Abstract
In the present investigation, treatment of metal cutting wastewater (MCW) using electrocoagulation (EC) process is designed and analyzed using response surface methodology (RSM). RSM is applied to optimize the operating variables viz. initial pH, current density, and operating time on the treatment of MCW in a batch mode by EC process using iron and aluminum electrodes. Quadratic models are developed for the responses such as chemical oxygen demand (COD), total organic carbon (TOC), and turbidity, and operating cost is calculated with respect to energy, electrode, and chemical consumptions. The actual COD, TOC, and turbidity removal efficiencies at optimized conditions are found to be 93.0%, 83.0%, and 99.8% for Fe electrode and 93.5%, 85.2%, and 99.9% for Al electrode, respectively, which agree well with the predicted response. The proposed model fits very well with the experimental data with R 2 adjusted correlation coefficients of 0.927 for COD, 0.924 for TOC, and 0.968 for turbidity removal for Al and 0.904 for COD, 0.976 for TOC, and 0.989 for turbidity removal for Fe electrodes, respectively. This study clearly shows that RSM is one of the suitable methods to optimize the operating conditions and maximize the COD, TOC, and turbidity removal efficiencies for both electrodes while keeping the operating costs to minimal (0.371 €/m3 for Fe and 0.337 €/m3 for Al electrodes).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metal cutting fluids (MCFs) are an essential component of heavy manufacturing facilities such as automotive engine, transmission, and stamping plants. MCFs have two important functions which are to lubricate between workpiece and tool and to remove heat generated during cutting and forming processes. Different types of oily wastes are generated, and the natures of these mainly depend on the working system and the type of machine used for each process. The main components in MCFs are refrigerant (water), lubricants (mineral, vegetable, and synthetic and semisynthetic oils), tensioactives (surfactants), inhibitors for corrosion (amines, borates, nitrites, etc.), biocides, and additives of high pressure, antiwear, and antifoam (Byers 2006; Anderson et al. 2003). MCFs are divided into two sections which are water-based and oil-based MCFs. Water content varies depending on the source, but oils in this group generally contain between 3% and 60% water. A wide variety of oil-contaminated waters and spent emulsions contains up to 15% mineral oils or combinations of mineral, vegetable, or animal oils. The main problem with MCFs is that they become contaminated with use, losing their properties and effectiveness, and have to be replaced by new ones, thus yielding metal cutting wastewater (MCW), which are generally high in chemical oxygen demand (COD), total organic carbon (TOC), and turbidity. The amount of MCWs generated from metal cutting operations increases every year, constituting a serious danger to the environment due to their high surface active and organic pollutant loads (Greeley and Rajagopalan 2004). These wastewaters require treatment prior to disposal to meet local sewer discharge standards. Various treatment methods for MCWs are microfiltration and ultrafiltration, adsorption, chemical coagulation, evaporation, chemical emulsion splitting and precipitation, and aerobic and anaerobic biological process (Byers 2006; Cheng et al. 2006; Hilal et al. 2004; Rios et al. 1998). The high organic content of most MCWs makes them good candidates for degradation by bacteria for waste treatment, but the high oil content of these fluids is typically eliminated using biological treatment as the primary treatment method. Biological treatment has been used to remove MCFs (i.e., to trap oil and solids from cutting fluids). However, these systems are prone to fouling by the free oils. In addition, the oil concentrate still needs to be disposed and a large volume of water must be treated to separate a small amount of oil (<5%). Thus, a chemical or physical treatment process almost always precedes the use of biological treatment for MCFs. However, biological treatment is an excellent secondary or tertiary treatment of MCFs for organics reduction. This process with chemical treatment is most economical for large users and especially in cases where there is not an option for sewer discharge.
Recently, there has been considerable interest in identifying new technologies that are capable of meeting more stringent treatment standards. For this purpose, electrocoagulation (EC) process has a more prominent role in the treatment of MCWs since it provides some significant advantages such as quite compact and easy operation and automation, no chemical additives, a shorter retention time, high sedimentation velocities, more easier dewatering, and reduced amount of sludge due to the lower water content. It can also prevent the production of unwanted side products (Chen 2004; Mollah et al. 2001). Therefore, EC has the potential to be the distinct economical and environmental choice for treatment of various industrial wastewaters and other related water management issues, and this treatment process has been applied to treat various types of industrial wastewaters (Kobya et al. 2006a, b, 2009, 2010; Kobya and Delipinar 2008; Kabdasli et al. 2009; Ponselvan et al. 2009; Arslan-Alaton et al. 2008; Barrera-Diaz et al. 2006; Can et al. 2003; and so on).
