, As, Cr, Zn, Pb…), P2O5%, SO3%, and SiO2% were determined using an X-ray fluorescence spectrometer. The FXL has been widely used for many years as a routine, rapid, and sophisticated analytical technique for quantifying minerals.
3.3 Multivariate Statistical Analysis
The multivariate statistical analysis (descriptive statistics, correlation matrix, PCA, HCA) is a useful tool in providing information about the relationship between nine different physical and chemical parameters to determine which parameters probably share the same origin, the possible source of TME, and to identify the geological processes that govern their distribution (adsorption, leaching…). Statistical analysis has been used across disciplines to address soil contamination problems such as geosciences, soil science, atmospheric research, environmental engineering, and chemometrics (Mostert et al., 2012).
Principal component analysis (PCA) simplifies the complexity of high-dimensional data while retaining trends and patterns. It does this by transforming the data into fewer dimensions, which act as summaries of features. The selection of significant principal components was based on the Kaiser criterion with an Eigen value higher than 1 (Kaiser, 1960). According to this criterion, only the first four principal components were retained. Hierarchical cluster analysis (HCA) is a strategy that seeks to build a hierarchy of clusters that has an established ordering from top to bottom (Köhn & Hubert, 2014). A “hierarchy of clusters” is usually represented by a dendrogram.
3.4 Pollution Indices
To assess the soil TME pollution level, it is indispensable to determine the contamination factor (CF) (Hakanson, 1980). CF evaluates the degree of anthropogenic contamination of sediments by a specific potentially toxic trace element. It is calculated by the equation (Eq. 2):
where CM is the concentration of metal in the sample and CB is the background concentration (in this study, we use element concentrations in the continental crust calculated from rock averages compiled by Wedepohl (1995) as ground concentration).
The levels of contamination were calculated and classified as either: CF < 1 the contamination is low; 1 < CF ≤ 3 the contamination is moderate; 3 < CF ≤ 6 the contamination is considerable; or finally C ≥ 6 the contamination is of very high level. The pollution load index (PLI) evaluates the degree to which the soil sediments associated with TME might impact the micro-flora and fauna of soil, as given by Eq. 3 (Tomlinson et al., 1980).
CF is contamination factor; n is the number of metals; C metal is the metal concentration in polluted sediments; and C background value is the background value of that metal.
According to the PLI value, the soil quality concerning TME safety was classified as null PLI—a background value, PLI ≥ 1—gradual degradation (non-polluted to moderately polluted), PLI ≥ 2—a moderate pollution, and PLI ≥ 4—high pollution.
4 Results and Discussion
4.1 Soil Properties and TME Concentrations in Soil
Table 2 shows that samples have widely varying pH values with depth. However, a change in the soil solution pH results in a corresponding change in the dominant retention mechanism of TME in the soils. Acid pH characterizes four samples: E3 (3.46), E6 (2.84), E2 (3.01), and E8 (5.75) of respective depths: 0–5 cm, 0–10 cm, 60–80 cm, and 170–180 cm. These samples are characterized by the maximum concentration of Cd, Zn, Sr, and Cr. As pH decreases, precipitation becomes less important, and cation exchange becomes dominant (Yong, 1993). Basic pH characterized the most of samples. At high pH values, precipitation mechanisms (e.g., precipitating as hydroxides and/or as carbonates) dominate (Yong, 1993). Total carbon (TC %) in soils is the sum of both organic and inorganic carbon. The samples show a maximum of TC% 19.95 at 80–90 cm depth in sub-sample E7. This value is accompanied by zero values of P2O5% and minimum values of SiO2%. Additionally, samples with low TC values (0–0.93 ms.cm−1) are characterized by high values of CaO% (19.56), P2O5% (0.93), and TME concentrations (Cd = 17.69 mg.Kg−1; Zn = 461.15 mg.Kg−1; Cr = 55.72 mg.Kg−1) (Table 2). The samples show a low P2O5% content with a maximum of 0.9%; this parameter is null in most of the samples, indicating low phosphorus content compared to that of the Tunisian phosphogypsum, which is equal to 1.2% (Tayibi et al., 2009). Silicon dioxide (SiO2%) is commonly presented as quartz. Its contents vary between 0 and 15.24 mg.Kg−1 registered in the control sample at 180–200 cm depth.
The mean values of Cd, Cr, Pb, Zr, Sr, and Zn in the soil of the concerned area showed a concentration above soil quality standards. The results show that the highest concentrations of TME in the samples of soils are (in descending order of abundance) Sr > Zr > Zn > Cr > Cd > Pb. Table 3 shows that soil TME concentrations were average of 7.04 mg.Kg−1 for Cd, 213.7 mg.Kg−1 for Zr, 0.70 mg.Kg−1 for Pb, 61.2 mg.Kg−1for Zn, 14.53 mg.Kg−1 for Cr, and 594.6 mg.Kg−1 for Sr, hence the great importance of comparing the surveyed concentration of TME with the background level.
The results showed that Zn, Cr, and Sr presented higher levels in the study area, while Cd and Pb presented the lowest values. In this study, soil TME concentrations of a non-polluted site (profile T) are considered as the background values. The maximum values of the TME concentrations for the study area are higher than the soil TME background. The average Sr, Zn, Cr, Cd, and Pb concentrations for the study area are 11.53, 61.2, 14.5, 3.97, and 0.7 times the soil TME background values, respectively (Table 4).
