Abstract
Pesticides are one of the main sources of pollution on the planet, affecting different environments. The aim of the study was to evaluate the chronic effect of atrazine (ATZ) on brain, gill, and liver histology and the clinical signs of intoxication in Hyphessobrycon eques fish and to determine the effect on the expression of proteins related to proliferation, damage, death, and cellular stress pathways. The chronic toxicity test was performed during 30 days of exposure to ATZ. Concentrations of 0.57, 1.14, 5.74, and 11.50 mg L−1 were applied and there was a control. Aquariums with a capacity of 5 L of water were used with aeration pumps containing 5 animals per replica, weighing between 1.0 ± 0.2 g per organism. At the end of the chronic toxicity tests of ATZ for H. eques, samples of the gills, brain, and liver were collected for histological analysis. The same organs were collected and stored in cryogen tubes in liquid nitrogen until the moment of extraction and then massaged mechanically for the Western-blot technique. In the histopathological analyses, the gills showed the greatest changes in relation to the other organs, such as the loss of support of the secondary lamellae, and the fish also showed a loss of swim bladder capacity. In conclusion, ATZ in the environment, in long-term exposure, can cause histological and biochemical effects, affecting the survival and proliferation of cell pathways.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides have negatively contributed to the pollution of aquatic environments by forming complex mixtures with heavy metals and other pollutants from various anthropogenic activities. The decline in fresh and marine water quality can be associated with the flow of these products from the terrestrial environment of agricultural production adjacent to water bodies (Fillols et al., 2020). Data from 2009 to 2018 show that the four best-selling pesticides in Brazil are glyphosate, 2,4-D, mineral oil, and atrazine (ATZ) (IBAMA, 2020), which are one of the main diffuse sources of pollution on the planet. Furthermore, the indiscriminate use of some molecules and the lack of monitoring of environmental dynamics can cause unpredictable effects on aquatic biota such as biochemical, physiological, and morphological changes in fish and lethality in aquatic organisms of different trophic levels (Shiogiri et al., 2016; Marques et al., 2021).
Among herbicides, ATZ belongs to the triazine group and inhibits photosystem II. It blocks electron transport in plant photosynthesis (Zajc et al., 1998) and the production of ATP (adenosine triphosphate), NADPH (electron acceptor coenzyme) (Phyu et al., 2011). It is used to control monocotyledonous and dicotyledonous weeds in sugarcane, wheat, conifers, sorghum, nuts, and corn (Zhao et al., 2017). ATZ ranks third among pesticides most often used in Brazil. It has a moderate toxicological level (Anvisa, 2016), and it has been banned in the European Union since 2004 (Montiel-León et al., 2019).
Environmental monitoring and assessments of the potentially harmful effects of chemicals released into the environment are critical in-depth understanding of the environmental dynamics of pesticides after use (Santos et al., 2015). Experiments with ATZ have been carried out for a long time with different species of aquatic organisms, which resulted in the ban in the European Union in 2004 (EC, 2004). However, in many regions, including Brazil, ATZ is still one of the most used herbicides. Therefore, ecotoxicological studies on this molecule are important, since the herbicide and its residues are still detected as persistent contaminants of aquatic environments for up to 100 days (Hou et al., 2017), e.g., in surface water at a concentration of 10.4 µg L−1 (Santos et al., 2015).
The histological characteristics of non-target organisms can express different changes resulting from adverse environmental conditions. Therefore, the evaluation of these histological parameters is crucial to determine cellular alterations that can occur in fish (Américo-Pinheiro et al., 2020).The use of biochemical techniques has been shown to be quite viable, as they offer high sensitivity and specificity for diagnosis of animal diseases (Talmi-Frank et al., 2006). Western blot is a technique that allows detection, characterization, and quantification of multiple proteins, especially those that are present in low amounts in a given sample (Kurien and Scofield, 2006), which is incubated with specific antibody markers for the protein of interest (Mahmood and Yang, 2012).
Responses are usually the first cells to occur within the biological and morphological changes in the animal (Carraschi et al., 2012).Thus, histopathology becomes a biomarker and logical integrator that allows the identification of biochemical and physiological changes in fish associated with water quality and the necessary care to prevent diseases in fish farms (Shiogiri et al. 2016; Erlacher-Reid, 2018). However, many studies on atrazine are performed with different species of organisms, but few studies of chronic toxicity and biomarkers, with the use of neotropical organisms, indicating a lack of information on this chemical in the Brazilian scenario.
Thus, the objectives of this study were to evaluate the chronic effect of ATZ on brain, gill and liver histology and the clinical signs of poisoning of the fish Hyphessobrycon eques and determine the effect on the expression of proteins related to proliferation pathways, as well as cellular damage, death, and stress.
