Abstract
The levels of persistent organic pollutants, polychlorinated biphenyls (PCBs), were investigated in influent, effluent, and sludge of five wastewater treatment plants (WWTPs) in Jordan. Concentrations of 12 dioxin-like PCBs were determined. The extraction of PCBs from wastewater samples was done by solvent extraction using dichloromethane/hexane 1:1 mixture. The concentrated extracts were sequentially subjected to multilayer silica gel, basic alumina, and florisil chromatography columns for further cleanup. Sludge samples were extracted in a Soxhlet apparatus, using petroleum ether. PCB determination has been completed using gas chromatography equipped with mass spectrometry detector. The total concentrations of PCBs in influent samples ranged from 34.65 to 228.3 ng L−1, in effluent samples from 16.1 to 123.88 ng L−1, and in the sludge samples from 51.1 to 223.85 ng kg−1. In three of the investigated wastewater treatment plants, the amount of PCBs in sludge exceeded the limit proposed by European Union legislation. The total removal efficiencies of the total PCBs were evaluated and ranged from 34.8 to 88.1% for Aqaba and Abu-Nsair WWTP, respectively. The values of incremental lifetime cancer risk due to exposure to PCBs in sludge samples were also estimated in this study, and they ranged from 2.415 × 10−7 to 1.193 × 10−6 for adults. The number of people suspected to have cancer due to the exposure to the sludge of the WWTPs in Jordan is between 4 and 20 out of ten million.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The increase in water demand worldwide due to the increase in human population and activity, in addition to the decrease in freshwater resources urge the need to recycle and reuse municipal wastewater. The outgoing wastewater that gains proper treatment in wastewater treatment plants (WWTPs) is a prominent candidate for many potential uses in irrigation, industry, or even for domestic uses (EPA 2012).
In Jordan, water scarcity is a reality, as the country is counted among the world’s most arid countries. The current per capita water supply in Jordan is 150 m3 per year which is almost one-third of the global average. Unfortunately, it is projected that per capita water availability will decline to 90 m3 by the year 2025 (Abdulla et al. 2016). Therefore, using treated wastewater is imperative in Jordan, especially in the field of agriculture. In fact, the reuse of treated wastewater in Jordan has reached one of the highest levels in the world due to the latest technologies employed in WWTPs that are spread all over the country (Al-Zahiri 2015).
In most countries, including Jordan, quantification of wastewater quality is based on the monitoring of traditional parameters that are regulated by the European Urban Wastewater Directive (91/271/EEC). These parameters include biochemical oxygen demand (BOD), chemical oxygen demand (COD), nitrates, phosphates, and total suspended solids (Directive 1991; Urbaniak and Wyrwicka 2017). However, these regulations do not include other substances that could pose threat to human health when released through WWTPs effluents such as polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAH) (Inglezakis et al. 2014).
Polychlorinated biphenyls (PCBs) have become available as industrial chemicals since 1930; their widespread application in the subsequent 50 years has resulted in their presence as persistent and ubiquitous environmental contaminants and/or pollutants (Breivik et al. 2007). The physicochemical properties of PCBs such as high lipophilicity and their high resistance to acidic and basic hydrolysis (WHO 1992) made them attractive for a variety of industrial purposes, for instance in manufacturing dielectrics in capacitor, transformers, and fire retardants. Over 800 million tons of PCBs have been produced until the late 1970s, when the production was banned in most countries. However, worldwide large-scale production continued up to the mid-1980s (Alawi and Heidmann 1991; Boersma and Lanting 2002).
Human exposure to PCBs is possible via several routes, including inhalation, dermal absorption, and food consumption (Font et al. 1996). PCBs were found to have potential carcinogenic, mutagenic, and teratogenic effects (Cortazar et al. 2002; Garcıa-Ruiz et al. 2001; Russo 2000; Travis et al. 1988).
PCBs are a group of 209 compounds or congeners of the formula C12H10-nCln, where n = 1–10. Toxicities and persistency of PCBs are dramatically dependent on the number and positions of chlorine substituents. If there is no chlorine at the ortho-positions, the biphenyl rings can be coplanar and structurally resemble dioxin (dioxin-like). Out of the 209 possible PCBs congeners, only 12 show toxicity similar to PCDD, which is the most toxic substance (Bruner-Tran and Osteen 2010).
