Abstract
In this study, the effect of the addition of natural (AT) and purified attapulgite (PAT) on the migration and transformation of phosphorus (P) in mariculture ponds sediments under static and hydrodynamic disturbance conditions was studied using sediment core incubation experiments. Whether under static or disturbance condition, the AT/PAT amendment suppressed the release of dissoluble reactive phosphorus (SRP) from sediments into the overlying seawater. The results showed that the addition of AT/PAT decreased the concentration of SRP in overlying seawater (maximum reduction rate: Rmax = 76.82%), the concentration of SRP in pore seawater (Rmax = 62.62%) and the diffusion flux of SRP at the sediment-water interface (SWI) (Rmax = 34.22%). Furthermore, adding AT/PAT to the top sediments (0–1 cm) not only reduces the content of Mobile-P in the added layer, but also reduces Mobile-P in the sediments (1–2 cm) below the added layer. In addition, under the condition of disturbance, the reduction rate of Mobile-P in the PAT added layer (42.16%) was higher than that of AT (21.12%). The reduction of Mobile-P in the top sediment, SRP in the pore seawater as well as the diffusion flux of SRP from the pore seawater to the overlying seawater across the SWI played a key role in the control of SRP release from sediments to the overlying seawater by the AT/PAT amendment. Results of this work indicate that the AT and PAT are very promising amendment for the control of SRP release from mariculture ponds sediments under static and hydrodynamic disturbance conditions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Human activities have produced a series of negative effects on marine ecosystems, among which the increasing problem of eutrophication in coastal waters is prominent particularly (Wang et al., 2021). Phosphorus is the key nutrient for plankton growth, and its high concentration level is one of the main factors causing eutrophication in coastal waters (Wu et al., 2017). Mariculture wastewater from inshore pond aquaculture, as an important point-source discharge, plays a crucial role in the transport of nutrients from surface runoff (Lang et al., 2020). Therefore, controlling the phosphorus concentration in the overlying seawater of the mariculture pond is helpful to control the occurrence of seawater eutrophication. When exogenous phosphorus is effectively controlled, controlling the release of endogenous phosphorus in sediments is an important means to reduce the phosphorus concentration in the overlying seawater of mariculture ponds. So far, researchers at home and abroad have developed a variety of techniques to control the release of endogenous phosphorus from sediments, including sediment dredging (Cheng et al., 2016), capping (Yin et al., 2020), and amending (Yin et al., 2016). Among them, the sediment amending technology, that is, the solid passivator is directly added to the sediment, can not only enhance the adsorption capacity of the sediment to the phosphorus in the overlying water, but also reduce the release risk of the phosphorus in the sediment to the overlying water, which has been widely studied in recent years (Fan et al., 2017; Yin et al., 2017; Wu et al., 2022). At present, researchers have carried out some beneficial exploration on lanthanum-modified bentonite clay (Spears et al., 2013), iron/aluminum co-modified calcite (Lei et al., 2022), heat-treated attapulgite (Gan et al., 2009), drinking water treatment residue (Wang et al., 2022), and other solid phosphorus deactivators.
Attapulgite is widely used to control the release of endogenous phosphorus in sediments as an in-situ phosphorus inactivation material, because it is characterized by high viscosity and surface area, porous structure, moderate layer charge and a large number of silanol groups on its surface (Gan et al., 2009). The adsorption capacity of natural attapulgite is relatively low (Kim et al., 2018; Fan et al., 2021). Through the purification and refinement of attapulgite, we have preliminarily investigated the feasibility of the purifying attapulgite to adsorb phosphorus in eutrophic seawater, and the effect of adding attapulgite on phosphorus adsorption characteristics, occurrence forms and release characteristics of phosphorus in sediments (Xu et al., 2022a; Xu et al., 2022b). However, previous studies have found that the hydrodynamic disturbance caused by wind wave disturbance and water flow can make sediment resuspending, and then have a significant impact on the phosphorus migration and transformation process in the sediment (Wu & Hua, 2014; Kang et al., 2022). Therefore, revealing the influence of attapulgite addition on phosphorus migration and transformation in sediments at different depths under static and hydrodynamic disturbance conditions is helpful to determine the control effect of attapulgite addition on phosphorus release from sediments of mariculture ponds, which is very important for applying attapulgite amending technology to control phosphorus release from sediments of mariculture ponds.
Therefore, in this paper, the sediment of mariculture pond is taken as the research object, and the hydrodynamic disturbance process of mariculture pond is simulated by stirring. The effects of natural and purified attapulgite addition on SRP in overlying seawater and pore seawater at different depths, SRP diffusion flux at the SWI and phosphorus occurrence forms in sediments at different depths were investigated under static and hydrodynamic disturbance conditions. Finally, the effects of attapulgite addition on phosphorus migration and transformation in the sediments of mariculture ponds under static and hydrodynamic conditions were revealed, so as to provide theoretical and practical basis for the application of attapulgite as a passivator to control the release of endogenous phosphorus in mariculture ponds.