2 Materials and Method
2.1 Pesticide
The pesticide used in the experiments was the herbicide ATZ (CAS No. 1912–24-9) in the commercial formulation Nortox 500.0 g L−1. The commercial formulation was diluted in deionized water to obtain the concentrations used in the toxicity tests. The study was evaluated by the Ethics Committee on the Use of Animals (CEUA/UNIFEB/Barretos) and was carried out according to the approved protocol of CEUA 04_2017.
2.2 Chronic Toxicity
The fish used were the red minor (H. eques) weighing between 1.0 ± 0.2 g per organism; they were acclimated in bioassay rooms, with temperature controlled at 25.0 ± 2.0 °C and with a photoperiod of 12 h of light, in aquariums with a capacity of 100.0 L of water, with continuous aeration. The fish were fed once a day with flake feed with 28% crude protein for ten days.
The chronic toxicity test for ATZ was carried out 30 days of exposure. Therefore, in aquariums with a capacity of 5.0 L of water with aeration pumps, 5 animals were transferred per replicate, with four replicates for each concentration tested, with a density of 1 g L−1 (ABNT, 2007).
The study concentrations were 0.57, 1.14, 5.74, and 11.50 mg L−1, defined on the basis of four quotients (5, 10, 50, and 100) applied to the value of LC50;48 h = 57.49 mg L−1 of ATZ to the H. eques, plus a control (no product was applied). The LC50;48 h value for the fish was determined in a previous assay under the same laboratory conditions as in the chronic test. The signs of intoxication (opercular beat, erratic swimming, agitation, permanence on the surface or the bottom of the aquarium, lethargy, eye irritation, corrosion of skin and fins) were evaluated according to Murty (1988).
The animals were fed once a day and the aquariums were siphoned every 7 days, with a renewal of 25.0% of the total water volume in use, during the 30 days of the experiment. During the chronic toxicity test for H. eques, water quality was evaluated at 7, 15, and 30 days during the experiment, using pH, electrical conductivity, temperature, and dissolved oxygen measuring equipment, was monitored according to the recommendations of ABNT (2007). During the 30 experimental days, the temperature remained between 25.9 and 26.5 °C; electrical conductivity between 122.5 and 127.9 µS cm−1; dissolved oxygen between 6.8 and 7.9 mg L−1, and pH between 7.6 and 7.9, thus maintaining the standards established by ABNT (2007). At the end of the experimental period, the No Observed Effect Concentration (NOEC) and the Observed Effect Concentration (OEC) were determined.
At the end of ATZ’s chronic toxicity tests for H. eques, the gill, brain, and liver samples were collected for histological and western blot analysis. For this purpose, the fish were euthanized in benzocaine (0.15 g L−1) and three animals were collected from each replicate and all samples were analyzed.
2.3 Histopathological Analysis
The organs were removed and immersed in a 10.0% formaldehyde buffered fixative solution for 24 h. After fixation, the fragments were dehydrated, cleared in xylene, and included in Histosec® (Merck). Then, microtomies were performed using a Lupetec semi-automatic microtome, and 5.0 μm-thick sections were made. They were stained with Hematoxylin–Eosin (HE) and Periodic acid-Schiff (PAS) (Behmer et al., 1976) and analyzed in a Panthera L-Motic light microscope.
2.4 Protein Expression Analyses
After collection, fish organs (100 mg of weight) were stored in cryovials, later placed in liquid nitrogen (at − 196 °C) until extraction. To evaluate the protein expression, the proliferation and damage pathways, death and stress of the cells, which would possibly be affected after the application of ATZ, were evaluated. Subsequently, the following components were added to the macerate: Tris buffer (50.0 mM, pH 7.6–8.0), NaCl (150.0 Mm), EDTA (5.0 mM), Na3VO4 (1.0 mM), NaF (10.0 mM), Na pyrophosphate (10.0 mM), NP-40 (1.0%), supplemented with DTT protease inhibitor cocktail (1.0 mM), Leupeptin (1.0 μg mL)−1, aprotinin (1.0 μg mL−1), PSMF (1.0 mM), EDTA (1.0 mM) for 1 h. The tissues were then centrifuged (13,000 rpm, 15 min, 4 °C) and the supernatant was stored (− 80 °C).
Soluble proteins were quantified by the Bradford method (Sigma), according to the manufacturer's recommendations. Afterwards, 20 µg of total proteins was separated through SDS-PAGE polyacrylamide (10.0%) and transferred to nitrocellulose membrane (Hybond-C, Amersham Biosciences, Little Chalfont, UK) using a mini Trans-Blot® Turbo Transfer System (BioRad). The membranes were blocked with powdered milk solution (5.0%) containing TBS and Tween (0.1%) (TBS-T; pH = 7.6) for 1 h at room temperature. After blocking, the membranes were incubated with the corresponding primary antibodies (12 h, 4 °C) prepared in TBS/Tween (0.1%) solution with BSA (5.0%).
