Abstract
Biochemical methane potential (BMP) and specific methanogenic activity (SMA) tests are performed to assess sludges’ ability to degrade substrates anaerobically. Traditionally, the Modified Gompertz model is applied to estimate methane production and to describe methanization kinetics. However, technical literature reports other models that can be used for these purposes and may be equally or much suitable. This work aimed to determine the BMP and SMA of four types of anaerobic sludges, conduct a kinetic study of methane production, apply six mathematical models in BMP assays, and evaluate which one best describes the methanization bioprocess. The tests were carried out in batch reactors (50 mL reaction volume) maintained at mesophilic temperature (35 °C) and under agitation (150 rpm). Glucose and a volatile fatty acids solution were used as substrates. As inoculants, sanitary sludge, brewery sludge, and two sludges from the swine wastewater treatment (SWS) were used, one of which was previously adapted to microaerobic conditions. SWS degraded the substrates more quickly and returned the highest BMP, SMA, and methane concentration values in the biogas. Thus, in studies of methane production and energy recovery, the application of SWS can be advantageous. Mathematical models that describe exponential functions, mainly the Fitzhugh model, were the most adequate to describe methane production kinetics from readily biodegradable substrates.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Given the paradigm shift to a circular bio-based economy in the context of massive waste generation, anaerobic digestion (AD) is a well-established biological process that has great potential to contribute to this change by providing efficient treatment of organic wastes and the energy generation from biogas (Rodrigues et al., 2019). The biogas generated in the AD, which is mainly composed of methane (CH4) and carbon dioxide (CO2), can be used to generate heat and electricity for industry and household use, being considered as a clean gas source to meet energy demand (Li et al., 2020). After purification and concentration, the CH4 obtained can be applied as a vehicle fuel, which adds value to the treatment and the product (Santos et al., 2020).
Adequate control of the full-scale anaerobic process requires knowledge about each applied substrate in biochemical methane potential (BMP), a parameter that reflects the CH4 yield (Rodrigues et al., 2019). The BMP is a crucial parameter for determining which substrates are most viable in methanization and assessing the microbial consortium’s ability to degrade substrates anaerobically. Besides that, the BMP test can provide the specific methanogenic activity (SMA) for an inoculum, which represents the rate at which methanogenic microorganisms can use the substrate to produce CH4 and CO2 (Huzir et al., 2019).
According to Raposo et al. (2011), the BMP assay is influenced by various factors (e.g., source of inoculum, substrate, biogas quantification method, and operational conditions). Among the factors that most influence the experimental BMP determination, the type of inoculum is one of the most important. The inoculum provides the initial syntrophic microbial population for anaerobic reactors start-up, and the start-up time depends on how well this initial pool is balanced (De la Rubia et al., 2018). Besides that, the inoculum may contain macronutrients that can affect the enzymatic activity of the microorganisms and, consequently, the CH4 yield in the AD process (Pellera & Gidarakos, 2016).
In this scope, it is essential to choose an inoculum with high BMP and SMA aiming at greater CH4 generation and reactor efficiency on removing organic matter in waste treatment plants. A range of works in the literature have already studied the effect of different sources of inoculum in the BMP assay, including sludges from anaerobic digesters treating agro-industrial or municipal wastes, animals manure, and landfill leachate (Barrena et al., 2018; Córdoba et al., 2016; Pozdniakova et al., 2012; Suksong et al., 2019; Man Zhou et al., 2019; Gu et al., 2014).
BMP test also allows obtaining the cumulative methane production curves (CMPC), where the cumulative specific CH4 production is plotted against experimental time. These kinetic curves depend on the substrate’s characteristics, the methanogenic microbial community activity, and the presence of inhibitory compounds on the media (Filer et al., 2019). Nevertheless, experimental BMP tests are very time consuming (e.g., 20 to > 100 days) (Da Silva et al., 2018; Ware & Power, 2017), in addition to not having a protocol fully established in the literature (Koch et al., 2019), which makes its use difficult by industrial treatment plants and consulting companies (Rodrigues et al., 2019).
The development of mathematical models to describe AD and the kinetic modeling of the process can overcome these limitations. In this way, kinetic modeling is a tool that provides an overview of AD reaction mechanisms and that helps to describe several specific parameters for monitoring the performance of digesters (Çetinkaya & Yetilmezsoy, 2019). Using the most suitable kinetic models for different real situations can improve the methanogenic process by predicting the system behavior, including conditions not empirically tested. Furthermore, it is also possible to optimize anaerobic reactors design and verify the technical–economic feasibility of adopting a specific substrate or inoculum in a full-scale AD plant for waste treatment, enabling more assertive technical decisions (Filer et al., 2019; Yang et al., 2016).
For these reasons, the literature already has a wide variety of studies evaluating different models to describe AD in different conditions (Mozhiarasi et al., 2020; Santos et al., 2020; Sun et al., 2015; Zan et al., 2019), mainly to verify the effect of different substrates in CH4 yield. In BMP tests, the Modified Gompertz mathematical model is traditionally applied to estimate CH4 production and describe methanization kinetics. However, technical literature reports other models, such as First-order exponential, Fitzhugh, Monomolecular, Logistic, and Transference, that may be equally or much suitable (Jijai et al., 2016; Sun et al., 2015). Also, research that promotes the study of kinetic modeling using different inoculants in the BMP assay is essential to investigate its use according to the need for different waste treatment plants aiming to optimize the CH4 yield in each situation. Besides, it is crucial to measure the effect of each inoculum in different conditions.
Therefore, this work aimed to determine the BMP and SMA of four types of anaerobic sludges, carry out a kinetic study of methane production, apply six mathematical models in BMP assays, and evaluate which one best describes the methanization bioprocess.
2 Material and Methods
2.1 Inoculum and Substrate
For a more robust analysis regarding the mathematical models that best describe CH4 production kinetics, two substrates (glucose—GL and volatile fatty acids—VFA) and four different microbial consortia were used in the BMP tests. Inocula with a more complex microbial composition were used, such as swine sludge and brewery sludge, and a sanitary sludge, a less complex inoculum.
