Abstract
Agricultural soil is easily polluted by heavy metal and recently by metal-based nanoparticles, which has been synthesized in lab and discarded to environment. The uptake and accumulation of them by crops in polluted soil may pose high risks for human health. Here, we investigated the fate and the fixation effect of Fe3O4-GO nanocomposites (NCs) to lead ions in the soil-leek system during four harvest lifecycle. The results showed within 100 days, 600-mg/kg Fe3O4-GO significantly decreased Pb(II) concentrations in leaves by 37.89%, 39.10%, 73.86%, and 47.17% compared with controls. When Fe3O4-GO was added into Pb(II)-polluted soil, a significant fixation effect of Pb(II) was found, and the reduce percentages were 47.29%, 66.60%, 78.04%, and 39.16% for leaves, stem, storage roots, and absorbing roots compared with controls. The scanning electron microscope images showed that the overall appearance of Fe3O4-GO has not been destroyed during the interaction with soil.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Among the environmental pollutants, heavy metal elements, such as Cd(II), Hg(II), Pb(II), Cr(VI), and Cu(II), have accumulation and concealment (Gu et al. 2018). These heavy metal elements would not only destroy the atmosphere, water, and soil ecological environment but also bring about serious problems in food safety through the enrichment of animals and plants, which would directly endanger human health (Liu et al. 2013).
According to reports, there have been too many heavy metal pollution incidents in the world, which have brought indelible harm (Inaba et al. 2005). Soil heavy metal pollution has become one of the urgent problems in China. In recent years, although China’s economy has continued to develop, the area of agricultural land pollution has gradually expanded (Huang et al. 2007). The situation of heavy metal pollution in China’s agricultural arable land is not optimistic. According to a preliminary report, the area of arable land polluted by heavy metals such as Pb(II), Cd(II), and As(V) accounted for about 20% of the country’s arable land (Bai et al. 2015). In the past 5 decades, about 800,000 t of Pb(II) has been released to the environment worldwide and most of them have been deposited in the soil, becoming a potential high risk (Yang et al. 2018). Heavy metals, deposited in farmland soil, could be accumulated into fruits and vegetables, especially the edible parts of leafy vegetables.
Many small-molecule compounds coupled with nanomaterials, such as Fe3O4, could absorb heavy metals and have been studied widely (Ghasemi et al. 2017; Sun et al. 2018). Among of them, Fe3O4-GO nanocomposites have unique physical and chemical properties, which make them widely used in the electronics industry, medical sciences, and chemistry (Liang et al. 2010). Numerous researches have reported that Fe3O4 or its functional materials could be used in in vivo imaging, targeted therapy, and heavy metal removal (Dan et al. 2015; Deng et al. 2008). There is a review which has summarized the characterization and mechanisms for the uptake of engineered nanoparticles by different plant (Rico et al. 2011a). However, Fe3O4-GO nanocomposites absorbing heavy metal in soil and fixing effect to reduce metal translocation into plant have been little reported. Zhu et al. have found no absorbing ability of Fe3O4 NPs in the soil-plant system and translocation in the plants grown due to their attachment with sand or soil grains (Zhu et al. 2008). However, a large number of nanomaterials can easily enter the water and soil system in the process of production, recycling, and processing, causing potential harm to agricultural cultivation. Therefore, it is urgent to evaluate their impact on the soil. CuO and Fe3O4 are two common metal oxides, and they can cause the bacterial community to be greatly affected by nanoparticles within few days (Simonin and Richaume 2015). Among them, CuO nanoparticle pollution can cause a greater impact, while Fe3O4 is less toxic to soil bacterial communities. At the same time, these two materials did not change the total amount of organic matter in the soil and had little effect on the macroscopic properties of the soil (Peng et al. 2017; Anderson et al. 2017). Multi-walled carbon nanotubes and silver nanoparticles could affect the germination rate, chlorophyll, and water-soluble protein content of rice seedlings (Park and Ahn 2016). TiO2 nanoparticles improved the growth of rice in phosphorus-deficient soils. The addition of TiO2 NPs increased the phosphorus content in rice roots, stem, and cereals, and the amino acid, palmitic acid, and glycerol content in cereals produced by plants (Zahra et al. 2017). TiO2 also increased spinach growth and biomass, improved photosynthesis of sage mustard, and inhibited the growth of broad bean roots (Sun et al. 2018; Zhao et al. 2019; Mu et al. 2015). Crops, particularly vegetables, could assimilate heavy metal from farmland and enriched elevated concentrations caused by human activities, such as wastewater irrigation. The above researches mainly studied the toxicity of the addition of nanomaterials to soil and plant growth; the impact of Fe3O4-based NPs in the soil and their effect on heavy metal migration in soil and plants are ignored.