Very high TME concentrations in a peri-urban area of M’Dhilla city were also reported in other studies. For example, Salhi (2017) found that the maximum Zn, Cr, Cd, and Pb content in the around discharges from the M’Dhilla washing plant was 443.5, 245.3, 48.92, and 19.90 mg.Kg−1. These suggest that a significant portion of the Zn, Cr, Cd, and Pb metals originated from non-crustal or anthropogenic processes. Results showed that the soil Cd levels in the peri-urban area of M’Dhilla city were much higher than those in the industrial areas of other cities such as agricultural soils in a typical peri-urban area in the southeast of China. Indeed, Huang et al. (2018) reported that the average levels of soil Cd were 0.80 mg.Kg−1. However, the concentrations of Cr and Zn (14.5 mg.Kg−1 and 61.2 mg.Kg−1, respectively) in a peri-urban area of M’Dhilla city were much lower than those shown in the agricultural soils located in a peri-urban area of China (69.11 mg.Kg−1 and 90.89 mg.Kg−1, respectively) (Huang et al., 2018).
4.2 Multivariate Statistical Analysis
Pearson’s correlation matrix is a measure of linear correlation between two sets of data. Table 5 shows that Cd has positive correlations with Cr, P2O5%, Zn, Sr, and Zr contents with coefficients 0.823, 0.750, 0.684, 0.421, and 0.237, respectively. Although Cd shows a negative correlation with SiO2% (− 0.399) and Pb (− 0.332) and TC (− 0.510), the Cd-Zn Cd-P2O5% and Cd-Cr correlation coefficients are considered statistically significant. Similarly, Cd and Cr seem to be interrelated parameters (0.823) despite the fact that they are a similar correlation with all parameters. Zn correlates positively with Cd, Cr, P2O5%, As, EC, and Sr and negatively with Pb, TC, and SiO2% (Table 5). The positive correlations between different parameters mean common sources, mutual dependence, and identical behavior during transport (Mao et al., 2013). Thus, the samples show a positive correlation between Cd, Cr, Zn, Sr, Zr, As, EC, and P2O5%. This means that they probably share a common origin, which is PG washing water. Contrarily, negative correlation with other parameters TC, SiO2% and Pb seem to be influenced by different processes.
4.3 Principal Components Analysis (PCA)
The results of PCA based on the most significant first principal components show four significant factors representing 81.580% of the total variance and are given in Table 6. The first factor (F1) accounts for 39.201% of the total variability and involves high positive loadings of Cd (0.888), Zn (0.782), Sr (0.618), P2O5% (0.824), and Cr (0.777) and relatively low loadings of Zr (0.0054). It indicates that these elements derive from a common origin, which is the anthropogenic activity of washing PG. SiO2% (− 0.544), TC (− 0.689), and Pb (− 0.518) behave oppositely from all other elements. They show a negative loading with F1 suggesting a geogenic source. Thus, in the factorial plan F1*F2 (Fig. 3a and Table 7), the 1st component (axis F1) describes the origin of the chemical elements Cd (0.888), Zn (0.782), Sr (0.618), P2O5% (0.824), As (0.351), Cr (0.777), SiO2% (− 0.544), TC (− 0.689), and Pb (− 0.518). From left to right, the F1 axis therefore reflecting the geogenic origin is manifested by a negative correlation with SiO2%, Pb, and EC (siliceous PG) and the anthropogenic origin of metallic trace elements in the study area (Khelifi et al., 2019). The second factor (F2) represents 17.664% of the total variance. It comprises positive loadings of As (0.646), Pb (0.290), Zr (0.767), SiO2% (0.368), and Zn (0.367) and negative loadings of Cr (− 0.126) and Sr (− 0.619). This factor is related to the texture of the soil (granulometry). The third factor that shows positive loadings of Pb (0.519), EC (0.859), and Zn (0.399) accounts for 15.343% of the total variance. The fourth factor (F4) accounts for 9.373% and comprises positive loadings of Cr (0.413), Zr (0.473), and Pb (0.227).
4.4 Cluster Analysis
PCA plot (Fig. 3a) displays the relationships between all 13 variables at the same time. The elements were hierarchically clustered based on the total metal concentrations in the soils. Thus, three distinct clusters were identified (G1, G2, G3). The first group (G1) involves Sr, CE, and Cr; the second group (G2) with Zn, Cd, P2O5, and Zr; and the third group (G3) with Pb, SiO2, and TC. This subdivision suggested that each group contains individuals sharing the same geochemical behavior, the same mineralogical phase, or are controlled by the same physical and chemical parameters. G2 and G3 are orthogonal to each other, so they are significantly uncorrelated (r close to 0). G1 is symmetrically opposite concerning the center with G3, so they are significantly negatively correlated (r close to − 1). The portioning of TME concentrations seems to be governed by several factors depending on the vertical distribution of the chosen samples. Based on the principal component values, PCA can explore multivariate relationships and reduce the number of variables across multiple subgroups while explaining the variance of the data (Fig. 3b) (Mouissi & Alayat, 2016; Missaoui et al., 2023).
The dendrogram of HCA shows three different clusters. The first cluster contains Cd, Zn, P2O5%, Sr, and EC, indicating a common origin and a common anthropogenic source which is PG waste. The second cluster includes SiO2%, Pb, and TC referring to the mineralogical soil phase. Zr and As form the third cluster, implying a natural source of those metals (Fig. 4). Finally, the analysis results are in concordance with those of PCA.
