Abstract
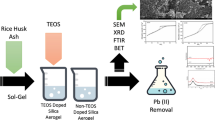
In this study, two silica aerogels were synthesized and compared of their morphology and lead adsorptive behavior. One silica aerogel was aged with organic solvents, ethanol and heptane (SG-OM), while the other was doped with tetraethyl orthosilicate (TEOS) (SG-TD). Both the materials were characterized using SEM, XRD, FTIR, and BET. As per results, SG-OM showed higher surface area (312 m2/g), higher pore volume (0.85 cm3/g), and higher porosity % (85%) than SG-TD with surface area (264 m2/g), higher pore volume (0.56 cm3/g), and higher porosity % (74.9%). Both the aerogels were highly mesoporous in their structure. Moreover, when the adsorbents were investigated for lead(II) removal from synthetic wastewater, SG-OM indicated higher removal with 98.58% (pH 6), 98% (5 ppm metal concentration), and 98% (0.1 g adsorbent dose) than SG-TD with removal of 65% (pH 6), 88% (5 ppm metal concentration), and 62% (0.1 g adsorbent dose). Moreover, the highest adsorption capacity (qe) indicated by SG-OM was 15.2 mg/g and SG-TD as 11.8 mg/g. It was revealed that aging with organic solvents (ethanol and heptane) resulted in an aerogel with enhanced morphological properties and lead(II) adsorptive efficiency than aerogel doped with TEOS. Finally, the obtained results were investigated for isotherms (Langmuir and Freundlich), and it was shown that both the adsorbents best fitted Langmuir isotherm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Silica aerogel is a lightweight, porous material with a high surface area and excellent thermal insulation properties. It is made by removing the liquid from a gel composed of silica particles, leaving behind a solid network of silica. One common method for making silica aerogel is through the use of tetraethyl orthosilicate (TEOS) as a precursor. TEOS is reacted with water and ethanol, creating a gel that can then be dried to form the aerogel (Gurav et al., 2010; Ibrahim et al., 2019). The use of ethanol in the production of silica aerogel is important because it acts as a solvent, helping to dissolve the TEOS and allowing it to react with water to form the gel. Heptane, a hydrocarbon solvent, can also be used in place of ethanol, but it may not be as effective in dissolving the TEOS and may also lead to the formation of a less porous aerogel (Wang et al., 2020). In addition to its thermal insulation properties, silica aerogel has been found to have excellent adsorption properties, particularly for lead ions. The high surface area and porous structure of the aerogel makes it an effective adsorbent, allowing it to bind lead ions from aqueous solutions (Akhter et al., 2021). This has potential applications in the removal of lead from contaminated water. It is worth noting that, while silica aerogel has many potential uses, it is also brittle and difficult to handle, which can limit its practical applications. Silica aerogel is a highly porous material with a high surface area, making it an effective adsorbent for a variety of contaminants, including lead ions. The porous structure of the aerogel allows it to effectively bind lead ions from aqueous solutions, making it a potential solution for the removal of lead from contaminated water (Terzioglu et al., 2018). Lead adsorption by silica aerogel has been widely studied, with researchers investigating various parameters such as pH, temperature, and initial lead ion concentration to optimize the adsorption process. It has been found that at pH 6–8, silica aerogel can effectively adsorb lead ions, with the highest adsorption capacity observed at pH 7. Additionally, it has been found that an increase in temperature can not only increase the rate of lead adsorption, but also decrease the total amount adsorbed. The mechanism of lead adsorption by silica aerogel is thought to involve both physical and chemical interactions. The high surface area of the aerogel allows for a large number of lead ions to be adsorbed through physical interactions such as electrostatic attraction and ion exchange (Falsafi et al., 2020). The chemical interactions, such as coordination between lead ions and functional groups present on the silica surface, also play a role in the lead adsorption process. Silica aerogel has been found to be an effective adsorbent for lead ions in comparison with other adsorbents such as activated carbon, zeolites, and metallic oxide. Silica aerogel has the advantage of high surface area, high porosity, low density, and low cost, which makes it an attractive adsorbent for lead ions.
Silica aerogel’s lead adsorption properties have potential for practical applications in the treatment of contaminated water. It could be used in the form of beads, granules, or monoliths, which are easy to handle, and can be easily separated from the treated water by filtration or centrifugation. Additionally, silica aerogel’s lead adsorption can be regenerated by using acid or chelating agents and can be reused multiple times. It's worth noting that, while silica aerogel’s lead adsorption properties are promising, further research is needed to optimize the adsorption process and to investigate the long-term stability and effectiveness of silica aerogel as an adsorbent for lead ions in real-world applications. Additionally, the cost of producing silica aerogel can be a significant limitation, so efforts to make it more cost-effective will be needed to make it a viable solution for large-scale water treatment applications (Akhter et al., 2021, 2022).
