Abstract
Since the beginning of the last century, there has been a high demand for renewable energy sources due to the decrease in fossil-based energy sources and the intense pollution of these energy types. Bioenergy recovery from sludge biomass is an attractive renewable energy source. Electrochemical treatment is one of the investigated physicochemical methods to increase methane production from waste activated sludge (WAS) biomass. It can be used as a new and efficient pretreatment for the hydrolysis of WAS. In this study, electrochemical technology with platinum/titanium (Pt/Ti) mesh electrodes was first applied for WAS before aerobic digestion. The effects of various operating conditions such as current intensity and initial pH of the sludge were investigated. The study showed that the amount of soluble COD of WAS increased with increasing current intensity. In addition, the results obtained showed that pH values higher or lower than the pH value of WAS (6.2) are favorable for the degradation of organic substances. An approximately 358% increase in COD solubility was detected at the end of the reaction time of 30 min at a current intensity of 25 A. The conducted study showed that electrochemical pretreatment is feasible and that increasing the methane production rate for anaerobic digester-containing wastewater treatment plants in Turkey has the potential to provide environmental and economic benefits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A large quantity of waste activated sludge (WAS) was generated from the biological wastewater treatment process, whose treatment and disposal were a huge challenge for many wastewater treatment plants (WWTPs) (Gherghel et al., 2019). These sludges can be classified as pre-precipitation sludge (PPS) and WAS. PPS generally originates from the pretreatment of raw wastewater containing physically precipitated organic matter with high biodegradability. WAS is produced biologically in the aeration unit in the activated sludge process. It mainly consists of biomass, extracellular polymeric substances (EPS) secreted by bacteria, resistant organics released from raw wastewater or bacterial decay, and inorganic substances from raw wastewater (Eddy et al., 2013).
Anaerobic digestion (AD) is widely applied for excessive sludge management for sludge stabilization, volume reduction, and energy recovery purposes. The organic contents in the sludge are broken down in anaerobic treatment with the help of four main microbial processes—hydrolysis, acidogenesis, acetogenesis, and the methanogenesis stage—which produce biogas rich in methane (CH4) for energy recovery (Macintosh, 2020). Application of AD is generally limited by slow rates of hydrolysis and poor biodegradability of WAS. Hydrolysis is catalyzed by extracellular enzymes produced by fermentative bacteria to break down carbohydrates, proteins, and lipids into their monomers, namely sugars, amino acids, and long-chain fatty acids (LCFA) (Sanders et al., 2000). Slow rates of hydrolysis in anaerobic treatment limit the sludge residence time (SRT), which determines the size or capacity of anaerobic reactors (Zhang et al., 2019). Pre-treatment of WAS to accelerate the hydrolysis stage may increase the efficiency of anaerobic treatment.
Although the organic content of sludge, measured as total chemical oxygen demand (COD), is high, the soluble fraction of COD, the organic material accessible to microorganisms and responsible for initiating the acidogenesis phase, is generally low. Therefore, sludge pretreatment is necessary to break down cell walls and facilitate the release of intracellular material into the aqueous phase to accelerate biodegradation and improve anaerobic decomposition. As a result, it has been shown that various sludge pretreatment technologies increase the hydrolysis or biodegradability of sludge in the anaerobic treatment process. Therefore, various technologies were applied to enhance the AD performance, mainly including WAS disintegration pretreatments (Bicakci & Eskicioglu, 2019; Luo et al., 2020; Wang et al., 2020), anaerobic co-digestion (AcD) with one or more low-cost organic wastes (Elalami et al., 2019), and application of various materials as a conductor to promote direct interspecies electron transfer (Arif et al., 2018; Li et al., 2019; Pan et al., 2020).