4.5 Vertical Distribution of TME in Soil
In order to quantify the TME accumulation in urban soil, it is essential to take soil samples below the surface layer (Linde et al., 2001). As shown in Fig. 5, profile D illustrates a high concentration of Cd (8.23 mg.Kg−1), Zn (22.71 mg.Kg−1), and Cr (10.46 mg.Kg−1) in topsoil at 0–10 cm of depth with extremely high pollution levels. The same thing for profile C, the concentration of the four TME (Cd, Sr, Pb, and Zr) was extremely high in the topsoil. While in the topsoil of profile B, the concentrations of the five TME, except for Cd (4.56 mg.Kg−1), did not exceed the threshold limits. All the four metals in profile C showed increasing content along with soil depth and increasing rates from 0–20 cm to 170–180 cm. A severe TME pollution also occurred in topsoil and in subsoil. For profile B, the TME concentrations in topsoil were much lower than those of profiles C and D. The concentration of Zn and Cr was not observed in the topsoil, and increased as the depth decreased, with the exception of Cd which was observed to have a high pollution level from the surface to the subsoil. Profile C showed a slight decrease in the concentration of Cd and Zr at 10 cm. Zr concentration continued to decrease until it reached its minimum at 30–40 cm. Additionally, Cd value increased again until it reached its maximum at 30–40 cm. Profile D showed a decreasing content with increasing soil depth and decreasing rates from 0–10 cm to 50–80 cm. The decreasing rates of Cr, Zn, and Zr from topsoil to subsoil were much higher than those of Cd, indicating that Cr, Zn, and Zr were more readily accumulated in topsoil than Cd. However, the concentrations of Cr at the soil depth of 10–190 cm were absent and increased all at once to the maximum value of 190–200 cm. The absence of Cr and Pb enrichment in the samples indicates that these metals are of natural source. The results show that Cd is still at a high pollution level in all the profiles, and at all depths. This may be explained by the high pollution level and the high exchangeable fraction of Cd. Cr concentration shows a heterogeneous distribution in profiles B and D, and it was absent in profile C. In contrast, Cd, Pb, and Zn seemed to have an even distribution in all profiles. The vertical distribution of Cd and Zr had a similar pattern in C and D profiles. Their concentrations were relatively high in 0–10 cm but relatively low in 20–80 cm. This distribution might be attributed to the large quantities of waste resulting from the washing discharges of PG from TCG on the one hand or from the liquid effluents from CPG on the other.
These results are conformed to the study of Salhi (2017), which concludes that the levels of TME on the soil surface are very high and do not comply with the discharge standards. They pose a problem with the toxicity of soil resources as well as on steppe rangelands. Furthermore, the contamination of the hydrographical network, by mining effluents that do not comply with Tunisian Discharge Standards, is mainly due to the phenomenon of leaching by rainwater with stocks of phosphate waste or by the direct discharge of muddy waste from laundries. Large-sized discharges are often deposited on the banks of wadis, while fine-sized discharges (of a muddy nature) are discharged directly into the flow bed of the wadis. These high levels are explained by the fact that the sludge has been discharged and accumulated in the hydrographical network for decades (about 30 years). Depending on their mobility, Zmemla et al. (2016) classified the TME contained in PG into three categories: high mobility (Sr, Zn), moderate mobility (As, Ba, Cd, and Cr), and low mobility (Cu, Ni, Pb, Se, V, Y, and Zr). Thus, PG leaching shows the ability of Cd to dissolve and contaminate the environment (Al-Masri et al., 2004). The lower pH accelerates the leaching of the TME. Acid pH characterizes four sub-samples: E11 (3.46), E1 (2.84), E2 (3.01), and E3 (5.75) of respective depths: 0–5 cm, 0–10 cm, 60–80 cm, and 170–180 cm. These samples are characterized by the maximum concentration of Cd, Zn, Sr, and Cr. In fact, Al-Masri et al. (2004) confirm this result for the leachability of Cd, and Zn in Syrian PG. The solubility of metals tends to increase at lower pH and decrease at basic pH. Indeed, results showed that Zn and Cd were the most easily transferred elements in the aqueous phase, with transfer percentages of 97 and 57%, respectively (using distilled water and varying the stirring time between 10 and 120 min). Likewise, sequential treatment of PG with concentrate H2SO4 showed that TME were dissolved differently: Cd (25%), Zn (10%), and Cu (10%). Thus, the increase of H2SO4 concentration caused an increase in Cd and Zn. However, acid pH characterizing the deep ground study area explained the higher concentration of TME at high depth. Actually, wastewater percolating from PG stock through the Wadi increases TME level, and high acid concentration increases TME solubility. With high TME solubility, its concentration increases with depth, but its style is relative with the mineralogical composition of the soil.
The difference in the vertical distributions of TME observed between profiles and compartments might be caused by many factors such as the influence of soil processes, combined with each other, on the distribution of these TME. Indeed, the pathogenesis involves redistributions of soil components (carbonates, clays, oxides, organic matter), often governed by water infiltration, in the soil profile.
4.6 Assessment of TME Pollution
According to the PLI value, the soil quality concerning TME safety was classified as follows: null PLI—a background value, PLI ≥ 1—gradual degradation (non-polluted to moderately polluted), PLI ≥ 2—moderate pollution, and PLI ≥ 4—high pollution.
The contamination factor, by which the contamination degree was evaluated, was mostly high for different sample types, especially for Cd. Accordingly, CF values of this metal ranged from 38.314 to 111.511. Thus, CF exceeded the established standards signifying a “very high contamination level” with CF > 6. Considerably high CF was noticed in profile E1. CF of Zn value ranged from 0.235 to 1.916 (Table 8) indicating that contamination of Zn was low to moderate in the total of samples. CF for Sr varied noticeably between nearly null values and considerable values. For further assessment of the pollution status of the studied site, the pollution load index (PLI) was determined. The PLI values of the twenty sites varied from 2.84 to 20.29. No soil sample had PLI ≤ 2. Two samples, E4 and E3, have PLI ≥ 2 (classified as moderate pollution) and the two other samples, E1and E2, with PLI ≥ 4 are classified as high pollution sites, with high contamination factors of Cd and Sr.