Various other studies in the past have used modifying agents to synthesize silica aerogels and investigated for lead(II) adsorption from synthetic wastewater, i.e., bispyrazole (Radi et al., 2017), AAAPTS (Fan et al., 2014), PEI (Delacour et al., 1999), Glutaraldehyde (Joshi & Srivastava, 2019), Thiamine (Deniz et al., 2017), and APTES (Faghihian et al., 2012); however, due to the inorganic nature, hazardous conduct and expensive cost, there was a need to use sustainable method and low cost organic solvents to synthesize silica aerogel with decent properties. Hence, in the present work, two variants of silica aerogel were synthesized and evaluated for lead(II) adsorption. One was modified and aged using organic solvents such as ethanol and heptane (SG-OM), while the second variant was doped with TEOS (SG-TD). Both aerogels were sustainable in nature and prepared using sodium silicate solution extracted from rice husk ash. After characterization, both the variants were analyzed for lead(II) adsorption from synthetic wastewater. The results were compared and analyzed for isotherm studies. Present work will add substantially towards synthesis of silica aerogels with decent properties and lead(II) adsorption using low cost organic solvents. Moreover, properties and adsorptive comparison among the prepared silica aerogels further emphasizes the overall improved performance by organically tailored silica aerogel over TEOS-doped aerogel.
2 Materials and Methods
The reagents used in the experiments were of the highest analytical grade and were used as is. The TEOS used was 99% pure, sourced from Daejung, Korea; the NaOH was 98% pure from Merck; and the lead nitrate was 99.95% pure from Merck. De-ionized water was used as both a solvent and as the adsorption medium. The silica aerogels produced were analyzed using SEM, XRD, FTIR, and BET methods. The metal solution samples after adsorption were analyzed using an atomic adsorption spectrometer (AAS).
2.1 Preparation of Rice Husk Ash
Initially, 100 g of rice husk was washed several times with distilled water to remove dirt and adhering impurities. The washed sample was dried in a drying oven at 110 °C until all the moisture was removed. Next, the dried rice husk was leached with 2 M HCl on a magnetic stirrer at 60 °C0 for 1 h to remove metallic impurities. Leached rice husk was washed with distilled water until neutral pH and dried in drying oven at 110 °C for a few hours until all moisture was removed. To remove the organic matter and produce rice husk ash, the moisture free leached rice husk was calcined at the temperature of 600 °C for 4 h. This resulted in amorphous rice husk ash, which was used to synthesize silica aerogels (Akhter, Soomro, et al., 2023).
2.2 Synthesis of Organically Modified Silica Aerogel (SG-OM)
A mixture of 5 g of rice husk ash and 1 molar NaOH solution was heated and stirred for an hour using a magnetic stirrer. The solution was then filtered using a filter paper to obtain a sodium silicate solution. The sodium silicate solution was neutralized using 1 molar HCl solution. The prepared hydrosol was left at room temperature for 24 h. The resulting hydrogel was then washed three times with de-ionized water, each time for 4 h, to remove any sulfates. The hydrogel was then soaked in an 80% ethanol and 20% water solution for 24 h to replace the water inside the pores of the gel with ethanol. The ethanol was then removed, and the gel was soaked in heptane for another 24 h. The heptane was removed, and the gel was dried in drying oven at 50 °Cfor 5 h and then at 120 °C until a constant weight was achieved (Akhter, Soomro, et al., 2023).
2.3 Synthesis of TEOS-Doped Silica Aerogel (SG-TD)
The process of synthesizing this variant begins with mixing 5 g of rice husk ash with 150 mL of 1 molar NaOH solution. The mixture is then heated and stirred for 1 h at 60 °C. The resulting solution is then filtered using a Whatman ashless filter paper 41 to separate the sodium silicate solution. The silicate solution is then neutralized using 1 molar HCl solution, followed by the addition of TEOS in the ratio of 1:10. The mixture is then aged for 24 h at room temperature, during which it transforms into a hydrogel through the condensation of silica. To remove any sulfates present, the gel is washed three times by aging in de-ionized water for 4 h each time. The de-ionized water is then disposed, and the gel is dried at 50 °C for 5 h and then at 120 °C until a constant weight is achieved (Akhter, Jamali, et al., 2023).