The electro-oxidation (EO) process is one of the treatment methods that have been successfully used for the removal of organic materials. The regent used in the electro-oxidation process is the electron. There is no need for an additional chemical substance. Electrons are environmentally friendly, and they are clean regents. Electrons do not need an additional process to remove them from the environment after organic matter removal. In EO, the pollutants are degraded by either a direct or an indirect oxidation process. In the direct anodic oxidation process, the pollutants are adsorbed on the anode surface and then destroyed by the anodic electron transfer reaction. In the indirect oxidation process, strong oxidants such as hypochlorite/chlorine, ozone, and hydrogen peroxide are electrochemically generated. The pollutants are then destroyed in a bulk solution by the oxidation reaction of the generated oxidant. All the oxidants are generated in situ and utilized immediately (Rajkumar & Palanivelu, 2007). The electro-oxidation process enables it to transform from particulate organic matter to soluble organic matter. The realization of this situation facilitates the formation of biogas. This is because the high hydrolysis capacity of electrochemical reactions creates short-lived and energy-rich free radicals that can break down microbial cell walls.
In the review study conducted by Zeng et al. in 2022, electro-oxidation studies used as pretreatment for anaerobic digestion were summarized. Electrodes used for sludge pretreatment are given as stainless steel, graphite, boron-coated diamond, metallic oxides plates, Ti coated with mixed metal oxide layer meshes, PbO2, IrO2, and RuO2. In this review, both laboratory scale and pilot scale studies with various electrodes were examined (Zeng et al. 2022). In this review, which examines the studies conducted from the beginning of the 2000s to the present, there are no studies conducted with titanium-coated platinum.
The present study was carried out to increase the dissolved organic matter of WAS by electro-oxidation process using titanium-coated platinum electrodes. The increase in dissolved organic matter will accelerate the hydrolysis stage of anaerobic treatment and increase the rate of methane production. In order to achieve this aim, WAS was treated by the EO process. In order to determine the optimum conditions, the initial pH value and current intensity parameters were investigated in a batch reactor. System performance was determined by analyzing soluble COD and suspended solids.
2 Materials and Methods
2.1 Sources of WAS
WAS were collected from a full-scale municipal WWTP in Erzurum, Turkey. After collection, the WAS was stored at 4 °C for less than 1 week before electro-oxidation treatment. The main characteristics of WAS are given in Table 1.
2.2 Batch-Mode EO Experiment
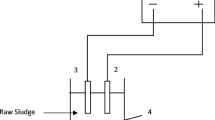
In the EO experiments, a reactor made of Plexiglas material with a volume of 1 L was used. The reactor has an inner diameter of 8 cm and a height of 22 cm. Platinum-coated titanium mesh electrodes were used as the anode material and pure titanium mesh electrodes were used as the cathode material. The total active surface area of the electrodes is approximately 1250 cm2. The distance between the electrodes is 5 mm. It was worked with a total of 6 electrodes, 3 anodes and 3 cathodes. The electrodes are positioned parallel to each other. Electricity was supplied to the system using a direct current power supply (Quassar 150 Switch Mode). The mixture of waste sludge was provided with the help of a peristaltic pump. Electrical conductivity and pH values were measured with the help of a WTW brand multimeter. The schematic view of the experimental setup is given in Fig. 1.
Chemical oxygen demand (COD) analyses were used to examine the parameters affecting the electro-oxidation process for WAS. COD analyses were conducted according to the closed system (reflux) method as specified in the standard methods 5220C-D. Raw WAS samples were first centrifuged at 6000 rpm for 10 min and then the supernatant was filtered through disposable filters with a pore size of 0.45 µm to analyze soluble COD (sCOD) concentrations. Suspended solids analyses were also performed according to standard methods 2540A (APHA, 2017). In the study, extraction processes were not applied for sCOD analysis. The sCOD amount of crude WAS was determined by filtering the filtrate obtained by centrifugation of the sample. The sludge pH was measured using a pH meter. Conductivity was determined by a conductivity meter. The following equations were used for calculation of the experimental data: Eq. 1-2
Calculation of treatment efficiencies:
Co is the initial pollutant concentration (mg/L) and Ct is the concentration of the pollutant remaining in the wastewater at time t (mg/L).