Due to natural factors (high-temperature rates) and management factors (excess of irrigation water), agricultural land is covered by saline soil and contains frequent gypsum crusts (Besser et al., 2021; Ncibi et al., 2020a, 2020b). Several environmental issues developed with the increasing exploitation of phosphate production. In fact, the mining industry produces large quantities of waste materials containing chemical elements, which are environmentally hazardous. Thus, chemical elements constitute a permanent pollution source for the different environmental components leading to the unhealthy functioning of the ecological system and raising the discomfort of the residents (Besser et al., 2018; Mokadem et al., 2012).
4.7 Air Pollution
In addition, samples show a high concentration of sulfur responsible for a large quantity training of SO2 and SO3. Thus, NOX (NO2 and NO) produced during the phosphate transformation activities are dispersed over long distances from the M’Dhilla TCG factories to neighboring regions. Longer exposures to the high concentration of NO2 irritate airways in the human respiratory system and may cause respiratory diseases, particularly asthma and lung cancer. NOx gases, particularly NO2, are emitted into the atmosphere, transported by wind, and interact with water, oxygen, and other chemicals in the atmosphere to form acid rain. In addition, wind can blow SOx and NOx over long distances, so acid rain becomes a widespread problem. Acid rain disrupts photosynthesis, hinders growth, and changes plant leaves morphology (Mabrouk et al., 2020; Mokadem et al., 2012). Moreover, in open-cast mining areas, there are concentrations of particles such as fly ash, fumes, and dust derived from coarse, fine, and ultrafine apatite particles released during the treatment activities, washing, and transport of phosphate. Results show that the study area is characterized by a high contamination factor of Cd and Sr. Thus, daily exposure to particles rich in TME and radioactive elements, Sr, Cd, Pb, Zn…, leads to respiratory and cardiovascular diseases, cancer, and infertility in young people (Hamed et al., 2014, 2022a; Khelifi et al., 2019).
4.8 Soil and Water Pollution
The use of the wet process, in the study area, is considered less safe to land and ground water, since 64% of dumped water is evaporated or infiltrated the soil (Maazoun & Bouassida, 2018). Although the water consumption in the wet process is almost 6 times as the dry one, it seems to be less dangerous than the dust emission when using the dry process.
In the attempt to develop a specific method to avoid the groundwater contamination and health risk, the use of geomembrane helps drain a significant portion of the evacuated water and reuses it in the industrial process. Therefore, wastewater recovery saves the environment and also leads to economizing the natural resources in the industry (Maazoun & Bouassida, 2018). Likewise, the use of olive mill wastewater, rich in organic matter, as a solvent constitutes an important solution to protecting the environment. In fact, the mixture of the olive mill wastewater and PG, during 60 days, ensures a supply of about 5% of organic matter. This product may be used in the agriculture as a fertilizer (Boughzala et al., 2015).
The study area is characterized by clay and silty soil. In this context, higher concentrations of TME are registered in the clay soil and with the presence of organic matter. This result confirmed many previous works which reported that muddy and silty soils retained much higher concentrations of TME (Koretsky et al., 2006; Singh et al., 2002). In fact, high CF was noticed in the study, which is marked by a soil cover of blackish to a grayish color. Pollutants in contaminated soil come mainly from the percolation of acidulated water and the phenomenon of leaching by rainwater. The percolation of contaminated sediments and sludge increases each year, and therefore, the Wadi perimeter increases along with contaminated surfaces (Fig. 7a, b). Acid pH increases the solubility of toxic compounds and thus facilitates their fixation or their exchanges with the soil. Hence, the degradation of agricultural soils and the contamination of camel pasture which consume polluted plants and water and therefore transfer these toxic metals to consumers (Fig. 6). In addition, the evaporation phenomenon is very important and marked by a high concentration of salt on the surface of the ground cover (Fig. 7e). Thus, only halophytes plants with great resistance against TME persist all around the Wadi (Fig. 7c) (Caçador et al., 2016; Sghaier et al., 2019; Sleimi & Abdelly, 2003). Vertical distributions of TME in the study area show a high concentration of Cd, Cr, Zn, and Sr in high depth. Moreover, the statistical level in the study area is very high (Fig. 7d). Thus, TME derived from phosphate ore and PG are mobilized from the soil to plants and shallow underground aquifers (Hamed et al., 2022b; Khelifi et al., 2020).
TME exposure in high concentration can cause severe neurological and developmental health effects (Hamed et al., 2022a; Khelifi et al., 2020). Because of its potential toxicity and high mobility in the environment, Cd is one of the most poisonous and extensively distributed pollutants (Kryštofová et al., 2012). Previous studies in the world aimed to assess the environmental and health impact of TME in the mining area. Chronic inhalation, even at low concentrations, causes kidney disease. Similarly, acute inhalation of high concentrations of Cd causes severe lung damage that can be fatal and could damage the kidneys and the bones (Khelifi et al., 2019). Recently, the study by Hamed et al. (2022a) aimed to assess the impact of TME pollution from phosphate mines in southern Tunisia. This study shows that the Cd could be transmitted to humans by inhalation, due to its high concentration in the environment. The age range affected the most seems to be the people over 60 years. Indeed, at least one family member of local residents working in the mining industry was affected, which reveals the level of pollution to which they have been exposed (Hamed et al., 2022a). Gafsa-M’Dhilla-Gabes constitute the triangle of death in Tunisia. These regions have the highest rate of people suffering from cancer. Additionally, cancer is considered the most dominant after lung diseases. Hammas et al. (2016) note that the highest number of cancer hospital patients come from the mining region of Gafsa.