A few literature studies have been available only for the treatment of metal cutting and oily wastewaters by EC (Kobya et al. 2008; Bensadok et al. 2008; Tir and Moulai-Mostefa 2008; Canizares et al. 2007, 2008; Khemis et al. 2005; Xu and Zho 2004; Sanchez-Calvo et al. 2003; Bergmann et al. 2003). EC treatment of MCWs has been tested on a laboratory scale, and good removal of COD, TOC, and turbidity at varying operating conditions is obtained. The process efficiency in the EC process is generally affected by many factors like pH, current density (CD), electrolyte concentration, electrode type, time, and operating cost. The optimization of these factors may significantly increase the process efficiency and practicality, and EC technology has been improved to minimize operating cost and maximize pollutant (i.e., BOD, COD, TOC, turbidity) effluent parameters. In conventional multifactor experiments, optimization is performed by varying a single factor and keeping the rest of the factors constant at a specific set of conditions. This method is very time consuming and incapable of producing an effective optimization (Olmez 2009; Prasad et al. 2008; Saravanathamizhan et al. 2007; Korbahti et al. 2007).
In this study, response surface methodology (RSM) is employed to optimize and to evaluate interactions of influencing factors on the treatment efficiency of MCW with a limited number of planned experiments. RSM focuses on effects and relationships of the main process variables to maximize COD, TOC, and turbidity treatment efficiencies and to minimize operating cost effect of third operational parameters: initial wastewater pH, current density, and operating time. Operating costs of the process at the optimum conditions are also evaluated for both electrodes.
2 Materials and Methods
2.1 Metal Cutting Wastewater
MCW was supplied by a local manufacturing company in Gebze (Turkey) which is involved in the machining of metallic pieces for the automotive sector. Two cubic meters of MCW per month is produced approximately for the effluent generated in the manufacturing processes. MCW used in this experimental work was maintained at 4°C to minimize microbial growth in the wastewater sample. The characteristics of MCW used in the study are given in Table 1.
2.2 Experimental Setup and Procedure
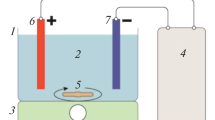
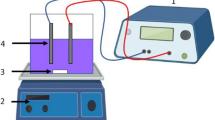
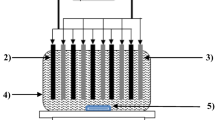
The characteristics of EC process and reactor are given in Table 2. The EC experiments are conducted in a 4.5-l Plexiglas reactor with a dimension of 255 mm × 195 mm × 90 mm. Aluminum (Al) or iron (Fe) plates (220 mm × 50 mm × 4 mm) are chosen as the anode/cathode pair for their performances, and the electrodes are situated 45 mm apart from each other and connected in a monopolar parallel mode, yielding a total effective electrode surface area of 660 cm2. Four electrodes are adopted in the electrolytic cell for all experimental run. The electrodes are connected to a digital DC power supply (Agilent 6675A model; 120 V, 18 A) operated at galvanostatic mode (Kobya et al. 2009; Kobya and Delipinar 2008). In each run, 4.0 l of the wastewater solutions is placed into the EC reactor. The current density is adjusted to a desired value and the EC is started. At the end of EC, the solution is filtered and then is analyzed. At the end of the run, the electrodes are washed thoroughly with water to remove any solid residues on the surfaces, dried and reweighted.
2.3 Experimental Design and Data Analysis
RSM is a collection of mathematical and statistical techniques, commonly used for improving and optimizing processes. It can be used to evaluate the relative significance of several affecting factors in the presence of complex interactions. When a combination of several independent variables and their interactions affects desired responses, RSM is an effective tool for optimizing the process (Myers and Montgomery 2002). RSM uses an experimental design such as the composite central design (CCD) to fit a model by least squares technique. This methodology optimizes for COD, TOC, and turbidity removals from MCWs in the EC process in terms of the process variables to provide more comprehensive interpretation and quantitative assessment of the treatment process.
The Design Expert 7.1.3 software (trial version) is used for the statistical design of experiments and data analysis and performed in duplicate. The three most important operating variables, initial wastewater pH (x 1), current density (x 2), and operating time (x 3), are optimized. The study ranges are chosen as initial wastewater pH 4–8, current density 30–100 A/m2, and operating time 10–40 min for the EC process using Al and Fe electrodes. Initial wastewater pH (x 1), current density (x 2), and operating time (x 3) are chosen as three independent variables in the EC process. Their range and levels are given in Table 3.