2.4 Sorption Studies
The study of lead (Pb) removal by silica aerogels was conducted using batch experiments. The experiments evaluated different parameters such as pH, initial metal concentration, and adsorbent dose. The lead solutions were prepared using de-ionized water and lead nitrate. The concentration of lead ions in the solutions after adsorption was measured using an atomic adsorption spectrometer (AAS). The efficiency of removal (R, %) and the adsorption capacity (qe, mg/g) were calculated using the appropriate equations:
3 Results and Discussion
3.1 Characterization of Silica Aerogels
Both the variants of silica aerogels (SG-OM and SG-TD) were characterized using SEM, FTIR, XRD, and BET. The results are discussed below.
3.2 Effect of Organic Solvents vs TEOS over Morphology
Figure 1 illustrates the morphology of the silica aerogel adsorbents employed in the current study. Both adsorbents presented a porous structure; however, there were variations in the size and uniformity of the pores. The adsorbent SG-OM displayed larger and more uniform pores when compared to SG-TD. This observation aligns with the results obtained from the BET analysis (Table 1). The enhanced porosity, pore size, and uniform distribution of SG-OM can be attributed to using organic solvents as modifying agents such as ethanol and heptane. Ethanol is known to enhance the pore features, whereas heptane improves the pores and gel network by suppressing the crack formation during ambient pressure drying (Akhter, Jamali, et al., 2023; Akhter, Soomro, et al., 2023; White et al., 2015). This suggests that the organic solvents lead to an improvement in the pore characteristics when compared to TEOS-doped silica aerogel. The larger and more uniform pores in the SG-OM adsorbent may enhance its surface area, which in turn can increase its adsorption capacity and efficiency.
3.3 Effect of Organic Solvents vs TEOS over Structure of Silica Aerogels
As shown in Fig. 2, both the aerogels indicated the sharp peaks lying between 21° and 22°, respectively. This illustrates the presence of silica network in the aerogel variants and their amorphous structure. The amorphous structure can be attributed to calcination of acid leached rice husk at 600° for 4 h prior to refluxing rice husk with NaOH solution (Akhter et al., 2021; Chen et al., 2021). This amorphous helped silica aerogels to achieve a highly mesoporous structure.
3.4 Effect of Organic Solvents vs TEOS over Functional Groups
Figure 3 illustrates the FTIR spectra of the two silica aerogel variants used as adsorbents in the current study. As seen from the figure, both adsorbents consist of a silica gel network, as indicated by the notable peaks at 1091 cm−1, 794 cm-−1, and 468 cm-−1, which are associated with siloxane (Si-O-Si) network vibration modes. These peaks confirm the presence of a silica network in the adsorbents. Additionally, we can observe the peaks at 966 cm−1 suggest the presence of ethanol as a result of aging with ethanol. The peaks at 3455 cm−1 and 1635 cm−1 are attributed to adsorbed water (O-H) which is present in very low amounts due to the exchange of pore fluid and ambient pressure drying of the respective silica aerogels. The peaks at 1250 cm−1 are likely due to C-O stretching, which could be associated with the presence of organic solvents such as ethanol and heptane used during the synthesis process (Al-Mothafer & Abdulmajeed, 2021). These results demonstrate that the FTIR spectra of the two silica aerogel variants are similar but with subtle variations in the peaks, which could be attributed to the different synthesis methodologies and aging techniques used.
3.5 Effect of Organic Solvents vs TEOS over Properties of Silica Aerogels
Figure 4 shows the N2 adsorption/desorption isotherms for both variants of silica aerogel. Table 1 shows the properties achieved by each of the adsorbent. BET results provide important information about the pore features and properties of an adsorbent. As can be seen (Fig. 4), both the adsorbents exhibit H1 hysteresis loop, indicating the cylindrical shape of the pores. Although, the pores in both adsorbents might be cylindrical in shape, a clear distinction in pore features can be seen from Table 1. The silica aerogel aged with organic solvents (SG-OM) showed higher surface area and much enhanced pore features than TEOS-doped aerogel (SG-TD). When we compare the properties of both the adsorbents, we see SG-OM with 15.38% enhanced surface area, 9.37% enhanced pore size, 34.11% enhanced pore volume, and 11.88% enhanced porosity than SG-TD variant. Such enhanced properties and pore features can be attributed to aging in organic solvents. Although, both the variants were highly mesoporous, however, organic solvents resulted in silica aerogel (SG-OM) to achieve much enhanced properties (Doke et al., 2021).