4.9 PG Geovalorisation
Several studies have shown that it is possible to geovalorize Tunisian PG. Nevertheless, PG can be used in different industrial fields, provided that the influence of all the impurities it may contain is controlled. Accordingly, in the cement industry, the natural gypsum is substituted by PG after a pre-treatment of this PG (Charfi-Fourati et al., 2000). On the other hand, the PG could be incorporated into the fired clay bricks used as a primary material in the chemical industry or as a construction material (Ajam et al., 2009, Saadaoui et al., 2017). The use of PG in road engineering is considered as a potential way to mobilize large quantities of this material (Sfar Felfoul et al., 2002). However, it poses environmental problems due to the solubility of the toxic or even radioactive constituents contained in the PG.
The performance of clay materials and the adsorption process of TME have been verified. Nevertheless, the effect of pH is the most critical parameter that influences the adsorption process of heavy metal ion (Bhattacharyya & Gupta, 2006). In fact, the adsorption of most heavy metals (Cu, Pb, Zn, and Cd.) decreases with the increase in pH above 6, due to the precipitation. On the other hand, the clay minerals have shown the applicability to remove metal pollutants from wastewater (Gu et al., 2019).
5 Conclusion and Recommendations
This study is an initial investigation to assess the TME vertical distribution and health risks in a typical peri-urban area in southwestern Tunisia (M’Dhilla basin) using geochemical and multivariate statistical analysis approaches. The assessments of the TME pollution using FXL experiments and multivariate statistical analysis (PCA and HCA) permit the following conclusion: The results showed that Cd, Sr, and Zn could be derived from an anthropogenic source, the PG water washing, while Cr and Pb are probably related to a natural phenomenon. TME contamination in agricultural soils in the study area was “moderate” to “severe” as shown by geochemical investigation of the vertical distribution of five TME (Cd, Cr, Zn, Pb, Zr, and Sr) in the peri-urban zone of M’Dhilla mining basin. The TME contents decrease respectively Sr > Zn > Cr > Cd > Pb. In summary, TME vertical distribution indicated that despite their behaviors to accumulate in the topsoil of long-term polluted areas, they could still move down to the deep soil layers gradually and cause deep soil pollution. Moreover, even if the topsoil is devoid of contaminants, TME are still in high concentration in the subsoil. The contamination factors proved that the studied area is definitely in a critical situation with a high contamination level in the most of sites. In this context, daily exposure to the contaminants cause serious problems for the inhabitants of the study area who suffer from many serious diseases and carcinogenic tumors affecting vital organs like the lungs, kidneys, and liver.
Additionally, the actual level of knowledge needs further evaluations of the radioactivity effect of PG and its environment and health risks. Thus, the best knowledge of PG radioactivity helps us to better choose the path of its geo-valorization and to make the right protective decisions.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Ajam, L., Ouezdou, M. B., Felfoul, H. S., & El Mensi, R. (2009). Characterization of the Tunisian phosphogypsum and its valorization in clay bricks. Construction and Building Materials, 23(10), 3240–3247. https://doi.org/10.1016/j.conbuildmat.2009.05.009
Al-Masri, M. S., & Al-Bich, F. (2002). Polonium-210 distribution in Syrian phosphogypsum. Journal of Radioanalytical and Nuclear Chemistry, 251(3), 431–435.
Al-Masri, M. S., Amin, Y., Ibrahim, S., & Al-Bich, F. (2004). Distribution of some trace metals in Syrian phosphogypsum. Applied Geochemistry, 19(5), 747–753. https://doi.org/10.1016/j.apgeochem.2003.09.014
Besser, H., & Hamed, Y. (2021). Environmental impacts of land management on the sustainability of natural resources in Oriental Erg Tunisia, North Africa. Environment, Development and Sustainability, 23(8), 11677–11705. https://doi.org/10.1007/s10668-020-01135-9
Besser, H., Mokadem, N., Redhaounia, B., Hadji, R., Hamad, A., & Hamed, Y. (2018). Groundwater mixing and geochemical assessment of low-enthalpy resources in the geothermal field of southwestern Tunisia. EUro-Mediterranean Journal for Environmental Integration, 3(1), 1–15. https://doi.org/10.1007/s41207-018-0055-z
Besser, H., Dhaouadi, L., Hadji, R., Hamed, Y., & Jemmali, H. (2021). Ecologic and economic perspectives for sustainable irrigated agriculture under arid climate conditions: An analysis based on environmental indicators for southern Tunisia. Journal of African Earth Sciences, 177, 104134. https://doi.org/10.1016/j.jafrearsci.2021.104134
Bhattacharyya, K. G., & Gupta, S. (2006). Adsorption of chromium (VI) from water by clays. Industrial & Engineering Chemistry Research, 45(21), 7232–7240. https://doi.org/10.1021/ie060586j
Boim, A. G. F., Melo, L. C. A., Moreno, F. N., & Alleoni, L. R. F. (2016). Bioconcentration factors and the risk concentrations of potentially toxic elements in garden soils. J. Envir. Manag., 170, 21–27. https://doi.org/10.1016/j.jenvman.2016.01.006
Boughzala, K., Jaouadib, A., Bouzouitac, K., Fattah, N., & Ben Hassine, H. (2015). Traitement et valorisation des rejets de phosphates de Gafsa. Revue Science Des Matériaux, 4, 13–31.
Caçador, I.; Duarte, B.; Marques, J.C.; Sleimi, N. (2016). Carbon mitigation: A salt marsh ecosystem service in times of change. In: Halophytes for Food Security in Dry Lands (M.A. Khan, M. Ozturk, B. Gul& M.Z. Ahmed Eds) Elsevier Academic Press: 83–110. https://doi.org/10.1016/B978-0-12-801854-5.00006-6.