In this table, the coded values for x 1, x 2, and x 3 are set at five levels −2, −1 (minimum), 0 (central), +1 (maximum), and + 2. Three dependent parameters are analyzed as responses, COD, TOC, and turbidity removals, in order to obtain optimum initial wastewater pH, current density, and operating time and to achieve highest removal of COD, TOC, and turbidity and lowest operating cost (as euros per cubic meter or euros per kilogram COD) for the treatment of MCW using the EC process. For Fe and Al electrodes, the actual design of this work is presented in Tables 4 and 5. These tables show the CCD in the form of a 23 full factorial design with three additional experimental trials (run numbers 1, 6, and 17) as replicates of the central point. Analysis of variance (ANOVA) is used for graphical analyses of the data to obtain the interaction between the process variables and the responses. The quality of the fit polynomial model is expressed by the coefficient of determination R 2, and its statistical significance is checked by the Fisher F test in the same program. Model terms are evaluated by the P value (probability) with 95% confidence level.
2.4 Chemical Analysis and Operating Costs
COD, TOC, and turbidity contents of liquid samples are monitored. All analyses are performed according to the standard method for water and wastewater analysis (Cleceri et al. 1998). COD is measured spectrophotometrically and analyzed by closed reflux colorimetric method (Perkin Elmer Lambda 35 UV/VIS spectrophotometer, USA). The TOC levels are determined through combustion of the samples at 680°C using a nondispersive IR source (Tekmar Dohrmann Apollo 9000). The turbidity (NTU) of samples is analyzed using a Mettler Toledo 8300 model turbidimeter control. The pH is measured using Mettler Toledo 2050 model pH control, and the conductivity is determined with Mettler Toledo 7100 model conductivity meter control system. The accuracy of all measured values for COD, TOC, and turbidity is estimated around 3%.
The operating cost (euros per cubic meter or euros per kilogram COD) for the EC process including mainly chemical, electrode, and electrical energy costs is calculated (Kobya et al. 2009, 2010), and results for both electrodes are shown in Tables 4 and 5.
3 Results and Discussion
3.1 Statistical Analysis
In the present work, the relationship between three variables (namely initial wastewater pH, current density, and operating time) and three important process responses (namely COD, TOC, and turbidity removal efficiencies) for the EC process using iron and aluminum electrodes is analyzed using RSM. Significant model terms are desired to obtain a good fit in a particular model. The CCD for Fe and Al electrodes in Tables 4 and 5 allows the development of mathematical equations where predicted results (y i ) are assessed as a function of x 1, x 2, and x 3 as the sum of a constant, three first-order effects (terms in x 1, x 2, and x 3), three interaction effects ((x 1·x 2), (x 1·x 3), (x 2·x 3)), and three second-order effects (\( x_1^2 \), \( x_2^2 \), and \( x_3^2 \)).
The adequacy of the RSM is justified through ANOVA. The ANOVA results for response parameters for Fe and Al electrodes are shown in Tables 6 and 7. Only terms found statistically significant are included in the model. For Fe and Al electrodes, Tables 6 and 7 illustrated the quadratic models in terms of coded factors and also showed other statistical parameters. F values from the ANOVA are 23.55 for COD, 22.67 for TOC, and 55.50 for turbidity removal for Al and 17.68 for COD, 72.12 for TOC, and 156.52 for turbidity removal for Fe electrodes, indicating that the model is significant. The large value of F indicates that most of the variation in the response could be explained by regression equation. The associated P value <0.01 indicates that the model is considered to be statistically significant. Values of Prob > F are less than 0.5 which shows that the model terms are significant. The R 2 coefficient gives the proportion of the total variation in the response variable accounted for by the predictors (x's) included in the model. A high R 2 value, close to 1, is desirable and has a reasonable agreement with adjusted R 2. A high R 2 coefficient ensures a satisfactory adjustment of the quadratic model to the experimental data. In the present study, the adjusted values of R 2 (Tables 6 and 7) for Al and Fe electrodes are 0.927 for COD, 0.924 for TOC, and 0.968 for turbidity removal and 0.904 for COD, 0.976 for TOC, and 0.989 for turbidity removal, respectively. The coefficient of variance (CV) as the ratio of the standard error of estimate to the mean value of the observed response (as a percentage) is a measure of reproducibility of the model. As shown in Tables 6 and 7, CV for COD, TOC, and turbidity removal is found to be 3.18%, 1.70%, and 3.73% for Fe electrode and 2.51%, 2.41%, and 0.26% for Al electrode, respectively. Adequate precision (AP) compares the range of the predicted values at the design points to the average prediction error. Ratios of CV > 4 indicate that adequate model is desirable. The AP values for Al and Fe electrodes used in the EC process are found to be 12.18 for COD, 28.54 for TOC, and 41.60 for turbidity removal and 16.8 for COD, 15.4 for TOC, and 25.5 for turbidity removal which indicate an adequate signal (Tables 6 and 7).