Figure 4 illustrates the results of N2 adsorption/desorption isotherms for two variants of silica aerogel. As can be observed from the figure, both adsorbents display H1 hysteresis loops, indicating the presence of cylindrical-shaped pores. However, a closer examination of the properties achieved by each adsorbent, as presented in Table 1, reveals a significant difference in the pore features of the two adsorbents. The silica aerogel aged with organic solvents (SG-OM) displayed a higher surface area, as well as improved pore size, volume, and porosity, when compared to the TEOS-doped aerogel (SG-TD). Specifically, SG-OM exhibited a 15.38% increase in surface area, 9.37% increase in pore size, 34.11% increase in pore volume, and 11.88% increase in porosity, compared to SG-TD. These enhanced properties and pore features can be attributed to the aging process in organic solvents. Despite both variants being highly mesoporous, the use of organic solvents resulted in a silica aerogel (SG-OM) with greatly improved properties, making it a more suitable adsorbent for various applications.
4 Sorption Studies
All sorption experiments were carried out in batch. The final lead concentrations in the solutions were determined using an atomic adsorption spectrometer (AAS).
4.1 Lead(II) Removal Efficiency of Adsorbents at Different pHs
PH plays an important role in determining the adsorption of metallic specie onto the adsorbent. At highly acidic pH range, there is an abundant presence of H+ ions than OH− ions in the solution, which form a positive layer onto the adsorbent. Such a positive layer creates an electrostatic repulsion, hence a reduced adsorption. Similarly, as acidic pH range is reduced towards basicity, more OH− ions are formed onto the adsorbent layer. This creates an electrostatic attraction between the negatively charged adsorbent surface and positively charged metallic specie such as Pb2+ in the present case (Akhter et al., 2021; Akhter, Jamali, et al., 2023).
The present batch study was conducted to investigate the Pb(II) removal efficiency by the two silica aerogel adsorbents (SG-OM and SG-TD). The pH ranges were selected as 2, 4, 6, 8, and 10. Higher pH than 10 would not give accurate results due to the precipitation. Other parameters were kept constant as adsorbent dose to 0.1 g, solution volume of 100 mL, initial pb concentration as 10 ppm, and time of 8 h, respectively.
Figure 5 shows the removal of lead(II) by both the adsorbents. As can be seen, silica aerogel synthesized via organic solvents (SG-OM) has shown much enhanced removal than TEOS-doped silica aerogel (SG-TD). This is clearly attributed to the higher surface area and enhanced pore features formed through aging in ethanol and heptane. When the adsorption pattern is taken into account, both the adsorbents have shown a similar pattern. At lower or acidic pH, a lower removal is observed. For SG-OM, the lowest removal was observed to be 25%, where as for SG-TD as mere 11%. The lower removal is a result of higher H+ ions in the solution. Furthermore, as the pH increases, lead removal by both the adsorbents increases as a result of OH− ions. At pH 4, SG-OM variant is shown to remove 79.87% of lead(II) ions, whereas SG-TD removes only 35%. As the pH approaches 6, both the adsorbents reach their highest removal and reach equilibrium. This hike in removal percentage could be attributed to decreased H+ ions and increased OH− ions, thereby resulting in electrostatic attraction between positively charged adsorbate ions and negatively charged OH ions. Furthermore, after pH 6, we see a decline in removal in the case of both the adsorbents which could be attributed to the binding sites of the adsorbents being fully occupied with metallic ions leaving behind no more available sites. SG-OM is shown to remove the highest removal of 98.58%, whereas SG-TD shows 65% removal at pH 6. This indicates that the optimum pH for both the adsorbents is pH 6. Moreover, the aging of silica aerogel with organic solvents (ethanol and heptane) has resulted in 33.58% enhanced lead removal than TEOS-doped silica aerogel. This indicates that modification with organic solvents enhance the properties of the synthesized aerogel and therefore results in higher lead removal than mere TEOS doping.