Charfi-Fourati, F., Bouaziz, J., & Belayouni, H. (2000). Valorisation du phosphogypse de Tunisie en vue de son utilisation comme substituant au gypse naturel dans la fabrication du ciment. Déchets Sciences Et Techniques, 20, 24–32. https://doi.org/10.4267/dechets-sciences-techniques.1550
Choura, M., Salhi, S., & Cherif, F. (2009). Mechanical behaviour study of soil polluted by crude oil: case of Sidi El Itayem oilfield Sfax Tunisia. Environmental Earth Sciences, 59(3), 573–580. https://doi.org/10.1007/s12665-009-0055-z
Dridi, N., Romdhane, L., Ferreira, R., & Sleimi, N. (2020). Fertilizer effect of composted sewage sludge and cattle manure on Pelargonium growth. J Water Sanitation and Hygiene for Development, 10(4), 1019–1025. https://doi.org/10.2166/washdev.2020.082
Garbaya, H., Jraba, A., Khadimallah, M. A., & Elaloui, E. (2021). The development of a new phosphogypsum-based construction material: A study of the Physicochemical. Mechanical and Thermal Characteristics. Materials, 14(23), 7369. https://doi.org/10.3390/ma14237369
Gu, H., Kang, X., Wang, L., Lichtfouse, E., & Wang, C. (2019). Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environmental Chemistry Letters, 17, 629–654. https://doi.org/10.1007/s10311-018-0813-9
Hachani, C., Lamhamedi, M. S., Abassi, M., Sleimi, N., & Béjaoui, Z. (2022). Effects of heavymetal-pollutedsoil (Pb, Zn, and Cd) on seedemergence, seedlinggrowth, and antioxidantactivity in four Fabaceaespecies. Water, Air, and Soil Pollution, 233(7), 263. https://doi.org/10.1007/s11270-022-05725-3
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A Sedimentological Approach. Water Research, 14(8), 975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Hamed, Y., Ahmadi, R., Hadji, R., Mokadem, N., Ben Dhia, H., & Ali, W. (2014). Groundwater evolution of the Continental Intercalaire aquifer of Southern Tunisia and a part of Southern Algeria: Use of geochemical and isotopic indicators. Desalination and Water Treatment, 52(10–12), 1990–1996. https://doi.org/10.1080/19443994.2013.806221
Hamed, Y., Hadji, R., Ncibi, K., Hamad, A., Ben Saad, A., Melki, A., Mokadem, N., Khelifi, F., & Mustafa, E. (2022b). Modelling of potential groundwater artificial recharge in the transboundary Algero-Tunisian Basin (Tebessa-Gafsa): The application of stable isotopes and hydroinformatics tools. Irrigation and Drainage, 71(1), 137–156. https://doi.org/10.1002/ird.2647
Hamed, Y.; Khelifi, F.; Besser, H.; Ben Sâad, A.; Ncibi, K.; Hadji, R.; Mliki, A. & Hamad, A. (2022a). Phosphate mining pollution in southern Tunisia: Environmental, epidemiological, and socioeconomic investigation. Environment, Development and Sustainability, 1–18.https://doi.org/10.1007/s10668-022-02606-x
Hammas, I., Horchani-Naifer, K., Férid, M., & Barca, D. (2016). Rare earths concentration from phosphogypsum waste by two-step leaching method. Intern. J. Mineral Processing, 149, 78–83. https://doi.org/10.1016/j.minpro.2016.02.011
Hentati, O., Abrantes, N., Caetano, A. L., Bouguerra, S., Gonçlves, F., Römbke, J., & Pereira, R. (2015). Phosphogypsum as a soil fertilizer: Ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. Journal of Hazardous Materials, 294, 80–89. https://doi.org/10.1016/j.jhazmat.2015.03.034
Huang, Y., Chen, Q., Deng, M., Japenga, J., Li, T., Yang, X., & He, Z. (2018). Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Envir. Management, 207, 159–168. https://doi.org/10.1016/j.jenvman.2017.10.072
Kaiser, H. F. (1960). The application of electronic computers to factor analysis. Edu. Psychol. Meas., 20, 141–151. https://doi.org/10.1177/001316446002000116
Khelifi, F., Besser, H., Ayadi, Y., Liu, G., Yousaf, B., Harabi, S., Bedoui, S., Zighmi, K., & Hamed, Y. (2019). Evaluation of potentially toxic elements’ (PTEs) vertical distribution in sediments of Gafsa-Metlaoui mining basin (Southwestern Tunisia) using geochemical and multivariate statistical analysis approaches. Environmental Earth Sciences, 78(2), 1–14. https://doi.org/10.1007/s12665-019-8048-z
Khelifi, F., Melki, A., Hamed, Y., Adamo, P., & Caporale, A. G. (2020). Environmental and human health risk assessment of potentially toxic elements in soil, sediments, and ore processing wastes from a mining area of southwestern Tunisia. Environmental Geochemistry and Health, 42, 4125–4139. https://doi.org/10.1007/s10653-019-00434-z
Khoualdia, B., Loungou, M., & Elaloui, E. (2018). Optimized activation of bentonite for adsorption of magnesium and cadmium from phosphoric acid. World, 3(4), 83–91. https://doi.org/10.11648/j.wjac.20180304.11
Köhn, H.F. & Hubert, L.J. (2014). Hierarchical cluster analysis. In Wiley Stats Ref: Statistics Reference Online, John Wiley & Sons, Ltd. 1–13. https://doi.org/10.1002/9781118445112.stat02449.pub2.