The following fitted regression models (equations in terms of coded values for the regressors) are used to quantitatively investigate the effects of initial pH, current density, and operating time on the characterization of the EC process for COD, TOC, and turbidity removal efficiency. Experimental results are evaluated using approximating functions of dependent variables COD (y 1), TOC (y 2), and turbidity (y 3) removal percents for the EC process using Fe and Al electrodes. These approximating functions are presented in Eqs. 1, 2, and 3 for Fe electrode and Eqs. 4, 5, and 6 for Al electrode:
The mathematical relationship between energy (ENC, kilowatt hour per cubic meter or kilowatt hour per kilogram COD) and electrode consumptions (ELC, kilogram Fe or Al per cubic meter or kilogram Fe or Al per kilogram COD), operating cost (OC, euros per cubic meter or euros per kilogram COD), and variables such as x 1, x 2, and x 3 is obtained (Tables 4 and 5).
The actual and the predicted COD, TOC, and turbidity removal efficiencies by EC process using Al and Fe electrodes are shown in Figs. 1 and 2. Actual values are the measured response data for a particular run, and the predicted values are evaluated from the model and generated by using the approximating functions. It is seen in Figs. 1 and 2 that the data points lie close to the diagonal line and the developed model is adequate for the prediction of each response.
Equations 1, 2, and 3 for Fe electrode and Eqs. 4, 5, and 6 for Al electrode in the EC process are used to visualize the effects of experimental factors on responses under optimized conditions in 3D graphs of Figs. 3 and 4. COD, TOC, and turbidity removal percentages are obtained with the operating parameters which are current density (30–100 A/m2), operating time (10–40 min), and pH (4–8), respectively (Figs. 3 and 4).
The COD, TOC, and turbidity removal efficiencies are found to increase with an increase in CD values, at any value of initial pH and operating time. Generally, it is known that COD, TOC, and turbidity removals increase with increasing Fe and Al dosages in chemical coagulation. It may be inferred from Figs. 3 and 4 that higher COD removal efficiency is achieved at higher CD and operating time (t EC) values. At higher CD, higher dissolution of electrode material (Faraday's law) with higher rate of formation of iron or aluminum hydroxides and some polymeric metal complexes resulted in higher COD, TOC, and turbidity removal efficiencies due to coprecipitation. In the case of Al electrode, monomeric species such as Al(OH)2+, Al(OH) +2 , Al2(OH) 4+2 , and Al(OH) −4 and polymeric species such as Al6(OH) 3+15 , Al7(OH) 4+17 , Al8(OH) 4+20 , Al13O4(OH) 7+24 , and Al13(OH) 5+34 are formed during the EC process (Can et al. 2003). In the case of Fe electrode, Fe(OH)2+, Fe(OH) +2 , Fe2(OH) 4+2 , Fe(OH) −4 , Fe(H2O) +2 , Fe(H2O)5OH2+, Fe(H2O)4(OH) +2 , Fe(H2O)8(OH) 4+2 , and Fe2(H2O)6(OH) 2+4 are produced (Kobya et al. 2009). Formation rates of these different species depend on pH of the medium and types of ions present and played an important role in the EC process. A decrease of the removal efficiency at a pH less than 4 and higher than 8 is observed and is attributed to an amphoteric behavior of Al(OH)3, Fe(OH)2, and Fe(OH)3 which led to soluble Al3+, Fe2+, and Fe3+ cations when the initial pH is low and to monomeric anions, Al(OH) −4 and Fe(OH) −4 when the initial pH is high. These soluble species are useless for water treatment. Therefore, initial pH in the EC process is kept in the range 4–9. Also, more sludge is produced from electrodes at higher CD values due to elevated dissolution rate of anode. Increased amount of sludge enhanced COD, TOC, and turbidity removal efficiencies due to sweep coagulation at higher CD and operating time values.