Calculation of energy consumptions:
I is the applied current intensity (A), V is the potential difference in the system (volt), t is the reaction time (min), and ϑ is the total wastewater volume (m3).
3 Results and Discussion
In this study, it was aimed to increase the soluble COD value of waste activated sludge. Initial pH value and current intensity were taken as parameters affecting solubility. Studies conducted for similar purposes have been examined in the literature. In Table 2, studies with different metal oxide–coated titanium sieve electrodes and BDD electrodes are compared.
When Table 2 is examined, electrode surface area, distance between electrodes, reaction time, initial TCOD, and SCOD amounts differ in studies using different metal oxide–coated titanium as anode electrodes. Although all of the mentioned studies were carried out with waste activated sludge, it is seen that the pollutant contents are different. The majority of the anode electrodes used are Ti/RuO2. Ti/IrO2 was used in one study. In the present study, Ti/Pt was used. The electrochemical oxidation capacities of mixed metal oxide electrodes and BDD electrodes are as follows (Table 3).
As can be seen from the table, the oxygen conversion potential values of the metal oxide–coated titanium electrodes are very close to each other, while the oxygen conversion potential of the BDD electrode is higher. This is clearly seen from the results of the studies given in the table. The theoretical information given below explains the difference in removal efficiencies between these electrode types.
In general, all electrodes are divided into active and non-active (Comninellis, 1994). The activity of electrodes is related to the interactions between hydroxyl radicals and anodes. At potentials that exceed the potential for oxygen evolution reactions (OER), water molecules undergo electrolytic discharge followed by physical adsorption of reactive ·OH radicals on the anode surface (Eq. 3).
Hydroxyl radicals adsorbed strongly on active electrodes can interact with the anode material forming higher oxides (MO), which then react with pollutant forming pollutant oxidation products, or release free oxygen (Martinez-Huitle & Ferro, 2006). Eq. 4, 5, and 6
This behavior is typical for anodes with a lower OER overpotential (Pt, IrO2, RuO2) and, as a result, lower activity towards organic oxidation. Non-active electrodes, such as BDD, have a high overpotential towards oxygen evolution reactions and weakly adsorb the ·OH radicals. This contributes to a decrease in oxygen evolution reactions, direct mineralization of organic compounds through Reaction 7 and, as a result, a higher pollutant degradation rate (Shestakova & Sillanpaa, 2017).
The fact that the oxygen overpotential value of the BDD electrode is higher than the value of the other Ti electrodes causes complete mineralization of the waste activated sludge. The results of the study with Ti electrodes increased the soluble COD value. This indicates that the electrochemical reaction remains in the intermediates. The decrease in the soluble COD value in the study with the BDD electrode shows that the electrochemical reaction continues until the final products, CO2 and H2O.
The effects of electrochemical treatment on WAS degradation were studied to evaluate initial pH value and current intensity to determine optimal pretreatment conditions in terms of organic matter solubilization.
3.1 Effect of Initial pH
It has been widely reported that pH played an important role in electro-oxidation (Chanikya et al., 2021; Barrera et al., 2021; Song et al., 2022). Experiments were carried out to determine the operating conditions for the electro-oxidation process depending on the initial pH value and current intensity of the waste sludge. Five different pH values were determined to measure the effect of the initial pH value. The pH values studied were 2, 4, 6, 8, and 10 (pH adjusted with sulfuric acid and sodium hydroxide). sCOD was determined to evaluate organic matter oxidation in the liquid phase. The current intensity was kept constant as 15 A. Depending on the initial pH values, the resulting pH values at the end of the 30-min reaction time were recorded. Experiments were carried out at room temperature. The change in pH, energy consumption, and temperature values is shown in Fig. 2.
