Abstract
The metal cutting wastewater was treated with electrocoagulation (EC) and electrochemical-Fenton (EF) method in a bipolar trickle tower reactor. In EC treatment, the COD removal efficiency was obtained as 81.3% with 89.0 kWh/m3 energy consumption using 0.7 mA/cm2 current density and 0.2 mM Na2SO4. With the EF method, 99.2% COD removal was achieved with 78.0 kWh/m3 energy consumption in optimum conditions (0.7 mA/cm2 current density, 90 mM H2O2, 0.2 mM Na2SO4 at pH 3) after 30-min treatment of wastewater. In EC and EF process, TOC and toxicity of the wastewater were decreased during the treatment period in parallel with COD removal as a result of response surface methodology (RSM) optimization. It was observed that the main metal and heavy metal contents were B, Al, Cr, Zn, Ni, Cu, Cd, and Pb when their concentrations were listed in descending order at the level of µg/L, and these heavy metal and boron contents were removed more efficiently in the EC process than EF treatment. The FTIR-ATR spectrum of the sludge observed the strong peaks around 875 cm−1 and 884 cm−1 related to vibrations of the Fe–O bonds in iron oxide after the EC and EF treatment. This study showed that metal cutting wastewater was successfully treated with EC and EF first time in the literature even if it had high COD, TOC, toxicity, and heavy metal contents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of metal cutting fluid has also increased with the development of the metal processing industry today. Cutting fluids are used in all areas of the industry to lubricate machinery and machine parts. Metal cutting fluids include pure oils, emulsions (boron oils), semi-synthetic products, and fully synthetic products. Purification of metal cutting fluids is an important problem. Electrochemical method stands to be a reasonable tool supplying effective organic matter, metal/heavy metal, and toxicity removal required for the legal discharge limit for metal industry wastewater.
The electrochemical wastewater treatment process consists of metal electrodes immersed in an electrolyte with electrical current applied to these electrodes (Mollah et al., 2001). The basis of the electrocoagulation (EC) treatment is precipitation, adsorption, and flotation processes in an electrochemical reactor (Ihara et al., 2004). Aluminum and iron electrodes are used in the EC process because of the high adsorption capacity of the ions produced from these metal electrode surface as a result of current application (Chen, 2004). In the EC, if aluminum or iron electrodes are used as anodes, these electrodes are dissolved and give Al3+, Fe2+, and Fe3+ ions to the solution; these ions also combine with hydroxyl ions in water; and they form metal hydroxides such as Al (OH) 3, Fe (OH) 2, and Fe (OH) 3. The adsorption abilities of metal hydroxide particles formed during EC are quite higher than chemical coagulation (Demirbas and Kobya, 2017). In EC treatment, the following two types of mechanisms are formed during the use of iron electrodes in wastewater (Govindan et al., 2020).
First mechanism:
Second mechanism:
At this stage, the pollutants in the water are deposited by clinging to the formed Fe(OH)2 and Fe(OH)3. Emulsifying agents, suspended solids, and colloidal materials can be stabilized by the EC successfully. When the particles are properly contacted with Fe(OH)2 and Fe(OH)3, the particles are neutralized, and different particles come together to form large flocs without coagulant addition from outside because coagulants are produced as in situ electrochemically (Larue et al., 2003). On the other hand, it is possible to remove the dissolved organic and inorganic contaminants by adsorbing on the surface of these flocs. During the electrochemical treatment, Fe+2 may be simultaneously or separately produced in the water or wastewater with in situ electrochemically, and then the Fenton reaction occurs with following reactions (Govindan et al., 2019).
This mechanism is called as electrochemical-Fenton (EF) method in literature. During the EF process, H2O2 can be produced electrochemically in situ (Gökkuş & Yıldız, 2016), but it generally added to the system from outside chemically because in situ H2O2 production requires high electrical potential application and long contact time (Klidi et al., 2019). The advantage of controlled hydroxyl radicals with promising results for non-biodegradable, persistent, and toxic compounds in the wastewater with EF methods, as compared to other conventional physical, mechanical, and biological treatment methods (Chang et al., 2004). It can be used for the following purposes in the wastewater treatment of the EC process: heavy metal removal, oil–water emulsion breakage, domestic wastewater treatment, drinking water treatment, biochemical oxygen demand (BOD), chemical oxygen demand (COD), nutrient removal like phosphate and nitrogen, suspended solids and colloid material removal, and complex organics removal. The metal cutting wastewaters including these listed pollutants simultaneously create serious environmental problems in aquatic systems, and the EC process is seen as capable and efficient method for this type mixed wastewaters in literature (Bensadok et al., 2008). Bensadok et al. (2008) conducted a study on the treatment of coolant and cutting fluid emulsion by EC method. The highest COD and turbidity removal efficiency was obtained at pH 6–7 when the pH of initial wastewater was carried out at 3, 5, 6, 7, 9, and 11. The optimum EC treatment conditions provided 92% COD and 99% turbidity removal. In another work, Bakırcı (2014) conducted a study on this type of wastewater by electrooxidation (EO) method using steel and EC treatment aluminum and iron electrodes. It was reported that turbidity removal efficiency increased as the voltage increased and the highest removal was obtained with steel electrodes (EO), while the lowest removal was achieved with an aluminum electrode (EC) obtaining more than 90% when the discharge efficiency increased as the number of electrodes increased. Aşkın (2011) carried out the removal of vegetal-based metal cutting fluid from wastewater by EC using a monopolar parallel electrode. As a result of this work, the highest removal efficiency was obtained as 90.6% COD and 99.3% turbidity under the following EC operation condition: pH 4, current density: 80 A/m2; electrolysis time: 30 min; and conductivity: 1 mS/cm. Kobya et al. (2008) carried out the treatment