Maximum COD, TOC, and turbidity removal efficiencies as well as operating variables (CD, t EC, and pH) at optimized values for treatment of MCW are 88.43%, 79.1%, 99.8%, 66.39 A/m2, 20.60 min, and 7.03 pH for Fe electrode and 90.11%, 81.30%, 99.9%, 62.67 A/m2, 24.39 min, and 5.01 pH for Al electrode, respectively. Operating costs for Fe and Al electrodes at optimum conditions are 0.80 €/m3 (or 0.22 €/kg COD) and 1.08 €/m3 (or 0.28 €/kg COD).
4 Conclusions
The statistical analysis shows that all the variables set in the preparation of the model, within the tested boundaries of the model, have significant effect on the model. Current density, initial pH, and operating time for the treatment of MCW influence the COD, TOC, and turbidity removal efficiencies. The optimal electrocoagulation conditions for maximum removal efficiencies of COD, TOC, and turbidity found by RSM are current density (66.39 A/m2), initial pH (7.03), and operating time of EC (20.60 min) for Fe electrode and current density (62.67 A/m2), initial pH (5.01), and operating time of EC (24.39 min) for Al electrode. It is characterized by desirability of 0.728 for Fe electrode and 0.819 for Al electrode. The actual COD, TOC, and turbidity removals at optimized conditions are found to be 93.0%, 83.0%, and 99.8% for Fe electrode and 93.5%, 85.2%, and 99.9% for Al electrode, respectively, which confirms close to the predicted response using RSM. Minimal operating costs for Fe and Al electrodes are 0.371 and 0.337 €/m3. A mathematical approach is useful for picturing the theoretical electrocoagulation of metal cutting wastewater treatment.
References
Anderson, J. E., Kim, B. R., Mueller, S. A., & Lofton, T. V. (2003). Composition and analysis of mineral oils and other compounds in metalworking and hydraulic fluids. Critical Reviews in Environmental Science and Technology, 33, 73–109.
Arslan-Alaton, I., Kabdaşlı, I., Hanbaba, D., & Kuybu, E. (2008). Electrocoagulation of a real reactive dyebath effluent using aluminum and stainless steel electrodes. Journal of Hazardous Materials, 150, 166–173.
Barrera-Diaz, C., Roa-Morales, G., Avila-Cordoba, L., Pavon-Silva, T., & Bilyeu, B. (2006). Electrochemical treatment applied to food-processing industrial wastewater. Industrial & Engineering Chemical Research, 45, 34–38.
Bensadok, K., Benammar, S., Lapicque, F., & Nezzal, G. (2008). Electrocoagulation of cutting oil emulsions using aluminium plate electrodes. Journal of Hazardous Materials, 152, 423–430.
Bergmann, H., Rittel, A., Iourtchouk, T., Schoeps, K., & Bouzek, K. (2003). Electrochemical treatment of cooling lubricants. Chemical Engineering and Processing, 42, 105–119.
Byers, J. P. (2006). Metalworking fluids. New York: CRC.
Can, O. T., Bayramoglu, M., & Kobya, M. (2003). Decolorization of reactive dye solutions by electrocoagulation using aluminum electrodes. Industrial & Engineering Chemistry Research, 42, 3391–3396.
Canizares, P., Martinez, F., Jimenez, C., Saez, C., & Rodrigo, M. A. (2008). Coagulation and electrocoagulation of oil-in-water emulsions. Journal of Hazardous Materials, 151, 44–51.
Canizares, P., Martinez, F., Lobato, J., & Rodrigo, M. A. (2007). Break-up of oil-in-water emulsions by electrochemical techniques. Journal of Hazardous Materials, 145, 233–240.
Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38, 11–41.
Cheng, C., Phipps, D., & Alkhaddar, R. M. (2006). Thermophilic aerobic wastewater treatment of waste metalworking fluids. Water and Environment Journal, 20, 227–232.
Cleceri, L. S., Greenberg, A. E., & Eaton, A. D. (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington DC: American Public Health Association.
Greeley, M., & Rajagopalan, N. (2004). Impact of environmental contaminants on machining properties of metal working fluids. Tribology International, 37, 327–332.
Hilal, N., Busca, G., Hankins, N., & Mohammad, A. (2004). The use of ultrafiltration and nanofiltration membranes in the treatment of metal-working fluids. Desalination, 167, 227–238.