4.2 Lead(II) Removal Efficiency of Adsorbents at Different Metal Concentrations
The initial metal concentration in a solution is a crucial factor in determining the efficiency of adsorption. Adsorption is the process by which a solid, liquid, or gas adsorbent binds to molecules of a solution, often through chemical or physical interactions. In the case of metal ions, adsorption is often used as a method of removing them from a solution through the use of an adsorbent, such as silica aerogel. The initial metal concentration in a solution is directly related to the amount of metal ions that can be adsorbed by the adsorbent. As the initial metal concentration increases, the adsorbent will reach its maximum adsorption capacity more quickly. In addition, the rate of adsorption will also increase as the initial metal concentration increases. However, it is important to note that as the initial metal concentration increases, the efficiency of adsorption may decrease. This is due to the fact that the adsorbent may become saturated with metal ions, resulting in a decrease in the number of available binding sites for the metal ions (Kushwaha et al., 2017; Leyva-Ramos et al., 2011). Therefore, it is important to optimize the initial metal concentration in order to achieve the most efficient adsorption.
In order to investigate the removal % by the adsorbents, 2 sets of batch experiments were designed for each adsorbent. The initial Pb concentration ranges were selected as 5, 10, 20, 30, and 40 ppm, respectively. Other parameters were kept constant such as pH at 6, solution volume as 100 mL, adsorbent dose as 0.1 g, and contact time of 24 h.
Figure 6 shows the removal % indicated by both the adsorbents. Table 2 shows the values along with adsorption capacity of each adsorbent. Overall, the graph indicates that as the initial metal concentration is increased, the removal % decreases. This is valid for both the adsorbents and is further emphasized by the removal % shown by each adsorbent. However, we see a significant difference in Pb removal and adsorptive trendline followed by each adsorbent. For both the adsorbents, the highest removal was indicated at the lowest Pb concentration of 5 ppm with 98% for SG-OM and 88% for SG-TD, respectively. The enhanced removal shown by SG-OM can be attributed to the improved morphological and physical properties achieved by using ethanol and heptane. Enhanced pore features and surface area provide with higher number of binding sites for metallic ions to attach, hence higher removal. This is valid at all the metallic concentrations as the adsorbent SG-OM showed higher removal % than SG-TD. For both the adsorbents, as the concentration is increased, the removal % significantly drops until at 40 ppm, where the removal % for SG-OM is 38% and for SG-TD, it is only 30%. This drop in the removal % is attributed to adsorption pattern of metallic ions onto the adsorbents. Adsorption depends upon the binding sites present on the surface and inside the material. Typically, a higher number of binding sites are available at the surface which results in metal ions being quickly adsorbed onto the surface. The more the surface area is, the higher the number of available binding sites. When the binding sites on the surface are occupied, the remaining adsorption takes place inside the material, which results in reduced mass transfer, hence reduced adsorption. Therefore, the adsorption trendline for both the adsorbents is the same; however, the higher removal by SG-OM is attributed to its enhanced surface area and pore features which provided higher number of binding sites for the adsorbate to attach. This is further validated by higher adsorption capacity indicated by SG-OM (Table 2) with the highest of 15.2 mg/g than SG-TD with 11.83 at 40 ppm.
4.3 Lead(II) Removal Efficiency of Adsorbents at Different Adsorbent Doses
Adsorbent dose plays a significant role in imparting the metallic removal in a solution. Higher doses of adsorbent provide with higher number of binding sites, hence a higher adsorption of metal ions onto the material (Martínez et al., 2020). This results in augmented removal of ions; however, it is important to determine the optimum dose. The ideal adsorptive behavior is when the lower adsorbent dose shows an efficient removal by adsorbing higher number of metal ions from the solution. Such an efficient behavior can be achieved if the material possesses remarkable morphological properties and provides higher number of binding sites.
To investigate the lead removal efficiency and optimum dose of both the adsorbents, a set of batch experiments were conducted. The adsorbent dose ranges were selected as 0.01, 0.05, 0.1, 0.2, and 0.3 g, respectively. The other parameters were kept at their optimum values determined from the previous experiments such as pH at 6, solution volume of 100 mL, initial metal concentration of 10 ppm, and contact time of 24 h.
Figure 7 shows the removal % achieved by each of the adsorbent. Both the adsorbents showed the lowest removal with lowest adsorbent dose of 0.01 g. However, as the dose is increased, the trendline followed by each adsorbent differs. In the case of SG-OM, we see a significant rise in removal when the dose is increased from 0.05 to 0.1 g with removal of just 49% at 0.05 g and the removal of 98% at 0.1 g. This sudden increase in adsorption is clearly attributed to more binding sites being available with higher doses. Also, the improved morphological features contribute significantly to this behavior. This is followed by the adsorbent attaining the equilibrium when all the sites are filled and the remaining adsorption taking place only inside the material. On the other hand, in the case of SG-TD, we see significantly gradual increase in removal % with increased adsorbent dose. In this, we see a significant jump in removal when the dose is increased from 0.1 to 0.2 g with 62% and 92%, respectively. This is followed by the adsorption reaching equilibrium. Comparatively lower removal than its companion adsorbent can be attributed to its lower surface area, pore volume, and porosity %. Finally, based on these results, it can be stated that SG-OM indicated higher removal % with 98% removal at optimum dose of 0.1 g, whereas SG-TD achieved higher removal of 92% at optimum dose of 0.2 g. Moreover, the enhanced removal at lower optimum dose of SG-OM is clearly attributed to aging with organic solvents such as ethanol and heptane, which resulted in material possessing improved surface area and pore characteristics than its companion adsorbent (SG-TD).