of metal cutting fluid wastewater EC method with iron and aluminum plate to obtain COD and total organic carbon (TOC) removal efficiency varying pH, current density and duration parameters. As a result of the study, the 93.0% COD and 78.0% TOC removal efficiency were observed at 60 A/m2 current density in 25-min treatment time at pH 5 for aluminum electrode, while 92.0% COD and 82.0% TOC removal efficiency was obtained at 60 A/m2 current density in 25-min treatment time at pH 7 for iron electrode under optimum conditions with 0.497 $/m3 and 0.768 $/m3 operating cost for iron and aluminum electrodes, respectively. In other study performed by the same researchers, the metal cutting fluid wastewater was treated by electrochemical precipitation method. As a result of the studies, the current density for iron electrode has been determined as 66.39 A/m2, pH 7.03, and the treatment time as 20.60 min as optimum conditions. For aluminum electrode, the best removal efficiency was achieved with the current density of 62.67 A/m2, pH 5.01, and a treatment time of 24.39 min. COD, TOC, and turbidity removal efficiencies for iron electrode were 93.0%, 83.0%, and 99.8% while COD, TOC, and turbidity removal efficiencies for aluminum electrode were 93.5%, 85.2%, and 99.9%, respectively (Kobya et al., 2011). Izquierdo et al. (2010) investigated the effect of soluble cutting liquids on the treatment efficiency of the electrolyte structure to be used in electrochemical precipitation studies using iron and aluminum electrodes. In electrochemical treatment studies using Al electrode, 0.75 kWh/m3 energy was spent using sodium chloride, and about 5 kWh/m3 energy was used in electrochemical treatment studies using Fe electrode. Sangal et al. (2013) worked at purification of metal cutting fluid with aluminum electrode by electrochemical precipitation method. The optimum value for pH is 6.50, 138.8 A/m2 for current density, and 40 °C for temperature, and 99% removal efficiency is obtained in this study.
As it was seen in the given studies above, although many electrochemical treatment studies are conducted for wastewater with this feature, it was not investigating in terms of heavy metals and toxic character. Also, the optimization of electrochemical treatment parameters such as pH, current density, and supporting electrolyte concentration was not studied in detail. In this study, treatment of metal cutting fluid wastewater was carried out by EC and EF processes, and the effect of pH, flow rate, current density, amount of support electrolyte on COD removal efficiency was optimized using response surface method (RSM) in parallel examining energy consumption of EC and EF processes. By treating metal cutting wastewaters with EC and EF in the bipolar trickle tower reactor under the optimum treatment conditions determined with RSM, COD, and TOC, heavy metals content and toxic character were successfully studied with a holistic approach first time in the literature.
2 Materials and Method
In this study, treatment of the metal cutting wastewater was carried out with electrocoagulation (EC) and electrochemical-Fenton (EF) method in a bipolar trickle tower (BTT) reactor with Rashing shaped iron electrodes operating recycled batch flow condition by means of optimizing EC and EF processes’ operating parameters. The TOC, boron, heavy metals, and cytotoxicity analysis of the treated wastewater were performed in the BTT reactor. For the characterization of sludge samples obtained by electrocoagulation (EC) and electrochemical-Fenton (EF) treatment, the morphological analysis was carried out via (FTIR). Details of these methods are described in the following sub-sections.
2.1 Electrochemical Treatment Setup
The BTT reactor used in electrochemical studies consisted of two intertwined glass pipes with the outer diameter of 4 cm and the inner diameter of 2.5 cm. The length of this column is 21.5 cm. The inside of the BTT reactor was filled with Rasching shaped iron electrode, and the BTT reactor consisted of 27 layers containing 5 electrodes in each layer. The placement and arrangement of the electrodes used in the reactor was made as given in the literature (Koparal et al., 2007). Insulating materials were placed between each layer; thus, the Rasching shaped iron electrodes were separated from each other, and bipolarity was provided for each electrode placed in the reactor. The operated experimental set is shown in Fig. 1. In the experimental studies, the BTT reactor with a volume of 125 mL was worked with recirculated batch operation using 50 mL/min flow rate.
2.2 Determination the Influent and Effluent Characteristic of the Wastewater
The real metal cutting wastewater characteristic used in this work is given in Table 1. The metal cutting wastewater with a volume of 500 mL was treated in the bipolar trickle tower (BBT) reactor.
Conductivity and pH values of the wastewater samples were measured with pH meter (Thermo Scientific Orion STAR A215). During the experiment, samples were taken from the reactor at certain time intervals during 30-min treatment time. These collected samples were centrifuged with the centrifuge (Nuve NF 800 R) for 7 min at 7000 rpm. The supernatant was analyzed to determine the removal efficiency of EC and EF methods by the utilization of chemical oxygen demand (COD). The COD values of wastewater were determined according to TS 2789 ISO 6060 Water Quality-Determination of Chemical Oxygen Demand standard during the treatment process using a thermo reactor (Merck Spectro quant TR 420).
The total organic carbon (TOC) values of the collected samples taken under batch conditions from EC and EF treatment process were evaluated with TOC analyzer (SHIMADZU, TOC-LCP, and ASI-L) for the metal cutting wastewater.
The heavy metal concentrations both of influent and effluent were analyzed with Inductively Coupled Plasma-Mass Spectrometer (Agilent 7800 ICP-MS) according to EPA 6020A 2007–02.
2.3 Determination the Reaction Kinetic of EC and EF Processes
The reaction kinetic of electrocoagulation (EC) and electrochemical-Fenton (EF) treatment was examined with first-order reaction kinetic model. The reaction constant was determined with linear regression coefficient (R2) between t given x-axis and \(In (\frac{{C}_{0}}{{{C}}_{t}})\) given in y-axis according to following Eq. 12 and Eq. 13:
where dC/dt is the COD removal rate, k is the first-order reaction constant, and C is the COD concentration of sample.