2 Materials and Methods
2.1 Experimental Materials and Reagents
The natural attapulgite clay used in this experiment came from a deposit in Xuyi County. It was dried at 105 °C for 2 h and mechanically pulverized to Φ<0.150 mm. In addition to attapulgite, the raw ore is mainly composed of quartz, albite, calcite, and muscovite and other trace components. Raw sediment (RS) cores were collected from the Litopenaeus vannamei aquaculture pond on the East China Sea coast of Shanghai using a Perspex core sampler (50 cm long, 8.8 cm in diameter). The sediment is passed through 50-mesh and 100-mesh sieves in turn (Yu et al., 2019), and the impurities are removed by sieving and fully mixed, and then stored at low temperature for future use. The KH2PO4, HCl, NaOH, NaCl, NH4Cl, and other chemical reagents used in the experiment are all produced by China National Pharmaceutical Group Chemical Reagent Co., Ltd. and are analytical pure.
2.2 Preparation of Purified Attapulgite
Because the raw ore contains calcite and other carbonate minerals and some colored impurities (e.g., Fe and Ti), it is difficult to remove by using the traditional method of gravity separation and purification with dispersant. Therefore, referring to the research method of Jin et al. (Jin et al., 2005; Tang et al., 2003; Zuo et al., 2005), the natural attapulgite clay was purified and the product with purity of 99.93% was obtained. Briefly, the first-step purified attapulgite (PAT-1st) was prepared by ultrasonic hydrothermal method, and then used 4% hydrochloric acid to remove the acid soluble matter in PAT-1st and activate attapulgite to obtain the second-step purified attapulgite (PAT-2nd). Finally, a reducing agent (sodium hydrosulfite) was added to PAT-2nd for complexation-reduction iron removal and bleaching, and the third-step purified attapulgite (PAT-3rd) can be obtained.
2.3 Long-term Sediment Phosphorus Control Core Incubation
In order to study the long-term control efficiency of phosphorus in sediment by adding AT/PAT under static and hydrodynamic disturbance conditions, the indoor columnar sediment culture experiment was carried out in a circular plexiglass container (d=8 cm, h=40 cm). The overlying seawater is prepared manually, with salinity of 35 psu, pH = 8.0–8.2 and temperature of 25±2 °C. The experiment is divided into two stages. The first stage is the experimental preparation stage. Six circular plexiglass containers were set up, each two containers in a group, and the experiments were conducted under static and disturbed conditions respectively. The containers that only add RS were marked as the control group, and the containers that both add RS and AT or PAT were marked as the addition group (Fig. 1). The two containers of the control group were added with 5 cm high RS respectively, while the four containers of the addition group were added with 4 cm high RS first, and then 1 cm high AT or PAT. Then, slowly add 1500 mL of configured overlying seawater to the reactor to avoid sediment suspension during water addition. After the sediment column is ready, it will enter the second stage, namely the sediment culture stage. In order to resuspend the sediment, a constant speed mixer was used to intermittently disturb the overlying seawater of one reactor in the control group and in the addition group, and the whole experimental process was carried out in an aerobic state (cycle 30 days). The propeller of the agitator is placed 0.5 cm below the water surface. The stirring speed is 120 rpm, stirring for 2 hours each time, and standing for 70 hours after stirring (Yu et al., 2019), and then the sampling test is carried out. A total of 10 times were stirred in this experiment. The dissolved oxygen (DO) and pH of the overlying seawater (10 cm above SWI) in the reactor were measured at regular intervals (72 h), and 50 mL of overlying seawater 10 cm above the SWI was collected for the determination of SRP, and then 50 mL of overlying seawater was slowly added along the pipe wall (Yu et al., 2019). The sediment was divided into 5 layers with a thickness of 1 cm after the culture experiment, and the layers were taken out and centrifuged in sequence. The resulting pore seawater was used for SRP analysis, and sediment samples were used for chemical extraction of P fractions.
2.4 Chemical Extraction of P Fractions
In this study, phosphorus fractions in raw and AT/PAT-amended sediments were extracted according to the method proposed by Rydin and Welch (Rydin & Welch, 1998). One gram of sample was put into a 50-mL centrifuge tube. Extract it successively with NH4Cl solution (1 mol/L), NaHCO3/Na2S2O4 solution (0.11 mol/L), NaOH solution (1 mol/L, normal temperature), HCl solution (0.5 mol/L), and H2SO4 solution (1 mol/L, 358 K). The volume of the extract is 25 mL, and the specific steps are shown in Table 1. After each step of extraction, the centrifuge was at 4500 rpm for 15 min, and the solution was immediately filtered through a 0.45-μm microporous membrane, and the phosphorus content was determined by molybdenum blue colorimetry. Mobile-P is the sum of Labile-P, BD-P and Org-P, and Total-P is the sum of phosphorus measured by all extraction fractions.