When the values are examined carefully, it is seen that the effluent pH values decrease for all initial pH values. The change in pH shows that WAS hydrolyzes electrochemically and turns into volatile fatty acids. This should be supported by soluble COD analyses. The increase in the effluent temperature value for each pH value examined can be explained by the electrical resistance depending on the potential difference value that occurs in the system under constant current intensity. In the pH 6 test, which has the lowest electrical conductivity value, the highest effluent temperature value is due to the highest electrical resistance in the system under constant current intensity.
Although a constant current of 15 A is applied for each initial pH value, different energy consumption values have emerged in accordance with Eq. 2, depending on the different potential difference values that occur in the system. The main reason for the emergence of different energy consumption values is the emergence of different electrical conductivity values for different pH values, depending on the change in the initial pH values. Since the changing electrical conductivity value will change the potential difference value applied to the system under constant current intensity, it is an expected result that the energy consumption values will change. Acid and base added for pH adjustment cause the conductivity value of the WAS to increase. In addition, it also causes the structure of WAS to change. This situation was also observed in the study conducted for the pretreatment of waste activated sludge (Xiao et al., 2015). Since the change in the structure of WAS affects electron transmission, this change leads to a change in energy consumption. The variation of dissolved organic matter occurring at each pH value examined is shown schematically in Fig. 3.
sCOD was determined as the main parameter for the evaluation of sludge solubilization. sCOD represents substances that can be readily used to produce methane during anaerobic digestion. Obviously, the amount of sCOD increased with increasing pH under alkaline condition or decreasing pH in the acid range. Higher dissolution efficiencies for soluble COD can be achieved at pH 2 or 10. The change of sCOD in acidic conditions is similar to the results obtained in studies in the literature (Barrios et al., 2015). Under these conditions, 108% and 101% efficiency were obtained, respectively. While the raw pH value of the WAS was neutral, 6.5, the soluble COD yield was 82%. That is, alkaline and acidic conditions can improve the electrochemical pretreatment of sludge. In alkaline conditions, hydroxy anions can destroy flock structures and cell walls. This can result in loss of natural shape of proteins, saponification of lipid, and hydrolysis of RNA. A similar situation was found in the study by Song et al. (Song et al., 2010). In acidic conditions, it is thought that particular organic substances are transformed into dissolved organic substances by the acidification process.
Under the constant current applied to the system, while large-molecule organic substances turn into dissolved organic substances, a part of the dissolved organic matter is exposed to electrochemical oxidation at the same time. This is not desirable for methane production in anaerobic treatment. However, electro-oxidation is inevitable for the dissolved organic matter in WAS to turn into final products such as H2O and CO2. When the total COD removal efficiency in Fig. 3 is examined, it is seen that the total COD removal efficiency is high in alkaline and acidic conditions where the soluble COD yield is high. The highest removal efficiency in total COD was obtained with 14% in pH 2 test, and the lowest removal efficiency was obtained with 5% in pH 6 test. Under basic conditions (pH 10), the total COD removal efficiency increased and reached 12%. In the study by Barrios et al., the highest sCOD removal was obtained at the lowest pH value (pH = 3). These results are not similar to the results obtained in this study. In addition, under acidic and basic conditions, the microbial cell may decompose into soluble organic matter form and into CO2 and H2O, the final oxidation product of organic matter. This may confirm the high removal efficiencies obtained in the study under acidic and basic conditions. However, since the difference between the highest efficiency in acidic conditions and the lowest efficiency in neutral conditions is not very high, it is not possible to clearly state the mechanism by which the microbial cell structure is destroyed. The known fact is that the reason for the increase in total COD yield in acidic conditions is due to electrochemical oxidation as well as acidification of WAS. Although the difference between total COD removal efficiencies is low, the soluble COD yield obtained in acidic conditions (pH = 2) is 108%, while this value is 82% at pH 6 and 101% under basic conditions (pH = 10). The authors believe that the difference in soluble COD values is predominantly due to the electrochemical reactions differing at different pH values, and also to the acidification of the WAS. Although the authors were not able to derive a mechanism in which both the electrochemical oxidation mechanisms and the degradation mechanisms resulting from acidification were explained together, such a mechanism could not be obtained from the literature.