2.4 Optimization of the Electrochemical Process Parameters with RSM
Response surface method (RSM) was used for optimization of electrochemical treatment processes parameter for modelling and analyzing to these parameters to show linear and non-linear interactions of the treatment parameters investigating COD removal efficiencies. Fractional design of experiment with 3 factors and 3 levels was made for RSM. The parameters affecting the electrochemical treatment studies, their code, and their levels used in the process are given in Table 2. The parameters were desired as the current density (mA/cm2), Na2SO4 concentration (mM), and initial pH. Minitab 19.0 was used for RSM statistical technique, and Pareto chart, surface plot/counter plot, and regression equation were obtained from RSM analysis.
2.5 Cytotoxicity Assays
The cytotoxicity experiments were conducted with Microtox test procedure using Vibrio fischeri bacteria comparing untreated model wastewater after the utilization of H2O2 for the optimum treatment condition obtained in EC and EF treatment studies. The Microtox system (Model 500 Analyzer) was supplied from Azur Environmental (Carlsbad, CA, USA). The lyophilized Vibrio fischeri bacteria (NRRL number B-11177, Microtox Acute reagent, Modern Water) with luminescence characteristics was used as test microorganism. The Vibrio fischeri bacteria were incubated with reconstitution solution (non-toxic ultra-pure water, Modern Water) and osmatic adjuster solution (22% sodium chloride, Modern Water) at 15 °C for 15 min in glass cells. Four serial dilutions of the sample were prepared with dilution solution (2% sodium chloride, Modern Water). The toxicity reduction was expressed as relative toxicity index (RTI) with lethal concentration required for 50% death of bacteria (LC50) of untreated and treated wastewater for EC and EF studies expressed as Eq. 14:
where RTI is the relative toxicity index (dimensionless), \({LC}_{50} t=0\) is the lethal concentration required for 50% population of bacteria at time 0 (%), and \({LC}_{50} t=t\) is the lethal concentration required for 50% population of bacteria at time t (%).
2.6 Sludge Characterization
The characterization of the resultant sludge samples was carried out by Fourier transform infrared spectrophotometer (FTIR-Shimadzu IRTracer-100) and attenuated total reflectance (ATR-Pike Tech). FTIR-ATR spectroscopy was allowed to analyze solid or liquid samples to be measured with small sample preparation without KBr disk requirement easily and fast. The sludge samples were dried at 80 ℃ for 1 h to remove water moisture. The prepared sludge samples were analyzed with FTIR-ATR with 4 cm−1 resolution between 4000 and 700 cm−1 wavelengths.
3 Results
3.1 The Results of Electrocoagulation (EC) and Electrochemical-Fenton (EF) Treatment
The results of process conditions of electrocoagulation (EC) and electrochemical-Fenton (EF) are given in Figs. 2 and 3, respectively.
COD removal efficiency and energy consumption of EC method. A The current density effect on the COD removal efficiency in EC, B current density effect on energy consumption in EC, C the effect of Na2SO4 concentration on COD removal efficiency in EC, D the effect of Na2SO4 concentration on energy consumption in EC, E initial pH on COD removal efficiency in EC, F the effect of initial pH on energy consumption in EC
The treatment results of EC and EF methods are summarized in Table 3 with the total energy consumption (kWh/m3), first-order reaction constant (min−1), and linearity.
The COD removal increases while the current density value increases up to 0.9 mA/cm2 from 0.2 mA/cm2 as a result of the EC conducted in Fig. 2 and Table 3. When the effect of current density change on energy consumption was examined, it was seen that energy consumption increased with increasing current as expected. In EC treatment, the COD removal efficiency was obtained as 81.3% with 89.0 kWh/m3 energy consumption under the optimum treatment conditions using 0.7 mA/cm2 current density and 0.2 mM Na2SO4 at pH 9.
It was seen that these results obtained in EC treatment studies are compatible with the literature. Tir and Moulai-Mostefa (2008) investigated the effect of pH, current density, and electrolysis time parameters on the treatment of oil industry wastewater by using EC method using an aluminum electrode with parallel plate design in the continuously stirred tank reactor. As a result of their study, 89.6% COD and more than 99.0% turbidity removal efficiency were obtained during the 22-min electrolysis period at pH 7 and applying 25 mA/cm2 current density. Unlike this previous study, the BTT reactor setup was operated in this paper, and similar COD removal efficiency was obtained at lower current density due to the increased electrode surface area in the BTT reactor setup. In addition, the BTT reactor was operated as recycled dispersed plug flow reactor, which is rarely reported in the literature. The main reason for the same removal efficiency at lower current density was that the removal utilization efficiency of dispersed plug flow reactors is higher than that of continuously stirred tank reactors. Similarly, Canizares et al. (2007) performed the purification of oil–water emulsion by electrochemical methods. As a result of the study, the initial pH of wastewater was reported as effective factor on the removal efficiency when the pH value varied between 5 and 9. As the current density raised, the COD removal efficiency increased with the same rate, while the COD removal efficiency decreases with increasing oil concentration. Likewise, Bensadok et al. (2008) conducted a study on the treatment of emulsion by EC method. The effect of current density was studied at values of 100, 150, and 200 A/m2, and it was concluded that as the current density increased, the COD and turbidity removal efficiency raised. PH studies were carried out at pH 3, 5, 6, 7, 9, and 11, and best COD and turbidity efficiency were obtained at pH 6–7. At the end of the study, the best conditions provided were COD and turbidity removal, respectively, of 92.0% and 99.0%. The data presented in this article was obtained from the BTT dispersed plug flow reactor, unlike the parallel plate completely stirred tank reactor generally used in literature.