2.5 Data Processing and Material Characterization
The collected overlying seawater and pore seawater were filtered by 0a .45-μm microporous membrane, and the SRP content in the filtrate was determined by molybdenum blue colorimetry. The average rate of phosphorus release from sediment to overlying seawater can be calculated according to the change of phosphorus concentration in overlying seawater during sediment culture experiment [J, mg·(m2·day)−1] (Zilius et al., 2015):
where V is the volume of overlying seawater (L). t is the time of sediment culture experiment (d). Ct and C0 are the mass concentration of phosphorus (mg·L−1) in the overlying seawater at time t and initial time, respectively. A is the area of the interface between sediment and overlying seawater (m2).
Using Fick’s first diffusion law and according to the mass concentration of phosphorus in overlying seawater and pore seawater, calculate the phosphorus diffusion flux at the interface between sediment and water [F, mg·(m2·day)−1] (Yu et al., 2017 (Yu et al., 2017)):
where φ is the porosity of sediment, and the value in this experiment was 0.8. Ds is the phosphorus diffusion coefficient of sediment (cm2·s−1), which can be calculated and determined according to the following formula. \(\frac{\partial \text{c}}{\partial Z}\left|{}_{Z=0}\right.\) is the mass concentration gradient of phosphorus at the sediment water interface [mg·(L·cm) −1]. This experiment is obtained by dividing the mass concentration difference between sediment pore seawater and overlying seawater by 1 cm.
where D0 is the diffusion coefficient of ions in the ideal dilution solution (cm2·s−1), and D0 is taken as 7.0×10-6 cm2·s−1 in this study.
For all sampling steps, field blanks for the analysis of the water properties were prepared using ultra-pure water and treated as the original water samples. In the laboratory, all samples were measured in parallel for 3 times. Excel 2007 software was used for data sorting, Origin software for mapping, SPSS 17.0 software for statistical analysis, and one-way ANOVA was used to compare the differences between the control group and the treatment group.
The pH and DO were measured by a dual-probe multiparameter meter (HQ40d, HACH, USA). The morphology and composition of materials were characterized by SEM, XRF, XRD, etc. The ASAP2020 specific surface area analyzer was used to analyze the N2 adsorption and desorption, pore size distribution and specific surface area of the material. The particle size of attapulgite was analyzed by using the Masterizer 2000 laser particle size detector. The cation exchange capacity (CEC) of attapulgite was determined by ammonium acetate method.
3 Results and Discussion
3.1 Physical and Chemical Properties of Natural and Purified Attapulgite
The mineral and chemical composition (determined by XRF and XRD) of AT and PAT are shown in Table 2 and Fig. 2. The physico-chemical properties such as specific surface area, pore volume, cation exchange capacity (CEC), and particle size (d90) are shown in Table 3. After the natural attapulgite was purified by steps, a high-grade product with attapulgite purity of 99.93%, ultra refine and easy to be re-dispersed in water was obtained. The specific surface area and pore volume of natural attapulgite were small, 152.076 m2/g and 0.212 cm3/g, respectively. After purification, the specific surface area and pore volume were increased to 203.254 m2/g and 0.395 cm3/g, respectively, which were significantly improved (P<0.05) (Table 3). The octahedral structure in attapulgite was not only not damaged, but also weakens the bonding force between the structural layers (Fig. 2). The pores in the structure were loosely expanded, the pore volume was greatly increased, and the surface activity was enhanced. Thus, the adsorption performance of attapulgite was improved, which provided the possibility for subsequent adsorption of phosphorus by attapulgite.
3.2 Changes in DO Concentration and pH Value in Overlying Seawater
The changes of DO concentration and pH value in the overlying seawater of the reactor during sediment culture are shown in Fig. 3. DO concentrations are greater than 4.0 mg/L, which indicates that the overlying seawater in various reactors in this study is in an aerobic state. When the sediment culture time was short (1–10 days), there was little difference (P>0.05) in the concentration of DO in the overlying seawater of the control group or the addition group under the two conditions of static and disturbance. When the sediment was cultured for a long time (15–30 days), the DO concentration of overlying seawater in a disturbed condition was significantly higher than that in a static condition. It can be seen that when the sediment culture time is long, the hydrodynamic disturbance tends to increase the DO concentration of overlying seawater. During sediment culture, the pH value of overlying seawater in various reactors had little difference and was in the range of slightly alkaline.