Sewage sludge from sludge treatment plants can be used for soil improvement as organic fertilizers or soil conditioners. Application of sludge to agricultural lands will reduce the amount of chemical fertilizers used, improve soil fertility, and recycle beneficiary waste products back into the soil and plants (Parkpian et al. 2002). The use of sewage sludge as a fertilizer is widespread. The amount of sludge used for agricultural purpose is 25% in Germany and up to 90% in Sweden (McLachlan et al. 1996).
Human adults and children may be exposed to PCB-contaminated soils (treated with WWTP sludge) through different intake pathways. The proposed EU standard for the use of sewage sludge as organic fertilizer as soil amendment is 800 ng g−1 dw for the sum of seven congeners (Katsoyiannis and Samara 200).
PCBs must be monitored in WWTP effluent to ensure that their level does not pose a threat to human or aquatic life. In the same context, the concentration of these compounds must be monitored in the sludge produced from WWTP as it could be used as a fertilizer and lead to direct human exposure.
In Jordan, few studies have been conducted in order to evaluate PCBs in the influent, effluents, and sludge of WWTPs (Al Nasir and Batarseh 2008).
In this study, the concentration of 12 dioxin-like PCBs will be determined in the influent, effluent, and sludge of five WWTPs located in different areas in Jordan. The efficiency of these WWTPs in the removal of PCBs will be evaluated. The toxic equivalency TEQ and the incremental lifetime cancer risk (ILCR) values will also be estimated in order to evaluate the potential health risk from exposure to PCBs in the WWTPs sludge.
2 Materials and Methods
2.1 Materials and Reagents
2.1.1 Solvents and Standards
The standard mixture contains (10 μg mL−1 in hexane) 12 of the PCB (dioxin-like) priority pollutants; isodrin as an internal standard; dichloromethane (HPLC-grade); n-Hexane (ACS grade >95%); Petroleum ether (ACS grade >95%).
2.2 Sampling
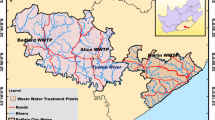
Samples were collected from the five main WWTPs located in southern, central, and northern regions of Jordan as shown in Fig. 1. Methods used for wastewater treatment usually incorporate primary and secondary steps. The primary steps include preliminary treatments such as skimming and screening to remove drips and large suspended solids, influent pumping, primary clarification, and coagulation. The secondary steps include waste stabilization ponds at Kherbet As-Samra and Aqaba, trickling filter-activated sludge technology at Irbid, rotating biological contractors at Abu-Nsair and activated sludge with extended aeration technologies Al-Salt WWTPs (Al-Khashman 2009; Al-Khashman et al. 2013). In July 2017, sampling was performed where samples from the influent and effluent of each WWTP were collected every 3 h from 9:00 to 18:00 to form three samples for each plant. Then, representative mixed samples were prepared by pooling the three individual samples. Wastewater samples were extracted and cleaned-up. All wastewater samples were collected in brown glass bottles, immediately transferred to the laboratory, filtered through 0.6-μm glass fiber filters to remove fine particulates, and then stored at 4 °C until time of extraction which was conducted within 24 h after. This sampling procedure is the same as that was followed by Al-Tarawneh et al. (2015).
Sites of studied WWTPs in Jordan (Al-Tarawneh et al. 2015)
The pH values of all the samples were directly measured at 25 °C after sampling and filtration. The values were between 7.0 (Irbid WWTP) and 8.0 (Kherbet AS-Samra WWTP) for the influent samples and between 5.8 (Aqaba WWTP) and 8.4 (Abu-Nsair WWTP) for effluent samples.
The sludge samples (about 0.5 kg) were collected at endpoints of the technical line of sewage sludge digestion. The collected samples were stored in prewashed (with dichloromethane) glass bottles and immediately transported to the laboratory. All sewage sludge samples were air-dried and milled to obtain representative samples.
