Abstract
Phthalates are endocrine-disrupting chemicals which affect endocrine system by bio-accumulation in aquatic organisms and produce adverse health effects in aquatic organisms as well as human beings, when come in contact. Present study focuses on occurrence and removal of two phthalates: diethylphthalate (DEP) and dibutylphthalate (DBP) in two full-scale wastewater treatment plants (WWTPs) i.e. sewage treatment plants (STPs) based on well-adopted technologies, activated sludge process (ASP) and sequencing batch reactor (SBR).Gas chromatography-mass spectrometry (GC–MS) analysis was performed for both wastewater and sludge sample for determination and identification of the concentration of these compounds in both STPs by monitoring the STPs for 9 months. It was observed that the concentration of DEP was less than DBP in the influent of ASP and SBR. Average concentrations of DEP and DBP in sludge sample of ASP were found to be 2.15 and 2.08 ng/g, whereas in SBR plant, these values were observed as 1.71 and 2.01 ng/g, respectively. Concerning the removal efficiency of DEP, SBR and ASP plants were found effective with removal efficiency of 91.51 and 91.03 %, respectively. However, in the case of DBP, SBR showed lower removal efficiency (85.42 %) as compared to ASP (92.67 %). Comparative study of both plants proposed that in ASP plant, DBP reduction was higher than the SBR. Fourier transformation infrared (FTIR) analysis also confirmed the same result of sludge analysis for both STPs. Sludge disposal studied with scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX) and thermo-gravimetric analysis (TGA) techniques confirmed that sludge of both STPs have high calorific value and can be used as fuel to make fuel-briquettes and bottom ash to make firebricks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalates are dialkyl or alkyl aryl esters of phthalic acids. These are prepared by reacting phthalic anhydride with alcohols and are commonly used for the softening of polyvinylchloride (PVC) resin to improve their flexibility, transparency, durability and workability by decreasing the glass transition temperature (Chen and Sung 2005; Adibi et al. 2008). These are chemically stable, odourless and colourless compounds that are slightly soluble in water (Clara et al. 2010; Medellin-Castillo et al. 2013) and are also used in insect repellents, adhesives, glues, paints, cosmetics, lubricants, gaskets, inks, rainwear, paper coatings, electrical wire insulation, roofing systems, pharmaceuticals, pesticides, etc. (Yuan et al. 2002; Lu et al. 2009; Na et al. 2012).
There are several non-point sources such as domestic and commercial discharges, street runoff, aerial deposition and point sources like agricultural and industrial discharges of phthalate compounds (Alatriste-Mondragon et al. 2003; Roslev et al. 2007). Due to their wide range of applications in industrial production, their production has rapidly grown in the last decades (Dargnat et al. 2009). Their widespread use and production cause direct entry of these compounds into the environment because they are not chemically bound to the polymeric matrix and migrate from plastic and other products during manufacturing processes and by leaching or evaporating from final products (Marttinen et al. 2003; Zeng et al. 2004). Most of the phthalates cause endocrine disrupting effect by altering endogenous hormones (Takeuchi et al. 2005), thus affecting reproductive and developmental physiology in the aquatic organisms and mammals (Lau et al. 2005). Therefore, these chemicals pose a potential threat to human and wildlife reproduction. Several regulatory bodies classified these compounds as chief environmental pollutants and as endocrine-disrupting compounds because of their toxic, carcinogenic and mutagenic properties (Cai et al. 2008; Rudel et al. 2010; Boonyaroj et al. 2012). When released to the environment, these compounds tend to adsorb on particles and therefore identified in rainwater, surface water, treated and untreated wastewater, sewage sludge, sediment and in storm water (Yang et al. 2005). Living beings are exposed to phthalates in two ways: first, indirect pathways like contamination in air, water and food; and second, direct contact with plastic products, mainly those intended as high contact products, like children’s toys and teethers (Ozer et al. 2012).
Literature survey suggested that DEP and DBP owing to their high water solubility have been most frequently identified phthalates in various environmental water samples (Xu et al. 2007). These are used in cosmetics and plastic products (Kolarik et al. 2008; Prasad and Suresh 2012). These compounds exhibit endocrine disrupting activity and cause adverse health effects in aquatic organisms and consequently to human beings due to which their determination and removal are of great concern (Mohan et al. 2007). The determination, identification and removal of these compounds in water and wastewater treatment plants have been reported in developed countries to a large extent but to a limited extent in India.