The antibodies anti-p-ERK/ERK, ATKt/anti-p-AKT (cell signaling) (v:v/1:1000) were used. The membranes were then washed with solution (TBS/Tween (0.1%)) and incubated with peroxidase-conjugated secondary antibody (Cell Signaling Technology). The protein β-tubulin was used as a control (v:v/1:2000 dilution). Detection was performed by chemiluminescence (ECL Western Blotting Detection Reagents, RPN2109, GE Healthcare) and the chemiluminescent signal was detected in a photodocumentation system (ImageQuant™ LAS 4000 mini (GE Healthcare)). The results were quantified by densitometry in the ImageJ software (version 1.41; National Institutes of Health).
3 Results and Discussion
3.1 Chronic Histopathological Effects
Endpoints of mortality, clinical signs of intoxication, histological changes, and western blot were used in the chronic toxicity test, with 30-day exposure of red minor (H. eques). NOEC was considered 0.57 mg L−1 and OEC was 1.14 mg L−1(Table 1).
During the chronic toxicity test, three fish died, one at the concentration of 11.50 mg L−1 with 18 days of exposure, another at 5.74 mg L−1 with 23 days of exposure and one at 1.14 mg L−1 with 28 days of exposure. At 7 days of exposure for all concentrations tested, the fish presented alteration in staining; in addition to this, sign of intoxication also occurred in 0.57 mg L−1 loss of swim bladder capacity; in 1.14 and 11.50 mg L−1, there was loss of swim bladder capacity and permanence at the bottom of the unit and in 5.74 mg L−1 agitation.
At 15 days on 1.14 mg L−1, there was change in color, the fish show loss of their original color, becoming sallow, fin corrosion, and permanence on the water surface, and at 5.74 mg L−1, there was change in color, fin corrosion, skin hemorrhage, and agitation. At 30 days, there was erratic swimming, change in color, lethargy, and permanence at the bottom of the aquarium at concentrations of 0.57 and 1.14 mg L−1. At the other concentrations, the most evident sign of intoxication was permanence at the bottom of the aquarium.
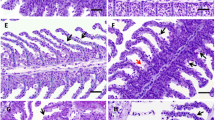
In the histopathological evaluation of the chronic effects of ATZ, the gills of the control fish had primary lamellae composed of the cartilage connective tissue, and the blood vessels within this tissue. Primary lamellae carry secondary lamellae lined with mucus-secreting and connective cells. In the spaces between the secondary lamellae, there were interlamellar spaces composed of chloride cells, mucus-secreting cells, and goblet cells (Fig. 1A).
At concentrations of 0.57 and 1.14 and mg L−1, there were no chronic effects of ATZ on the histological structure of the gills. At 5.74 mg L−1, there was an increase in the interlamellar lining epithelium and in the secondary lamellae lining cells, which reduces the gas exchange area of secondary lamellae, an action that may have a reversible effect. At 11.50 mg L−1, there was a decrease in the interlamellar epithelium and the mucous cells lining the secondary lamellae, loss of lamellar support, and apical fusion of the lamellae in some regions (Fig. 1B).
The exposure of Tilapia (Oreochromis niloticus) to 1.0 and 2.0 mg L−1 of ATZ for 7 and 15 days caused gill hemorrhage, secondary lamella hypertrophy, epithelial enlargement, and aneurysm in secondary lamellae (Oliveira et al., 2018). The gills are important indicators of water quality in studies of environmental contamination (Américo-Pinheiro et al., 2020). They are considered to be the first of entry for pesticides into fish (Velmurugan et al., 2007). Also, they are essential for breathing and osmoregulation (Mcdonald, 1983); therefore, changes in gill morphology can impair blood flow, gas exchange, and fish homeostasis (Melletti et al., 2004). Increased interlamellar epithelium and disorganization in gill structure increase the distance from water-dissolved oxygen to the blood (Américo-Pinheiro et al., 2020).
Tissue changes in the gills indicate the impact of pesticides on fish that can cause irreparable damage to the gills by increasing and modifying regulatory cells of the physiological system, in an attempt to minimize the effects of the contaminant (Andrade Vieira and da Silva, 2019).
In the control group, the liver showed hepatocytes arranged in cord-like organization in comparison to the central veins. There were small hepatocytes whose central nuclei were somewhat displaced to the periphery, and slightly acidophilic cytoplasm. Sinusoidal capillaries border hepatocytes from the central veins, giving them a cord-like appearance. Cells from the exocrine pancreas were present in some central veins. Control hepatocytes have glycogen granules within the cytoplasm (Fig. 2A).