2.3 Sample Extraction and Purification
2.3.1 Extraction of Wastewater Samples
A 100 mL of influent and effluent wastewater samples was filtered using 0.6-mm glass fiber filters to remove fine particulates, then extracted three times under ultrasonic conditions using liquid-liquid extraction method with 200 mL of dichloromethane/hexane for 1 h (1:1, v/v). The total extracted volume was 180 mL of dichloromethane/hexane (1: 1, v/v), and the extracts were concentrated to 1 mL and subjected to a solvent exchange to hexane by a rotary evaporator. The concentrated extracts were sequentially subject to multilayer silica gel, basic alumina, and florisil chromatography columns for further clean-up, following the published procedures (Liu et al. 2006). The multilayered silica gel column was packed from bottom to top with 1 g of activated silica, 4 g of basic silica (1.2% w/w), 1 g of activated silica, 8 g of acid silica (30% w/w), 2 g of activated silica, and 4 g of anhydrous sodium sulfate. The columns were preserved in dichloromethane/hexane (1:1, v/v) before loading the extracts. Elution of the samples was carried out at a rate of 1 mL min−1 under vacuum. Then the eluent was collected and evaporated to dryness with a rotary evaporator. The final residue was dissolved in 1.0 mL of n-hexane containing 100 ng L−1 of isodrin (I.S) and then 2 μl of the reconstituted solution was injected onto the GC/MS.
2.3.2 Extraction of Sludge Samples
One-gram dry weight (dw) sludge samples was mixed homogeneously with appropriate anhydrous sodium sulphate. Then, the samples were extracted in a Soxhlet apparatus, for 8 h (about 15 min for each cycle) using 150 mL of petroleum ether. The solvent was then evaporated using a rotary evaporator at 40 °C and 100 mbar to ca. 3 mL. This method was performed following Alawi and Heidmann (1991) with slight modification. A first column (30 × 1.6 cm) was prepared as follows: 2 g of anhydrous sodium sulfate, 10 g of sulfuric acid-impregnated silica gel (40% w/w), and 2 g of anhydrous sodium sulfate. A second column (30 × 1.6 cm) was prepared as follows: 25 g of Florisil® (3% w/w deionized water) (60–100 mesh) was filled into the column, and then 1 cm of anhydrous sodium sulfate was added. The first column was washed with 100 mL petroleum ether. Then, the residue from the extraction step was added to this column and eluted with 100 mL dichloromethane. After that, the extract was added to the second column and eluted with 60 mL dichloromethane and 240 mL of petroleum ether. The eluate was concentrated at 40 °C and 100 mbar to ca. 1 mL and then evaporated to dryness using a gentle stream of nitrogen. Finally, the residue was dissolved in 1.0 mL n-hexane containing 100 ng mL−1 isodrin (I.S), and then 2 μl of the reconstituted solution was injected into the GC/MS.
2.4 GC/MS Working Conditions
Gas chromatograph (Agilent 6890 series II, with auto sampler injector series 7683) was used for PCBs analysis: injected volume, 1 μL/splitless; column, BPX-5, 30 cm × 0.25 mm i.d. × 0.25-μm film thickness; carrier gas, helium (99.999%) (8 psi); temperature program, 100 °C (10 min), 100–160 °C (25 °C/min), 160–270 °C (5 °C/min), and 270 °C isotherm (27 min); detector, mass selective quadrupole – detector, Agilent 5973 N (MSD); auxiliary (transfer line), 280 °C; electron impact ionization, ionization energy 70 eV; calibration substance, perfluorotributylamine (PFTBA); tuning masses, 69/219/502 m/z; acquisition mode, selective ion monitoring (SIM) mode; and data analysis, HP MSD productivity Chemstation software.
Mass was scanned at a range of 50 to 500 amu at 0.5-s scan rate in total ion chromatogram (TIC) mode to identify the compounds in the standard mixture. For retention time designation purposes, mass spectrum for each analyte was compared with that published on databases and also compared with the NIST mass spectral reference library for more confirmation. In sample analysis, SIM-mode was applied, where two ions were monitored for each analyte at its retention time range. The ratio between the ions abundances was used for identification purposes, while one of them was used for the quantitative analysis. The GC-Mass, SIM-mode resolution profiles for the standard mixture of the 12 PCB congeners, in addition to the internal standard is shown in Fig. 2.