Fe3O4-GO, as a new type of carbon nanomaterial derived from graphene, is widely used in the removal of heavy metals and organic fields due to its rich oxygen-containing functional groups, large specific surface area, and easy separation (Xiao et al. 2017). Leek is an edible vegetable found throughout Asia, North America, North Africa, and Europe, which has advantages of strong adaptability to the environment, relatively low requirements for cultivation conditions, short growth cycles, continuous harvesting, and heavy metal enrichment (Gonzalez et al. 2003). It has been used as a model plant for study of heavy metal migration from soil-plant system (S-w et al. 2007).
In this paper, pot-culture experiment was used to study the effect of Fe3O4-GO in the soil on the migration of Pb(II). Characterization of synthesized Fe3O4-GO was finished through scanning electron microscope (SEM), transmission electron microscope (TEM), and Fourier transform infrared spectrometer (FTIR). Interaction of Fe3O4-GO with Pb(II) in soil was revealed by the analysis of Pb(II) and Fe(III) concentration change in the content of Pb(II) and Fe(III) in the leaves, stem, and roots of leek and subcellular fraction of leaves. This is, to the best of our knowledge, the first report on effects of Fe3O4-GO application on hindrance Pb(II) translocation into leek over an extended contact period (100 days) under soil conditions.
2 Materials and Methods
2.1 Soil Source and Characteristic
Organic farm soil was obtained from Nanjing. The soil was sieved through mesh sizes of 20, air-dried at room temperature, and stored at 4 °C before use. Sieved soil was also test for their heavy metal concentration and pH.
2.2 Synthesis of Fe3O4-GO and Characterization
GO was prepared by using the Hummers method (Chen et al. 2013). In this experiment, Fe3O4-GO was synthesized by using the co-precipitation method, which has been reported before (Sun et al. 2018). The characterization referred to the previous report (Sun et al. 2018). TEM and SEM were used to observe the surface morphological characteristics. FTIR was used to analyze the chemical groups, and the scanning range was 4000–400 cm−1.
2.3 Spiking of Soil with Fe3O4-GO and Lead
Fe3O4-GO was mixed as a powder into soil mechanically 20 min with a mixer. Four pots, named A, B, C, and D, were added with soil containing 0-, 600-, 0-, and 600-mg/kg (dry mass/air-dried soil mass basis) Fe3O4-GO separately. Commonly, 1500 g of soil was placed in each plastic flower pot. After 24 h, 500-mg/kg lead (dry mass/air-dried soil mass basis) dissolved in 1-L water was added into pots C and D for 20 days to simulate long-term pollution. The soil/nanoparticle mixtures were stored at 4 °C before planting.
2.4 Leek Cultivation in Soil with Fe3O4-GO and Lead
The leek was first cultivated and grown in natural soil. Then, the upper part of leek was cut off, leaving a 5-cm stem on the ground. Then, the leek was uprooted and washed with tap water. The root part was reserved 3–4 cm to promote the development of new roots. The leek was then transferred to pots A, B, C, and D. In each pot, 15 leeks were planted. The leek is watered every 2 days and harvested every 25 days. There are harvests four times for all the experiments. Finally, Fe3O4-GO added in the soil is separated by magnet separation and characterized by TEM, SEM, and FTIR.
2.5 Pb(II) Concentration in Subcellular Components of Leaves and Organ of Leek
Leaves, stem, and roots were collected and digested with HNO3-H2O2. The digestion process followed the method of GB 5009.268-2016, and the concentration of Pb(II) and Fe(III) were determined by ICP-MS.