There were any other studies where the BTT reactor was used for the treatment of metal cutting wastewater, but the efficiency of BTT reactor was examined to remove the toxic pollutants such as dye and phenol in the literature. Koparal et al. (2007) distinguished Basic Red 29 removal with electrochemical oxidation in BTT reactor with boron-doped diamond electrodes. They examined the effect of dye concentration, supporting electrolyte concentration, current density, flow rate, and pH on chemical oxygen demand (COD) and color removal efficiency. As a result, they obtained 97.2% color and 91.0% COD removal efficiency at 1 mA/cm2 current density, 36.3 mL/min flow rate, 5.8 pH value, and 0.01 M Na2SO4 concentration. These results differed from the fallouts obtained in this presented article since the wastewater characteristics and the input organic pollution load in terms of COD were different. In the similar study, Yavuz et al. (2008) performed the phenol removal by electrochemical oxidation in BTT plug flow reactor with boron-doped diamond electrodes. They attained the 99.5% phenol and over 95.0% COD removal efficiency at 200 mg/L initial phenol concentration, in the presence of 0.03 M NaCl with 60-min treatment period (Yavuz et al., 2008). The same research group investigated Basic Blue3 (BS3) removal with the same method and same BTT reactor of another study. Within the scope of their study, the removal efficiency was examined based on the color, temperature, flow rate, support electrode concentration, dye concentration, and COD. As a result, 99% color and 86.7% COD removal efficiency were obtained under the optimum treatment conditions (0.875 mA/cm2 current density, 30 °C temperature, 109.5 mL/min flow rate, and 0.01 M support electrode concentration (Yavuz et al., 2011). Achieving high removal efficiency in these studies for the removal of toxic pollutants such as dye and phenol indicated that the 99.2% COD obtained in our study was consistent for metal cutting wastewater. It is known that the supporting electrolyte type and concentration affect the treatment efficiency of electrochemical treatment as indicated in Fig. 2 and Table 3. Na2SO4 was used in this present article since the permissible concentration in water is allowed to rise up to 250 mg/L, and it creates less side reactions in this present article.
As could be seen in the studies summarized above, the EC system is a very complex process, and optimization for the current density, the support electrolyte concentration, and the initial wastewater pH is required for each wastewater with different characteristics to be treated. The effect of these three parameters must be investigated with the COD removal response as a function of the current density versus Na2SO4 concentration and the COD removal response as a function of and pH versus Na2SO4 concentration because of the complexity of EC treatment. When EC treatment studies were examined, it was seen that the degradation mechanism changes depending on the experimental parameters. The current density (0.3, 0.7, and 0.9 mA/cm2), the addition of sodium sulfate as the support electrolyte concentrations (0.1, 0.2, and 0.3 M Na2SO4), and initial pH (3, 6, and 9) were investigated according to factorial design randomly. It was clearly stated that the effects of these electrochemical treatment parameters and which parameters were more effective on COD removal for this type of wastewater in the Pareto chart given in Fig. 3. The regression equation expressing the effects of these parameters on removal efficiency was given in Eq. 15, where A is the current density (mA/cm2), the supporting electrolyte concentration of Na2SO4 (mg /L), and C is initial pH to express in a more comprehensible mode.
It was determined that the concentration of Na2SO4 (B) is more effective than current density (A) and initial pH (C) on EC treatment when the removal efficiency response showed the linear terms of the current density (A) and the concentration of Na2SO4 (B) and the initial pH (C). The surface plot and the contour plot of the COD removal as a function of Na2SO4 concentration versus the current density and Na2SO4 concentration versus initial pH are shown in Fig. 4 and obtained from RSM analysis with the fractional design of EC treatment.
The RSM analysis of EC (the surface plot (A) and the contour plot (B) of the COD removal response as a function of the current density versus Na2SO4 concentration, and the surface plot(C) and the contour plot (D) of the COD removal response as a function of and initial pH versus Na2SO4 concentration)
As a result of RSM analysis with the fractional design of the treatment, it was investigated that the most effective parameter of EC treatment was the current density. The main reason why the current density was the most effective parameter of them was thought that the current density played an effective role in the Fe+2 dissolved from the anode passing current. It was examined that Na2SO4 addition altered the COD removal efficiency, while the initial pH of wastewater had any significant effect on the COD removal. The initial pH value of wastewater increased to about pH 9 in a very short time (about 5 min) regardless of the input pH value due to OH− producing from cathode as seen as Eq. 7, so the pH of wastewater stayed basic condition during EC treatment. Similarly, Gökkuş et al. (2014) reported that Taguchi method was useful to determine the optimum treatment conditions such as initial pH, Fe2+ and H2O2 concentrations, initial dye concentration, and mixing rate for color and COD removal with Fenton oxidation process. The main objective of this presented study was determining the optimum treatment conditions of EC process and then enhancing COD removal efficiency by EF method for the treatment of the metal cutting wastewater.
The EC experimental setup created by using iron electrodes could be easily converted to the EF method by adding additional H2O2 from the outside because Fe+2 has already produced on site dissolving from the anode passing current the amount. To improve the treatment performance, the efficiency of EF method on the metal cutting wastewater is also investigated by adding H2O2 to EC system, and the effect of EF method on COD removal and energy consumption is illustrated Fig. 5.