Representative chromatogram for a standard mixture solution containing 250 ng L−1 of PCBs; 1-isodrin (I.S.), 2–3,3′,4,4′-tetrachlorobiphenyl 3–3,4,4′,5-tetrachlorobiphenyl 4–2,3,3′,4,4′-pentachlorobiphenyl 5–2,3,4,4′,5-pentachlorobiphenyl 6–2,3′,4,4′,5-pentachlorobiphenyl 7–2′,3,4,4′,5-pentachlorobiphenyl 8–3,3′,4,4′,5-pentachlorobiphenyl 9–2,3,3′,4,4′,5 hexachlorobiphenyl 10–2,3,3′,4,4′,5′-hexachlorobiphenyl 11–2,3′,4,4′,5,5′-hexachlorobiphenyl 12–3,3′,4,4′,5,5′-hexachlorobiphenyl 13–2,3,3′,4,4′,5,5′-heptachlorobiphenyl
2.5 Preparation of Standard, Internal Standard, Stock, and Working Solutions
A 10 mg L−1 of PCBs standard (12 congeners) has been already prepared. Then, through serial dilutions, the series of working solutions were prepared as follows: 100, 500, and 1000 ng L−1. The internal standard isodrin 1000 ng L−1 was prepared by dissolving the exact 1.0 mL volume into an exact volume of 1000 mL of cyclohexane. Then, serial dilution steps were followed to prepare the working solutions with the concentration of 250 ng L−1 of the internal standard.
2.6 Method Validation
2.6.1 Linear Range
For the calculation of the performance data, a calibration was carried out with five concentration levels for PCBs 25, 100, 250, 500, and 750 ng L−1. From the resulting calibration curves, the regression coefficients, which characterize the linearity of the calibration function, were calculated. The regression coefficients were between 0.995 and 1.00, indicating a good linearity of the calibration function in these concentration ranges.
2.6.2 Detection Limit and Limit of Quantitation of PCBs
The instrument limits of detection (LOD) were obtained by diluting the standard mixture solution until the ratio of signal to noise S/N equaled three, while the lower limits of quantitation (LLOQ) were calculated when S/N ratio equaled ten. Table 1 shows LOD and LLOQ values for PCBs compounds.
The method limit of detection (MLOD) and method lower limit of quantitation (MLLOQ) were calculated when S/N ratio equaled three and ten, respectively. Table 2 shows MLOD and MLLOQ values for PCBs compounds.
2.6.3 Instrument Precision of PCBs
The precision of the instrument was measured through the injection of standard solutions (50, 250, 500 ng L−1) five times each. The percentage relative standard deviation (RSD%) was found to be less than the accepted limit value for trace analysis (RSD < 15%) (González and Herrador 2007); these results show that we have a good instrument precision.
2.6.4 Extraction Recoveries for Influent and Effluent Wastewater Samples
Three samples, each has 100 mL of deionized water, were used as the blank and spiked with the PCB standard mixture to give the concentrations of 100, 250, and 500 ng L−1. These samples were mixed thoroughly, extracted, and analyzed applying the abovementioned method. The recovery tests were done thrice at different times and were injected three times.
The results gave the recovery range of 85–117% for the 100 ng L−1concentration, 82–94% for the 250 ng L−1concentration, and 87–112% for the 500 ng L−1 concentration. All recoveries were found to lie within the acceptable range for trace analysis of 80–120% (González and Herrador 2007).
2.6.5 Extraction Recoveries for Sludge Samples
Three samples, each of 1 g of pure sand soil, were used as the blank and spiked with the PCBs standard mixture to give the concentrations of 100, 250, and 500 ng kg−1. These samples were mixed thoroughly, extracted, cleaned-up, and analyzed according to the abovementioned method. The recovery tests were done in thrice at different times and injected three times. The results gave the recovery range of 86–105% for the 100 ng kg−1 concentration, 84–96% for the 250 ng kg−1 concentration, and 94–115% for the 500 ng kg−1 concentration. All recoveries were found to lie within the acceptable range for trace analysis of 80–120% (González and Herrador 2007).
2.7 Calculations of Toxic Equivalency and Risk Assessment
Incremental lifetime cancer risk ILCR is the probability of developing cancer as the result of exposure to a specific carcinogen, and the value of ILCR is used to assess cancer risk. If the estimated ILCR is 1 × 10−6 (US Environmental Protection Agency 2005). Cancer risks will be considered essentially negligible. Canadian guidelines specify ILCR value of 1 × 10−5 (Canadian Council of Ministers of the Environment 2008). However, if the ILCR is greater than 1 × 10–6, risk management measures should be taken.
In order to assess the cancer risk associated with the exposure to PCB in sludge in this study, we treated the sludge as soil since the sludge is expected to be used in soil fertilization as its rich in nutrients. Therefore, we followed the calculation method of ILCR for soil proposed by USEPA (National Research Council 2006). We also considered soil ingestion as the main exposure pathway of the intake of PCBs in humans.