GL and VFA are synthetic substrates well-established in the literature and very easily biodegradable carbon sources (Angelidaki et al., 2009). The VFA solution consisted of acetic acid (HAc), propionic acid (HPr), and butyric acid (HBu), in the proportion of 1:1:1 g chemical oxygen demand (COD). Inocula were characterized in terms of total solids (TS), total volatile solids (TVS), and total fixed solids (TFS). Brewery sludge (BS) (82.4 ± 2.0 gTS L−1; 34.5 ± 0.9 gTVS L−1; and 47.9 ± 1.1 gTFS L−1) was collected from an upflow anaerobic sludge blanket reactor (UASB) that treated brewery wastewater. Sanitary sludge (SS) (55.5 ± 0.8 gTS L−1; 37.9 ± 0.6 gTVS L−1; 17.6 ± 1.4 gTFS L−1) was obtained from a UASB reactor in a sewage treatment plant located in Fortaleza, Ceará, Brazil.
The same sanitary sludge was subsequently acclimated to swine wastewater (SW) treatment and used in the BMP tests. Sanitary sludge was adapted to the SW in two different conditions: anaerobic (anaerobic swine sludge—SWSAN) and microaerobic (microaerobic swine sludge—SWSMI). The adaptation was carried out under mesophilic temperature (35 °C) in two lab-scale UASB reactors (3.5 L). In sludge collection, both reactors were operated for 90 days, and one of them received micro-aeration by supplying atmospheric air (0.5 LO2 L−1 day−1). The SWSAN had a characterization of 51.3 ± 0.6 gTS L−1, 35 ± 2.0 gTVS L−1, and 16.3 ± 2.6 gTFS L−1; and SWSMI presented 51.5 ± 0.8 gTS L−1, 36.9 ± 5.0 gTVS L−1, and 14.6 ± 1.7 gTFS L−1.
2.2 BMP and SMA Tests
Three batch assays were used for each substrate, and three endogenous controls (blank assays) were used for each inoculum. The endogenous controls contained only inoculum with basal medium and buffer, i.e., without a carbon source from the substrate, assessing residual CH4 production due to endogenous decay. The BMP was determined by subtracting the CH4 gross output from the endogenous controls (Holliger et al., 2016).
The borosilicate glass reactors had 110 mL total volume, with 50 mL for the reaction volume and 60 mL for the headspace. The substrate/microorganism ratio (S/X) applied was 0.53 ± 0.03 gCOD gVS−1. The nutrient solutions used in the BMP tests to promote optimal microbial metabolism (Bertolino et al., 2008) were macronutrients (mg L−1)—NH4Cl (500); KH2PO4 (650); K2HPO4 (150); MgCl2 (100); CaCl2.2H2O (100); Na2S.7H2O (50); FeCl3.6H2O (2), and micronutrients (mg L−1)—ZnCl2 (0.05); CuCl2.2H2O (0.03); MnCl2.4H2O (0.5); (NH4)6Mo7O24.4H2O (0.05); CoCl2.6H2O (2); NiCl2.6H2O (0.05); H3BO3 (0.01); AlCl3.6H2O (0.05), and HCl solution (1 mL L−1). The initial pH was corrected to 7.0 with 1 N NaOH, and sodium bicarbonate (NaHCO3) was added as a buffer in the proportion of 1 g of buffer for each 1 g of COD (Santos et al., 2020).
The bottles were sealed with butyl rubber stoppers and aluminum crimps. Gaseous nitrogen (N2) was used as a purge gas for 1 min for each reactor (Çetinkaya & Yetilmezsoy, 2019; Santos et al., 2020). The reactors were maintained at mesophilic temperature (35 ± 1 °C) under orbital agitation of 150 rpm until the CH4 production was constant (Filer et al., 2019). The experiment was completed only when it was observed that the daily CH4 production for three consecutive days was less than 1% of the accumulated CH4 volume, which occurred between the 28th and 35th (Holliger et al., 2016).
Volumetric CH4 quantification was performed using a manometric pressure transmitter (Warme LTDA, Brazil). At the experiment’s beginning and end of the experiment, analyses of pH, series of solids, and total, particulate, and soluble chemical oxygen demand (COD) were performed. The physical–chemical analyses were performed according to Standard Methods for the Examination of Water and Wastewater (APHA, 2017). At the end of the batch test, a 1 mL biogas sample from each batch reactor was analyzed by gas chromatography to determine the biogas composition (CH4, CO2, H2S, N2). Biogas samples were collected with gas-tight syringes (SampleLock syringe, Hamilton Company, USA). The biogas composition was analyzed by gas chromatography-barrier ionization discharge (GC BID-2010 Plus, Shimadzu Corporation, Japan) equipped with a GS GASPRO column (60 m × 0.32 mm) (Agilent Technologies Inc., USA). The oven, injector, and detector temperatures were 250, 50, and 100 °C, respectively. Helium gas was used as the carrier gas in a flow of 2 mL min−1, and the run time of the method was 9 min (Morais et al., 2020a).
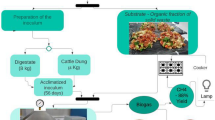
The reactor’s internal pressure was converted into methane volume using the normal conditions of temperature and pressure as a reference [Pref = 1 atm (1013.25 mbar) and Tref: 273 K (0 °C)]. Thus, we used methane percentage in the biogas, the pressure recorded on the manometer (mbar), and a conversion factor (mL mbar−1) to obtain the methane volume. These calculations were performed using Equations S1 and S2 (Supplementary Material). BMP, biogas potential production (BPP), and SMA were calculated according to Equations S3, S4, S5, and S6. Kinetic modeling using mathematical models, such as Modified Gompertz, Logistic, and Transference, was used to estimate the maximum methane production rate (µm − NLCH4 kgVS−1 day−1) and determine SMA (gCODCH4 gVS−1 day−1). BMP experimental assay’s schematic is presented in Fig. 1.