At concentrations of 0.57, 1.14, and 5.74 mg L−1, there were no chronic effects on the histological structure of the liver. At 11.50 mg L−1, there was a total disarrangement of the cord-like structure of hepatocytes, and an increase in sinusoidal capillaries. This fact indicates a possible accumulation of red blood cells in the liver as a result of the detoxification, which can be reversible in fish (Fig. 2B).
Chronic exposure of pacu (Piaractus mesopotamicus) for 96 h to 28.58 mg L−1 of ATZ showed more severe effects on the liver, with morphological disruption and nuclear and cytoplasmic vacuolation in hepatocytes (Paiva et al., 2017), while exposure of lambari (Astyanax altiparanae) to 0.5–10.0 µg L−1 of ATZ for 30 days caused increased vascularization of sinusoidal capillaries and leukocyte infiltration (Destro et al., 2021). The differences in histopathological effects may be due to the concentration tested and the exposure times used in each study. However, the chronic effects of ATZ have been shown to occur on the liver in fish.
Histological studies are important to describe and compare tissues in organs, especially environmental ones associated with changes in conditions, such as the liver. What is considered the central organ of xenobiotic metabolism in fish, and changes in this organ may be useful as biomarkers that indicate exposure to environmental stressors (Dane and Sisman, 2017; Van Dyk et al., 2012).
The analysis of the brains of the control fish showed a molecular layer with few cells that had rounded nuclei and evident nucleoli dispersed in an abundant, slightly acidophilic extracellular matrix. In the vicinity of the granular layer, there is a greater accumulation of cells with more basophilic nuclei, in some regions. In most regions, cell type and cell distribution are similar to those of the molecular layer. There is a large number of cells in the granular layer. The histological structure of the brain of red minor (H. eques) did not show the chronic effect of ATZ at any study concentration (Fig. 3).
Agrochemicals, especially organophosphates and carbamates, are compounds known to be capable of altering the activity of the enzyme acetylcholinesterase (Payne et al., 1996). Previous studies have shown that fish subjected to sublethal concentrations of pesticides can show changes and inhibition concerning the activity of this enzyme (Ferrari et al., 2007; Miron et al., 2005; Modesto and Martinez, 2010). However, ATZ had no effects on acetylcholinesterase activity in the brain of Prochilodus lineatus, according to Santos (2010).
3.2 Effect of ATZ on Protein Expression
In the chronic exposure of H. eques to ATZ, there was an increase in the relative expression (%) of the activated form of the AKT protein (p-AKT) in the gills of H. eques at all evaluated concentrations in comparison to the control, especially at 11.50 mg L−1 (Fig. 4).
The active form of the ERK protein (p-ERK) also showed a higher relative expression at 0.57 mg L−1 in the gills, while in the other concentrations, such expression was lower than in the control (Fig. 4).
In the brain, there was a dose-dependent increase in p-AKT expression (Fig. 5) compared to the control. On the other hand, in general, the expression of p-ERK was inhibited in the brain at most of the study concentrations; the highest expression occurred at 5.74 mg L−1. At 0.57 mg L−1, there was no expression of this protein; at 1.14 and 11.50 mg L−1, the protein was inhibited, in comparison to the control (Fig. 5).
The analysis of p-AKT expression in the liver showed that greater expression occurred at 11.50 mg L−1 and at 5.74 mg L−1. At 5.74 mg L−1, there was no protein expression while at 0.57 mg L−1, the expression values were similar to those of the control (Fig. 6). For p-ERK, expressions were similar, but lower than those of the control at concentrations of 0.57, 1.14, and 11.50 mg L−1 (Fig. 6). At 5.74 mg L−1, there was an increase in p-ERK expression in comparison to the control (Fig. 6).
Another essential function of AKT is the regulation of glycogen synthesis through phosphorylation and inactivation of GSK-3α and β (Hajduch et al., 2001; Cross et al., 1995). AKT may also play a role in insulin stimulation of glucose transport (Hajduch et al., 2001). AKT is also involved in cell cycle regulation by preventing GSK-3β-mediated phosphorylation and cyclin D1 degradation and by downregulating cyclin-dependent kinase inhibitors p27 Kip and p21 Waf1 (Zhou et al., 2001).
AKT also plays a critical role in cell growth through direct phosphorylation of mTOR (Navé et al., 1999). This protein also acts on the phosphorylation and inactivation of tuberin (TSC2), an mTOR inhibitor within the mTOR raptor complex (Inoki et al., 2002). The inhibition of mTOR disrupts the protein synthesis machinery as a result of the inactivation of its effector, the p70 S6 kinase, and the activation of ukaryotic initiation factor 4E 1-binding protein (4E-EP1), a translation inhibitor (Inoki et al., 2002; Manning et al., 2002).
In general, after analysis of the organs, especially the gills, brain, and liver, the activity of the AKT protein (p-AKT) showed an increase in expression compared to the control, which suggests activation of cell growth and survival factors of fish exposed to ATZ.