Toxic equivalency factors (TEFs) with reference to 2,3,7,8-TCDD have been assigned by World Health Organization (Van den Berg et al. 2006) for the twelve dioxin-like PCBs for toxicity assessment, and the TEF values are given in Table 5. The toxicity of PCBs is expressed as a toxic equivalency (TEQ) value. Toxic equivalency of carcinogenic dioxin-like PCBs was calculated using toxicity equivalency factors (TEFs) using Eq. 1:
where Ci is the concentration of individual PCB (ng kg−1), and TEFi is the corresponding toxic equivalency factor.
Incremental lifetime cancer risk (ILCR) for PCBs was calculated using Eq. 2 (Kumar et al. 201):
where IR is the soil ingestion rate (mg day−1) which estimated the number of consumption of soil per day, and this number is usually higher for children compared with adults (Phillips 2017), F is a conversion factor, ED is exposure frequency (years), CSF is a number that reflects how cancer risk rises with increased exposure to a certain compound, BW is body weight, and AT is averaging time which is the number of days for the proposed 70 lifetime for an adult and 12 years for a child.
Input parameters for risk estimates are mentioned in Table 3 (Agency, U. E. P. 1996; Kumar et al. 2014).
2.8 Determination of PCBs in Wastewater and Sludge Samples
Each of the 10 wastewater samples and the five sludge samples was extracted and cleaned up twice, and then each extract was injected twice into the GC/MS column. The net result concentrations were calculated as the average of the four values.
The qualitative analysis of the sample was done using the SIM mode to determine the PCBs. Two ions (quantification and identification ions) for each analyte were monitored, and then the ratio between their area signals was calculated and compared with that generated from standard mixture. These ions and ratios were summarized in Table 4. For quantitative calculations, only the signal of the quantification ion was used for each analyte. Peak area for the monitored ion was compared with the internal standard ion peak area, and then relative peak area was calculated. For each analyte, multipoint calibration method was applied to estimate the analyte concentration using its PRA value and regression equation of its calibration curve.
3 Results and Discussion
3.1 Concentration of PCBs in Samples
Tables 5 and 6 show the analytical results of the wastewater samples of the five WWTP and the five sludge samples, respectively. Results are presented as the average ± standard deviation for each compound. Furthermore, TEQ for each compound calculated using Eq. 1 is stated in the Tables. The summations of TEQ for all PCBs in each WWTP were calculated, and the values are presented in Tables 5 and 6 for the influent, effluent, and the sludge of the five WWTPs.
Seven out of the twelve investigated PCBs were found in the influent of studied WWTP samples, which have been categorized as priority pollutants by USEPA (EPA 2003). The total concentration of PCBs in the influent ranged from 34.65 ng L−1 at Aqaba WWTP to 228.3 at Abu-Nsair WWTP. The most appearing congener of the tested PCBs was the pentacongener PCB 126 (with the highest toxicity) which appeared in all the samples with the highest concentration among all congeners. On the other hand, none of the tested hexachlorobiphenyl congeners was detected in any of the samples. Comparison of the result of this study with other studies is summarized in Table 7. And it reveals that our results are sometimes comparable, higher or lower than the results reported in other countries. The influent samples with high COD values (COD values are shown in Table 8) were found to have high PCB content, which is expected as PCBs are part of the COD in the wastewater. Other factors such as the pH or the source of wastewater could affect the levels of PCBs in the influent samples. The variation of PCBs values in the effluent from different WWTPs could be attributed to different treatment technologies employed in each plant. The initial characteristics of the influent wastewater could also have an effect on the final concentration of PCBs. The amounts of PCBs in the sludge samples were always high with respect to those of the effluent of WWTP (as shown in Fig. 3) due to the lipophilic nature of both PCBs and sludge. And that causes the concentration of these substances in the sludge.
3.2 Removal Efficiencies of PCBs from WWTPs
The removal efficiencies (RE %) of PCBs during the treatment process in the WWTPs were calculated using the following general equation:
where Cinf is the concentration of PCBs in the influent, and Ceff is the concentration of PCBs in the effluent. The results are summarized in Table 8.