Kabdasli, I., Arslan, T., Ölmez-Hanci, T., Arslan-Alaton, I., & Tünay, O. (2009). Complexing agent and heavy metal removals from metal plating effluent by electrocoagulation with stainless steel electrodes. Journal of Hazardous Materials, 165, 838–845.
Khemis, M., Tanguy, G., Leclerc, J. P., Valentin, G., & Lapicque, F. (2005). Electrocoagulation for the treatment of oil suspensions. Trans IChemE, Part B, Process Safety and Environmental Protection, 83, 50–57.
Kobya, M., Ciftci, C., Bayramoglu, M., & Sensoy, M. T. (2008). Study on the treatment of waste metal cutting fluids using electrocoagulation. Separation and Purification Technology, 60, 285–291.
Kobya, M., & Delipinar, S. (2008). Treatment of the baker’s yeast wastewater by electrocoagulation. Journal of Hazardous Materials, 154, 1133–1140.
Kobya, M., Demirbas, E., & Akyol, A. (2009). Electrochemical treatment and operating cost analysis of textile wastewater using sacrificial iron electrodes. Water Science and Technology, 60, 2261–2270.
Kobya, M., Demirbas, E., Dedeli, A., & Sensoy, M. T. (2010). Treatment of rinse water from zinc phosphate coating by batch and continuous electrocoagulation processes. Journal of Hazardous Materials, 173, 326–334.
Kobya, M., Hiz, H., Senturk, E., Aydiner, C., & Demirbas, E. (2006b). Treatment of potato chips manufacturing wastewater by electrocoagulation. Desalination, 190, 201–211.
Kobya, M., Senturk, E., & Bayramoglu, M. (2006a). Treatment of poultry slaughterhouse wastewaters by electrocoagulation. Journal of Hazardous Materials, 133, 172–176.
Korbahti, B. K., Aktas, N., & Tanyolac, A. (2007). Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. Journal of Hazardous Materials, 148, 83–90.
Mollah, M. Y. A., Schennach, R., Parga, J. P., & Cocke, D. L. (2001). Electrocoagulation (EC)—science and applications. Journal of Hazardous Materials, 84, 29–41.
Myers, R. H., & Montgomery, D. C. (2002). Response surface methodology: Process and product optimization using designed experiments (2nd ed.). USA: Wiley.
Olmez, T. (2009). The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. Journal of Hazardous Materials, 162, 1371–1378.
Ponselvan, F. I. A., Kumar, M., Malviya, J. R., Srivastava, V. C., & Mall, I. D. (2009). Electrocoagulation studies on treatment of biodigester effluent using aluminum electrodes. Water, Air, and Soil Pollution, 199, 371–379.
Prasad, R. K., Kumar, R. R., & Srivastava, S. N. (2008). Design of optimum response surface experiments for electro-coagulation of distillery spent wash. Water, Air, and Soil Pollution, 191, 5–13.
Rios, S., Pazos, C., & Coca, J. (1998). Destabilization of cutting oil emulsions using inorganic salts as coagulants. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 138, 383–389.
Sanchez-Calvo, L., Leclerc, J.-P., Tanguy, G., Cames, M. C., Paternotte, G., Valentin, G. (2003). An electrocoagulation unit for the purification of soluble oil wastes of high COD. Environmental Progress, 22, 57–65.
Saravanathamizhan, R., Mohan, N., Balasubramanian, N., Ramamurthi, V., & Basha, C. A. (2007). Evaluation of electro-oxidation of textile effluent using response surface methods. Clean, 35, 355–361.
Tir, M., & Moulai-Mostefa, N. (2008). Optimization of oil removal from oily wastewater by electrocoagulation using response surface method. Journal of Hazardous Materials, 158, 107–115.
Xu, X., & Zho, X. (2004). Treatment of refectory oily wastewater by electrocoagulation process. Chemosphere, 56, 889–894.
Acknowledgements
This research work is a part of TUBITAK project. The authors thank TUBITAK for their financial support of this work under contract TUBITAK-CAYDAG-104Y267.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobya, M., Demirbas, E., Bayramoglu, M. et al. Optimization of Electrocoagulation Process for the Treatment of Metal Cutting Wastewaters with Response Surface Methodology. Water Air Soil Pollut 215, 399–410 (2011). https://doi.org/10.1007/s11270-010-0486-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0486-x