2.3 Data Processing
The experiment was conducted using a completely randomized design (CRD), with a 4 × 2 factorial, being studied four different sludges (SS, BS, SWSAN, SWSMI) fed with GL or VFA as the only carbon source, which totaled eight balanced treatments (SS + GL; SS + VFA; BS + GL; BS + VFA; SWSAN + GL; SWSAN + VFA; SWSMI + GL. and SWSMI + VFA), in three repetitions, resulting in 24 experimental plots that were randomly allocated in the incubator to promote spatial randomness. The response variables for the BMP and SMA assays were (1) specific methanogenic activity (SMA, gCODCH4 gVS−1 day−1), (2) biochemical methane potential (BMP, NLCH4 kgVS−1), (3) biogas potential production (BPP, NLBIOGAS kgVS−1), (4) COD removal efficiency by CH4 generation (RECH4, %), and (5) biogas composition (CH4 and CO2, %).
SISVAR software version 5.6 (Ferreira, 2019) was applied to statistically analyze this work results through analysis of variance (one-way ANOVA) with a 95% confidence level and 5% probability (p < 0.05) and Tukey’s tests to compare treatments. According to the same response variable analyzed, the same letters’ average values belong to the same statistical group at the 5% probability level within the Tukey’s test.
2.4 Kinetic modeling study
The mathematical models selected to describe CH4 production were First-order exponential, Monomolecular, Fitzhugh, Modified Gompertz, Logistic, and Transference (Pellera & Gidarakos, 2016; Ware & Power, 2017). A description of these models and their equations is presented in Table 1. The model’s goodness of fit to the CH4 production profile and the coefficient of determination values (R2), the normalized root-mean-square error (NRMSE), and the Akaike Information Criterion (AIC) were determined according to other publications (Morais et al., 2020a, 2021). The higher the value of R2 and the lower the NRMSE and AIC, the greater the estimated data adequacy to the experimental data. R2, NRMSE, and AIC were calculated according to Eqs. S7, S8, and S9. Data obtained from kinetic modeling were applied to plot each process curve using MATLAB® software version R2016b (Coelho et al., 2020a, 2020b).
Mt, methane accumulated during the incubation period (NLCH4 kgVS−1); M, volume of methane generated during the experiment (NLCH4 kgVS−1); k, first-order methane production rate constant (day−1); t, digestion time (d); n, shape constant (dimensionless); e, Euler number (dimensionless); λ, time of the lag phase (d); µm, maximum rate of methane production (NLCH4 kgVS−1 day−1).
3 Results and Discussion
3.1 Study of Methane Production Kinetics
The cumulative methane production curve (CMPC) of the SS + GL was exponentially shaped (Fig. 2a) with a non-existent delay time (λ = 0.0 day), indicating the microorganism’s affinity to the substrate. However, low values of CH4 maximum rate production (14–23 NLCH4 kgVS−1 day−1) were estimated, resulting in low SMA values (Table 2). The microbial community present produced 80% of the CH4 total volume in approximately 11 days, a period called T80. This parameter is widely used to analyze the anaerobic reactor’s performance and estimate the hydraulic retention time (HRT) of continuous reactors (Córdoba et al., 2018). Thus, the lower the T80, the greater the inoculum efficiency in the substrate methanization process (Sanjaya et al., 2016).
Cumulative methane production curves (CMPC) for each treatment and the most suitable models in the kinetic modeling. a SS + GL and Fitzhugh model. b SS + VFA and Fitzhugh model. c BS + GL and Fitzhugh model. d BS + VFA and Gompertz model. e SWSAN + GL and Fitzhugh model. f SWSAN + VFA and Fitzhugh model. g SWSMI + GL and Fitzhugh model. h SWSMI + VFA and Fitzhugh model
For the SS + VFA treatment, the CMPC had a sigmoidal shape (Fig. 2b), resulting in a long delay period (λ = 1.0–7.7 days) and a slower and more progressive CH4 production over time, promoting lower values of μm (4.8–5.9 NLCH4 kgVS−1 day−1) compared to the values obtained with GL. The T80 obtained for the SS + VFA treatment was 26 days, corroborating the low SMA values.
The sigmoidal-type curve is expected in the methanization of substrates that are difficult to hydrolyze, such as those rich in lipids or lignocellulosic substances. However, this behavior can also be observed when the inoculum is not adapted to a given substrate (Ware & Power, 2017). As GL and VFA are substrates readily biodegradable and are standard in BMP tests, and all inocula were subjected to the same experimental conditions, it is possible to infer that the SS has a low anaerobic performance. The best models to describe CH4 production from SS were the Fitzhugh (with GL) and Logistic (with VFA) models, which returned higher R2 values, lower error values (NMRSE), and lower AIC values (Table 2).
The exponential CMPC generated by the BS + GL treatment also showed a non-existent lag phase (λ = 0.0 days), and CH4 production occurred more quickly when compared to treatments with SS, as can be confirmed by the lower T80 value (7 days) and higher μm value (31.9–48.7 NLCH4 kgVS−1 day−1) obtained (Table 2 and Fig. 2c). The BS + VFA CCPM was sigmoidal (Fig. 2d). Nevertheless, it presented a shorter delay time (λ = 0.55–1.77 days) in the CH4 formation when compared to the SS + VFA treatment, resulting in a higher value of μm (13–19 NLCH4 kgVS−1 day−1) and lower T80 value (13 days) (Table 2).
The CMPC generated in the treatments with the SWS were exponential, with a non-existent delay time (λ = 0.0 day), low T80 (approximately 7 day), and high μm values (Table 3 and Fig. 2e–h). The Fitzhugh model was the most adequate to describe the CH4 production from this inoculum. The Transference model was the mathematical model that presented the best fit compared to the other sigmoidal models (Modified Gompertz and Logistic) used. Therefore, its μm values were used to calculate SMA.
Therefore, mathematical models that describe exponential functions, mainly the Fitzhugh model, were the most suitable to describe the kinetics of CH4 production from readily biodegradable substrates, such as GL and VFA. In contrast, mathematical models expressing logistic growth were more reliable in describing a slower methanization process, gradual time, and a high lag phase time (λ). Thus, based on the data expressed in Tables 2 and 3, the Fitzhugh model presented the lowest NRMSE and AIC values, making it more suitable than the Modified Gompertz model to describe the methane production from readily biodegradable substrates, whose methanization has a short or no lag phase. However, the Fitzhugh model does not estimate the latency phase time nor the maximum methane production rate (µm). For these estimates, and using the same analysis criterion (lower NRMSE and AIC values), the Transference model was more effective than the Modified Gompertz model.