3.3 Changes in SRP Concentration in Overlying Seawater
As shown in Fig.4 a, the sediments used in this study released SRP into the overlying seawater under both static and hydrodynamic disturbance conditions. Moreover, hydrodynamic disturbance and the addition of AT/PAT significantly changed the exchange behavior of SRP between sediment and overlying seawater (P<0.05). For the control group at static condition, the SRP concentration in the overlying seawater increased significantly from 0 to 0.157 mg/L during sediment culture. For the disturbed control group, the SRP concentration in the overlying seawater first increased and then decreased and reached equilibrium. When the culture time was 15 days, the SRP concentration reached the maximum (0.104 mg/L). In addition, when the sediment culture time was in the early stage (< 15 days), the SRP concentration in the overlying seawater in the disturbed condition was higher than that in the static condition. This is because when the sediment is disturbed and resuspended, a large amount of SRP is released into the pore seawater, resulting in the rapid increase of SRP concentration in the overlying seawater. When the sediment culture time was long (15–30 days), the SRP concentration in the overlying seawater in the disturbed condition was significantly lower than that in the static condition. It shows that multiple intermittent disturbances can inhibit the migration of phosphorus form sediments to the overlying seawater. This is because with the extension of culture time, disturbance accelerates the reoxygenation process and increases the DO concentration of the seawater (Fig. 3). Originally, the reduced iron manganese oxide in the suspended solids oxidized and transformed into oxidized iron manganese oxide with strong phosphorus adsorption capacity, which enhanced the adsorption of suspended solids on SRP in the overlying seawater and settled into the sediment with the action of gravity (Zheng et al., 2013). In this study, after multiple gap disturbances, the amount of SRP in the overlying seawater adsorbed by the suspended solids are obviously greater than the amount of SRP released from the pore seawater, resulting in a significant decrease in the concentration of SRP in the overlying seawater after the disturbance.
a Changes in SRP concentration of overlying seawater in control and amendment columns. b Average release rates of SRP from sediments to the overlying seawater in control and amendment columns. Different lowercase letters represent significant differences between different culture conditions at the same time (P<0.05)
As can also be seen from Fig. 4 a, in the static condition, the SRP concentrations in the overlying seawater of the AT and PAT addition groups were 0.038–0.058 mg/L and 0.036–0.048 mg/L respectively, which were significantly lower than that of the control group (0.048–0.157 mg/L), and the reduction rates were 3.26–70.46% and 20.24–76.82% respectively. This shows that the addition of AT/PAT can greatly reduce the release of phosphorus from sediment to overlying seawater. In addition, in the hydrodynamic disturbance condition, the SRP concentrations in the overlying seawater of AT and PAT addition groups were 0.044–0.062 mg/L and 0.038–0.052 mg/L respectively, which were also significantly lower than 0.075–0.104 mg/L in the control group, and the reduction rates were 27.02–49.21% and 40.26–57.81% respectively. This shows that the addition of AT/PAT in the hydrodynamic disturbance condition can also significantly reduce the migration of phosphorus from sediments to overlying seawater. Furthermore, for the addition group, the SRP concentration in the overlying seawater in the disturbed condition was higher than that in the static condition. This shows that the disturbance reduces the efficiency of AT/PAT addition to controlling the release of sediment phosphorus to the overlying seawater. It should be noted that although the disturbance has a certain negative impact on the effect of AT/PAT addition on controlling phosphorus release in sediments, AT/PAT addition can still keep the SRP concentration in overlying seawater at a very low level under the disturbance condition. Moreover, the addition of AT and PAT can significantly reduce the release rate of phosphorus from sediment to overlying seawater under both static and hydrodynamic disturbance conditions (Fig. 4b).
3.4 Changes in SRP Concentration in Pore Seawater
In the control group, the sediments at different depths release a certain amount of phosphorus into the pore seawater, and the SRP concentration in the pore seawater increases with the increase in depth (Fig. 5). The penetration depth of O2 in sediments is usually less than 1 cm (Liu et al., 2018). In the simulation experiment, most sediments are in an anoxic state. Ferric oxide or hydroxide is reduced to ferrous oxide or hydroxide, while phosphorus adsorbed by ferric oxide or hydroxide is easily released into pore seawater (Meis et al., 2013). The deeper the sediment, the lower the redox potential is expected, which may cause more phosphorus to be released into the pore seawater through the dissolution of ferric oxide or hydroxide, resulting in the increase of SRP concentration in the pore seawater with the increase in depth. Actually, the process of phosphorus release from sediment to overlying seawater includes phosphorus release from sediment to pore seawater and phosphorus diffusion from pore seawater to overlying seawater. The distribution of phosphorus was controlled by transport and reaction processes and the phosphorus diffusion should be one of the major reasons for the above results. In addition, under hydrodynamic disturbance, the contact opportunity between AT/PAT and SRP in pore seawater is increased, and the adsorption of SRP in pore seawater by AT/PAT is promoted. At the same time, the disturbance leads to the increase of O2 penetration depth and redox potential in the sediment, which transforms Fe2+ to Fe3+, and then forms iron phosphorus compounds, which reduces the concentration of SRP in the pore seawater of surface sediment (0–2 cm).