The AKT protein, also referred to as PKB or Rac, with 60 kDa, plays a critical role in controlling survival and apoptosis (Franke et al., 1995; 1997), as this protein kinase is activated by insulin and various growth and survival factors. AKT promotes cell survival by inhibiting apoptosis by phosphorylating and inhibiting several targets, including Bad, forkhead transcription factors (Brunet et al., 1999), c-Raf (Zimmermann and Moelling, 1999), and Caspase-9. In the case of the gills and brain of fish exposed to ATZ, the increased expression of the active form of these proteins suggests the occurrence of a stimulus for cell survival, compared to the control (Fig. 4 and 5).
For the p-ERK signaling pathway, there was a reduction in activity in comparison to the control. In the gill samples, p-ERK showed phosphorylation in the control and at the concentration of 11.50 mg L−1; in the brain, it showed phosphorylation in the control and at a concentration of 5.74 mg L−1; in the kidney, it showed a greater increase in concentrations compared to the control; and in the liver, its expression was inhibited. This protein is related to cell survival, growth, and differentiation processes. Once activated, ERK1/2 kinases directly phosphorylate their substrates, e.g., transcription factors, ion channels, membrane proteins, cytoskeletal proteins, transporters, other kinases, adapter proteins, growth factor, and estrogen receptors (Mattos, 2011; Roux and Blenis, 2004). Depending on duration, magnitude, and subcellular location, ERK activation controls various cellular responses, such as proliferation, migration, differentiation, and death (Murphy and Blenis, 2006).
According to Cavalli et al. (2013), the involvement of the MAPK pathway in glyphosate-induced toxicity in testicular cells was confirmed using the western-blot technique, since ERK1/2 and p38 were activated/phosphorylated in testicles of prepubertal rats exposed to the pesticide. The results for ATZ for H. eques were similar to those of glyphosate, as it induced an increase in the phosphorylation of two of the MAPK pathways, ERK 1/2 and JNK 1/2, thus indicating their activation. The participation of the AKT/GSK-3β pathway was also evaluated, and in both of them, there was a significant increase in the phosphorylation of these enzymes when compared to the control group. These data demonstrate the participation of MAPKs and AKT/GSK-3β signal transduction pathways in the modulation of glyphosate mechanisms (Cattani et al., 2014).
Notably, oxidative stress is commonly cited as a potential mechanism of carcinogenesis for various environmental exposures, including pesticides (Alavanja et al., 2013; Guyton et al., 2015; Loomis et al., 2015). ATZ can induce oxidative stress in rodents such as Rattus norvegicus and Mus musculus (Abarikwu, 2014; Jin et al., 2014; Zhao et al., 2014). According to Hu et al. (2016), ATZ at 0.01 and 0.1 μM promoted the proliferation of RM1 cells (Prostate Lineage) and accelerated cell cycle progression in RM1 cells.
4 Conclusion
Chronic exposure of H. eques to the herbicide ATZ for 30 days causes severe histological and cellular damage to the fish. The damage observed in the gills, such as loss of gill support and apical fusion of the secondary lamellae, is irreversible. In the liver, one of the main effects was the accumulation of red blood cells due to the detoxification process. These changes in different tissues compromise the biochemical functions and cellular pathways of protein expression in fish. The biomarkers presented in our article are fundamental and prove that even at low concentrations for long periods, ATZ is an environmental contaminant that compromises the health and survival of fish. Therefore, we recommend the use of histological analyses and protein expression as sensitive biomarkers of fish exposure to pesticides.
Data Availability
Data generated from the experiment.
References
Abarikwu, So. (2014). Protective effect of quercetin on atrazine-induced oxidative stress in the liver, kidney, brain, and heart of adult wistar rats. Toxicology International, 21, 148–155.
ABNT (Associação Brasileira de Normas Técnicas). (2007). NBR 15499 (1° ed. 21). Rio de Janeiro: Ecotoxicologia aquática – Toxicidade crônica de curta duração – Método de ensaio com peixes.
Alavanja, M. C., Ross, M. K., & Bonner, M. R. (2013). Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA: Cancer Journal Clinicians, 63, 120–42.
Américo-Pinheiro, J. H. P., Machado, A. A., Cruz, C., Aguiar, M. M., Ferreira, L. F. R., Torres, N. H., & Machado-Neto, J. G. (2020). Histological changes in targeted organs of Nile Tilapia (Oreochromis niloticus) exposed to sublethal concentrations of the pesticide carbofuran. Water, Air, and Soil Pollution, 231, 1–8.
Andrade Vieira, J. A. R., & da Silva, G. S. (2019). Avaliação dos efeitos do Roundup® e da hipóxia sobre os parâmetros hematológicos e histologia branquial de Colossoma macropomum (Cuvier, 1818), Scientia Amazonia, v. 8, n.2, CAm16-CAm28.