Subcellular components of leaves were also separated and determined (Allfrey 1959). A 4 °C pre-cooled extraction buffer (250-mM sucrose, 1-mM dithiothreitol, and 50-mM pH 7.5 Tris-HCl solution) was added to fresh leaf sample and has undergone rapid milling at 4 °C. The solid-to-liquid ratio was 1:10. The obtained homogenate was rinsed with extraction buffer. The homogenate was centrifuged at 300 r/min for 30 s. The bottom sediment contained the cell wall of the leaves. The upper suspension was further centrifuged at 20000 r/min for 45 min. The obtained precipitate contained the organelle of the leaves, and the upper solution contained soluble substances, including inorganic ions, organic substances in vacuoles, and cytoplasm. The collected three parts were measured by ICP-MS as described before. All the measurements were repeated 3 times.
3 Results and Discussion
3.1 Recycle and Characterization of Fe3O4-GO in Soil
Fe3O4-GO, which has been mixed in the soil for 100 days, still maintained high magnetic properties and could be separated from the soil. As shown in Fig. 1, the color of Fe3O4-GO separated from soil (Fig. 1b) indicated that Fe3O4-GO has adsorbed Fe oxides existed in soil. After cleaning with ultra-pure water several times, the color of recycled Fe3O4-GO (Fig. 1c) changed to pristine color, but the Fe oxides was still adsorbed with Fe3O4-GO.
The synthesized Fe3O4-GO (Fig. 1a and Fig. 2b) was a complex of Fe3O4 and GO. As shown in Fig. 2a, GO was a smooth and two-dimensional nanomaterial. Multiple wrinkles could be observed on its surface. The magnetic nanomaterial (Fe3O4) with a diameter of 15–20 nm was attached to the GO surface in the synthetic process, as shown in Fig. 2b. The morphology of Fe3O4-GO was also characterized by SEM in Fig. 2c. The SEM image of Fe3O4-GO showed that a large amount of Fe3O4 nanoparticles were tightly attached to the surface of the GO sheet. After recycle and wash, the SEM of Fe3O4-GO indicated that the morphology and magnetic properties have been maintained (Fig. 2d). However, there were some impurities, which have magnetic properties and mixed with Fe3O4-GO. On the other hand, some studies have found that the addition of nanoparticles had little impact on the macroscopic properties of the soil (Ben-Moshe et al. 2013). This is due to the low nanoparticle addition and low mobility of them.
3.2 FTIR Analysis of Fe3O4-GO and GO
As shown in Fig. 3, FTIR spectra of GO, Fe3O4-GO, and Fe3O4-GO recycled from the soil were obtained. There were some obvious differences among these spectra. The strong absorption peak at 3417 cm−1 was due to the stretching vibration of -OH, which was blue shifted compared with 3424 cm−1. The stretching vibration of aromatic C=C double bond was found at 1631 cm−1. Compared with Fe3O4-GO and GO, the symmetric stretching vibration of O=C-O at 1404 cm−1 was more obvious. There is a new absorption peak at 1031 cm−1, which is assigned to Si-O and Si-O-Si stretching vibrations (Rico et al. 2011b). This peak indicated that muscovite existed in recycled Fe3O4-GO. Relative to 879 cm−1, a significant red shift occurred at a peak of 893 cm−1. The 893 cm−1 and 797 cm−1 belonged to the C=O stretching vibration or C-H bending vibration. The peak at 584 cm−1 in Fig. 3B and 583 cm−1 in Fig. 3C corresponded to the stretching vibration mode of Fe-O in Fe3O4, indicating that the recycled Fe3O4-GO maintained the original structure.
According to the above results, it could find that the structure of Fe3O4-GO nanomaterials recycled from the soil has not been destroyed and some impurities could not be cleaned as they also have magnetic properties. The SEM of Fe3O4-GO showed that the microspheres and the sheet structure were not damaged. The FTIR results showed the absorption peak at 583 cm−1 of Fe-O, indicating that Fe3O4-GO in the soil still maintains the physical and chemical characteristics of Fe3O4 (G-y et al. 2008). Depended on soil conditions, the fate of nanoparticles was different.