Koretsky, C. M., Haas, J. R., Ndenga, N. T., & Miller, D. (2006). Seasonal variations in vertical redox stratification and potential influence on trace metal speciation in minerotrophic peat sediments. Water, Air, and Soil Pollution, 173(1), 373–403. https://doi.org/10.1007/s11270-006-9089-y
Kryštofová, O., Zítka, O., Křížková, S., Hynek, D., Shestivska, V., Adam, V., Hubálek, J., Macková, M., Macek, T., Zehnálek, J., Babula, P., Havel, L., & Kizek, R. (2012). Accumulation of cadmium by transgenic tobacco plants (Nicotianatabacum L.) carrying yeast metallothione in gene revealed by electrochemistry. Int. J. Electrochem. Sci., 7(2), 886–907.
Linde, M., Bengtsson, H., & Öborn, I. (2001). Concentrations and pools of heavy metals in urban soils in Stockholm, Sweden. Water, Air and Soil Pollution, 1(3), 83–101. https://doi.org/10.1023/A:1017599920280
Maas, S., Scheifler, R., Benslama, M., Crini, N., Lucot, E., Brahmia, Z., Benyacoub, S., & Giraudoux, P. (2010). Spatial distribution of heavy metal concentrations in urban suburban and agricultural soils in a Mediterranean city of Algeria. Environmental Pollution, 158(6), 2294–2301. https://doi.org/10.1016/j.envpol.2010.02.001
Maazoun, H. & Bouassida, M. (2018). Phosphogypsum management challenges in Tunisia. In International Congress and Exhibition “Sustainable Civil Infrastructures: Innovative Infrastructure Geotechnology” (88–104). Springer, Cham. https://doi.org/10.1007/978-3-030-01941-9_7
Mabrouk, L., Mabrouk, W., & Ben Mansour, H. (2020). High leaf fluctuating asymmetry in two native plants growing in heavy metal-contaminated soil: The case of Metlaoui phosphate mining basin (Gafsa, Tunisia). Environmental Monitoring and Assessment, 192, 406. https://doi.org/10.1007/s10661-020-08385-0
Manta, D. S., Angelone, M., Bellanca, A., Neri, R., & Sprovieri, M. (2002). Heavy metals in urban soils: A case study from the city of Palermo (Sicily). Italy. Science of the Total Environment, 300(1–3), 229–243. https://doi.org/10.1016/S0048-9697(02)00273-5
Mao, L., Mo, D., Guo, Y., Fu, Q., Yang, J., & Jia, Y. (2013). Multivariate analysis of heavy metals in surface sediments from lower reaches of the Xiangjiang River, southern China. Environmental Earth Sciences, 69(3), 765–771. https://doi.org/10.1007/s12665-012-1959-6
Mekki, A., & Sayadi, S. (2017). Study of heavy metal accumulation and residual toxicity in soil saturated with phosphate processing wastewater. Water, Air and Soil Pollution, 228(215), 1–15. https://doi.org/10.1007/s11270-017-3399-0
Missaoui, R., Abdelkarim, B., Ncibi, K., Hamed, Y., Choura, A., & Essalami, L. (2022). Assessment of groundwater vulnerability to nitrate contamination using an improved model in the Regueb Basin, Central Tunisia. Water, Air, and Soil Pollution, 233(322), 1–16.
Missaoui, R., Ncibi, K., Abdelkarim, B., Bouajila, A., Choura, A., Hamdi, M., & Hamed, Y. (2023). Assessment of hydrogeochemical characteristics of groundwater: Link of AHP and PCA methods using GIS approach in semi-arid region. Central Tunisia: Euro-Mediterranean Journal for Environmental Integration. https://doi.org/10.1007/s41207-023-00345-7
Mokadem, N., Hamed, Y., Ben Sâad, A., & Gargouri, I. (2012). Atmospheric pollution in North Africa (ecosystems-atmosphere interactions): A case study in the mining basin of El Guettar-M’Dhilla (Southwestern Tunisia). Arabian J: Geosciences. https://doi.org/10.1007/s12517-013-0852-2
Mostert, M. M., Ayoko, G. A., & Kokot, S. (2012). Multi-criteria ranking and source identification of metals in public playgrounds in Queensland, Australia. Geoderma, 173, 173–183. https://doi.org/10.1016/j.geoderma.2011.12.013
Mouissi, S., & Alayat, H. (2016). Use of the principal component analysis (PCA) for physico-chemical charcterization of an aquatic ecosystem waters: Case of Oubeira Lake (extreme north eastern Algeria). J. Materials and Environmental Science, 7(6), 2214–2220.
Ncibi, K., Chaar, H., Hadji, R., Baccari, N., Sebei, A., Khelif, F., Abbes, M., & Hamed, Y. (2020a). A GIS-based statistical model for assessing groundwater susceptibility index in shallow aquifer in Central Tunisia (Sidi Bouzid basin). Arabian J. Geosciences, 13(98), 1–21. https://doi.org/10.1007/s12517-020-5112-7
Ncibi, K.; Hadji, R.; Hamdi, M.; Mokadem, N.; Abbes, M.; Khelif, F.; Zighmi, K. & Hamed, Y. (2020b). Application of the analytic hierarchy process to weight the criteria used to determine the Water Quality Index of groundwater in the northeastern basin of the Sidi Bouzid region, Central Tunisia. Euro-Mediterranean J. for Environ. Integr, 5(1). https://doi.org/10.1007/s41207-020-00159-x.