Present study is focused on identification, removal and fate of these compounds in two full-scale STPs based on ASP and SBR technologies. For these purpose, two phthalates i.e. diethylphthalate (DEP) and dibutylphthalate (DBP) were studied in two full-scale wastewater treatment plants based on activated sludge process (ASP) and sequencing batch reactor (SBR) located in Jagjeetpur, Hardwar (Uttarakhand), India.
Material and methods
Chemicals and sample collection
Standards of DEP (CAS 84-66-2) and DBP (CAS 84-72-2) bearing high purity (>99 %) were purchased from Sigma-Aldrich Chemie GmbH (Germany). All other solvents used for extraction and dilution of phthalates were of HPLC & Spectroscopy grade. Glasswares were rinsed with dichloromethane (DCM) and then dried in oven. Filter papers employed in extraction procedure were also dried before use.
Influent and effluent samples were collected from the selected wastewater treatment plants (WWTPs) viz 18 million litres per day (MLD) activated sludge process (ASP) and 27 MLD sequencing batch reactor (SBR) located in Jagjeetpur, Hardwar (Uttarakhand), India. The sewage inflowing to WWTPs is of domestic type. Capacity of ASP and SBR treatment plants was 18 and 27 MLD, respectively. Composite samples were collected monthly throughout the study period from both the WWTPs. Sludge sample was also collected from the aeration tanks (activated sludge) of both plants. Sampling bottles were cleaned and rinsed with sample two to three times before collection of sample. For microbial analysis, samples were taken in autoclaved amber glass bottles. All the samples were preserved at 4 °C before analysis. Typical characteristics of DEP and DBP are summarized in Table 1.
Extraction method for wastewater and sludge
The reference method 606 mentioned in “Environmental Protection Agency: methods for organic chemical analysis of municipal and industrial wastewater” (EPA 2002), was employed for extraction of influent and effluents samples. One litre of wastewater was taken in an amber glass bottle and extracted three times with 100 mL of a solvent mixture of n-hexane and DCM in the ratio of 85:15, with the help of mechanical shaking for 20 min. The combined extracts were concentrated under a gentle stream of nitrogen to 1 mL and purified with florisil column and finally eluted with 25 mL of a solvent mixture of n-hexane/diethylether in the ratio of 80:20. The fraction was evaporated to 1 mL under N2 gas streams and was analyzed using a gas chromatography–mass spectrometer (GC–MS) (Dargnat et al. 2009). Culatti crusher was used for the fine crushing of dried samples. Five grams of samples were extracted with 100 mL of DCM in a volatilization/condensation device for 24 h. The extracts were centrifuged for 15 min, concentrated and transferred to hexane, and again centrifuged and evaporated to 1 mL. The cleanup method was same as described for wastewater.
Analytical methods
The concentrations of DEP and DBP were analyzed with Varian 450 gas chromatography coupled to an Varian 240 mass spectrometer (GC–MS), Varian Technologies, 4330 EA Middelburg, The Netherlands, operating in External EI mode, and a VF-5 MS capillary column (30 m length × 0.25 mm inner diameter, 0.25-μm film thickness) for chromatographic separation. The extracts (2.0 μL) were injected onto GC–MS in EI mode, and the injection port and detection temperatures were 280° and 300 °C, respectively. The temperature of column was initiated at 60 °C for 7.0 min and increased to 180 °C at a rate of 5 °C/min, held for 5.0 min and finally increased up to 280 °C at the same rate and time. The flow rate of the carrier gas (helium) was maintained constant at 1 mL/min. Identification of phthalates was based on their retention times relative to the standards, while quantitative determination was based on peak areas in relation to standards, and linear 8-point calibration curves. Thereafter, the removal efficiency was calculated by using the following formula (Xian et al. 2010):
where I n is the measured concentration in the influent of the plant and E f is the measured concentration in the final effluent of the plant
Physicochemical parameters i.e. chemical oxygen demand (COD), biochemical oxygen demand (BOD), total suspended solids (TSS), volatile suspended solids (VSS), total nitrogen (TN), ammonium nitrogen (NH4-N), nitrate nitrogen (NO3-N), total phosphorus (TP) and microbiological parameters like total coliform (TC) and faecal coliform (FC) were analyzed according to Standard Methods (APHA 2005).