Values of the CH4 production rate constant (k) obtained confirmed the higher speed in CH4 production in the tests conducted with GL (Tables 2 and 3). For most tests, as kGL > kVFA, it is inferred that the acidogenic community of the evaluated inocula has a more satisfactory performance than the acetogenic community. In the tests performed with GL, kBS > kSWSmi > kSWSan > kSS, with values of 0.34, 0.29, 0.28, 0.16 day−1, respectively, and in the treatments with VFA, kSWSmi > kSWSan > kBS > kSS, with values of 0.32, 0.28, 0.10, and 0.05 day−1, respectively (Tables 2 and 3). SWS inoculants degraded the substrates and produced CH4 more quickly than the other evaluated inoculants, justifying the higher SMA values found for these sludges. Therefore, the higher the k, the greater the SMA. The SS showed the lowest CH4 production rate, consistent with the high T80 and low SMA values obtained for this inoculum.
3.2 Study of Specific Methanogenic Activity
The low values obtained for the coefficient of experimental variation (CV) in the variance analysis indicate the tests’ experimental precision at the level of 5% probability (Supplementary Material—Table S1). According to the F test, statistically significant differences were found between treatments identified by comparing the eight treatments’ response variables by applying the Tukey’s test (Table 4). As the tests were conducted under the same operational conditions (agitation and temperature) and media culture (basal medium, buffer, and S/X ratio), it is understood that any significant statistical difference between treatments is due to the inocula microbial and biochemical characteristics.
As shown in Table 4, the SS presented the lowest SMA for the other inocula evaluated. For the SS + GL treatment, SMA (0.057 gCODCH4 gVS−1 day−1) was superior to that obtained in the SS + VFA treatment (0.010 gCODCH4 gVS−1 day−1), which indicates that the acidogenic microbial community performed better than the acetogenic community in the process of anaerobic digestion (Angelidaki et al., 2009). Therefore, it can be inferred that this inoculum has low acetogenic and syntrophic activity in short-chain carboxylic acids (propionate and butyrate) conversion into acetate and in acetate conversion to CH4 (Aquino et al., 2007).
The low inocula methanogenic activity may be related to its storage at 3 °C for 1 month before the tests and also to the S/X ratio adopted. According to Kotch et al. (2019), the dilution of the inoculum, low S/X ratio, and storage at low temperatures are the main factors that contribute to the lag phase increase and directly impact kinetics and methane generation. Furthermore, we ruled out the possibility of compromising the microbial consortium activity due to reactor acidification, as sodium bicarbonate was used as a buffer, and the initial and final pH of the reaction medium was close to neutrality (pH 6.8–7.2) in all reactors.
The low SMA values obtained for the SS are the following studies carried out by Bertolino et al. (2008), who reported an SMA value of 0.0579 gCODCH4 gVS−1 day−1. The authors used SS as inoculum and sanitary sewage as a substrate, obtained from a UASB reactor that treated sewage from a university campus. The test was performed in triplicate using reactors of 200 mL work volume and 50 mL headspace, under a controlled temperature (35 °C), and adopting an S/X ratio of 0.225 gCOD gVS−1.
BS showed higher SMA values than SS (Table 4), 0.123 gCODCH4 gVS−1 day−1 for GL and 0.050 gCODCH4 gVS−1 day−1 for VFA. These values were lower than those reported by Longaretti et al. (2019), which obtained an SMA for a BS of 0.33 gCODCH4 gVS−1 day−1 using GL as a substrate. The authors conducted their experiments in reactors with a maximum volume of 2.5 L, with 2 L reaction volume and 0.5 L headspace, under mesophilic temperature (35 ± 1 °C), 5.0 gVS L−1 biomass concentration, and 4.0 gCOD L−1 substrate. The lower SMA values found in this study are possibly due to the difference in the anaerobic sludge’s physical–chemical and microbiological characteristics. Compared with the inocula adapted to swine wastewater treatment (SWs), the BS showed a lower SMA value when the VFA solution was the substrate and statistically equal amounts when the GL was used (Table 4).
The highest SMA values were obtained for the inocula adapted for the SW treatment. Notably, the treatments carried out with VFA presented greater SMA than those carried out with GL, indicating high acetogenic activity. SWSMI + VFA had the highest SMA value, but SWSMI + GL had SMA statistically equal to BS + GL and SWSAN + GL (Table 4). However, it is possible to infer that micro-aeration contributed to the microbial community syntrophic activity, mainly in the tests performed with VFA, resulting in higher values of SMA, BMP, BPP, and CH4 concentration in biogas values about SWSAN + VFA. Nonetheless, in the GL tests, the response variables’ values for both SWS were statistically similar.
Micro-aeration has been the subject of study in several studies aimed at increasing hydrolysis, removing H2S from biogas, increasing CH4 production, and even removing emerging micropollutants and recalcitrant compounds in anaerobic reactors (Krayzelova et al., 2014; Lim & Wang, 2013; Meng et al., 2019; Ruan et al., 2019). However, it was not possible to state the hydrolytic community’s better performance developed in the microaerophilic reactor (SWSMI sludge) because a more complex substrate (e.g., cellulose) was not evaluated in the BMP tests.
The SMA values for the SW treatment inoculants obtained in this study were higher than those reported by Alves et al. (2005). They conducted SMA tests with inoculants obtained from a swine manure biodigester and a bovine manure biodigester, using a VFA solution as substrate and an S/X ratio of 1.0 gCOD gVS−1. The results obtained by the authors were 0.0331 gCODCH4 gVS−1 day−1 and 0.0565 gCODCH4 gVS−1 day−1 for swine and bovine sludges, respectively. According to Angelidaki et al. (2009), inocula must have SMA values equal to or greater than 0.10 gCODCH4 gVS−1 day−1 to be suitable for carrying out BMP tests with real substrates. In this sense, the BS and SWS proved to be ideal for this purpose. They are also much suitable as sludge sources for feeding anaerobic systems used for domestic, agro-industrial, or industrial wastes treatment and in biogas production and energy recovery studies.