4.4 Isotherm Studies
Isotherms are important in the field of adsorption, as they provide a way to quantify the relationship between the amount of a substance adsorbed on a surface and the pressure or concentration of that substance in the surrounding environment. The Langmuir and Freundlich isotherms are two common models used to describe this relationship. The Langmuir isotherm assumes that adsorption occurs on a homogeneous surface with a limited number of adsorption sites, while the Freundlich isotherm assumes that adsorption occurs on a heterogeneous surface with a varying adsorption energy. Both isotherms have been widely used to describe adsorption behavior in a variety of systems and have been found to accurately describe adsorption in many cases. Understanding the adsorption behavior of a substance is important in many areas such as water treatment, air purification, chemical separation, and surface catalysis (Dhaouadi et al., 2021; Sharifpour et al., 2018).
To investigate the isotherms, the results of both the adsorbents were analyzed using Langmuir and Freundlich isotherms. As can be seen from Figs. 8, 9, 10 and 11, both the adsorbents follow Langmuir isotherms with R2 value of 0.94 for SG-OM and 0.92 for SG-TD, respectively. It can be stated that Langmuir isotherm best fits the adsorption of lead ions onto both the adsorbents. Table 3 shows the Langmuir isotherm constants for both the adsorbents.
5 Conclusion
This study involved the synthesis of two types of silica aerogels. One was modified and aged with organic solvents; ethanol and heptane (SG-OM), while the other one was only doped with TEOS. A comparative study was conducted to differentiate the characteristics, properties, and lead adsorptive behavior by both the adsorbents. The materials were analyzed via SEM, XRD, FTIR, and BET. As per results, the silica aerogel modified with organic solvents (SG-OM) indicated higher surface area, higher pore volume, higher porosity, and highly mesoporous structure than silica aerogel doped with TEOS (SG-TD). Moreover, when the adsorption experiments were conducted, silica aerogel modified with organic solvents (SG-OM) showed significantly higher removal in all sets of experiments (pH, metal concentration, and adsorbent dose). This can clearly be attributed to aging of the gel with ethanol and heptane. Such aging resulted in material achieving enhanced morphological features and lead adsorption than mere TEOS doping. The highest adsorption capacities achieved by SG-OM and SG-TD were 15.2 mg/g and 11.8 mg/g, respectively.
Besides, the present work carries its own limitations in terms of properties of adsorbents, synthesis time, and performance in real wastewater scenario. The variant doped with TEOS (SG-TD) showed lower properties and removal than its companion adsorbent, which requires further research of trying different TEOS ratios to enhance the properties. The enhanced properties are expected to indicate a higher removal. Likewise, although the organically tailored silica aerogel (SG-OM) showed higher removal, adsorption capacity, and enhanced properties, yet the synthesis time is much higher than TEOS-doped silica aerogel (SG-TD). Further research is needed to modify the sol-gel aging process and experiment different ratios of the respective organic solvents to reduce the synthesis time. Finally, there is a need to investigate the efficiency of the respective adsorbents in real wastewater scenario and exploit the optimum parameters for maximum removal.