3.3 Pb(II) Concentration in Leek Leaves Obtained by Four Harvests
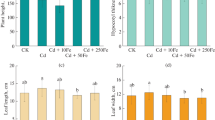
First, we have investigated the Pb(II) concentration of leek grown in natural soil. The concentration of Pb(II) was 21.39 mg/kg. The pH of the soil was 7.27. Figure 4 presented the content of Pb(II) in leek leaves harvested at 25, 50, 75, and 100 days without Pb(II) artificial pollution. The results showed that the soil added with Fe3O4-GO will affect the Pb(II) translocation of plant on it. The highest Pb(II) concentration detected in the four harvests was 1.2 mg/kg (fresh weight), and the addition of Fe3O4-GO make the Pb(II) concentration less than 0.3 mg/kg. The primary Pb(II) concentration was near 21 mg/kg, most of them could not be enriched by leaves. This indicated that the original Pb(II) in the soil was uneasy to be absorbed by leek (Rieuwerts et al. 2006). Overall, Pb(II) concentration of leaves from pot A was significantly lower than that from pot B. As shown in Fig. 4, Pb(II) concentration in leaves harvest on the 50th day was maximum, which was 1.37 times than that on the 25th day. For leaves obtained from pot B (Fe3O4-GO was added), a gradual decline of Pb(II) concentration was observed. In conclusion, Fe3O4-GO has a fixation effect on Pb(II) in soil and hindered their transportation. For pots C and D, Pb(II) was added to soil by spraying for 20 days. The Pb(II) concentration in leaves is detected and shown in Fig. 5. Apparently, a sharp decline trend of Pb(II) concentration for leaves could be observed. As free lead ions could be easily enriched by plant, the concentration of Pb(II) in first harvest leaves was around 600 mg/kg. And Fe3O4-GO containing soil in pot D could prevent a lot of free lead ions be transferred from roots to leaves. The leaves obtained from pot D showed less than 400-mg/kg Pb(II). The concentration difference between the two samples was extremely significant (P ˂ 0.01). Compared with the samples obtained from pots C and D on the 25th, 50th 75th, and 100th days, Pb(II) concentrations were reduced by 37.89%, 39.10%, 73.86%, and 47.17%, respectively. The results indicated that Fe3O4-GO could prevent part of lead ions to be enriched. Pb(II) was bound to the surface of Fe3O4-GO by formation of Pb-O bonds. Considering that the soil could also interact with Pb(II) and the proportion of Fe3O4-GO in soil was 0.06%, this fixation effect was remarkable as shown in Fig. 5. The application of Fe3O4-GO reduced the mobility of Pb(II) in the soil obviously, which was consistent with an earlier report (Chlopecka and Adriano 1997).
Fe3O4-GO was a nanocomposite containing Fe3O4 and GO. The mechanism of metal adsorption on Fe3O4-GO included electrostatic attraction and surface complexation. Electrostatic attraction play an important role for Fe3O4 in the adsorption process, and the surface of Fe3O4 adsorbents undergoes protonation/deprotonation with pH change. GO has high affinity for many metal ions through surface complexation due to the abundant oxygen-containing functional group on the surfaces of graphene oxide (Sun et al. 2018). Several studies have found that selenium exhibited protective effects against many toxic element heavy metals in the process of plant growth. However, this antagonistic effect was dosage- and speciation-dependent.
Selenium (Se) has been used to counteract the toxicity of cadmium and lead in soil. In soil, Se may influence the formation of iron plaque on rice root surface and then affect Cd uptake (Qingqing et al. 2019). Hu et al. have applied selenium fertilization in soils and found that metal mobility and bioavailability decreased significantly. The translocation factor of Cd and Pb from soil to iron plaque decreased significantly (Hu et al. 2014). Hg-Se complexes could also be formed in soil and reduce the Hg levels in grain, depending on the chemical speciation of Se (Wang et al. 2016).