Furthermore, the addition of AT/PAT reduced the concentration of SRP in pore seawater in the depth range of 0–4 cm under static and hydrodynamic disturbance conditions (Fig. 5). Under the static condition, at the depth of 0–1, 1–2, 2–3, and 3–4 cm, the SRP concentration in pore seawater of the control group (Static, RS) were 0.466, 0.593, 0.898, and 1.398 mg/L, respectively. The SRP concentration in pore seawater of the AT group decreased by 50.06%, 35.12%, 45.27%, and 62.62% respectively, and that of the PAT group decreased by 57.99%, 49.88%, 50.47%, and 60.53% respectively. Under the disturbance condition, at the depth of 0–1, 1–2, 2–3, and 3–4 cm, the SRP concentration in pore seawater of the control group (disturbance, RS) were 0.340, 0.389, 0.941, and 1.460 mg/L, respectively. The SRP concentration in pore seawater of the AT group decreased by 34.34%, 2.50%, 46.31%, and 13.45% respectively, and that of the PAT group decreased by 45.79%, 26.54%, 50.44%, and 59.94% respectively. This means that the addition of AT/PAT can effectively inhibit the release of phosphorus from sediments to pore seawater, whether at static or under disturbance.
3.5 The Effects of Attapulgite Addition on SRP Diffusion Flux at the Sediment-Water Interface
Under static and hydrodynamic disturbance conditions, the SRP diffusion fluxes at the SWI in the control group were 1.470 mg/(m2·day) and 1.423 mg/(m2·day), while those in the AT addition group were 1.073 mg/(m2·day) and 1.297 mg/(m2·day), and those in the PAT addition group were 1.051 mg/(m2·day) and 0.936 mg/(m2·day), respectively. Obviously, the addition of AT/PAT reduced the diffusion flux of phosphorus at the SWI. Considering that the consistent influence trends of AT/PAT addition on SRP concentration in overlying seawater, SRP concentration in pore seawater and phosphorus diffusion flux at the SWI. The reduction of SRP concentration in pore seawater and phosphorus diffusion flux at the SWI play a very important role in controlling the release of sediment phosphorus to overlying seawater by AT/PAT improved technology (Yu et al., 2019). It is worth noting that although the hydrodynamic disturbance reduces the SRP diffusion flux at the SWI in the addition group, the disturbance slightly increases the SRP concentration in the overlying seawater of the addition group and the rate of SRP release from sediments to the overlying seawater (Fig. 4b). This may because of the resuspension of sediments caused by the disturbance and the release of some weakly adsorbed phosphorus in suspended solids and SRP in pore seawater, resulting in the increase of SRP concentration in overlying seawater to a certain extent.
3.6 The Effects of Attapulgite Addition on Phosphorus Speciation in Sediment
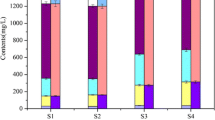
As shown in Fig. 6 a, after long-term incubation experiment, the content of Labile-P in sediment was very small and can be ignored. Compared with the control group, when AT/PAT was added to the surface 0–1cm sediment in the static and disturbance conditions, the content of BD-P in the surface 0–1cm sediment of the AT addition group decreased by −20.19% and 25.12%, respectively, and the content of BD-P in the 0–1cm sediment of the PAT addition group decreased by 16.84% and 60.29% respectively. Except for the static group (AT), other experimental groups, whether disturbed or not, led to the decrease of BD-P content in the added layer. This may be because after the combination of Fe and P in AT, a large part of them exist as BD-P. In addition, the content of Org-P in 0–1cm surface sediments of the addition group also decreased to a certain extent. On the contrary, under static and disturbance conditions, compared with the control group, the content of NaOH-rP in 0–1cm surface sediments of the AT addition group increased by 1.16% and 2.46%, respectively, and the content of NaOH-rP in 0-1 cm surface sediments of the PAT addition group increased by 4.63% and 7.99% respectively. At the same time, the contents of HCl-P and Res-P in the addition layer also increased to a certain extent. For the AT addition group, the increase rates in the static condition were 1.42% and 8.81%, respectively, those in the disturbance condition were 0% and 4.41% respectively. For the PAT addition group, the increase rates in the static condition were 1.42% and 4.41% respectively, and those in the disturbance condition were 8.48% and 17.61% respectively. Moreover, adding AT/PAT to the 0-1 cm sediment on the surface layer will also increase the contents of NaOH-rP and Res-P in the 1–2cm sediment below the addition layer to a certain extent.