ANVISA. Brazilian Health Surveillance Agency. (2016). Available at: http://portal.anvisa.gov.br/resultado-de-busca?p_p_id=101&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_101_struts_action=%2Fasset_publisher%2Fview_content&_101_assetEntryId=2838557&_101_type=document. Accessed 11 June 2021
Behmer, A. O., Talosa, C. M. E., & Neto, F. G. A. (1976). Manual De Técnicas Para Histologia Normal e Patológica (p. 239). São Paulo: Edart.
Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., Anderson, M. J., Arden, K. C., Bleins, J., & Grrenberg, M. E. (1999). Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell, 96, 857–868.
Carraschi, S. P., Cruz, C., Machado Neto, J. G., Ignácio, N. F., Barbuio, R., & Machado, M. R. F. (2012). Histopathological biomarkers in pacu (Piaractus mesopotamicus) infected with Aeromonas hydrophila and treated with antibiotics. Ecotoxicology Environmental Safety, 83, 115–120.
Cattani, D., Cavalli, V. L. L. O., Reig, C. E. H., Domingues, J. T., Dal-Cim, T., Tasca, C. I., Silva, F. R. M. B., & Zamoner, A. (2014). Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: Involvement of glutamate excitotoxicity. Toxicology, 320, 34–45.
Cavalli, V. L. L. O., Cattani, D., Reig, C. E. H., Pierozan, P., Zanatta, L., Parisotto, E. B., Filho, D. W., Silva, F. R. M. B., Pessoa-Pureur, R., & Zamoner, A. (2013). Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radical Biology and Medicine., 65, 335–346.
Cross, D. A. E., Alessi, D. R., Cohen, P., Andjelkovich, M., & Hemmings, B. A. (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature, 378, 785–789.
Dane, H., & Sisman, T. (2017). A histopathological study on the freshwater fish species chub (Squalius cephalus) in the Karasu River, Turkey. Turkish Journal of Zoology, 41(1), 1–11. https://doi.org/10.3906/zoo-1509-21
Destro, A. L. F., Silva, S. B., Gregório, K. P., Oliveira, J. M., Lozi, A. A., Zuanon, J. A., Salaro, A. L., Matta, S. L. P., Gonçanves, R. V., & Freitas, M. B. (2021). Effects of subchronic exposure to environmentally relevant concentrations of the herbicide atrazine in the Neotropical fish Astyanax altiparanae. Ecotoxicology and Environmental Safety, 208, 111601.
EC, European Commission. Commission decision of 10 March 2004 concerning the non-inclusion of atrazina in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this active substance, 2004/248/EC [displayed 10 March 2015]. Available at http://ec.europa.eu/food/plant/protection/evaluation/existactive/oj_atrazina.pdf
Erlacher-Reid, C. D. (2018). Considerations for treatment of large zoologic collections: Fish. Vet Clin Exot Anim, 21, 311–325.
Ferrari, A., Venturino, A., & D’Angelo, A. M. P. (2007). Muscular and brain cholinesterase sensitivities to azinphos methyl and carbaryl in the juvenile rainbow trout Oncorhynchus mykiss. Comparative Biochemistry and Physiology, 146C, 308–313.
Fillols, E., Davis, A. M., Lewis, S. E., & Ward, A. (2020). Combining weed efficacy, economics and environmental considerations for improved herbicide management in the Great Barrier Reef catchment area. Science of the Total Environment, 720, 137481.
Franke, T. F., Kaplan, D. R., & Cantley, L. C. (1997). PI3K: Downstream AKT ion blocks apoptosis. Cell, 88, 435–437.
Franke, T. F., Yang, S. I., Chan, T. O., Datta, K., Kazlauskas, A., Morrison, Kaplan, D. R., & Tsichlis, P. N. (1995). The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell, 81, 727–36.
Guyton, K. Z., Loomis, D., Grosse, Y., El Ghissassi, F., Benbrahim-Tallaa, L., Guha, N., Scoccianti, C., Mattock, H., & Straif, K. (2015). Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. The Lancet Oncology, 16, 490–491.
Hajduch, E., Litherland, G. J., & Hundal, H. S. (2001). Protein kinase B (PKB/Akt)–a key regulator of glucose transport. FEBS Letters, 492, 199–203.
Hou, X., Huang, X., Ai, Z., Zhao, J., & Zhang, L. (2017). Ascorbic acid induced atrazina degradation. Journal of Hazardous Materials, 327, 71–78.
Hu, K., Tian, Y., Du, Y., Huang, L., Chen, J., Li, N., & Liu, W. (2016). Atrazina promotes proliferation of prostate cancer cells. International Journal of Oncology., 48, 2166–2174.
Inoki, K., Li, Y., Zhu, T., Wu, J., & Guan, K. L. (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biology, 4, 648–657.