The total removal efficiencies range from 34.8 to 88.1%. Removal efficiencies of the WWTPs were in the following order: Abu-Nsair ˃ Al-Salt ˃ Kherbet AS-Samra ˃ Irbid ˃ Aqaba. The differences in the removal efficiencies between the different WWTPs depended strongly on the treatment process technology employed and the initial chemophysical properties of the treated water. As the results indicated, using a rotary biological contactor as a technology for treatment gave the highest removal efficiency. On the other hand, waste stabilization ponds gave the lowest efficiency. Employing activated sludge technology shows high efficiency in PCBs removal as can be seen from the values of Al-Salt WWTP. However, the values are lower in Irbid WWTP, which uses the same technology as a result of high organic load represented by its high COD values. Most technologies rely on biological treatment, which involve biological degradation of organic matter in wastewater. Nevertheless, the slow biodegradability of PCBs makes it hard to achieve complete removal. Therefore, coupling with tertiary treatment step, such as adsorption, could enhance the efficiency of removal of PCBs from wastewater.
3.3 Risk Assessment
The optimal TEQ value of PCBs for a soil should be below 5 ng kg−1 (Antunes et al. 2008; Basler 1994; EC 2010; Weber et al. 2018). TEQ values greater than 100 ng kg−1 are considered to be relatively high and reduce the potential of using the soil due to contamination and level of toxicity. In our study, ƩTEQ values remain below the limits for all the sludge samples from the investigated WWTPs as shown in Table 9. Therefore, using this type of sludge in soil fertilization should not pose a health risk in terms of exposure to PCBs.
Risks for human adults and children posed by PCBs were calculated in terms of incremental lifetime cancer risk (ILCR) using Eq. 2. The estimated ILCR values ranged from 2.42 × 10−7 to 1.19 × 10−6 for adults and from 1.41 × 10−6 to 8.28 × 10−6 for children. The highest ILCR was identified in the sludge sample from Abu-Nsair WWTP, while the lowest ILCR was found in the sludge sample from Aqaba WWTP as shown in Table 9.
In Jordan, guidelines for PCBs in soils have not been established yet. Thus, recommended guidelines from USEPA were taken into consideration. According to USEPA guidelines in regulatory terms, an estimated cancer risk of 10−6 or less denotes virtual safety. On the other hand, an estimated cancer risk greater than 10−4 denotes potentially high risk (EPA U 1989; Kumar et al. 2014). Therefore, the results of all WWTPs fall below the limit of great cancer risk.
By multiplying the estimated cancer risks of exposure to sludge-PCBs sample by 106, it is possible to determine the theoretical number of cancer cases per million of people. For example, ILCR of 8.28 × 10−6 in Abu-Nsair sample means that the number of people who are suspected of cancer due to exposure to PCBs in this sample is eight out of every million. Therefore, if a person was exposed to the highest level of PCBs in Abu-Nsair for 70 years, he has 8 in a million increased chance of developing cancer from this exposure. This is the highest estimate of the risk, but the actual risk is likely lower. The total estimated cancer risks associated with the exposure to sludge samples-PCBs for adults and children in all WWTPs were found to be acceptable. Even under the worst-case scenario, the estimated cancer risk for adults and children in all sites are within the acceptable range of excess cancer risk of 1.0 × 10−4 to 1.0 × 10−6, specified by the USEPA (Kumar et al. 2014; US Environmental Protection Agency 2005).
4 Conclusion
The present study gives insight into the level of twelve dioxin-like PCBs in the influent, effluent, and sludge of five WWTPs in Jordan. The removal efficiencies of PCBs in the investigated WWTP were calculated. Variation in the obtained results was observed due to different characteristics of the raw wastewater in the effluent as well as the different treatment technologies employed in the investigated plant. Abu-Nsair WWTP, which uses rotating biological contactor in the treatment, shows the highest efficiency with respect to the removal of total PCBs. In three of the investigated wastewater treatment plants, the amount of PCBs in sludge exceeded the limit proposed by European Union legislation, which suggests that this sludge should be subjected to further treatment process in order to be safe for use as fertilizer or for human exposure. The total estimated cancer risk of exposure to PCBs in the studied sludge samples ranged from 4.1 × 10−7 to 2.42 × 10−6 for adults. The number of people suspected to develop cancer due to the exposure to the sludge of the WWTPs in Jordan is between 4 and 20 out of ten million.
References
Abdulla, F., Alfarra, A., Abu Qdais, H., & Sonneveld, B. (2016). Evaluation of wastewater treatment plants in Jordan and suitability for reuse. Academia Journal of Environmental Sciences, 4(7), 111–117.
Agency, U. E. P (1996). PCBs: cancer dose-response assessment and application to environmental mixtures: National Center for Environmental Assessment Washington, DC.