As can be seen in Table 1 and Fig. 5, the real wastewater of metal cutting with a high COD concentration is successfully treated for the first time in the literature with the EC and EF methods. With the EF method, 99.2% COD removal was achieved with 78.0 kWh/m3 energy consumption in optimum conditions (0.7 mA/cm2 current density, 90 mM H2O2, 0.2 mM Na2SO4 at pH 3) after 30-min treatment of wastewater. The COD reduction mechanism of wastewater was explained in first-order reaction kinetics in the EC and EF treatment. The reaction rate constant was found as 0.0568 min−1 in EC treatment and 0. 1445 min−1 in EF treatment for the optimum treatment condition as seen in Table 3. Sangal et al. (2013) reported that the turbidity removal at different current densities for the EC treatment of the soluble oil–water emulsion showed first-order kinetics obtaining 0.02923 min−1, 0.03082 min−1, 0.105128 min−1, and 0.040314 min−1 rate constant for 59.2 A/m2, 99.92 A/m2, 138.8 A/m2, and 179.8 A/m2 current density, respectively. Dirany et al. (2012) reported first-order reaction kinetic for EF treatment under the optimum treatment conditions (50 mM Na2SO4 and 0.2 mM Fe+2, at 60 mA, pH 3.0 using a Pt/carbon electrodes). The reaction kinetics found in our study was compatible with the literature when compared to the electrochemical studies.
In the EF method, high COD removal efficiency could be attained in the treatment of high pollution load wastewater in the literature like metal cutting wastewater. Similarly, in this presented work, Chachou et al. (2015), the treatment of oily water emulsions was purified by the EF method, and they carried out using two parallel plate electrodes in the form of stainless steel, graphite, and Ti/Pt electrodes adding Fe2SO4 from outside and producing in situ H2O2. The highest COD removal efficiency with 93.6% was obtained at pH 3, 0.15 A current value, 0.05 M Na2SO4 amount, 0.015 M Fe2SO4 amount, and 180 min electrolysis with graphite electrode. Since the producing H2O2 in situ electrochemically takes time in this reverse EF production method conducted, the longer hydraulic retention time was applied in the previous study. In this presented study, the hydraulic retention time and energy consumption were reduced by adding H2O2 from the outside. In this process presented in this paper addition, the operating cost can be decreased since Ti and Pt electrodes are more expensive than Fe and Al electrodes.
The total organic carbon (TOC) is a potential alternative to both the COD and the BOD5 tests and has the advantage of being both faster and potentially more precise than the COD test. The effects of EC and EF treatment on TOC removal efficiency are given in the Fig. 6.
Comparison of COD and TOC Removal Efficiency of EC and EF method (EC was conducted at optimum treatment condition following 0.7 mA/cm2 current density and 0.2 mM Na2SO4 at pH 9, and EF was conducted at optimum treatment condition following 0.7 mA/cm2 current density, 90 mM H2O2, 0.2 mM Na2SO4 at pH 3)
Wastewater contains organic matters, and these organic matters in wastewater can be biodegradable and non-biodegradable. These organic substances can be measured in the biochemical oxygen demand for 5 days (BOD5), the chemical oxygen demand (COD) test, and the total organic carbon (TOC) test. Chemical oxygen demand includes carbon-based BOD, non-biodegradable organic material, and biomass (Eq. 16). Generally, COD value is higher than the BOD5 due to non-biodegradable organic material ingredients for industrial wastewater.
There are a few types of complex organic compounds that cannot be broken down even in the COD test. In this situation, TOC measurement could be more effective to determine organic ingredients of wastewater. TOC measures the total organic ingredients of wastewater using a high precision thermo catalytic oxidation with a high temperature TOC analyzer. TOC could be used as an alternative measurement to the COD test for monitoring the wastewaters and effluents. TOC and COD comparison given in Fig. 5 of influent and effluent samples of metal cutting wastewater shows that this wastewater contained the complex organic compounds that cannot be broken down even in the COD test. TOC values of the samples were reported higher than their COD values resulting complex organic compounds during the EC and EF treatments. Also, it could be inferred that the complex organic ingredients of metal cutting wastewater could not treated effectively due to lower TOC removal efficiency obtained EC and EF processes. EF process is more effective on the complex organic ingredients of metal cutting wastewater than EC treatment according to Fig. 6. The difference between TOC and COD value could lead to toxicity because of the complex organic ingredients.
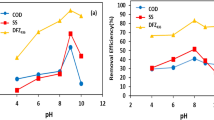
Metal cutting wastewater can contain boron compounds as inorganic borate salts dissolved in oil, fatty acid amide, 2,2'-oxycehanol, sodium sulfonate, alkyl amide, tall oil fatty acid, N, N, methylenebismorpholine, isopropanol, and heavy metal resulting cutting process of metal (Shah, 2009). In this study, boron and heavy metal concentration of metal cutting wastewater were investigated during the EC and EF treatments in addition to organic content. The boron concentration in the wastewater obtained with ICP-MS is given in Fig. 7. The heavy metal concentration in the wastewater obtained with ICP-MS is given in Fig. 8.
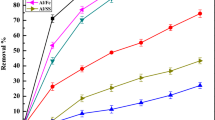
As a result of ICP-MS, it was determined that the boron content was approximately 1000 mg/L, and it could be removed effectively with EC and EF treatment (Fig. 7). According to Fig. 8, it is observed that the main metal and heavy metal contents were Al, Cr, Zn, Ni, Cu, Cd, and Pb when their concentrations were listed in descending order at the level of µg/L. The ICP-MS results showed that the metal and heavy metal content were removed more efficiently in the EC process than EF treatment. Even if there is no legal limit for boron discharge to the receiving water environment, the metal cutting wastewater does not meet the discharge limit set for the color parameter due to the milky color given by the boron-containing emulsion. Therefore, it is necessary to ensure boron removal as well as organic pollution of wastewater considering the metal and heavy metal content.