Instituto Brazileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) – IBAMA. (2020). Relatórios de comercialização de agrotóxicos. 2019. Available at http://www.ibama.gov.br/relatorios/quimicos-e-biologicos/relatoriosdecomercializacao-de-agrotoxicos#historicodecomercializacao. Accessed 4 Jul 2021
Jin, Y., Wang, L., Chen, G., Lin, X., Miao, W., & Fu, Z. (2014). Exposure of mice to atrazina and its metabolite diaminochlorotriazine elicits oxidative stress and endocrine disruption. Environmental Toxicology and Pharmacology., 37, 782–790.
Kurien, B. T., & Scofield, R. H. (2006). Western blotting. Methods. San Diego. 38, 283-293.
Loomis, D., Guyton, K., Grosse, Y., El Ghissasi, F., Bouvard, V., Benbrahim-Tallaa, L., Guha, N., Mattock, H., & Straif, K. (2015). International Agency for Research on Cancer Monograph Working Group, IARC, Lyon France. Lancet Oncology, 16, 891–892.
Mahmood, T., & Yang, P.-C. (2012). Western blot: Technique, theory, and trouble shooting. North American Journal of Medical Science, 4, 429–434.
Manning, B. D., Tee, A. R., Logsdon, M. N., Bleins, J., & Cantley, L. C. (2002). Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Molecular Cell, 10, 151–162.
Marques, M. B. L., Brunetti, I. A., Faleiros, C. A., Cruz, C., Iqbal, H. M. N., Bilal, M., & Américo-Pinheiro, J. H. P. (2021). Ecotoxicological assessment and environmental risk of the insecticide chlorpyrifos for aquatic neotropical indicators. Water, Air, and Soil Pollution, 232, 428. https://doi.org/10.1007/s11270-021-05369-9
Mattos, G.E. (2011). Vias de sinalização e efeito biológico da corticotropina (ACTH), do peptídeo NH2-terminal da próopiomelanocortina (N-POMC) e do fator de crescimento de fibroblastos (FGF2) em culturas primárias de células da suprarenal de ratos. Tese de doutorado, USP-SP.
Mcdonald, D. G. (1983). The effects of H+ upon the gill of fresh water fish. Canadian Journal of Zoology, 61, 691–703.
Melletti, P.C., Rocha, O., & Martinez, C. B. R. (2004) Avaliação da degradação ambiental na Bacia do Rio Mogi-Guaçu por meio de testes de toxicidade com sedimento e análises histopatológicas em peixes. In: Brigante J, Espíndola ELG. Limnologia fluvial: um estudo no rio Mogi-Guaçu. 1 ed. Editora Rima, p. 249–280.
Miron, D., Crestani, M., Schetinger, M. R., Morsch, V. M., Baldisserotto, B., & Tierno., M. A., Moraes, G., Vieira, V. L. P. (2005). Effects of the herbicides clomazone, quinclorac, and metsulfuron methyl on acetylcholinesterase activity in the silver catfish (Rhamdia quelen) (Hepatapteridae). Ecotoxicology and Environmental Safety, 61, 398–403.
Modesto, K. A., & Martinez, C. B. R. (2010). Roundp® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere, 78(3), 294–299.
Montiel-León, J. M., Munoz, G., Duy, S. V., Do, D. T., Vaudreuil, M. A., Goeury, K., Guillemette, F., Amyot, M., & Sauvé, S. (2019). Widespread occurrence and spatial distribution of glyphosate, atrazine, and neonicotinoids pesticides in the St Lawrence and Tributary Rivers. Environmental Pollution, 250, 29–39.
Murphy, L. O., & Blenis, J. (2006). MAPK signal specificity: The right place at the right time. Trends in Biochemical Sciences, 31, 268–275.
Murty, A.S. (1988). Toxicology of pesticide to fish. Boca Raton, Editora: CRC Press.1, 129.
Navé, B. T., Ouwens, D. M., Withers, D. J., Alessi, D. R., & Shepherd, P. R. (1999). Mammalian target of rapamycin is a direct target for protein kinase B: Identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. The Biochemical Journal, 344(2), 427–431.
Oliveira, S. E., Costa, P. M., Nascimento, S. B., Castro, W. V., Ribeiro, R. I. M. A., Santos, H. B., & Thomé, R. G. (2018). Atrazine promotes immunomodulation by melanomacrophage centre alterations in spleen and vascular disorders in gills from Oreochromis niloticus. Aquatic Toxicology, 202, 57–64.
Paiva, P. P., Delcorso, M. C., Matheus, V. A., Queiroz, S. C. do N., Collares-Buzato, C. B., & Arana, S. (2017). Acute toxicity of comercial atrazine in Piaractus mesopotamicus: histophatologival, ultrastructural, molecular and genotoxic evaluation. Veterinary World, 10(9), 1008–1019.