3.4 Effect of Fe3O4-GO on Pb(II) Distribution in Subcellular Components of Leaves
Pb(II) concentration in the cell wall, organelles, and soluble fractions of leek harvested on the 25th day is detected and presented in Fig. 6. The Pb(II) concentration in unpolluted leaves was around 100 ng; however, the value was more than 4000 ng for Pb(II)-polluted sample. The Pb(II) distribution followed the order cell wall ˃ organelles ˃ soluble substance. An important result was that Pb(II) concentration of soluble matter was almost very low and only existed in polluted leek, which indicated that the leek was seriously polluted. The Pb(II) distribution in subcellular components of leaves could be regarded as the existence of exogenous pollution stress. The addition of Fe3O4-GO in soil lead Pb(II) concentration decrease 8.15~37.72% in leaves, stem, storage roots, and absorbing roots. The addition of Fe3O4-GO has inhibited the transfer of Pb(II) from soil to roots and no in harm plant. Figure 7 showed the Fe(III) concentration in the cell wall, organelles, and soluble parts. For unpolluted leek, the Fe(III) distribution followed the order organelles > cell wall > soluble matter, which was different from Pb(II) distribution. For polluted leek, the Fe(III) distribution followed the order cell wall > organelles > soluble matter.
Based on the analysis of first harvest leek, Fe(III) was found in three subcellular components of unpolluted leaves, and the addition of Fe3O4-GO reduced the Fe(III) concentration in the cell wall, organelles, and soluble parts by 55.81%, 66.12%, and 24.29%, respectively. The reason might be that Fe3O4-GO absorbed some Fe elements or very high concentration of Pb(II) in the first leek, decreasing the efficiency of Fe(III) transportation. This lead Pb(II) concentration increased, and the Fe(III) concentration reduced in soluble matter.
3.5 Effect of Fe3O4-GO on Pb(II) Distribution in Leek Organ
Commonly, roots have stronger enrichment effect than leaves. The concentration of Pb(II) in leaves, stem, storage roots, and absorbing roots, which were harvested on 100th day, was shown in Table 1. No matter if Fe3O4-GO was added or not, the Pb(II) distribution trend was consistent in the four experiments. The Pb(II) concentration followed the order absorbing roots > storage roots > stem> leaves. As shown in Table 1, Pb(II) concentration in stem and roots obtained from pot D was also obviously lower than that from pot C. This proved again that Fe3O4-GO has a significant fixation effect on Pb(II) in the soil under high Pb(II) pollution condition. The reduce percentages were 47.29%, 66.60%, 78.04%, and 39.16% for leaves, stem, storage roots, and absorbing roots. For no Pb(II) pollution leek (pots A and B), the Pb(II) transfer coefficient decreased from 34.91 to 13.24%. For artificial polluted leek, the Pb(II) transfer coefficient decreased from 18.60 to 16.25%. These results revealed that the addition of Fe3O4-GO in soil could prevent the migration of Pb(II) from soil to plant. Some other materials, such as rice straw-derived biochar, have been studied before and owned the ability to immobilize Cu(II), Pb(II), and Cd(II) in a simulated polluted Ultisol (Jiang et al. 2012).
On the other side, we have detected Fe(III) concentration in leaves, stem, storage roots, and absorbing roots. As shown in Table S1, slight Fe(III) increase was observed in roots and stem, which was probably caused by the degradation of Fe3O4 on GO. However, the Fe(III) concentration increased only in stem and roots, and the transfer coefficient of Fe(III) was lower than 10%. Combined with the SEM results, we can conclude that Fe3O4-GO could not enter the soil-plant ecosystem. These results were consistent with a survey, which reveal that Fe3O4 could not enter wheat plants.
4 Conclusion
The application of Fe3O4-GO reduced Pb(II) mobility in soil and enrichment of Pb(II) in roots, stem, and leaves compared with the control.
The recycled nanoparticles showed that their structure and morphology did not changed significantly. The fate and the potential co-transport risks need more new evidence in spite of some reports have shown that nanoparticles could be transported in the soil-to-plant system. After analysis of four harvest leek, results showed that Pb(II) contents in leaves decreased dramatically after the fourth harvest. This revealed that it is reasonable to abandon the first few harvest leek in tradition.
References
Allfrey, V. (1959). The isolation of subcellular components (pp. 193–290). The Cell: Elsevier.