Comparison of heavy metal removal efficiency of EC and EF Method (EC was conducted at optimum treatment condition following 0.7 mA/cm2 current density and 0.2 mM Na2SO4 at pH 9, and EF was conducted at optimum treatment condition following 0.7 mA/cm2 current density, 90 mM H2O2, 0.2 mM Na2SO4 at pH 3)
3.2 The Results of Cytotoxicity Assays
The of cytotoxicity reduction of metal cutting wastewater is shown in Fig. 9 using the relative toxicity index in the EC and EF treatment.
The results in Fig. 9 constitute a proof for the efficiency and usability of EC and EF method for the treatment of metal cutting wastewater. In the proposed EC and EF method, it attained toxicity removal in parallel with high COD, TOC, and metal and heavy metal removal within 60 min of treatment. Although this level of treatment efficiency can be satisfactory for discharge, the cytotoxicity test is necessary for the protection of aquatic life. Also electrooxidation processes like EF tend to turn into more toxic forms of heavy metals and permanent organic compounds with toxic properties that these type wastewaters have. Libralato et al. (2008) reported the ethanolamines toxicity in oily wastewater with evaporation and air-stripping to assess via Microtox bioassay investigating the monoethanolamine, diethanolamine, and triethanolamine specifically. Such analysis is crucial due to the possibility of treated wastewater to contain cytotoxins that might have adverse effects to human health and ecosystem in case of the more toxic intermediate by-products could be formed especially in EF process. It is seen that the toxicity decreased below the initial toxicity of the wastewater during the treatment period in Fig. 9. The summarized treatment results of EC and EF treatment are compared with the legal national discharge limit for metal industry wastewater including metal cutting, grinding, and sanding facilities in Turkey in the Table 4 (Su Kirliliği Kontrolü Yönetmeliği, 2004).
According to the compared results given in the Table 4, EC and EF method stands to be a reasonable tool with its high COD, TOC, metal/heavy metal, and toxicity removal efficiency compared to legal discharge limit for metal industry wastewater including metal cutting, grinding, and sanding facilities in Turkey (Su Kirliliği Kontrolü Yönetmeliği, 2004).
3.3 The Results of Sludge Characterization
For the characterization of sludge produced by the EC and EF treatment, the resultant sludge samples were analyzed with Fourier transform infrared spectrophotometer (FTIR-Shimadzu IRTracer-100) and attenuated total reflectance (ATR-Pike Tech). FTIR-ATR spectrum of sludge formed in EC and EF treatment is given in Fig. 10 and the related FTIR spectrum must be placed after this paragraph) with 4 cm−1 resolution between 4000 and 700 cm−1 wavelengths.
Comparison of FTIR-ATR spectrum of sluge samples formed in EC (
 line) and EF treatment (
line) and EF treatment (
 line) (EC was conducted at optimum treatment condition following 0.7 mA/cm2 current density and 0.2 mM Na2SO4 at pH 9, and EF was conducted at optimum treatment condition following 0.7mA/cm2 current density, 90 mM H2O2, 0.2 mM Na2SO4 at pH 3)
line) (EC was conducted at optimum treatment condition following 0.7 mA/cm2 current density and 0.2 mM Na2SO4 at pH 9, and EF was conducted at optimum treatment condition following 0.7mA/cm2 current density, 90 mM H2O2, 0.2 mM Na2SO4 at pH 3)
As seen from FTIR spectra of spectrum of sludge samples formed in EC and EF treatment, the peaks of 2954 cm−1, 2920 cm−1, 2850 cm−1, 2113 cm−1, 1919 cm−1, 1562 cm−1, 1454 cm−1, 1377 cm−1, 1087 cm−1, 875 cm−1, and 884 cm−1 peaks are presented in the Fig. 10. The major peak was 1087 cm−1 EC treatment, while 1056 cm−1 and 999 cm−1 were presented in the EF treatment. Peaks at 2954 cm−1, 2920 cm−1, and 2850 cm−1 were corresponded to C-H stretching mode of saturated C–C bonds, and these peaks showed the presence of hydrocarbons in the sludge (Aswathy et al., 2016). Also, C–H stretching vibrations as asymmetric and symmetric were observed at 2920 cm−1 and 2850 cm−1, respectively, indicating methylene groups presented in the sludge in EC and EF treatment (Nidheesh & Gandhimathi, 2014). The peak at 2113 cm−1 and 1919 cm−1 indicated the C = C stretching vibrations of aromatic compounds. The peaks of 1562 cm−1, 1454 cm−1, 1377 cm−1, 1087 cm−1, 875 cm−1, and 884 cm−1 were presented in the FTIR spectra of Fe sludge sample attributing to O–H stretching which implies the Fe floc formation containing oxyhydroxides (Gönder et al., 2017). The other peaks at 1087 cm−1, 875 cm−1, and 884 cm−1 were also reported due to C-N, C-O, and C–C groups, respectively (Danial et al., 2019). The strong band produced around 1087 cm−1 can be attributed to C–O–C vibrations and bands at 875 cm−1 and 884 cm−1 are related to vibrations of the Fe–O bonds in iron oxide. After the EE and EF treatment, the produced sludge contained the wastewater ingredients and supporting electrolyte solution. Similar sludge characterization studies were carried out since the properties of the sludge formed after treatment would determine the disposal or sludge treatment methods and their cost. Although less sludge formation listed among the advantages of the EF method, elemental characterization of formed sludge would be necessary as it could be a possible option to reuse that sludge in the appropriate area instead of its treatment or disposal. In our study, Fe+2 ion dissolved from electrode surface converted iron oxide in sludge according to FTIR results.
4 Conclusion
This work presented the results of the metal cutting wastewater treatment with electrocoagulation (EC) and electrochemical-Fenton (EF) method in a bipolar trickle tower reactor with Rashing shaped iron electrodes operating recycled batch flow condition. The results were summarized in the following list.