Payne, J. F., Mathieu, A., Melvin, W., & Fancey, L. L. (1996). Acetylcholinesterase, an old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundlan. Marine Pollution Bulletin, 32, 225–231.
Phyu, Y. L., Palmer, C. G., Warne, M. J., Hose, G. C., Chapman, J. C., & Lim, R. P. (2011). A comparison of mixture toxicity assessment: Examining the chronic toxicity of atrazine, permethrin and chlorothalonil in mixtures to Ceriodaphnia cf. dubia. Chemosphere, 85, 1568–1573.
Roux, P.P., & Blenis, J. (2004). ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiology and molecular biology reviews, June, 320–344.
Santos, E. A., Cruz, C., Carraschi, S. P., Silva, J. R. M., Botelho, R. G., Velini, E. D., & Pitelli, R. A. (2015). Atrazina levels in the Jaboticabal water stream (São Paulo State, Brazil) and its toxicological effects on the pacu fish Piaractus mesopotamicus. Archives of Industrial Hygiene and Toxicology, 66, 73–82.
Santos, T. G. (2010). Biomarcadores bioquímicos e genéticos para a detecção dos efeitos do herbicida atrazina no peixe neotropical Prochilodus lineatus. Universidade Estadual de Londrina.
Shiogiri, N. S., et al. (2016). Effects of azithromycin on tilapia (Oreochromis niloticus): health status evaluation using biochemical, physiological and morphological biomarkers. Aquaculture Research, p. 1–15.
Talmi-Frank, D., Strauss-Ayali, D., Jaffe, C. L., & Baneth, G. (2006). Kinetics and diagnostic and prognostic potential of quantitative Western Blot analysis and antigenspecific enzyme-linked immunosorbent assay in experimental canine leishmaniasis. Clinical and Vaccine Immunology. Oxford., 3, 271–276.
Van Dyk, J. C., Cochrane, M. J., & Wagenaar, G. M. (2012). Liver histopathology of the sharptooth catfish Clarias gariepinus as a biomarker of aquatic pollution. Chemosphere, 87(4), 301–311. https://doi.org/10.1016/j.Chemosphere.2011.12.002
Velmurugan, B., Selvanayagam, M., Cengiz, E. I., & Unlu, E. (2007). Histopathology of lambda-cyhalothrin on tissues (gill, kidney, liver and intestine) of Cirrhinus mrigala. Envrionmental Toxicology and Pharmacology, 24, 286–291.
Zajc, A., Neuefeind, T., Prade, L., Reinemer, P., Huber, R., & Bieseler, B. (1998). Herbicide detoxification by glutathione S-transferases as implicated from X-ray structures. Pesticide Sciences., 55, 248–252.
Zhao, F., Li, K., Zhao, L., Liu, J., Suo, Q., Zhao, J., Wang, H., & Zhao, S. (2014). Effect of Nrf2 on rat ovarian tissues against atrazina-induced anti-oxidative response. International Journal of Clinical and Experimental Pathology., 7, 2780–2789.
Zhao, X., Wang, L., Ma, F., Bai, S., Yang, J., & Qi, S. (2017). Pseudomonas sp, ZXY-1 a nwly isolated and highly afficient atrazina-degrading bacterium and optimization of biodegradation using response surface methodology. Journal Environmental Science., 54, 152–159.
Zhou, B. P., Liao, Y., Xia, W., Spohn, B., Lee, M. H., & Hung, M. C. (2001). Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nature Cell Biology., 3, 245–252.
Zimmermann, S., & Moelling, K. (1999). Phosphorylation and regulation of Raf by Akt (protein kinase B). Science, 286, 1741–1744.
Funding
Isabella Alves Brunetti received grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Proc. 2015/03420–6). This work is funded by the Laboratory of Ecotoxicology and Pesticide Efficacy of the Barretos—Brazil. Juliana Heloisa Pinê Américo-Pinheiro thanks Brazil University for the Institutional Research Development and Support Grant.
Author information
Authors and Affiliations
Contributions
Isabella Alves Brunetti: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization, funding acquisition. Pâmela Castro Pereira: methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft. Danilo Sagrillos Oliveira: methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft. Viviane Aline Oliveira Silva: methodology, software, validation, formal analysis, investigation, resources, writing—original draft. Rui Manuel Reis: methodology, software, validation, formal analysis, investigation, resources, writing—original draft. Juliana Heloisa Pinê Américo-Pinheiro: formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration. Claudinei da Cruz: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brunetti, I.A., Pereira, P.C., Oliveira, D.S. et al. Histological Biomarkers and Protein Expression in Hyphessobrycon eques Fish Exposed to Atrazine. Water Air Soil Pollut 233, 97 (2022). https://doi.org/10.1007/s11270-022-05569-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05569-x