3.2 Effect of Current Intensity
Current intensity is a key parameter that has a direct impact on the efficiency of the electro-oxidation process. The effect of current intensity on electrochemical treatment efficiency was investigated for different wastewater types (Du et al., 2021; Luu, 2020). When adequate electrical power supply is provided, WAS, like other organic compounds, decomposes into smaller molecules at the anode (Barrios et al., 2015, 2017). Five different current intensity values were determined to measure the effect of the current intensity. The current intensity values studied were 5, 10, 15, 20, and 25 A. In the experiments where the effects of current intensity were examined, the initial pH value was chosen as 6.2, the value with the lowest total COD removal efficiency. The conversion rate of particulate organic matter to dissolved organic matter was obtained at higher rates at acidic and alkaline pH values. However, it was observed that the total COD removal efficiencies were high at these pH values. Since this situation will reduce methane production in anaerobic treatment, raw pH value was chosen as the initial pH value to examine the effects of current intensity. Depending on the current intensity values, the resulting pH values at the end of the 30-min reaction time were recorded. Experiments were carried out at room temperature. The change in pH, energy consumption, and temperature values is shown in Fig. 4.
The initial pH value of WAS was chosen as 6 in all experiments where the current intensity was examined. When Fig. 4 is examined, the increase in current intensity caused a decrease in the effluent pH value. While the effluent pH value was 4.9 at 5 A, the effluent pH value decreased to 3.55 at 25 A. It was also seen in the study by Anglada et al. that the increasing current intensity leads to a decrease in the effluent pH value (Anglada et al., 2009). The increase in current intensity caused a significant increase in the effluent temperature values. While an effluent temperature of 24 °C was obtained at 5 A, this value was determined as 67 °C at 25 A. Since the increase in current intensity increases the electrical resistance, some of the electrical energy supplied to the system is transformed into heat energy. This conversion caused the electrolyte to heat up. The greater the current intensity, the greater the portion converted into heat energy. This is clearly seen from Fig. 4.
When Eq. 2 is carefully examined, it is seen that the most important parameter affecting the energy consumption value is the current intensity and the applied potential difference. The increase in the current intensity caused an increase in the potential difference values applied to the system depending on the constant electrical conductivity value of the WAS. The increase in both values is the reason for the energy consumption values to reach very high values. The energy consumption value, which was 23 kW-h/m3 at 5 A, reached 76 kW-h/m3 when the current intensity was increased to 25 A. The effects of current intensity on total and soluble COD are shown in Fig. 5.
These results confirmed that the efficiency of soluble COD increases with increasing current intensity. It is consistent with previous studies that the current intensity is an important parameter controlling the removal efficiency in the electro-oxidation process (Öztürk et al., 2021). It was determined that the rate of degradation of organic pollutants increased as the current intensity increased. This effect is explained by the formation of higher amounts of oxidant species (Ozyonar & Korkmaz, 2022). It is supported by the existing studies in the literature that the increase in current intensity increases the electrochemical decomposition rate of organic matter (Barrios et al., 2015; Can et al., 2019; Veli et al., 2021). Based on the experimental results obtained, it was determined that the current intensity plays an important role in the degradation of WAS. The electro-oxidation process provided a maximum of 198% COD dissolution under these operating conditions (initial pH = 6.2, 25-A current intensity, 18 ± 2 °C temperature, and 30-min reaction time). When the results are evaluated in terms of total COD, they are similar to the results obtained in soluble COD values. Since increasing current intensity increases the amount of oxidant produced electrochemically, it is expected that the total COD removal efficiency will increase. However, the situation that needs attention is the change in the soluble COD (sCOD) and total COD (tCOD) ratio of raw and treated WAS. This change is given in Table 4.