Al Nasir, F., & Batarseh, M. (2008). Agricultural reuse of reclaimed water and uptake of organic compounds: pilot study at Mutah University wastewater treatment plant, Jordan. Chemosphere, 72(8), 1203–1214.
Alawi, M., & Heidmann, W. (1991). Analysis of polychlorinated biphenyls (PCB) in environmental samples from the Jordan Valley. Toxicological & Environmental Chemistry, 33(1–2), 93–99.
Al-Khashman, O. (2009). Chemical evaluation of Ma’an sewage effluents and its reuse in irrigation purposes. Water Resources Management, 23(6), 1041–1053.
Al-Khashman, O., Al-Hwaiti, M., Al-Khatib, L., & Fraige, F. (2013). Assessment and evaluation of treated municipal wastewater quality for irrigation purposes. Research Journal of Environmental and Earth Sciences, 5(5), 229–236.
Al-Tarawneh, I., El-Dosoky, M., Alawi, M., Batarseh, M., Widyasari, A., Kreuzig, R., & Bahadir, M. (2015). Studies on human pharmaceuticals in Jordanian wastewater samples. Clean: Soil, Air, Water, 43(4), 504–511.
Al-Zahiri, A. (2015). Assessment of performance of wastewater treatment plants in Jordan. International Journal of Civil and Environmental Engineering, 1–6.
Antunes, P., Viana, P., Vinhas, T., Capelo, J., Rivera, J., & Gaspar, E. M. (2008). Optimization of pressurized liquid extraction (PLE) of dioxin-furans and dioxin-like PCBs from environmental samples. Talanta, 75(4), 916–925.
Basler, A. (1994). Regulatory measures in the Federal Republic of Germany to reduce the exposure of man and the environment to dioxins. Organohalogen Compounds, 20, 567.
Boersma, E., & Lanting, C. (2002). Environmental exposure to polychlorinated biphenyls (PCBs) and dioxins Short and long term effects of breast feeding on child health (pp. 271–287). Springer.
Breivik, K., Sweetman, A., Pacyna, J., & Jones, K. (2007). Towards a global historical emission inventory for selected PCB congeners—a mass balance approach: 3. An update. Science of the Total Environment, 377(2–3), 296–307.
Bruner-Tran, K., & Osteen, K. (2010). Dioxin-like PCBs and endometriosis. Systems Biology in Reproductive Medicine, 56(2), 132–146.
Canadian Council of Ministers of the Environment (2008). Canadian Soil Quality Guidelines for Carcinogenic and Other Polycyclic Aromatic Hydrocarbons (PAHS): environmental and human health effects: scientific supporting document. Canadian Council of Ministers of the Environment.
Chevreuil, M., Granier, L., Chesterikoff, A., & Létolle, R. (1990). Polychlorinated biphenyls partitioning in waters from river, filtration plant and wastewater plant: the case for Paris (France). Water Research, 24(11), 1325–1333.
Cortazar, E., Zuloaga, O., Sanz, J., Raposo, J., Etxebarria, N., & Fernandez, L. (2002). MultiSimplex optimisation of the solid-phase microextraction–gas chromatographic–mass spectrometric determination of polycyclic aromatic hydrocarbons, polychlorinated biphenyls and phthalates from water samples. Journal of Chromatography A, 978(1–2), 165–175.
Directive, E. U. W. (1991). Council Directive of 21. May 1991 concerning urban waste water treatment (91/271/EEC). Journal of the European Communities, 34, 40.
EC (2010). Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee on the implementation of the Community Strategy for dioxins, furans, and polychlorinated biphenyls (COM (2001) 593)–Third progress report/* COM/2010/0562 final*.
EPA (2003). Appendix A to 40 CFR, Part 423–126 Priority Pollutants.
EPA (2012). 2012 Guidelines for water reuse.
EPA, U. (1989). Risk assessment guidance for superfund. Human Health evaluation manual part A.
Font, G., Manes, J., Molto, J., & Pico, Y. (1996). Current developments in the analysis of water pollution by polychlorinated biphenyls. Journal of Chromatography A, 733(1–2), 449–471.
Garcıa-Ruiz, C., Martın-Biosca, Y., Crego, A., & Marina, M. (2001). Rapid enantiomeric separation of polychlorinated biphenyls by electrokinetic chromatography using mixtures of neutral and charged cyclodextrin derivatives. Journal of Chromatography A, 910(1), 157–164.