The Fourier transformation infrared (FTIR) spectra were obtained at wave number range of 400–4000/cm on a PerkinElmer GX FTIR system equipped with OMNIC software. Before application of sludge sample to FTIR instrument, sample was grinded with the help of grinder to reduce the average particle size 1–2 μ as homogenous mixture gives best results. After proper grinding, about 5–10 g of finely ground samples were then placed onto the face of a KBr plate, a small drop of mineral oil was added and the second window was placed on top. A gentle circular and back-and-forth rubbing motion of the two windows evenly distributes the mixture between the plates. The mixture should appear slightly translucent, with no bubbles, when properly prepared. The sandwiched plates were placed in the spectrometer and a spectrum was obtained.
Phthalate compounds are used in many kinds of cleansing products and cosmetics; therefore, these compounds may cause risk of sample contamination during analysis, so it is advisable to take extreme precautions to avoid sources of contamination in the laboratory equipment during handling and analysis. Therefore, use of plastic equipment throughout the whole procedure of sample preparation was avoided to minimize the risk of contamination (Tingting et al. 2013). Some other appropriate steps should be taken to avoid this problem like all glasswares were washed and rinsed in DCM and air dried, blank samples of the whole process should be analyzed after every set of ten samples and spiked water samples also analyzed periodically to evaluate the performance of method.
Results and discussion
The quality of wastewater was determined with the help of physicochemical and microbiological analysis. All the selected parameters were determined to identify the extent of pollution strength of treated and untreated water samples of both sewage treatment plants (STPs) and their removal efficiency. The average values and standard deviation of all parameters for this study are shown in Table 2. The average removal efficiency of COD, BOD and TSS was found ∼83.22, 82.52 and 85.31 % in ASP and 88.02, 90.53 and 88.31 % in SBR plant. Similarly, average removal efficiency of TN, TC and FC are 94.41, 90.03 and 91.33 % for ASP and 93.91, 91.04 and 94.50 % for SBR plant. The removal efficiency of other parameters like NH4-N, NO3-N and TP was almost in the same range. The obtained results of above parameters indicate that both the plants are working properly, but the removal efficiency of SBR plant is high in comparison of ASP plant. The effectiveness of wastewater treatment plants in relation to the removal of microbiological pollution was measured with the help of determination of number of TC and FC in wastewater samples (Kazmi et al. 2008). Results revealed that the removal efficiency of both treatment plants is appreciable (For SBR DEP > 91 %, DBP > 85 % and for ASP DEP > 91 %, DBP > 92 %.
Occurrence of phthalates in wastewater
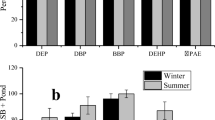
Previous studies reported that phthalate compounds undergo bacterial degradation and adsorbed on sludge particles which cause removal of these compounds from wastewater (Carballa et al. 2004). Figure 1a, b shows the GC–MS results of both influent and effluent samples of ASP and SBR plants, and the respective results of the concentration of DEP and DBP are shown in Fig. 2a, b. The removal of DEP and DBP was varying from 83.50 to 94.72 and 82.81 to 97.54 % in ASP plant and 81.74 to 98.31 and 67.62 to 96.53 % in SBR plant during a monitoring period of 9 months from April to December. The removal of DEP was higher in SBR plants, whereas the removal of DBP was higher in ASP plant. The removal of DEP was highest in September for ASP plant and in November for SBR plant. Similarly, removal of DBP was high during May and December for ASP plant and during May, July, August, September and December for SBR plant. The probable reason of variation in concentration may be due to variation in pH and temperature and also because of some other factors like plant configurations, quality of influents, solids retention time (SRT), hydraulic retention time (HRT), etc. (Chang et al. 2005; Zhou et al. 2010). The average value and standard deviation of TSS indicate that high removal efficiency of phthalates during the study period was due to high concentration of TSS. Statistical analysis of performance data showed that there is an appreciable correlation between removal of phthalates and other physicochemical parameters such as BOD, COD, TSS and TC (Chang et al. 2004; Lu et al. 2009; Chatterjee and Karlovsky 2010).