-
In EC treatment, the COD removal efficiency was obtained as 81.3% with 89.0 kWh/m3 energy consumption using 0.7 mA/cm2 current density and 0.2 mM Na2SO4.
-
With the EF method, 99.2% COD removal was achieved with 78.0 kWh /m3 energy consumption in optimum conditions (0.7 mA/cm2 current density, 90 mM H2O2, 0.2 mM Na2SO4 at pH 3) after 30-min treatment of wastewater.
-
The COD reduction mechanism of wastewater was explained first-order reaction kinetics in the EC and EF treatment. The reaction rate constant was found as 0.0568 min−1 in EC treatment and 0. 1445 min−1 in EF treatment.
-
As a result of RSM analysis with the fractional design of the treatment, it was determined that current density more effective than the concentration of Na2SO4 and initial pH on EC treatment, and the removal efficiency response showed the linear terms of the current density and the concentration of Na2SO4 and the initial pH.
-
TOC values of the samples were reported higher than their COD values resulting complex organic compounds during the EC and EF treatments. EF process was more effective on the complex organic ingredients of metal cutting wastewater than EC treatment.
-
In EC and EF process, it was seen that the cytotoxicity decreased below the initial toxicity of the wastewater during the treatment period.
-
It was observed that the main metal and heavy metal contents were Al, Cr, Zn, Ni, Cu, Cd, and Pb when their concentrations were listed in descending order at the level of µg/L. The ICP-MS results showed that the metal and heavy metal content were removed more efficiently in the EC process than EF treatment
-
In the FTIR-ATR spectrums of the major peak was 1087 cm−1 EC treatment, while 1056 cm−1 and 999 cm−1 were presented in the EF treatment. Peaks at 2954 cm−1, 2920 cm−1 and 2850 cm−1 were corresponded to C-H stretching mode of saturated C–C bonds, and these peaks showed the presence of hydrocarbons in the sludge. The strong band produced around 1087 cm−1 can be attributed to C–O–C vibrations, and bands at 875 cm−1 and 884 cm−1 are related to vibrations of the Fe–O bonds in iron oxide. After the EE and EF treatment, the produced sludge contained the wastewater ingredients, and supporting electrolyte solution.
Availability of Data and Materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request with following link: https://drive.google.com/drive/folders/12sA17XZcT3wB2NyFLqqcWlB5MozL3vrf?usp=sharing.
References
Aswathy, P., Gandhimathi, R., Ramesh, S. T., & Nidheesh, P. V. (2016). Removal of organics from bilge water by batch electrocoagulation process. Separation and Purification Technology, 159, 108–115.
Aşkın, Ş. (2011). Bitkisel Tabanlı Metal Kesme Sıvılarının Elektrokimyasal çöktürme Yöntemi İle Giderimi. Kocaeli: Gebze Yüksek Teknoloji Enstitüsü, Mühendislik ve Fen Bilimleri Enstitüsü.
Bakırcı, B. (2014). Kesme Sıvılarının Karakterizasyonu Ve Elektrokimyasal çöktürme ile Atıksulardan Arıtımı. Zonguldak: Bülent Ecevit Üniversitesi, Fen Bilimleri Enstitüsü.
Bensadok, K., Benammar, S., Lapicque, F., & Nezzal, G. (2008). Electrocoagulation of cutting oil emulsions using aluminium plate electrodes. Journal of Hazardous Materials, 152(1), 423–430.
Canizares, P., Martínez, F., Lobato, J., & Rodrigo, M. A. (2007). Break-up of oil-in-water emulsions by electrochemical techniques. Journal of Hazardous Materials, 145(1–2), 233–240.
Chachou, L., Gueraini, Y., Bouhalouane, Y., Poncin, S., Li, H. Z., & Bensadok, K. (2015). Application of the electro-Fenton process for cutting fluid mineralization. Environmental Technology, 36(15), 1924–1932.
Chang, P. H., Huang, Y. H., Hsueh, C. L., Lu, M. C., & Huang, G. H. (2004). Treatment of non-biodegradable wastewater by electro-Fenton method. Water Science and Technology, 49(4), 213–218.
Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38(1), 11–41.
Danial, R., Sobri, S., Abdullah, L. C., & Mobarekeh, M. N. (2019). FTIR, CHNS and XRD analyses define mechanism of glyphosate herbicide removal by electrocoagulation. Chemosphere, 233, 559–569.
Demirbas, E., & Kobya, M. (2017). Operating cost and treatment of metalworking fluid wastewater by chemical coagulation and electrocoagulation processes. Process Safety and Environmental Protection, 105, 79–90.
Dirany, A., Sirés, I., Oturan, N., Özcan, A., & Oturan, M. A. (2012). Electrochemical treatment of the antibiotic sulfachloropyridazine: Kinetics, reaction pathways, and toxicity evolution. Environmental Science & Technology, 46(7), 4074–4082.
Govindan, K., Suresh, A. K., Sakthivel, T., Murugesan, K., Mohan, R., Gunasekaran, V., & Jang, A. (2019). Effect of peroxomonosulfate, peroxodisulfate and hydrogen peroxide on graphene oxide photocatalytic performances in methyl orange dye degradation. Chemosphere, 237, 124479.
Govindan, K., Sumanasekara, V. D., & Jang, A. (2020). Mechanisms for degradation and transformation of β-blocker atenolol via electrocoagulation, electro-Fenton, and electro-Fenton-like processes. Environmental Science: Water Research & Technology, 6(5), 1465–1481.
Gökkuş, Ö., Çoşkun, F., Kocaoğlu, M., & Yıldız, Y. Ş. (2014). Determination of optimum conditions for color and COD removal of Reactive Blue 19 by Fenton oxidation process. Desalination and Water Treatment, 52(31–33), 6156–6165.