González, A. G., & Herrador, M. Á. (2007). A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. TrAC Trends in Analytical Chemistry, 26(3), 227–238.
Inglezakis, V. J., Zorpas, A. A., Karagiannidis, A., Samaras, P., Voukkali, I., & Sklari, S. (2014). European Union legislation on sewage sludge management. Fresenius Environmental Bulletin, 23(2 A), 635–639.
Katsoyiannis, A., & Samara, C. (2004). Persistent organic pollutants (POPs) in the sewage treatment plant of Thessaloniki, northern Greece: occurrence and removal. Water Research, 38(11), 2685–2698.
Kumar, B., Verma, V., Singh, S., Kumar, S., Sharma, C., & Akolkar, A. (2014). Polychlorinated biphenyls in residential soils and their health risk and hazard in an industrial city in India. Journal of public health research, 3(2).
Liu, H., Zhang, Q., Cai, Z., Li, A., Wang, Y., & Jiang, G. (2006). Separation of polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and dibenzo-furans in environmental samples using silica gel and florisil fractionation chromatography. Analytica Chimica Acta, 557(1–2), 314–320.
McLachlan, M., Horstmann, M., & Hinkel, M. (1996). Polychlorinated dibenzo-p-dioxins and dibenzofurans in sewage sludge: sources and fate following sludge application to land. Science of the Total Environment, 185(1–3), 109–123.
National Research Council. (2006). Health risks from dioxin and related compounds: evaluation of the EPA reassessment. National Academies Press.
Parkpian, P., Klankrong, K., DeLaune, R., & Jugsujinda, A. (2002). Metal leachability from sewage sludge-amended Thai soils. Journal of Environmental Science and Health, Part A, 37(5), 765–791.
Pham, T., Proulx, S., Brochu, C., & Moore, S. (1999). Composition of PCBs and PAHs in the Montreal urban community wastewater and in the surface water of the St. Lawrence River (Canada). Water, Air, and Soil Pollution, 111(1–4), 251–270.
Phillips, L. (2017). Exposure factors handbook chapter 5 (update): soil and dust ingestion.
Russo, M. (2000). Diol sep-pak cartridges for enrichment of polychlorobiphenyls and chlorinated pesticides from water samples; determination by GC-ECD. Chromatographia, 52(1–2), 93–98.
Travis, C., Hattemer-Frey, H., & Arms, A. (1988). Relationship between dietary intake of organic chemicals and their concentrations in human adipose tissue and breast milk. Archives of Environmental Contamination and Toxicology, 17(4), 473–478.
Urbaniak, M., & Wyrwicka, A. (2017). PCDDs/PCDFs and PCBs in wastewater and sewage sludge. Physico-Chemical Wastewater Treatment and Resource Recovery, 109.
US Environmental Protection Agency (2005). Guidelines for carcinogen risk assessment. In Risk Assessment Forum. Washington, DC: US EPA.
Van den Berg, M., Birnbaum, L., Bosveld, A., Brunström, B., Cook, P., Feeley, M., & Kennedy, S. (1998). Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environmental Health Perspectives, 106(12), 775–792.
Van den Berg, M., Birnbaum, L. S., Denison, M., De Vito, M., Farland, W., Feeley, M., & Haws, L. (2006). The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicological Sciences, 93(2), 223–241.
Vogelsang, C., Grung, M., Jantsch, T., Tollefsen, K., & Liltved, H. (2006). Occurrence and removal of selected organic micropollutants at mechanical, chemical and advanced wastewater treatment plants in Norway. Water Research, 40(19), 3559–3570.
Weber, R., Herold, C., Hollert, H., Kamphues, J., Blepp, M., & Ballschmiter, K. (2018). Reviewing the relevance of dioxin and PCB sources for food from animal origin and the need for their inventory, control and management. Environmental Sciences Europe, 30(1), 42.
WHO (1992). Polychlorinated biphenyls, polychlorinated terphenyls (PCBs and PCTs: health and safety guide.11.
Funding
The authors thank Al-Balqa Applied University for providing financial aids for this project.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on this submission had made substantial contributions to the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
The authors declare that all the data in this work are original and not reused from other sources.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abu-Shmeis, R.M., Tarawneh, I.N., Al-qudah, Y.H. et al. Evaluation of the Removal Efficiency of PCBs from Five Wastewater Treatment Plants in Jordan. Water Air Soil Pollut 231, 114 (2020). https://doi.org/10.1007/s11270-020-04482-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04482-5