Nriagu, J. O., & Pacyna, J. M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333(6169), 134–139. https://doi.org/10.1038/333134a0
Pen-Mouratov, S., Shukurov, N., & Steinberger, Y. (2008). Influence of industrial heavy metal pollution on soil free-living nematode population. Environ. Pollu., 152(1), 172–183. https://doi.org/10.1016/j.envpol.2007.05.007
Pérez-López, R., Nieto, J. M., López-Coto, I., Aguado, J. L., Bolívar, J. P., & Santisteban, M. (2010). Dynamics of contaminants in phosphogypsum of the fertilizer industry of Huelva (SW Spain): From phosphate rock ore to the environment. Applied Geochemistry, 25, 705–715. https://doi.org/10.1016/j.apgeochem.2010.02.003
Rutherford, P. M., Dudas, M. J., & Arocena, J. M. (1995). Radioactivity and elemental composition of phosphogypsum produced from three phosphate rock sources. Waste Manage. & Res., 13(5), 407–423. https://doi.org/10.1016/S0734-242X(05)80021-7
Saadaoui, E., Ghazel, N., Ben Romdhane, C., & Massoudi, N. (2017). Phosphogypsum: potential uses and problems–a review. International Journal of Environmental Studies, 74(4), 558–567. https://doi.org/10.1080/00207233.2017.1330582
Salhi, B. (2017). Socio-spatial and environmental changes in the mining basin of Gafsa (South West of Tunisia): Contribution of geomatics tools. University of Maine.
Sey, E., & Belford, E. J. D. (2019). Levels of heavy metals and contamination status of a decommissioned tailings dam in Ghana. Intern. J. Environ. Qual., 35, 33–50. https://doi.org/10.6092/issn.2281-4485/9060
Sfar Felfoul, H., Ouertani, N., Clastres, P., & Benouezdou, M. (2002). Amélioration des caractéristiques du phosphogypse en vue de son utilisation en technique routière. Déchets - Revue Francophone D’écologie Industrielle., 28, 21–25. https://doi.org/10.4267/dechets-sciences-techniques.2405
Sghaier, D. B., Bankaji, I., Pedro, S., Caçador, I., & Sleimi, N. (2019). Photosynthetic behaviour and mineral nutrition of Tamarixgallica cultivated under aluminum and NaCl combined stress. Phyton-International Journal of Experimental Botany, 88(3), 239–252. https://doi.org/10.32604/phyton.2019.06887
Singh, M., Müller, G., & Singh, I. B. (2002). Heavy metals in freshly deposited stream sediments of rivers associated with urbanisation of the Ganga Plain, India. Water, Air and Soil Pollution, 141(1), 35–54. https://doi.org/10.1023/A:1021339917643
Sleimi, N., Kouki, R., Hadj Ammar, M., Ferreira, R., & Perez-Clemente, R. (2021). Barium effect on germination, plant growth, and antioxidant enzymes in Cucumissativus L. plants. Food Science & Nutrition, 9(4), 2086–2094. https://doi.org/10.1002/fsn3.2177
Sleimi, N. & Abdelly, C. (2003). Salt-tolerance strategy of two halophytes species: Spartinaalterniflora and Suaedafruticosa. In: Tasks for Vegetation Science (Cash Crop Halophytes, H. Lieth & M. Mochtchenko eds). Kluwer Academic Publishers, Netherland. 38: 79–85 https://doi.org/10.1007/978-94-017-0211-9
Sleimi, N.; Bankaji, I.; Dallai, M. & Kefi O. (2014). Accumulation des éléments traces et tolérance au stress métallique chez les halophytes colonisant les bordures de la lagune de Bizerte. Rev. Ecol. (Terre Vie), 69(1): 49–59. https://hal.archives-ouvertes.fr/hal-03530492/document.
Tayibi, H., Choura, M., López, F. A., Alguacil, F. J., & López-Delgado, A. (2009). Environmental impact and management of phosphogypsum. J. Environ. Manag., 90(8), 2377–2386. https://doi.org/10.1016/j.jenvman.2009.03.007
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen, 33(1), 566–575. https://doi.org/10.1007/BF02414780
Walker, W. E., Harremoës, P., Rotmans, J., Van Der Sluijs, J. P., Van Asselt, M. B. A., Janssen, P., & Krayer von Krauss, M. P. (2003). Defining uncertainty: A conceptual basis for uncertainty management in model-based decision support. Integrated Assessment, 4(1), 5–17. https://doi.org/10.1076/iaij.4.1.5.16466
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica Et Cosmochimica Acta, 59(7), 1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
Yong, R.N. (1993). Water management in East (China) and South-East Asia: Report prepared for URB GPI regional definition workshop. Environment and Natural Resources Division, IDRC Research Results.URI: http://hdl.handle.net/10625/30298.
Zmemla, R., Chaurand, P., Benjdidia, M., Elleuch, B., & Bottero, J. Y. (2016). Characterization and pH dependent leaching behavior of Tunisian phosphogypsum. Am. Acad. Sci. Res. J. Engin. Techn. Sci., 24(1), 230–244.
Acknowledgements
The authors are thankful to the anonymous reviewers and editorial handling for their contribution to the improvement of the manuscript. All the authors would like also to thank the International Association of Water Resources in the Southern Mediterranean Basin, Tunisia, for the support provided.
Author information
Authors and Affiliations
Contributions
Conceptualization: Younes Hamed and Noomene Sleimi; Methodology: Naima Hidouri and Rim Missaoui; Formal analysis and investigation: Naima Hidouri, Abderraouf Jbara, Noomene Sleimi, and Younes Hamed; Writing—original draft preparation: Naima Hidouri and Rim Missaoui; Writing—review and editing: Naima Hidouri, Rim Missaoui, Youssef Balal, Noomene Sleimi, and Younes Hamed; Resources: Noomene Sleimi and Younes Hamed; Supervision: Younes Hamed. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hidouri, N., Missaoui, R., Jraba, A. et al. Assessing the Vertical Distribution and Health Risks of Trace Metal Elements in Phosphogypsum-Enriched Agricultural Soils of Typical Peri-Urban Areas in Southwestern Tunisia. Water Air Soil Pollut 234, 332 (2023). https://doi.org/10.1007/s11270-023-06335-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06335-3