Anderson, A. J., McLean, J. E., Jacobson, A. R., & Britt, D. W. (2017). CuO and ZnO nanoparticles modify interkingdom cell signaling processes relevant to crop production. Journal of Agricultural and Food Chemistry.
Bai, X., Feng, R., Hua, Z., Zhou, L., & Shi, H. (2015). Adsorption of 17β-estradiol (E2) and Pb(II) on Fe3O4/graphene oxide (Fe3O4/GO) nanocomposites. Environmental Engineering Science, 32, 370–378.
Ben-Moshe, T., Frenk, S., Dror, I., Minz, D., & Berkowitz, B. (2013). Effects of metal oxide nanoparticles on soil properties. Chemosphere., 90, 640–646.
Chen, J., Yao, B., Li, C., & Shi, G. (2013). An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon., 64, 225–229.
Chlopecka, A., & Adriano, D. (1997). Influence of zeolite, apatite and Fe-oxide on Cd and Pb uptake by crops. The Science of the Total Environment, 207, 195–206.
Dan, Y., Zhang, W., Xue, R., Ma, X., Stephan, C., & Shi, H. (2015). Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma–mass spectrometry analysis. Environmental Science & Technology, 49, 3007–3014.
Deng, Y., Qi, D., Deng, C., Zhang, X., & Zhao, D. (2008). Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. Journal of the American Chemical Society, 130, 28–29.
Ghasemi, E., Heydari, A., & Sillanpää, M. (2017). Superparamagnetic Fe3O4@ EDTA nanoparticles as an efficient adsorbent for simultaneous removal of Ag (I), Hg (II), Mn (II), Zn (II), Pb (II) and Cd (II) from water and soil environmental samples. Microchemical Journal, 131, 51–56.
Gonzalez, M., Miglioranza, K. S., Aizpún de Moreno, J. E., & Moreno, V. J. (2003). Organochlorine pesticide residues in leek (Allium porrum) crops grown on untreated soils from an agricultural environment. Journal of Agricultural and Food Chemistry, 51, 5024–5029.
Gu, Q., Yang, Z., Yu, T., Yang, Q., Hou, Q., & Zhang, Q. (2018). From soil to rice–a typical study of transfer and bioaccumulation of heavy metals in China. Acta Agriculturae Scandinavica Section B Soil and Plant Science, 68, 631–642.
G-y, L., Y-r, J., K-l, H., Ding, P., & Chen, J. (2008). Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. Journal of Alloys and Compounds, 466, 451–456.
Hu, Y., Norton, G. J., Duan, G., Huang, Y., & Liu, Y. (2014). Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant and Soil, 384, 131–140.
Huang, S., Liao, Q., Hua, M., Wu, X., Bi, K., Yan, C., et al. (2007). Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province. China Chemosphere, 67, 2148–2155.
Inaba, T., Kobayashi, E., Suwazono, Y., Uetani, M., Oishi, M., Nakagawa, H., et al. (2005). Estimation of cumulative cadmium intake causing itai–itai disease. Toxicology Letters, 159, 192–201.
Jiang, J., Xu, R.-K., Jiang, T.-Y., & Li, Z. (2012). Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. Journal of Hazardous Materials, 229-230, 145–150.
Liang, J., Xu, Y., Sui, D., Zhang, L., Huang, Y., Ma, Y., et al. (2010). Flexible, magnetic, and electrically conductive graphene/Fe3O4 paper and its application for magnetic-controlled switches. The Journal of Physical Chemistry C., 114, 17465–17471.
Liu, X., Song, Q., Tang, Y., Li, W., Xu, J., Wu, J., et al. (2013). Human health risk assessment of heavy metals in soil–vegetable system: a multi-medium analysis. The Science of the Total Environment, 463, 530–540.
Mu, L., Gao, Y., & Hu, X. (2015). l-Cysteine: a biocompatible, breathable and beneficial coating for graphene oxide. Biomaterials, 52, 301–311.
Park, S., & Ahn, Y.-J. (2016). Multi-walled carbon nanotubes and silver nanoparticles differentially affect seed germination, chlorophyll content, and hydrogen peroxide accumulation in carrot (Daucus carota L.). Biocatalysis and Agricultural Biotechnology, 8, 257–262.