Change history
12 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11270-024-07337-5
References
Akhter, F., Jamali, A. R., & Khan, W. (2023). TEOS-doped vs non-TEOS silica aerogels: A comparative study of synthesis, characterization, isotherm studies and performance evaluation for Pb (II) removal from synthetic wastewater. Water, Air, and Soil Pollution, 234(1), 32. https://doi.org/10.1007/S11270-022-06051-4/TABLES/3
Akhter, F., Rao, A. A., Abbasi, M. N., Wahocho, S. A., Mallah, M. A., Anees-ur-Rehman, H., & Chandio, Z. A. (2022). A comprehensive review of synthesis, applications and future prospects for silica nanoparticles (SNPs). Silicon, 14(14), 8295–8310. https://doi.org/10.1007/s12633-021-01611-5
Akhter, F., Soomro, S. A., & Inglezakis, V. J. (2021). Silica aerogels; a review of synthesis, applications and fabrication of hybrid composites. Journal of Porous Materials, 28(5), 1387–1400. https://doi.org/10.1007/s10934-021-01091-3
Akhter, F., Soomro, S. A., Jamali, A. R., & Inglezakis, V. J. (2023). Structural, morphological and physiochemical analysis of SiC8H20O4/C2H5O/C7H16 modified mesoporous silica aerogels. Physical Chemistry Research, 11(1), 1–8. https://doi.org/10.22036/PCR.2022.332609.2044
Al-Mothafer, Z. H. A., & Abdulmajeed, I. M. (2021). Comparative study in use sodium silicate instead of nh4oh as an alkaline basic catalyst to gelation unmodified silica aerogel based on tetraethoxysilane (Teos). Journal of Ovonic Research, 17(4), 373–382 https://www.researchgate.net/profile/Inaam-M-Abdulmajeed/publication/354031262_Comparative_study_in_use_sodium_silicate_instead_of_NH_4_OH_as_an_alkaline_basic_catalyst_to_gelation_unmodified_silica_aerogel_based_on_tetraethoxysilane_TEOS/links/611fbd4c16
Chen, Y. X., Sepahvand, S., Gauvin, F., Schollbach, K., Brouwers, H. J. H., & Yu, Q. (2021). One-pot synthesis of monolithic silica-cellulose aerogel applying a sustainable sodium silicate precursor. Construction and Building Materials, 293, 123289. https://doi.org/10.1016/J.CONBUILDMAT.2021.123289
Delacour, M. L., Gailliez, E., Bacquet, M., & Morcellet, M. (1999). Poly(ethylenimine) coated onto silica gels: Adsorption capacity toward lead and mercury. Journal of Applied Polymer Science, 73(6), 899–906. https://doi.org/10.1002/(SICI)1097-4628(19990808)73:6<899::AID-APP6>3.0.CO;2-O
Deniz, S., Tasci, N., Yetimoglu, E. K., & Kahraman, M. V. (2017). New thiamine functionalized silica microparticles as a sorbent for the removal of lead, mercury and cadmium ions from aqueous media. Journal of the Serbian Chemical Society, 82(2), 215–226. https://doi.org/10.2298/JSC160816098D
Dhaouadi, F., Sellaoui, L., Reynel-Ávila, H. E., Landín-Sandoval, V., Mendoza-Castillo, D. I., Jaime-Leal, J. E., Lima, E. C., Bonilla-Petriciolet, A., Lamine, A., & ben. (2021). Adsorption mechanism of Zn2+, Ni2+, Cd2+, and Cu2+ ions by carbon-based adsorbents: Interpretation of the adsorption isotherms via physical modelling. Environmental Science and Pollution Research, 28(24), 30943–30954. https://doi.org/10.1007/s11356-021-12832-x
Doke, S. D., Patel, C. M., & Lad, V. N. (2021). Improving physical properties of silica aerogel using compatible additives. Chemical Papers, 75(1), 215–225. https://doi.org/10.1007/s11696-020-01281-4
Faghihian, H., Nourmoradi, H., & Shokouhi, M. (2012). Performance of silica aerogels modified with amino functional groups in PB(II) and CD(II) removal from aqueous solutions. Polish Journal of Chemical Technology, 14(1), 50–56. https://doi.org/10.2478/v10026-012-0059-4
Falsafi, M. H., Moghaddas, M., & Moghaddas, J. (2020). Removal of heavy metals from synthetic wastewater using silica aerogel-activated carbon composite by adsorption method. Journal of Applied Research in Water and Wastewater, 7(1), 90–96 (Vol. 13, Issue 1). https://arww.razi.ac.ir/article_1471_0.html
Fan, H. T., Sun, X. T., Zhang, Z. G., & Li, W. X. (2014). Selective removal of lead(II) from aqueous solution by an ion-imprinted silica sorbent functionalized with chelating N-donor atoms. Journal of Chemical and Engineering Data, 59(6), 2106–2114. https://doi.org/10.1021/JE500328T