All these parameters are useful in increasing the removal efficiency of phthalate compounds. The extent, to which a treatment plant removes suspended solids, as well as BOD, determines the efficiency of the treatment process and the extent of phthalate removal. Therefore, the results obtained from this study indicate that the overall removal efficiency, i.e. disappearance of these compounds from water phase, is 91.51 % (DEP) and 85.42 % (DBP) in SBR plant while 91.03 % (DEP) and 92.67 % (DBP) in ASP plant.
Statistical analysis
Correlations (r Pearson) were established between various physicochemical parameters (BOD, COD, TSS, TC, pH and temperature) and targeted pollutants, i.e. DBP and DEP for SBR and ASP plant as shown in Tables 3 and 4, respectively. Statistical analysis (Table 3) shows that removal of DBP had a strong correlation with COD, TSS and TC removal, while DEP removal was found to be linked with BOD and TC removal. Temperature has shown insignificant effect on removal of DBP and DEP for both of the plants. Hence, efficiency of plants is independent of temperature. From Table 4, it was observed that removal of DBP in ASP plant has a strong correlation with TC removal. Also, a slight dependency was observed between BOD and DBP removal. In addition, BOD removal was found to be having good correlation with log TC removal but not as good with DBP removal. On the other side, removal of DEP was found to be linked with pH in reactor and TC removal.
Occurrence of phthalates in sludge
A large amount of phthalate compounds undergo degradation by activated sludge, small amount is discharged in the effluent, small amount gets accumulated in the system and a very small amount remains in the waste sludge (Marttinen et al. 2004; Huang et al. 2010). Small concentration of phthalate compounds was also found in sludge samples because phthalate compounds get adsorbed on sludge particles (Medellin-Castillo et al. 2013) which cause removal of these compounds from wastewater and presence in sludge samples. GC–MS graph of sludge samples of ASP and SBR plants is given in Fig. 1c, and concentrations of these compounds in sludge samples are given in Fig. 2c. The average concentration and standard deviation of DEP and DBP in sludge samples of ASP plant were 2.08 ± 0.53 and 2.08 ± 0.48 ng/g, respectively. However, the average concentration and standard deviation of DEP and DBP in sludge sample of SBR plant were 1.71 ± 0.20 and 2.01 ± 0.28 ng/g, respectively. Concentration of DEP is more during June, August and October, and concentration of DBP is more during May, July and November in ASP sludge. Similarly, concentration of DEP is more during April and November and that of DBP during May, July and November in SBR sludge. It is observed from above results that adsorption is greater in these months in comparison of other months. It may be because of variation in temperature, pH and other climatic conditions (Chang et al. 2005). Degradation and adsorption of phthalates is also affected by properties of compounds like alkyl branch chains, hydrophobicity, dispersion, and boiling points (Zeng et al. 2004; Mohan et al. 2007; Gao et al. 2014).
Sludge analysis
Fourier transforms infrared spectroscopy of samples
The removal efficiency of compounds during both ASP and SBR treatment process can be understood by FTIR analysis of sludge (solid residue) generated after the treatment (Singh et al. 2013). Figure 3 shows that the FTIR result of sludge generated during the treatment process of both of the plants. Stretching and bending vibration frequencies of sludge analysis are summarized in Table 3. Absorption band at 609 and 1142, and 612 and 1128/cm in both plants sludge shows the presence of C-H stretching frequency of -CH3 group and C-O stretching of C-O-C group coupled with adjacent C-C group indicating the presence of alkane residues and ether groups, respectively. Peak at 1646 and 1640/cm is characteristic peak of C = C stretching frequency due to the presence of aromatic ring. Peaks at 2852, 2926 and 2960/cm in both plants sludge suggest the presence of aliphatic methylene groups. Table 5 show a comparative analysis of various broad, strong and sharp peaks of FTIR spectra of both plants.
Figure 3 shows that bands at 1406, 1456 and 1468/cm can be assigned to the presence of aromatic ring in the sludge of both plants sample. The broad band at 3428.86 and 3429.25/cm was assigned to the presence of O-H stretching (Silva et al. 2012; Parida et al. 2014). Peaks at 1536.22/cm in SBR sludge sample indicate the presence of -NO2 group, and peaks at 1332 to 1353/cm in both plants sludge due to C-C and C-H stretching band indicate the presence of methylene group. The results were compared with those obtained by GCMS analysis. Therefore, it was observed that phthalates during bacterial degradation by both (ASP and SBR) processes get decomposed into phthalic acid, benzonic acid, phenol and at the end of processes finally converted into carbon dioxide and water under aerobic conditions (Zeng et al. 2004; Cai et al. 2007).