Gökkuş, Ö., & Yıldız, Y. Ş. (2016). Application of electro-Fenton process for medical waste sterilization plant wastewater. Desalination and Water Treatment, 57(52), 24934–24945.
Gönder, Z. B., Balcıoğlu, G., Vergili, I., & Kaya, Y. (2017). Electrochemical treatment of carwash wastewater using Fe and Al electrode: Techno-economic analysis and sludge characterization. Journal of Environmental Management, 200, 380–390.
Ihara, I., Kanamura, K., Shimada, E., & Watanabe, T. (2004). High gradient magnetic separation combined with electrocoagulation and electrochemical oxidation for the treatment of landfill leachate. IEEE Transactions on Applied Superconductivity, 14(2), 1558–1560.
Izquierdo, C. J., Canizares, P., Rodrigo, M., Leclerc, J., Valentin, G., & Lapicque, F. (2010). Effect of the nature of the supporting electrolyte on the treatment of soluble oils by electrocoagulation. Desalination, 255(1–3), 15–20.
Klidi, N., Proietto, F., Vicari, F., Galia, A., Ammar, S., Gadri, A., & Scialdone, O. (2019). Electrochemical treatment of paper mill wastewater by electro-Fenton process. Journal of Electroanalytical Chemistry, 841, 166–171.
Kobya, M., Ciftci, C., Bayramoglu, M., & Sensoy, M. (2008). Study on the treatment of waste metal cutting fluids using electrocoagulation. Separation and Purification Technology, 60(3), 285–291.
Kobya, M., Demirbas, E., Bayramoglu, M., & Sensoy, M. (2011). Optimization of electrocoagulation process for the treatment of metal cutting wastewaters with response surface methodology. Water, Air, & Soil Pollution, 215(1–4), 399–410.
Koparal, A. S., Yavuz, Y., Gürel, C., & Öğütveren, Ü. B. (2007). Electrochemical degradation and toxicity reduction of CI Basic Red 29 solution and textile wastewater by using diamond anode. Journal of Hazardous Materials, 145(1–2), 100–108.
Larue, O., Vorobiev, E., Vu, C., & Durand, B. (2003). Electrocoagulation and coagulation by iron of latex particles in aqueous suspensions. Separation and Purification Technology, 31(2), 177–192.
Libralato, G., VolpiGhirardini, A., & Avezzù, F. (2008). Evaporation and air-stripping to assess and reduce ethanolamines toxicity in oily wastewater. Journal of hazardous materials, 153(3), 928–936.
Mollah, M. Y. A., Schennach, R., Parga, J. R., & Cocke, D. L. (2001). Electrocoagulation (EC)—science and applications. Journal of Hazardous Materials, 84(1), 29–41.
Nidheesh, P. V., & Gandhimathi, R. (2014). Effect of solution pH on the performance of three electrolytic advanced oxidation processes for the treatment of textile wastewater and sludge characteristics. RSC Advances, 4(53), 27946–27954.
Sangal, V. K., Mishra, I., & Kushwaha, J. (2013). Electrocoagulation of soluble oil wastewater: Parametric and kinetic study. Separation Science and Technology, 48(7), 1062–1072.
Shah, F.U. (2009). Boron compounds as additives to lubricants: Synthesis, characterization and tribological optimization. Swedish: Luleå University of Technology, Environmental and Natural Resources Engineering, Sustainable Process Engineering.
Su Kirliliği Kontrolü Yönetmeliği (2004). TC Çevre ve Şehircilik Bakanlığı, Ankara.
Tir, M., & Moulai-Mostefa, N. (2008). Optimization of oil removal from oily wastewater by electrocoagulation using response surface method. Journal of Hazardous Materials, 158(1), 107–115.
Yavuz, Y., Koparal, A. S., & Öğütveren, Ü. B. (2008). Phenol degradation in a bipolar trickle tower reactor using boron-doped diamond electrode. Journal of Environmental Engineering, 134(1), 24–31.
Yavuz, Y., Koparal, A. S., & Öğütveren, Ü. B. (2011). Electrochemical oxidation of Basic Blue 3 dye using a diamond anode: Evaluation of colour, COD and toxicity removal. Journal of Chemical Technology & Biotechnology, 86(2), 261–265.
Author information
Authors and Affiliations
Contributions
Tuğçe GEDİK: Electrochemical experiments, data curation, validation, and visualization; Fadime KARAER ÖZMEN: TOC analysis, ICP-MS analysis, toxicity analysis, FTIR analysis, formal analysis, writing-original draft preparation, visualization, investigation. Filiz BAYRAKCI KAREL: Supervisor, conceptualization, methodology, and investigation. Ali Savaş KOPARAL: Conceptualization, methodology, investigation, reviewing, proof reading, and editing.
Corresponding author
Ethics declarations
Ethics Approval
The authors have consulted the Ethical Responsibilities stated in the Instructions for Authors in preparing the submitted manuscript. The authors hereby declare that this article has been submitted only to this, has not been published already, and is not under consideration for publication or in press elsewhere.
Ethical Conduct
The authors hereby declare that this article was prepared with ethical responsibilities. The plagiarism report was supplied with data and material availability link. Results were presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gedik, T., Özmen, F.K., Karel, F.B. et al. Treatment of Metal Cutting Wastewaters in Bipolar Trickle Tower Reactor by Electrocoagulation and Electrochemical-Fenton methods: Reduction of Organic Matter, Boron, Heavy Metals, and Toxicity with Sludge Characterization. Water Air Soil Pollut 232, 481 (2021). https://doi.org/10.1007/s11270-021-05423-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05423-6