To sum up, the addition of AT/PAT to 0–1cm surface sediment results in the decrease of Mobile-P (Labile-P, BD-P, and Org-P) content and the increase of NaOH-rP, HCl-P, and Res-P contents in the added layer. At the same time, it also changes the occurrence form of phosphorus in the sediment 1–2cm below the addition layer, and transforms into a more stable form of phosphorus. This is further proved in the proportion of different forms of phosphorus in the total extractable phosphorus (Fig. 6b). For the Mobile-P content in the AT addition layer, it is reduced by 18.39% and 21.12% under static and hydrodynamic disturbance conditions, respectively. For the Mobile-P content in the PAT addition layer, it is reduced by 12.71% and 42.16% under static and hydrodynamic disturbance conditions, respectively. This means that the addition of AT/PAT lead to the conversion of Mobile-P to stable phosphorus (NaOH-rP, HCl-P, and Res-P) in the sediment of the addition layer, whether at static or hydrodynamic disturbance conditions. At the same time, the downward migration of AT/PAT in the addition layer also promotes the same transformation of phosphorus forms in the sediments 1–2cm below the addition layer to a certain extent. This further confirms that the addition of AT/PAT to 0–1cm surface sediments reduces the potential mobility of phosphorus in the added layer and the sediments immediately adjacent to the added layer. Obviously, this is conducive to the release control of phosphorus in sediments by AT/PAT improved technology. In deep sediments (2–5 cm), the proportion of different forms of phosphorus in the total extractable phosphorus has almost no changes, which means that adding AT/PAT to 0–1cm surface sediments can only regulate the distribution of phosphorus forms in the added layer and the sediments adjacent to the added layer. It has little effect on the distribution of phosphorus forms in deep sediments, which may be due to the limited ability of AT/PAT to migrate downward in the addition layer (Yin et al., 2016; Rydin et al., 2017).
4 Conclusion
Under static and hydrodynamic disturbance conditions, the addition of AT/PAT reduces the SRP concentration in the overlying seawater and pore seawater at different depths, and the diffusion flux of SRP at the SWI. The addition of AT/PAT to the top sediments (0–1 cm) promotes the great transformation of Labile-P, BD-P, and Org-P to NaOH-rP, HCl-P, and Res-P in the added layer, which not only reduces the Mobile-P in the added layer, but also in the sediments (1–2 cm) below the added layer. Compared with the static condition, hydrodynamic disturbance can enhance the passivation effect of AT/PAT on SRP in the pore seawater of top sediments and the reduction effect of SRP diffusion flux at the SWI. The reduction rate of Mobile-P in the PAT added layer (42.16%) is higher than that of AT (21.12%), indicating that the purified attapulgite has a better practical application prospect. The reduction of Mobile-P in the top sediment, SRP in the pore seawater as well as the diffusion flux of SRP from the pore seawater to the overlying seawater across the SWI play a key role in the control of SRP release from sediments to the overlying seawater by the AT/PAT amendment.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Zilius, M., Giordani, G., Petkuviene, J., Lubiene, I., Ruginis, T., & Bartoli, M. (2015). Phosphorus mobility under short-term anoxic conditions in two shallow eutrophic coastal systems (Curonian and Sacca di Goro lagoons). Estuarine, coastal and shelf Science, 164, 134–146. https://doi.org/10.1016/j.ecss.2015.07.004
Fan, T., Wang, M., Wang, X., Chen, Y., Wang, S., Zhan, H., Chen, X., Lu, A., & Zha, S. (2021). Experimental study of the adsorption of nitrogen and phosphorus by natural clay minerals. Adsorption Science & Technology, 2021, 4158151. https://doi.org/10.1155/2021/4158151
Fan, Y., Li, Y. W., Wu, D. Y., Li, C. J., & Kong, H. N. (2017). Application of zeolite/hydrous zirconia composite as a novel sediment capping material to immobilize phosphorus. Water Research, 123, 1–11. https://doi.org/10.1016/j.watres.2017.06.031
Gan, F., Zhou, J., Wang, H., Du, C., & Chen, X. (2009). Removal of phosphate from aqueous solution by thermally treated natural palygorskite. Water Research, 43(11), 2907–2915. https://doi.org/10.1016/j.watres.2009.03.051
Jin, Y. L., Chen, J., Qian, Y. H., Yao, H. Q., & Wu, L. L. (2005). Choice of pure dispersant for preparing ultrafine and pure attapulgite. China Mining Magazine, 01, 80–82 in Chinese.