When Table 4 is examined, it is seen that the sCOD/tCOD ratio of raw WAS is 0.093. It is seen that this ratio increases as the current intensity applied to WAS increases. Although the increase in the sCOD/tCOD ratio is not of great importance for the treatment carried out by the electro-oxidation process, it is very important for the anaerobic treatment to be carried out after this process. Increasing the soluble COD value will cause a decrease in the reaction time in the hydrolysis stage, which is one of the anaerobic treatment stages. The reduction of the hydraulic phase leads to a reduction in the reactor volume. In addition, the amount of heat needed to provide mesophilic or thermophilic conditions will be lower. This will result in a decrease in operating costs in addition to a decrease in the investment cost of anaerobic treatment. As a result, the obtained results show that the electro-oxidation process should be used as a pretreatment in order to treat the WAS with anaerobic treatment and to obtain a larger amount of methane gas.
4 Conclusions
With the experimental study, the optimum operating conditions required to increase the soluble COD amount in the WAS by the electro-oxidation process were determined. In the first stage of the study, the effects of the initial pH value on the change in the amount of soluble COD were investigated. It was observed that the amount of soluble COD increased in acidic and alkaline conditions. There are two reasons for this to happen; firstly, the added acid and base changed the structure of the WAS, facilitating electron transfer. Secondly, the difference in the type and amount of hydroxyl radicals formed in the electrochemical reactor in this pH range affected the oxidation capacity. In pH experiments, although the highest soluble COD efficiency occurs at pH 2, the effluent pH value creating very acidic conditions such as 1.7 will create a negative effect for anaerobic treatment. Likewise, the neutral pH range revealed in the pH 10 experiment is not suitable for anaerobic treatment. Therefore, the raw pH value of the WAS was used as the initial pH value in the current intensity studies. In the second stage, where the effects of current intensity were examined, the highest soluble COD efficiency was obtained at 25-A current intensity. This value is approximately 198%. The effluent pH value in this study is approximately 3.8. It is a suitable value for anaerobic treatment. The fact that the effluent temperature is approximately 65 °C indicates that a certain part of the heat energy required for anaerobic treatment can be met with the help of the electro-oxidation process. Due to the decrease in the amount of particulate solids in WAS, the amount of power required for the mixing process used in anaerobic treatment will also decrease. Increasing current intensity increased the sCOD/tCOD ratio. This situation provides very suitable conditions for anaerobic treatment. As a result, the pre-treatment of WAS with electro-oxidation process under optimum conditions such as initial pH value of 6 and current intensity of 25 A has led to high soluble COD values. The results obtained show that the study achieved its goals.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Anglada, A., Urtiaga, A., & Ortiz, I. (2009). Pilot scale performance of the electro-oxidation of landfill leachate at boron-doped diamond anodes. Environmental Science and Technology, 43, 2035–2040.
APHA, 2017, Standard methods of examination of water and wastewater, 23rd. Edition, Chemical oxygen demand (COD), D-closed reflux colorimetric method, 5–21
Arif, S., Liaquat, R., & Adil, M. (2018). Applications of materials as additives in anaerobic digestion technology. Renewable and Sustainable Energy Reviews, 97, 354–366.
Barrera, H., Nunez, F. U., Barrios, J. A., Becerril, E., Uribe, B. A. F., & Díaz, C. E. B. (2021). Degradation of nonylphenol ethoxylate 10 (NP10EO) in a synthetic aqueous solution using a combined treatment: Electrooxidation-gamma irradiation. Fuel, 283, 118929.
Barrios, J. A., Becerril, E., De León, C., Barrera-Diaz, C., & Jiménez, B. (2015). Electrooxidation treatment for removal of emerging pollutants in wastewater sludge. Fuel, 149, 26–33.
Barrios, J. A., Duran, U., Cano, A., Cisneros-Ortiz, M., & Hernandez, S. (2017). Sludge electrooxidation as pre-treatment for anaerobic digestion. Water Science Technology, 75(4), 775–781.
Bicakci, G. K., & Eskicioglu, C. (2019). Recent developments on thermal municipal sludge pretreatment technologies for enhanced anaerobic digestion. Renewable and Sustainable Energy Reviews, 110, 423–433.