Peng, W., Li, H., Liu, Y., & Song, S. (2017). A review on heavy metal ions adsorption from water by graphene oxide and its composites. Journal of Molecular Liquids, 230, 496–504.
Qingqing, H., Yiyun, L., Xu, Q., Lijie, Z., Xuefeng, L., & Yingming, X. (2019). Selenite mitigates cadmium-induced oxidative stress and affects Cd uptake in rice seedlings under different water management systems. Ecotoxicology and Environmental Safety, 168, 486–494.
Rico, C. M., Majumdar, S., Duarte-Gardea, M., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2011a). Interaction of nanoparticles with edible plants and their possible implications in the food chain. Journal of Agricultural and Food Chemistry, 59, 3485–3498.
Rico, C. M., Majumdar, S., Duarte-Gardea, M., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2011b). Interaction of nanoparticles with edible plants and their possible implications in the food chain. Journal of Agricultural and Food Chemistry, 59, 3485–3498.
Rieuwerts, J. S., Ashmore, M. R., Farago, M. E., & Thornton, I. (2006). The influence of soil characteristics on the extractability of Cd, Pb and Zn in upland and moorland soils. Science of the Total Environment, 366, 864–875.
Simonin, M., & Richaume, A. (2015). Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: a review. Environmental Science and Pollution Research, 22, 13710–13723.
Sun, M., Li, P., Jin, X., Ju, X., Yan, W., Yuan, J., et al. (2018). Heavy metal adsorption onto graphene oxide, amino group on magnetic nanoadsorbents and application for detection of Pb(II) by strip sensor. Food and Agricultural Immunology, 29, 1053–1073.
S-w, H. U. A. N. G., B-w, H. A. N., A-l, H. E., J-y, J. I. N., C-j, L. I., S-l, X. I. N. G., et al. (2007). Spatial variability of heavy metals in soils and Chinese leeks in a vegetable production area beside highway of suburb. Acta Agriculturae Boreali-Sinica, S2.
Wang, Y., Dang, F., Evans, R. D., Zhong, H., Zhao, J., & Zhou, D. (2016). Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: the key role of antagonism in soil. Scientific Reports, 6, 19477.
Xiao, R., Zhang, X., Zhang, X., Niu, J., Lu, M., Liu, X., et al. (2017). Analysis of flavors and fragrances by HPLC with Fe3O4@ GO magnetic nanocomposite as the adsorbent. Talanta, 166, 262–267.
Yang, Q., Li, Z., Lu, X., Duan, Q., Huang, L., & Bi, J. (2018). A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Science of the Total Environment, 642, 690–700.
Zahra, Z., Waseem, N., Zahra, R., Lee, H., Badshah, M. A., Mehmood, A., et al. (2017). Growth and metabolic responses of rice (Oryza sativa L.) cultivated in phosphorus-deficient soil amended with TiO2 nanoparticles. Journal of Agricultural and Food Chemistry, 65, 5598–5606.
Zhao, X., Zhao, C., Du, X., & Dong, D. (2019). Detecting and mapping harmful chemicals in fruit and vegetables using nanoparticle-enhanced laser-induced breakdown spectroscopy. Scientific Reports, 9, 906.
Zhu, H., Han, J., Xiao, J. Q., & Jin, Y. (2008). Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. Journal of Environmental Monitoring, 10, 713–717.
Funding
This research was supported by National Natural Science Foundation of China (Grant No. 31601547).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The fate and the fixation effect of Fe3O4-GO nanocomposites to Pb(II) in the soil-leek system during four harvest lifecycle were investigated.
• After addition of Fe3O4-GO into Pb(II)-polluted soil, a significant fixation effect of Pb(II) was found based on the analysis of leaves, stem, storage roots, and absorbing roots.
• The scanning electron microscope images showed that the overall appearance of Fe3O4-GO has not been destroyed during the interaction with soil.
Electronic Supplementary Material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Sun, M., Chu, C. et al. Fixation Effect of Fe3O4-GO to Hinder Pb(II) Translocation into Leek. Water Air Soil Pollut 231, 326 (2020). https://doi.org/10.1007/s11270-020-04694-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04694-9