Kang, T. W., Yang, H. J., Han, J. H., Han, Y. U., Kim, M. S., Kim, J., Hwang, S. H., Na, E. H., & Chang, Y. S. (2022). Identifying pollution sources of sediment in Lake Jangseong, Republic of Korea, through an extensive survey: Internal disturbances of past aquaculture sedimentation. Environmental Pollution, 306, 119403. https://doi.org/10.1016/j.envpol.2022.119403
Kim, M. J., Lee, J. H., Lee, C. G., & Park, S. J. (2018). Thermal treatment of attapulgite for phosphate removal: a cheap and natural adsorbent with high adsorption capacity. Desalination and Water Treatment, 114, 175–184. https://doi.org/10.5004/dwt.2018.22409
Lang, Z. C., Song, X. R., Song, G., Han, L. J., Zhang, Q. Z., & Zhou, M. H. (2020). Comprehensive treatment of marine aquaculture wastewater by a cost-effective flow-through electro-oxidation process. Science of The Total Environment, 722, 137812. https://doi.org/10.1016/j.jece.2022.107262
Lei, J. J., Lin, J. W., Zhan, Y. H., Wen, X., & Li, Y. Q. (2022). Effect of sediment burial depth on the control of sedimentary phosphorus release by iron/aluminum co-modified calcite and strategy for overcoming the negative effect of sediment burial. Science of The Total Environment, 838(3), 156467. https://doi.org/10.1016/j.scitotenv.2022.156467
Liu, Q., Ding, S. M., Chen, X., Sun, Q., Chen, M. S., & Zhang, C. S. (2018). Effects of temperature on phosphorus mobilization in sediments in microcosm experiment and in the field. Applied Geochemistry, 88, 158–166. https://doi.org/10.1016/j.apgeochem.2017.07.018
Meis, S., Spears, B. M., Maberly, S. C., & Perkins, R. G. (2013). Assessing the mode of action of Phoslock® in the control of phosphorus release from the bed sediments in a shallow lake (Loch Flemington, UK). Water Research, 47(13), 4460–4473. https://doi.org/10.1016/j.watres.2013.05.017
Rydin, E., Kumblad, L., Wulff, F., & Larsson, P. (2017). Remediation of a Eutrophic Bay in the Baltic Sea. Environmental Science & Technology, 51(8), 4559–4566. https://doi.org/10.1021/acs.est.6b06187
Rydin, E., & Welch, E. B. (1998). Aluminum dose required to inactivate phosphate in lake sediments. Water Research, 32(10), 2969–2976. https://doi.org/10.1016/S0043-1354(98)00055-4
B.M. Spears, M. Lürling, S. Yasseri, A. T. C.C.M. Gibbs, S. Meis, C. McDonald, J. McIntosh, D. Sleep, F.V. Oosterhout, Lake responses following lanthanum - modified bentonite clay (Phoslock®) application: An analysis of water column lanthanum data from 16 case study lakes, Water Research 47(15) (2013) 5930-5942. 10.1016/j.watres.2013.07.016.
Tang, Q. G., Shen, S. Y., Zhou, X. L., & Jiang, Y. (2003). Study on purification and properties of banqiao palygorskite. Non-Metallic Mines, 05, 38–40 in Chinese.
Cheng, L., Shao, S., Shen, Q., Fan, C., Zhang, L., & Zhou, Q. (2016). Effects of riverine suspended particulate matter on the post-dredging increase in internal phosphorus loading across the sediment-water interface. Environmental Pollution, 211, 165–172. https://doi.org/10.1016/j.envpol.2015.12.045
Wu, Z., Liu, Y., Liang, Z. Y., Wu, S. F., & Guo, H. C. (2017). Internal cycling, not external loading, decides the nutrient limitation in eutrophic lake: A dynamic model with temporal Bayesian hierarchical inference. Water Research, 116, 231–240. https://doi.org/10.1016/j.watres.2017.03.039
Wang, Y., Liu, D., Xiao, W., Zhou, P., Tian, C., Zhang, C., Du, J., Guo, H., & Wang, B. (2021). Coastal eutrophication in China: Trend, sources, and ecological effects. Harmful Algae, 107, 102058. https://doi.org/10.1016/j.hal.2021.102058
Wang, C. H., Hao, Z., Huang, C. H., Wang, Q. H., Yan, Z. S., Bai, L. L., Jiang, H. L., & Li, D. D. (2022). Drinking water treatment residue recycled to synchronously control the pollution of polycyclic aromatic hydrocarbons and phosphorus in sediment from aquatic ecosystems. Journal of Hazardous Materials, 431, 128533. https://doi.org/10.1016/j.jhazmat.2022.128533
Wu, D., & Hua, Z. L. (2014). The effect of vegetation on sediment resuspension and phosphorus release under hydrodynamic disturbance in shallow lakes. Ecological Engineering, 69, 55–62. https://doi.org/10.1016/j.ecoleng.2014.03.059