Gurav, J. L., Rao, A. V., Nadargi, D. Y., & Park, H. H. (2010). Ambient pressure dried TEOS-based silica aerogels: Good absorbents of organic liquids. Journal of Materials Science, 45(2), 503–510. https://doi.org/10.1007/S10853-009-3968-8
Ibrahim, A. M., Chiad, B. T., Twej, W. A. A., & Mohammed, R. A. (2019). Effects of TEOs aerogel particles size of TEOs aerogel on its mesoporous structure and thermal behavior via supercritical drying and high temperature. Iraqi Journal of Science, 60(January), 119–128. https://doi.org/10.24996/ijs.2019.60.S.I.18
Joshi, S., & Srivastava, R. K. (2019). Adsorptive removal of lead (Pb), copper (Cu), nickel (Ni) and mercury (Hg) ions from water using chitosan silica gel composite. Environmental Monitoring and Assessment, 191(10), 1–9. https://doi.org/10.1007/s10661-019-7777-5
Kushwaha, A. K., Gupta, N., & Chattopadhyaya, M. C. (2017). Adsorption behavior of lead onto a new class of functionalized silica gel. Arabian Journal of Chemistry, 10, S81–S89. https://doi.org/10.1016/j.arabjc.2012.06.010
Leyva-Ramos, R., Berber-Mendoza, M. S., Salazar-Rabago, J., Guerrero-Coronado, R. M., & Mendoza-Barron, J. (2011). Adsorption of lead(II) from aqueous solution onto several types of activated carbon fibers. Adsorption, 17(3), 515–526. https://doi.org/10.1007/S10450-010-9313-3
Martínez, M. G. A., de Jesús Pérez Bueno, J., Reza, E. M., & López, M. L. M. (2020). Lead adsorption in manganese oxides as powders and coatings supported on silica gel beads and tin inverse opal-like structures. Current Analytical Chemistry, 17(6), 831–838. https://doi.org/10.2174/1573411016666200116095505
Radi, S., el Massaoudi, M., Bacquet, M., Degoutin, S., Adarsh, N. N., Robeyns, K., & Garcia, Y. (2017). A novel environment-friendly hybrid material based on a modified silica gel with a bispyrazole derivative for the removal of ZnII, PbII, CdII and CuII traces from aqueous solutions. Inorganic ChemistryFrontiers, 4(11), 1821–1831. https://doi.org/10.1039/c7qi00322f
Sharifpour, E., Khafri, H. Z., Ghaedi, M., Asfaram, A., & Jannesar, R. (2018). Isotherms and kinetic study of ultrasound-assisted adsorption of malachite green and Pb2+ ions from aqueous samples by copper sulfide nanorods loaded on activated carbon: Experimental design optimization. Ultrasonics Sonochemistry, 40, 373–382. https://doi.org/10.1016/j.ultsonch.2017.07.030
Terzioglu, P., Temel, T. M., İkizler, B. K., & Yucel, S. (2018). Preparation of nanoporous silica aerogel from wheat husk ash by ambient pressure drying process for the adsorptive removal of lead from aqueous solution. Journal of Bioprocessing & Biotechniques, 8(01). https://doi.org/10.4172/2155-9821.1000315
Wang, C., Kim, J., Tang, J., Na, J., Kang, Y., Kim, M., Lim, H., Bando, Y., Li, J., & Yamauchi, Y. (2020). Large-scale synthesis of MOF-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents. Angewandte Chemie, 132(5), 2082–2086. https://doi.org/10.1002/ANGE.201913719
White, L. S., Bertino, M. F., Kitchen, G., Young, J., Newton, C., Al-Soubaihi, R., Saeed, S., & Saoud, K. (2015). Shortened aerogel fabrication times using an ethanol-water azeotrope as a gelation and drying solvent. Journal of Materials Chemistry A, 3(2), 762–772. https://doi.org/10.1039/C4TA04633A
Data and Code Availability
The data in this manuscript is available with the corresponding author and can be provided on reasonable request.
Funding
This work was supported by Researchers Supporting Project number (RSP2023R100), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Faheem Akhter, Heera Lal Soni, Sobhy M. Ibrahim: abstract, introduction, and materials and methods; Faheem Akhter Fernanda Miranda, Sobhy M. Ibrahim: results and discussion and conclusion
Corresponding author
Ethics declarations
Ethics Approval
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akhter, F., Soni, H.L., Jamali, A.R. et al. Efficient Adsorption of Lead(II) Ions from Artificial Wastewater by Organically Tailored vs TEOS-Doped Silica Aerogels: Synthesis, Characterization and Isotherm Studies. Water Air Soil Pollut 234, 180 (2023). https://doi.org/10.1007/s11270-023-06204-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06204-z