Hence, FTIR analysis of solid residues of both plants indicates that most of the phthalate compounds were degraded into small compounds during bacterial degradation. The peaks observed in the FTIR spectra may also be due the presence of some other compounds like lipid, carbohydrate, fats and proteins because these compounds remain in sewage due to microbial degradation (Grube et al. 2006).
Scanning electron microscope and energy dispersive X-ray analysis
The morphology and elemental distribution in the sludge sample were determined with the help of a scanning electron microscopy and energy dispersive X-ray spectroscopy (SEM-EDX). Figure 4a, b shows the SEM-EDX analysis of ASP and SBR sludge. SEM indicates that surface texture of the sludge is amorphous having different pores and sizes. EDX analysis of sludge samples of both plants was used to evaluate the elemental composition (Fig. 4a′, b′). Sludge element composition was found to be 60.98 and 56.07 % carbon, 33.95 and 38.28 % oxygen, 0.84 and 0.47 % magnesium, 1.17 and 0.69 % aluminium, 2.12 and 2.06 % silicon, 0.65 and 0.42 % phosphorous, and 0.85 and 0.60 % calcium respectively for ASP and SBR plant. It was observed that ASP sludge contains high amount of carbon i.e. 60.98 % indicating the highest COD removal in ASP as compared to SBR.
Thermo-gravimetric and differential thermal analysis of sludge samples
Thermo-gravimetric analysis (TGA) and differential thermal analysis (DTA) of sludge samples were carried out from 20 to 1000 °C at a heating rate of 10 °C/min in the air atmosphere. Quantitative determination of any weight changes associated with thermally induced transitions and changes in the chemical composition of the substances means degradation kinetics of sludge samples was carried out with the help of this analysis (Mondal et al. 2012). Endothermic peaks indicate that these curves are also involved in phase changes (Singh et al. 2013). Figure 5a, b shows TGA–DTA curves of the both plants sludge samples. The degradation characteristics mean that weight loss with temperature, peak temperature and enthalpy change are shown in Table 6. TGA pattern of sludge samples of both plants found that 4.90 and 4.11 % weight loss of adhered moisture and evolution of some volatile matter from 19 to 239 and 28 to 200 °C temperature ranges for ASP and SBR, respectively.
The TGA of sludge sample of ASP showed two degradation zones between 239–350 and 450–995 °C with the maximum rates of weight loss of 0.34 and 2.12 mg/min at 265 and 479 °C. Similarly, TGA of sludge sample of SBR indicates the two degradation zones between 200–300 and 400–996 °C with the maximum rates of weight loss of 0.31 and 1.45 mg/min at 269 and 486 °C temperature. Different exothermic peaks could be observed on the DTA curves for both plants sludge samples (Fig. 5b). Overall, weight loss of sludge for ASP and SBR was found to be 78.70 and 70.53 %, respectively. The average calorific value of Indian coal is 18.80 MJ/kg, and the heating values of ASP and SBR sludge were found to be 8.50 and 9.50 MJ/kg, respectively. Hence, these sludge residues may be used as a fuel along with high calorific value fuels like agricultural and industrial wastes in boilers. Bottom ash thus obtained can be used for construction purposes with cementitious material.
Conclusions
On the basis of the present studies, the following conclusions can be drawn:
-
Removal efficiency of DEP and DBP was found to be satisfactory from both the wastewater treatment plants (ASP and SBR). GC–MS analysis for both STPs wastewater sample has shown that maximum removal efficiency of DEP was obtained in SBR-based STPs, whereas maximum removal efficiency of DBP was obtained in ASP-based STP. Similarly, GC–MS results of samples extracted from the sludge derived after the treatment from both STPs indicate the presence of a small fraction of phthalates in both STPs.
-
Results of FTIR analysis of sludge of both STPs confirmed that phthalates compounds were degraded into small and easily decomposable molecules during the treatment processes.
-
Physicochemical analysis of both plants confirmed the decrease of COD concentration and others parameters indicating the decrease in concentration of organic contaminants (phthalates) during both the treatment process.
-
SEM/EDX and TGA/DTA analysis of sludge confirmed that sludge residues have high calorific value and can be used to make fuel-briquettes.