Can, O. T., Gengeç, E., & Kobya, M. (2019). TOC and COD removal from instant coffee and coffee products production wastewater by chemical coagulation assisted electrooxidation. Journal of Water Process Engineering, 28(2019), 28–35.
Chanikya, P., Nidheesh, P. V., Babu, D. S., Gopinath, A., & Kumar, M. S. (2021). Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Separation and Purification Technology, 254, 117570.
Comninellis, C. (1994). Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochimica Acta, 39, 1857–1862.
Du, X., Mo, Z., Li, Z., Zhang, W., Luo, Y., Nie, J., Wang, Z., & Liang, H. (2021). Boron-doped diamond (BDD) electro-oxidation coupled with nanofiltration for secondary wastewater treatment: Antibiotics degradation and biofouling. Environment International, 146, 106291.
Eddy, M., Burton, F., Tchobanoglous, G., Tsuchihashi, R. (2013) Wastewater engineering: Treatment and resource recovery. McGraw-Hill Education, New York, NY, USA.
Elalami, D., Carrere, H., Monlau, F., Abdelouahdi, K., Oukarroum, A., & Barakat, A. (2019). Pretreatment and co-digestion of wastewater sludge for biogas production: Recent research advances and trends. R Renewable and Sustainable Energy Reviews, 114, 109287.
Gherghel, A., Teodosiu, C., & Gisi, S. D. (2019). A review on wastewater sludge valorization and its challenges in the context of circular economy. Journal of Cleaner Production, 228, 244–263.
Huang, H., Zeng, Q., Heynderickx, P. M., Chen, G. H., & Wu, D. (2021). Electrochemical pretreatment (EPT) of waste activated sludge: Extracellular polymeric substances matrix destruction, sludge solubilisation and overall digestibility. Bioresource Technology, 330, 125000.
Li, Y., Chen, Y. G., & Wu, J. (2019). Enhancement of methane production in anaerobic digestion process: A review. Applied Energy, 240, 120–137.
Luo, J. Y., Zhang, Q., Zhao, J. N., Wu, Y., Wu, L. J., Li, H., Tang, M., Sun, Y. Q., Guo, W., Feng, Q., Cao, J. S., & Wang, D. B. (2020). Potential influences of exogenous pollutants occurred in waste activated sludge on anaerobic digestion: A review. Journal of Hazardous Materials, 383, 121176.
Luu, T. L. (2020). Tannery wastewater treatment after activated sludge pre-treatment using electro-oxidation on inactive anodes. Clean Technologies and Environmental Policy, 22, 1701–1713.
Macintosh, C.L. (2020) Anaerobic Co-digestion: Micro to Full-scale.
Martinez-Huitle, C., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chemical Society Reviews, 35, 1324–1340.
Ozturk, D., Yilmaz, A. E., & Ayas, Z. Ş. (2021). Electrochemical mineralization of abattoir wastewater with continuous system. International Journal of Environmental Science and Technology, 18, 3761–3776.
Ozyonar, F., & Korkmaz, M. U. (2022). Sequential use of the electrocoagulation-electrooxidation processes for domestic wastewater treatment. Chemosphere, 290, 133172.
Pan, C., Fu, X. D., Lu, W. J., Ye, R., Guo, H. W., Wang, H. T., & Chusov, A. (2020). Effects of conductive carbon materials on dry anaerobic digestion of sewage sludge: Process and mechanism. Journal of Hazardous Materials, 384, 121339.
Rajkumar, D., & Palanivelu, K. (2007). Electrochemical treatment of industrial wastewater. Journal of Hazardous Materials, 113, 123–129.
Sanders, W. T. M., Geerink, M., Zeeman, G., & Lettinga, G. (2000). Anaerobic hydrolysis kinetics of particulate substrates. Water Science and Technology, 41(3), 17–24.