In this context, SMA tests provide essential information for the operation, maintenance, and management of anaerobic reactors (Hussain & Dubey, 2017). The SS showed low SMA values (Table 4), suggesting that the anaerobic reactor in which this inoculum was collected has low efficiency in removing organic matter through the CH4 formation (RECH4). Thus, the data obtained in BMP tests can contribute to identifying operational problems in anaerobic systems. Besides, the determination of kinetic parameters can contribute to the resolution of the issues recognized, determine the most appropriate models, and determine the reactors’ design and operational parameters, such as volume, hydraulic retention time (HRT), solids retention time (SRT), and volumetric organic loading rate (Santos et al., 2020).
3.3 Analysis of BMP and Biogas Quality
The SWS were the inoculants that presented the highest BMP and BPP (Table 4). Analyzing the BMP tests performed with GL, SWS provided higher values compared to the other inocula. The same occurs in tests with VFA, but, in this case, the BMP of SWSMI was statistically superior to that of SWSAN (Fig. 3). The inoculants that presented the highest SMA value also produced the most significant amount of CH4 and biogas (Table 4). SMA is a parameter that represents the microbial capacity to transform substrates into CH4, hydrogen (H2), and carbon dioxide (CO2); thus, the higher the SMA, the higher the BPP and BMP of the inoculum (Aquino et al., 2007).
Methane and biogas production potential in the studied experimental configurations. Different bar letters indicate a significant difference with a < 0.05, following variance analysis. Lower case letters were used to express the BMP variable’s statistical groups and upper case letters to represent the BPP variable’s statistical groups
SW is an agro-industrial residue rich in nutrients that are favorable to the growth of anaerobic microorganisms, presenting a high organic matter concentration, mainly in the form of proteins and lipids, suspended solids, and alkalinity, which gives it buffering capacity, and an increased presence of pathogenic and non-pathogenic microorganisms (Córdoba et al., 2016). The SW characteristics may have contributed to the development of a specialized microbial community in the inoculum. A high syntrophic activity among hydrolytic, acidogenic, and methanogenic microorganisms was established, which favored the methanization process (Morais et al., 2020b).
Pereira et al. (2009) studied the impact of operational parameters on a UASB reactor’s microbial consortium. They reported that the anaerobic sludge formed in SW treatment has a dense and granular structure, high morphological diversity, and ecological balance between hydrogenotrophic and acetoclastic archaea. For these reasons, the SWS sludge proved to be efficient in the GL and VFA methanization.
Schneiders et al. (2013) determined the BMP of granular anaerobic sludge from a UASB reactor operated by treating wastewater from the food industry. The tests were conducted in reactors with 400 mL of reaction volume kept at 33 °C. The authors reported a BMP of 337 NLCH4 kgVS−1 when a mixture of VFA was used as a substrate in an S/X ratio of 1 gCOD gVS−1. Longaretti et al. (2019) evaluated the BMP of five different anaerobic inoculants from UASB reactors that treated agro-industrial wastewater. The experiment was carried out in reactors of 2-L reaction volume maintained at mesophilic temperature (35 ± 1 °C). The reactors were inoculated with 5.0 gVS.L−1, and 4.0 gCOD L−1 of GL was used as a substrate. The BMP found for each evaluated sludge was 208 NLCH4 kgVS−1 for the brewery industry sludge, 196 NLCH4 kgVS−1 for the grain-processing industry sludge, 165 NLCH4 kgVS−1 for the dairy industry sludge, and 180 NLCH4 kgVS−1 for the leather industry sludge. The BMP values (121–197 NLCH4 kgVS−1) found for the inocula evaluated in this study were lower than those reported by Schneiders et al. (2013) and by Longaretti et al. (2019). This was probably due to differences in the physical–chemical and microbial characteristics of each biomass evaluated.
According to Table 4 and Fig. 4, the SWS showed a higher amount of CH4 in the biogas than the other inocula. According to Longaretti et al. (2019), sludge adapted to anaerobically degrade animal waste generally produces biogas with higher CH4 content, reaching 90% CH4 values in biogas. The amount of CH4 in the biogas found in this work (49–63%) follows the German International Standard for Anaerobic Digestion (VDI-4630), which indicates that biogas from carbohydrate degradation, such as GL, generally has 50% CH4 and 50% CO2, which may differ according to the inoculum used (VDI, 2006).
Biogas composition in the studied experimental configurations. Different bar letters indicate a significant difference with a < 0.05, following variance analysis. Lower case letters were used to express the BMP variable’s statistical groups and upper case letters to represent the BPP variable’s statistical groups
The biogas generated in the reactors was predominantly composed of CH4 and CO2 and non-quantifiable remains of H2S and N2. Compared with the tests performed with GL, when VFA was used as a substrate, higher biogas quality was obtained, which showed 77–92% CH4 (Table 4 and Fig. 4). This result is expected since the acidogenic bacteria generate CO2 when metabolizing GL to produce simpler organic compounds. When the substrate for methanization is a VFA solution composed of acetate, propionate, and butyrate, oxidation of propionate and butyrate occurs for acetate production and direct use of acetate by acetoclastic methanogenic archaea for CH4 and CO2 production in the methanogenesis stage (Miaomiao Zhou et al., 2018). In this process, the CO2 accumulation is lower due to the suppression of the acidogenic stage.
As the CH4 concentration in biogas was higher when using VFA as a substrate, for these treatments, the efficiency of removing organic matter through the generation of CH4 (RECH4) was also higher when compared to those obtained with GL (Table 4). The SS showed the lowest efficiencies, the BS intermediate values, and the SWS the highest. These results corroborate with the SMA and BMP obtained for these inocula. According to Table 4, the RECH4 range in the GL tests was 61–81% and 52–94% in the VFA tests.