References
Adibi, J. J., Whyatt, R. M., Williams, P. L., Calafat, A. M., Camann, D., Herrick, R., Nelson, H., Bhat, H. K., Perera, F. P., Silva, M. J., & Hauser, R. (2008). Characterization of Phthalate Exposure among Pregnant Women Assessed by Repeat Air and Urine Samples. Environmental Health Perspectives, 116(4), 467–473.
Alatriste-Mondragon, F., Iranpourb, R., & Ahringa, B. K. (2003). Toxicity of di-(2-ethylhexyl) phthalate on the anaerobic digestion of wastewater sludge. Water Research, 37(6), 1260–1269.
APHA. (2005), Standards Methods for the Examination of Water and Wastewater (21stedn.).Washington, DC: APHA, AWWA and WEF.
Boonyaroj, V., Chiemchaisri, C., Chiemchaisri, W., Theepharaksapan, S., & Yamamoto, K. (2012). Toxic organic micro-pollutants removal mechanisms in long-term operated membrane bioreactor treating municipal solid waste leachate. Bioresource Technology, 113, 174–180. doi:10.1016/j.biortech.2011.12.127.
Cai, Q. Y., Mo, C. H., Wu, Q. T., Zeng, Q. Y., & Katsoyiannis, Y. (2007). Occurrence of organic contaminants in sewage sludges from eleven wastewater treatment plants, China. Chemosphere, 68(9), 1751–1762. doi:10.1016/j.chemosphere.2007.03.041.
Cai, Q. Y., Mo, C. H., Wu, Q. T., & Zeng, Q. Y. (2008). Polycyclic aromatic hydrocarbons and phthalic acid esters in the soil–radish (Raphanussativus) system with sewage sludge and compost application. Bioresource Technology, 99(6), 1830–1836. doi:10.1016/j.biortech.2007.03.035.
Carballa, M., Omil, F., Lema, J. M., Llompart, M., Garcia-Jares, C., Rodriguez, I., Gomez, M., & Ternes, T. (2004). Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Research, 38(12), 2918–26. doi:10.1016/j.watres.2004.03.029.
Chang, B. V., Liao, C. S., & Yuan, S. Y. (2005). Anaerobic degradation of diethyl phthalate, di-n-butyl phthalate, and di-(2-ethylhexyl) phthalate from river sediment in Taiwan. Chemosphere, 58(11), 1601–1607. doi:10.1016/j.chemosphere.2004.11.031.
Chang, B. V., Yang, C. M., Cheng, C. H., & Yuan, S. Y. (2004). Biodegradation of phthalate esters by two bacteria strains. Chemosphere, 55(4), 533–538. doi:10.1016/j.chemosphere.2004.11.031.
Chatterjee, S., & Karlovsky, P. (2010). Removal of the endocrine disrupter butyl benzyl phthalatefrom the environment. Applied Microbial Biotechnology, 87(1), 61–73. doi:10.1007/s00253-010-2570-y.
Chen, W. L., & Sung, H. H. (2005). The toxic effect of phthalate esters on immune responses of giant freshwater prawn (Macrobrachiumrosenbergii) via oral treatment. Aquatic Toxicology, 74(2), 160–171. doi:10.1016/j.aquatox.2005.05.008.
Clara, M., Windhofer, G., Hartl, W., Braun, K., Simon, M., Gans, O., Scheffknecht, C., & Chovanec, A. (2010). Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere, 78(9), 1078–1084. doi:10.1016/j.chemosphere.2009.12.052.
Dargnat, C., Tail, M. J., Chevreuil, M., & Blanchard, M. (2009). Phthalate removal throughout wastewater treatment plant Case study of Marne Aval station (France). Science of the Total Environment, 407(4), 1235–1244. doi:10.1016/j.scitotenv.2008.10.027.
EPA Appendix A to part 136. (2002). Method for organic chemical analysis of municipal and industrial wastewater. Method 606— Phthalate ester.
Gao, D., Li, Z., Wen, Z., Ren, N. (2014). Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the Songhua River in China. Chemosphere, 95, 24–32.doi.org/10.1016/j.chemosphere.2013.08.009.
Grube, M., Lin, J. G., Lee, P. H., & Kokorevicha, S. (2006). Evaluation of sewage sludge-based compost by FTIR spectroscopy. Geoderma, 130(3-4), 324–333. doi:10.1016/j.geoderma.2005.02.005.