Shestakova, M., & Sillanpaa, M. (2017). Electrode materials used for electrochemical oxidation of organic compounds in wastewater. Reviews in Environmental Science and Biotechnology, 16, 223–238.
Son, L. J., Zhu, N. W., Yuan, H. P., Hong, Y., & Ding, J. (2010). Enhancement of waste activated sludge aerobic digestion by electrochemical pre-treatment. Water Research, 44, 4371–4378.
Song, L. J., Zhu, N. W., Yuan, H. P., Hong, Y., & Ding, J. (2010). Enhancement of waste activated sludge aerobic digestion by electrochemical pre-treatment. Water Research, 44, 4371–4378.
Song, P., Sun, C., Wang, J., Ai, S., Dong, S., Sun, J., & Sun, S. (2020). Efficient removal of Cu-EDTA complexes from wastewater by combined electrooxidation and electrocoagulation process: Performance and mechanism study. Chemosphere, 287, 131971.
Veli, S., Arslan, A., İşgören, M., Bingöl, D., & Demiral, D. (2021). Experimental design approach to COD and color removal of landfill leachate by the electrooxidation process. Environmental Challenges, 5, 100369.
Wang, S. Q., Yu, S. N., Lu, Q. H., Liao, Y. Y., Li, H. C., Sun, L. P., Wang, H. T., & Zhang, Y. (2020). Development of an alkaline/acid pre-treatment and anaerobic digestion (APAD) process for methane generation from waste activated sludge. Science of the Total Environment, 708, 134564.
Xiao, B., Liu, C., Liu, J., & Guo, X. (2015). Evaluation of the microbial cell structure damages in alkaline pretreatment of waste activated sludge. Bioresource Technology, 196, 109–115.
Xu, J., Yuan, H., Lin, J., & Yuan, W. (2014). Evaluation of thermal, thermal-alkaline, alkaline and electrochemical pretreatments on sludge to enhance anaerobic biogas production. Journal of the Taiwan Institute of Chemical Engineers, 45, 2531–2536.
Yang, H. G., Chun, H. Y., & Pak, D. (2014). Improvement of sludge anaerobic degradability by combined electro-flotation and electro-oxidation treatment. Biochemical Engineering Journal, 90, 44–48.
Ye, C., Yuan, H., Dai, X., Lou, Z., & Zhu, N. (2016). Electrochemical pretreatment of waste activated sludge: Effect of process conditions on sludge disintegration degree and methane production. Envıronmental Technology, 37(22), 2935–2944.
Yuan, H., Yu, B., Cheng, P., Zhu, N., Yin, C., & Ying, L. (2016). Pilot-scale study of enhanced anaerobic digestion of waste activated sludge by electrochemical and sodium hypochlorite combination pretreatment. International Biodeterioration and Biodegradation, 110, 227–234228.
Zhang, L., Duan, H., Ye, L., Liu, L., Batstone, D. J., & Yuan, Z. (2019). Increasing capacity of an anaerobic sludge digester through FNA pre-treatment of thickened waste activated sludge. Water Research, 149, 406–413.
Zhen, G., Lu, X., Li, Y. Y., & Zhao, Y. (2014). Combined electrical-alkali pretreatment to increase the anaerobic hydrolysis rate of waste activated sludge during anaerobic digestion. Applied Energy, 128, 93–102.
Zheng, Q., Huang, H., Tan, Y., Chen, G., & Hao, T. (2022). Emerging electrochemistry-based process for sludge treatment and resources recovery: A review. Water Research, 209, 117939.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Alper Erdem YILMAZ, Beyhan KOCADAĞISTAN, and Emine Cansu ARARGÜÇ. The first draft of the manuscript was written by Alper Erdem YILMAZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erdem YILMAZ, A., KOCADAĞISTAN, B. & ARARGÜÇ, E.C. The Investigation of the Parameters Affecting the Solubility of Waste Activated Sludge in the Electro-oxidation Process. Water Air Soil Pollut 233, 189 (2022). https://doi.org/10.1007/s11270-022-05665-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05665-y