4 Conclusions
Mathematical models that describe exponential functions, mainly the Fitzhugh model, were the most adequate to describe methane (CH4) production kinetics from readily biodegradable substrates, with reduced or non-existent lag phase time (λ). The mathematical models representing logistic growth functions, such as Modified Gompertz and Logistic, were more reliable to describe the methanization bioprocess of the test carried out with brewery sludge (BS), with volatile fatty acids (VFA), and the test conducted with sanitary sludge (SS) with VFA. Such behavior was due to the CH4 production curve presenting a sigmoidal shape, expressing a slower CH4 production, and justifying a more extended λ than the other tests. Based on these results, it is understood that the Modified Gompertz model has been used indiscriminately in the technical literature and does not always present the most accurate results. Here, we propose other models that can be satisfactorily applied for a better understanding of substrate biomethanization.
Through the kinetic analysis, it was possible to conclude that swine sludges (SWS) could degrade the substrates and generate CH4 quickly compared to the other evaluated sludges, justifying the higher specific methanogenic activity (SMA) values found. SWS showed the highest biochemical potential of methane (BMP) values (153–197 NLCH4 kgVS−1), SMA (0.117–0.157 gCODCH4 gVS−1 day−1) values, and the highest concentrations of CH4 in biogas (53–82%). In addition, the SS showed the lowest BMP and SMA values, allowing us to conclude that BS and SWS are more advantageous to be applied in CH4 production and energy recovery studies.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Alves, R. G. C. de M., Filho, P. B., Philippi, L. S., Henn, A., & Monteiro, L. W. da S. (2005). Digestores anaeróbios para tratamento de dejetos suínos—Avaliação de partida de diferentes configurações de reatores. In: 23° Congresso Brasileiro de Engenharia Sanitária e Ambiental, Associação Brasileira de Engenharia Sanitária, Campo Grande, Brasil.
Angelidaki, I., Alves, M., Bolzonella, D., Borzacconi, L., Campos, J. L., Guwy, A. J., et al. (2009). Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Science and Technology, 59(5), 927–934. https://doi.org/10.2166/wst.2009.040
APHA. (2017). – American Public Health Association. Standard methods for the examination of water and wastewater (2017) 23nd edition. APHA/AWWA/WEF, Washington.
Aquino, S. F., Chernicharo, C. A. L., De, M., Florêncio, L., Santos, D., & Monteggia, L. O. (2007). Metodologia para determinação da atividade metanogênica específica (AME) em lodos anaeróbios. Engenharia Sanitária e Ambiental, 12(2), 192–201. https://doi.org/10.1590/S1413-41522007000200010.Accessed13September2016
Barrena, R., Traub, J. E., Gil, C. R., Goodwin, J. A. S., Harper, A. J., Willoughby, N. A., et al. (2018). Batch anaerobic digestion of deproteinated malt whisky pot ale using different source inocula. Waste Management, 71, 675–682. https://doi.org/10.1016/j.wasman.2017.06.025
Bertolino, S. M., Carvalho, C. F., & Aquino, S. F. (2008). Caracterização e biodegradabilidade aerobia e anaeróbia dos esgotos produzidos em campus universitário. Engenharia Sanitária e Ambiental, 13(3), 271–277. https://doi.org/10.1590/s1413-41522008000300005
Çetinkaya, A. Y., & Yetilmezsoy, K. (2019). Evaluation of anaerobic biodegradability potential and comparative kinetics of different agro-industrial substrates using a new hybrid computational coding scheme. Journal of Cleaner Production, 238, 20219934189. https://doi.org/10.1016/j.jclepro.2019.117921
Coelho, M. M. H., Morais, N. W. S., Ferreira, T. J. T., Silva, F. S. S., Pereira, E. L., & dos Santos, A. B. (2020a). Carboxylic acids production using residual glycerol as a substrate in anaerobic fermentation: A kinetic modeling study. Biomass and Bioenergy, 143, 105874. https://doi.org/10.1016/j.biombioe.2020.105874
Coelho, M. M. H., Morais, N. W. S., Pereira, E. L., Leitão, R. C., & dos Santos, A. B. (2020b). Potential assessment and kinetic modeling of carboxylic acids production using dairy wastewater as substrate. Biochemical Engineering Journal, 156(December 2019), 107502. https://doi.org/10.1016/j.bej.2020.107502
Córdoba, V., Fernández, M., & Santalla, E. (2016). The effect of different inoculums on anaerobic digestion of swine wastewater. Journal of Environmental Chemical Engineering, 4(1), 115–122. https://doi.org/10.1016/j.jece.2015.11.003
Córdoba, V., Fernández, M., & Santalla, E. (2018). The effect of substrate/inoculum ratio on the kinetics of methane production in swine wastewater anaerobic digestion. Environmental Science and Pollution Research, 25(22), 21308–21317. https://doi.org/10.1007/s11356-017-0039-6
Da Silva, C., Astals, S., Peces, M., Campos, J. L., & Guerrero, L. (2018). Biochemical methane potential (BMP) tests: Reducing test time by early parameter estimation. Waste Management, 71, 19–24. https://doi.org/10.1016/j.wasman.2017.10.009
De la Rubia, M. A., Villamil, J. A., Rodriguez, J. J., & Mohedano, A. F. (2018). Effect of inoculum source and initial concentration on the anaerobic digestion of the liquid fraction from hydrothermal carbonisation of sewage sludge. Renewable Energy, 127, 697–704. https://doi.org/10.1016/j.renene.2018.05.002
Donoso-Bravo, A., Pérez-Elvira, S. I., & Fdz-Polanco, F. (2010). Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chemical Engineering Journal, 160(2), 607–614. https://doi.org/10.1016/j.cej.2010.03.082
Ferreira, D. F. (2019). Sisvar: A computer analysis system to fixed effects split plot type designs. Revista Brasileira De Biometria, 37(4), 529–535. https://doi.org/10.28951/rbb.v37i4.450
Filer, J., Ding, H. H., & Chang, S. (2019). Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water, 11(5), 921. https://doi.org/10.3390/w11050921
Groot, J. C. J., Cone, J. W., Williams, B. A., Debersaques, F. M. A., & Lantinga, E. A. (1996). Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Animal Feed Science and Technology, 64(1), 77–89. https://doi.org/10.1016/S0377-8401(96)01012-7
Gu, Y., Chen, X., Liu, Z., Zhou, X., & Zhang, Y. (2014). Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresource Technology, 158(2014), 149–155. https://doi.org/10.1016/j.biortech.2014.02.011
Holliger, C., Alves, M., Andrade, D., Angelidaki, I., Astals, S., Baier, U., et al. (2016). Towards a standardization of biomethane potential tests. Water Science and Technology, 74(11), 2515–2522. https://doi.org/10.2166/wst.2016.336
Hussain, A., & Dubey, S. K. (2017). Specific methanogenic activity test for anaerobic degradation of influents. Applied Water Science, 7(2), 535–542. https://doi.org/10.1007/s13201-015-0305-z
Huzir, N. M., Mahmood, N. A. N., Muhammad, S. A. F. S., Umor, N. A., & Ismail, S. (2019). Effect of specific methanogenic activity (SMA) of anaerobic sludge under high salinity. Journal of Advanced Research in Applied Sciences and Engineering Technology, 16(1), 35–40.