Huang, M. Z., Ma, Y. W., Wang, Y., Wan, J. Q., & Zhang, H. P. (2010). The fate of di-n-butyl phthalate in a laboratory-scale anaerobic/anoxic/oxic wastewater treatment process. Bioresource Technology, 101(20), 7767–7772. doi:10.1016/j.biortech.2010.05.028.
Kazmi, A. A., Tyagi, V. K., Trivedi, R. C., & Kumar, A. (2008). Coliforms removal in full-scale activated sludge plants in India. Journal of Environmental Management, 87(3), 415–419. doi:10.1016/j.jenvman.2007.01.017.
Kolarik, B., Bornehag, C. G., Naydenov, K., Sundell, J., Stavova, P., & Nielsen, O. F. (2008). The concentrations of phthalates in settled dust in Bulgarian homes in relation to building characteristic and cleaning habits in the family. Atmospheric Environment, 42(37), 8553–8559. doi:10.1016/j.atmosenv.2008.08.028.
Lau, T. K., Chu, W., & Graham, N. (2005). The degradation of endocrine disruptor di-n-butyl phthalate by UV irradiation: a photolysis and product study. Chemosphere, 60(8), 1045–1053. doi:10.1016/j.chemosphere.2005.01.022.
Lu, Y., Tang, F., Wang, Y., Zhao, J., Zeng, X., Luo, Q., & Wang, L. (2009). Biodegradation of dimethyl phthalate, diethyl phthalate and di-n-butyl phthalate by Rhodococcussp. L4 isolated from activated sludge. Journal of Hazardous Materials, 168(2-3), 938–943. doi:10.1016/j.jhazmat.2009.02.126.
Marttinen, S. K., Kettunen, R. H., Sormunen, K. M., & Rintala, J. A. (2003). Removal of bis(2-ethylhexyl) phthalate at a sewage treatment plant. Water Research, 37(6), 1385–139.
Marttinen, S. K., Ruissalo, M., & Rintala, J. A. (2004). Removal of bis(2-ethylhexyl) phthalate from reject water in a nitrogen-removing sequencing batch reactor. Journal of Environmental Management, 73(2), 103–109. doi:10.1016/j.jenvman.2004.05.011.
Medellin-Castillo, N.A. Ocampo-Perez, R., Leyva-Ramos, R., Sanchez-Polo M., Rivera-Utrilla, J., & Mendez-Diaz, J. D. (2013). Removal of diethyl phthalate from water solution by adsorption, photo-oxidation, ozonation and advanced oxidation process (UV/H2O2, O3/H2O2 and O3/activated carbon). Science of the Total Environment, 442, 26–35.doi.org/10.1016/j.scitotenv.2012.10.062.
Mohan, S. V., Shailaja, S., Krishna, M. R., & Sarma, P. N. (2007). Adsorptive removal of phthalate ester (Di-ethyl phthalate) from aqueous phase by activated carbon: a kinetic study. Journal of Hazardous Materials, 146(1-2), 278–282. doi:10.1016/j.jhazmat.2006.12.020.
Mondal, B., Srivastava, V. C., & Mall, I. D. (2012). Electrochemical treatment of dye-bath effluent by stainless steel electrodes: multiple response optimization and residue analysis. Journal of Environmental Science and Health, Part A, 47(13), 2040–2051. doi:10.1080/10934529.2012.695675.
Na, S., Ahn, Y. G., Cui, M., & Khim, J. (2012). Significant diethyl phthalate (DEP) degradation by combined advanced oxidation process in aqueous solution. Journal of Environmental Management, 101, 104–110. doi:10.1016/j.jenvman.2012.01.028.
Ozer, E.T., Osman, B., Kara, A., Bes, N., Irli, S., Gucer, E., & Sözeri, H. (2012). Removal of diethyl phthalate from aqueous phase using magnetic poly (EGDMA–VP) beads. Journal of Hazardous Materials, 229– 230, 20– 28. doi.org/10.1016/j.jhazmat.2012.05.037.
Parida, S., Panda, M., Parija, A., & Das, S. C. (2014). Thermal studies of different agrowaste reinforced novolac composites prepared under isothermal conditions. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 5, 1580.
Prasad, B., & Suresh, S. (2012). Biodegradation of dimethyl phthalate, diethyl phthalate, dibutyl phthalate and their mixture by VariovoraxSp. International Journal of Environmental Science and Development, 3(3), 283–288.