Wu, Y. H., Song, L., Shi, M. F., Gu, C. H., Zhang, J. X., Lv, J. Y., & Xuan, L. L. (2022). Ca/Fe-layered double hydroxide–zeolite composites for the control of phosphorus pollution in sediments: Performance, mechanisms, and microbial community response. Chemical Engineering Journal, 450(3), 138277. https://doi.org/10.1016/j.cej.2022.138277
Xu, C., Feng, Y., Li, H., Yang, Y., & Wu, R. (2022a). Adsorption and immobilization of phosphorus from eutrophic seawater and sediment using attapulgite - Behavior and mechanism. Chemosphere, 313, 137390. https://doi.org/10.1016/j.chemosphere.2022.137390
Xu, C., Feng, Y., Li, H., Yang, Y., & Wu, R. (2022b). Research progress of phosphorus adsorption by attapulgite and its prospect as a filler of constructed wetlands to enhance phosphorus removal from mariculture wastewater. Journal of Environmental Chemical Engineering, 10(6), 108748. https://doi.org/10.1016/j.jece.2022.108748
Yin, H., Du, Y., Kong, M., & Liu, C. (2017). Interactions of riverine suspended particulate matter with phosphorus inactivation agents across sediment-water interface and the implications for eutrophic lake restoration. Chemical Engineering Journal, 327, 150–161. https://doi.org/10.1016/j.cej.2017.06.099
Yin, H., Han, M., & Tang, W. (2016). Phosphorus sorption and supply from eutrophic lake sediment amended with thermally-treated calcium-rich attapulgite and a safety evaluation. Chemical Engineering Journal, 285, 671–678. https://doi.org/10.1016/j.cej.2015.10.038
Yin, H., Yang, P., Kong, M., & Li, W. (2020). Use of lanthanum/aluminum co-modified granulated attapulgite clay as a novel phosphorus (P) sorbent to immobilize P and stabilize surface sediment in shallow eutrophic lakes. Chemical Engineering Journal, 385, 123395. https://doi.org/10.1016/j.cej.2019.123395
Yu, J. H., Ding, S. M., Zhong, J. C., Fan, C. X., Chen, Q. W., Yin, H. B., Zhang, L., & Zhang, Y. L. (2017). Evaluation of simulated dredging to control internal phosphorus release from sediments: Focused on phosphorus transfer and resupply across the sediment-water interface. Science of The Total Environment, 592, 662–673. https://doi.org/10.1016/j.scitotenv.2017.02.219
Yu, Y., Lin, J. W., Zhan, Y. H., He, S. Q., Wu, X. L., Wang, Y., Zhao, Y. Y., Lin, Y., & Liu, P. Q. (2019). Effect of zirconium-modified zeolite addition on migration and transformation of phosphorus in river sediments under static and hydrodynamic disturbance conditions. Environmental Science, 40(03), 1337–1346 in Chinese.
Zheng, X. L., Zhang, J. J., & Chen, L. (2013). Advances in the study of migration and transformation mechanisms of endogenous phosphorus via sediment resuspension. Advances in Water Science, 24(2), 287–295 in Chinese.
Zuo, X. Z., Zhu, Z. H., & Zheng, M. S. (2005). Technical discussion on bleaching and iron-removing of attapulgite clay. Non-Metallic Mines, 06, 50–52 in Chinese.
Funding
This research was supported by the Major Science and Technology Program for Water Pollution Control and Treatment under Grant No. 2015ZX07205-003, China Ocean Mineral Resources R&D Association under Grant No.DY135-B2-15.
Author information
Authors and Affiliations
Contributions
Chenglong Xu: investigation, data curation, writing - original draft, writing - review & editing. Yali Feng: conceptualization, supervision, funding acquisition. Haoran Li: methodology, supervision, funding acquisition. Yi Yang: software. Shiwei Jiang: validation. Ruifeng Wu: validation.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Purified attapulgite was used to control P release from sediment of mariculture ponds.
• The effects of static and disturbance on P transport and transformation in sediments are different.
• Attapulgite can transform mobile P into stable P in the sediment.
• Nature and purified attapulgite capping reduced risk of sediment-P release.
• Purified attapulgite capping has a good application prospect.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Feng, Y., Li, H. et al. The Effect of Purified Attapulgite Addition on Migration and Transformation of Phosphorus in Mariculture Ponds Sediments Under Static and Disturbance Conditions. Water Air Soil Pollut 234, 137 (2023). https://doi.org/10.1007/s11270-023-06154-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06154-6