Jijai, S., Srisuwan, G., O-thong, S., Norli, I., & Siripatana, C. (2016). Effect of substrate and granules/inocula sizes on biochemical methane potential and methane kinetics. Iranica Journal of Energy & Environment, 7(2), 94–101.
Kafle, G. K., & Chen, L. (2016). Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Management, 48, 492–502. https://doi.org/10.1016/j.wasman.2015.10.021
Koch, K., Hafner, S. D., Weinrich, S., & Astals, S. (2019). Identification of critical problems in biochemical methane potential (BMP) tests from methane production curves. Frontiers in Environmental Science, 7(November), 1–8. https://doi.org/10.3389/fenvs.2019.00178
Krayzelova, L., Bartacek, J., Kolesarova, N., & Jenicek, P. (2014). Microaeration for hydrogen sulfide removal in UASB reactor. Bioresource Technology, 172, 297–302. https://doi.org/10.1016/j.biortech.2014.09.056
Li, Y., Xu, H., Hua, D., Zhao, B., Mu, H., Jin, F., et al. (2020). Two-phase anaerobic digestion of lignocellulosic hydrolysate: Focusing on the acidification with different inoculum to substrate ratios and inoculum sources. Science of the Total Environment, 699, 134226. https://doi.org/10.1016/j.scitotenv.2019.134226
Lim, J. W., & Wang, J. Y. (2013). Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Management, 33(4), 813–819. https://doi.org/10.1016/j.wasman.2012.11.013
Lima, D. R. S., Adarme, O. F. H., Baêta, B. E. L., Gurgel, L. V. A., & de Aquino, S. F. (2018). Influence of different thermal pretreatments and inoculum selection on the biomethanation of sugarcane bagasse by solid-state anaerobic digestion: A kinetic analysis. Industrial Crops and Products, 111(November 2017), 684–693. https://doi.org/10.1016/j.indcrop.2017.11.048
Longaretti, G., Meira, J. A., Sganderla, I. B., Magro, J. D., Fiori, M. A., Maria, J., & Mello, M. D. (2019). Estudo da atividade metanogênica específica de diferentes lodos anaeróbios. Engenharia Sanitária e Ambiental, 24(6), 1139–1146. https://doi.org/10.1590/S1413-41522019140730
Meng, J., Li, J., He, J., Li, J., Deng, K., & Nan, J. (2019). Nutrient removal from high ammonium swine wastewater in upflow microaerobic biofilm reactor suffered high hydraulic load. Journal of Environmental Management, 233(August 2018), 69–75. https://doi.org/10.1016/j.jenvman.2018.12.027
Morais, N. W. S., Coelho, M. M. H., Ferreira, T. J. T., Pereira, E. L., Leitão, R. C., & dos Santos, A. B. (2020a). A kinetic study on carboxylic acids production using bovine slaughterhouse wastewater: A promising substrate for resource recovery in biotechnological processes. Bioprocess and Biosystems Engineering, 44(0123456789), 271–282. https://doi.org/10.1007/s00449-020-02440-3
Morais, N. W. S., Coelho, M. M. H., de Silva, A., & S. e., Pereira, E. L., Leitão, R. C., & dos Santos, A. B. . (2020b). Kinetic modeling of anaerobic carboxylic acid production from swine wastewater. Bioresource Technology, 297, 122520. https://doi.org/10.1016/j.biortech.2019.122520
Morais, N. W. S., Coelho, M. M. H., de Silva, A., & S. e., Silva, F. S. S., Ferreira, T. J. T., Pereira, E. L., & dos Santos, A. B. . (2021). Biochemical potential evaluation and kinetic modeling of methane production from six agro-industrial wastewaters in mixed culture. Environmental Pollution, 280, 116876. https://doi.org/10.1016/j.envpol.2021.116876
Mozhiarasi, V., Weichgrebe, D., & Srinivasan, S. V. (2020). Enhancement of methane production from vegetable, fruit and flower market wastes using extrusion as pretreatment and kinetic modeling. Water, Air, and Soil Pollution, 231, 3. https://doi.org/10.1007/s11270-020-04469-2
Pellera, F. M., & Gidarakos, E. (2016). Effect of substrate to inoculum ratio and inoculum type on the biochemical methane potential of solid agroindustrial waste. Journal of Environmental Chemical Engineering, 4(3), 3217–3229. https://doi.org/10.1016/j.jece.2016.05.026
Pereira, E. L., Campos, C. M. M., & Moterani, F. (2009). Efeitos do pH, acidez e alcalinidade na microbiota de um reator anaeróbio de manta de lodo (UASB) tratando efluentes de suinocultura. Revista Ambiente & Água - An Interdisciplinary. Journal of Applied Science, 4(3), 157-168. c.