Roslev, P., Vorkamp, K., Aarup, J., Frederiksen, K., & Nielsen, P. H. (2007). Degradation of phthalate esters in an activated sludge wastewater treatment plant. Water Research, 41(5), 969–976. doi:10.1016/j.watres.2006.11.049.
Rudel, R. A., Dodson, R. E., Morello-Frosch, L. J. P. R., Zuniga, D. E. C. M. M., Yau, A. Y., Just, A. C., & Brody, J. G. (2010). Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two Northern California Communities. Environmental Science Technology, 44(17), 6583–6590.
Silva, J. D. O., Filho, G. R., Ribeiro, S. D., Vieira, J. G., & Cerqueira, D. A. (2012). Thermal analysis and FTIR studies of sewage sludge produced in treatment plants. The case of sludge in the city of Uberlândia-MG, Brazil. ThermochimicaActa, 528, 72–75. doi:10.1016/j.tca.2011.11.010.
Singh, S., Srivastava, V. C., & Mall, I. D. (2013). Mechanistic study of electrochemical treatment of basicgreen 4 dye with aluminum electrodes through zetapotential, TOC, COD and color measurements, andcharacterization of residues. RSC Advanced, 3(37), 16426–16439. doi:10.1039/c3ra41605d.
Takeuchi, S., Iida, M., Kobayashi, S., Jin, K., Matsuda, T., & Kojim, H. (2005). Differential effects of phthalate esters on transcriptional activities via human estrogen receptors and androgen receptor. Toxicology, 210(2-3), 223–233. doi:10.1016/j.tox.2005.02.002.
Tingting, M. A., Teng, Y., Christie, P., Luo, Y., Chen, Y., Ye, M., & Huang, Y. (2013). A new procedure combining GC-MS with accelerated solvent extraction for the analysis of phthalic acid esters in contaminated soils. Frontiers of Environmental Science & Engineering, 7(1), 31–42. doi:10.1007/s11783-012-0463-2.
Xian, Q., Hu, L., Chen, H., Chang, Z., & Zou, H. (2010). Removal of nutrients and veterinary antibiotics from swine wastewater by a constructed macrophyte floating bed system. Journal of Environmental Management, 91(12), 2657–2661. doi:10.1016/j.jenvman.2010.07.036.
Xu, B., Gao, N. Y., Sun, X. F., Xia, S. J., Rui, M., Simonnot, M. O., Causserand, C., & Zhao, J. F. (2007). Photochemical degradation of diethyl phthalate with UV/H2O2. Journal of Hazardous Materials B, 139(1), 132–139. doi:10.1016/j.jhazmat.2006.06.026.
Yang, G. P., Zhao, X. K., Sun, X. J., & Lu, X. L. (2005). Oxidative degradation of diethyl phthalate by photochemically-enhanced Fenton reaction. Journal of Hazardous Materials, B 126(1-3), 112–118. doi:10.1016/j.jhazmat.2005.06.014.
Yuan, S. Y., Liu, C., Liao, C. S., & Chang, B. V. (2002). Occurrence and microbial degradation of phthalate esters In Taiwan river sediments. Chemosphere, 49(10), 1295–1299.
Zeng, F., Cui, K., Li, X., Fud, J., & Sheng, G. (2004). Biodegradation kinetics of phthalate esters by pseudomonas fluoresences FS1. Process Biochemistry, 39(9), 1125–1129. doi:10.1016/S0032-9592(03)00226-7.
Zhou, H., Huang, X., Wang, X., Zhi, X., Yang, C., Wen, X., Wang, Q., Tsuno, H., & Tanaka, H. (2010). Behaviour of selected endocrine-disrupting chemicals in three sewage treatment plants of Beijing, China. Environmental Monitoring Assessment, 161(1-4), 107–121. doi:10.1007/s10661-008-0731-6.
Acknowledgments
Authors are thankful to the Department of Science and Technology (DST), India, for providing financial help for carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saini, G., Pant, S., Singh, S.O. et al. A comparative study of occurrence and fate of endocrine disruptors: diethyl phthalate and dibutyl phthalate in ASP- and SBR-based wastewater treatment plants. Environ Monit Assess 188, 609 (2016). https://doi.org/10.1007/s10661-016-5617-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5617-4