Pitt, R. E., Cross, T. L., Pell, A. N., Schofield, P., & Doane, P. H. (1999). Use of in vitro gas production models in ruminal kinetics. Mathematical Biosciences, 159(2), 145–163. https://doi.org/10.1016/S0025-5564(99)00020-6
Pozdniakova, T. A., Costa, J. C., Santos, R. J., Alves, M. M., & Boaventura, R. A. R. (2012). Anaerobic biodegradability of Category 2 animal by-products: Methane potential and inoculum source. Bioresource Technology, 124, 276–282. https://doi.org/10.1016/j.biortech.2012.08.022
Raposo, F., Fern, V., De Rubia, M. A., Borja, R., B, F., Fern, M., , et al. (2011). Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. Journal of Chemical Technology and Biotechnology, 86, 1088–1098. https://doi.org/10.1002/jctb.2622
Redzwan, G., & Banks, C. (2004). The use of a specific function to estimate maximum methane production in a batch-fed anaerobic reactor. Journal of Chemical Technology and Biotechnology, 79(10), 1174–1178. https://doi.org/10.1002/jctb.1107
Rodrigues, R. P., Rodrigues, D. P., Klepacz-Smolka, A., Martins, R. C., & Quina, M. J. (2019). Comparative analysis of methods and models for predicting biochemical methane potential of various organic substrates. Science of the Total Environment, 649, 1599–1608. https://doi.org/10.1016/j.scitotenv.2018.08.270
Ruan, D., Zhou, Z., Pang, H., Yao, J., Chen, G., & Qiu, Z. (2019). Enhancing methane production of anaerobic sludge digestion by microaeration: Enzyme activity stimulation, semi-continuous reactor validation and microbial community analysis. Bioresource Technology, 289, 121643. https://doi.org/10.1016/j.biortech.2019.121643
Sanjaya, A. P., Cahyanto, M. N., & Millati, R. (2016). Mesophilic batch anaerobic digestion from fruit fragments. Renewable Energy, 98, 135–141. https://doi.org/10.1016/j.renene.2016.02.059
dos Santos, L. A., Valença, R. B., da Silva, L. C. S., de Holanda, S. H., & B., Silva, A. F. V. da, Jucá, J. F. T., & Santos, A. F. M. S. . (2020). Methane generation potential through anaerobic digestion of fruit waste. Journal of Cleaner Production, 256, 120389. https://doi.org/10.1016/j.jclepro.2020.120389
Schneiders, D., Till, A., Lapa, K. R., & Pinheiro, A. (2013). Atividade metanogênica específica (AME) de lodos industriais provenientes do tratamento biológico aeróbio e anaeróbio. Revista Ambiente & Água - an Interdisciplinary Journal of Applied Science, 8(2), 135–145. https://doi.org/10.4136/1980-993X
Schulin-Zeuthen, M., Kebreab, E., Gerrits, W. J. J., Lopez, S., Fan, M. Z., Dias, R. S., & France, J. (2007). Meta-analysis of phosphorus balance data from growing pigs. Journal of Animal Science, 85(8), 1953–1961. https://doi.org/10.2527/jas.2006-715
Suksong, W., Mamimin, C., Prasertsan, P., Kongjan, P., & O-Thong, S. (2019). Effect of inoculum types and microbial community on thermophilic and mesophilic solid-state anaerobic digestion of empty fruit bunches for biogas production. Industrial Crops and Products, 133(August 2018), 193–202. https://doi.org/10.1016/j.indcrop.2019.03.005
Sun, C., Cao, W., & Liu, R. (2015). Kinetics of methane production from swine manure and buffalo m anure. Applied Biochemistry and Biotechnology, 177, 985–995. https://doi.org/10.1007/s12010-015-1792-y
VDI. (2006). VDI-4630 Guideline. Fermentation of organic materials. Characterization of the substrate, sampling (Collection of material data, fermentation tests). Düsseldorf, Germany.
Vieira, S., & Hoffmann, R. (1977). Comparison of the logistic and the Gompertz growth functions considering additive and multiplicative error terms. Applied Statistics, 26(2), 143. https://doi.org/10.2307/2347021
Ware, A., & Power, N. (2017). Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renewable Energy, 104, 50–59. https://doi.org/10.1016/j.renene.2016.11.045
Yang, H., Deng, L., Liu, G., Yang, D., Liu, Y., & Chen, Z. (2016). A model for methane production in anaerobic digestion of swine wastewater. Water Research, 102, 464–474. https://doi.org/10.1016/j.watres.2016.06.060
Zan, F., Zeng, Q., Hao, T., Ekama, G. A., Hao, X., & Chen, G. (2019). Achieving methane production enhancement from waste activated sludge with sulfite pretreatment: Feasibility, kinetics and mechanism study. Water Research, 158, 438–448. https://doi.org/10.1016/j.watres.2019.04.048
Zhou, M., Yang, H., Zheng, D., Pu, X., Liu, Y., Wang, L., & Zhang, Y. (2019). Methanogenic activity and microbial communities characteristics in dry and wet anaerobic digestion sludges from swine manure. Biochemical Engineering Journal, 152, 107390. https://doi.org/10.1016/j.bej.2019.107390
Zhou, M., Yan, B., Wong, J. W. C., & Zhang, Y. (2018). Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresource Technology, 248, 68–78. https://doi.org/10.1016/j.biortech.2017.06.121
Funding
This study received support from the National Council for Scientific and Technological Development—CNPq, the Coordination for the Improvement of Higher Education Personnel—CAPES, the Minas Gerais State Research Support Foundation—FAPEMIG, and the National Institute of Science and Technology in Sustainable Sewage Treatment Stations—INCT Sustainable ETFs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•The Fitzhugh model best described the methanization kinetics of the substrates.
•Modified Gompertz Model does not always present the most accurate results.
•SWS inoculants produced CH4 more quickly than the other inoculants.
•SW characteristics may have contributed to the methanization process.
•Micro-aeration promoted higher values of SMA and BMP.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morais, N.W.S., Coelho, M.M.H., de Oliveira, M.G. et al. Kinetic Study of Methanization Process Through Mathematical Modeling in Biochemical Methane Potential Assays from Four Different Inoculants. Water Air Soil Pollut 232, 423 (2021). https://doi.org/10.1007/s11270-021-05387-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05387-7