Abstract
Agricultural land use is widely accepted to elicit changes on surrounding environment and neighboring ecosystems. Meanwhile, the impact of different types of agricultural land use likely cause a variety of impacts on nearby ecosystems and the organisms that inhabit them. Freshwater systems support a wide range of organisms—from infaunal or epifaunal invertebrates to mobile pelagic and littoral fish species. The focus of this study was to determine how agricultural activity in the upstream catchment influences sediment properties and the resulting ability of three distinct invertebrate species to survive and reproduce in these different sediments. This will be the first study that evaluates the utility of the sediment quality triad when assessing the impact of agricultural activity on invertebrate growth, reproduction, and survival. In analyzing sediment and water chemistry, as well as metal and pesticide levels, none of the predictor variables were able to adequately explain the variation seen in any of the biological endpoints (reproduction, mortality, growth, or biomass). Although none of the factors measured in this experiment could explain the variation seen in biological endpoints, the experimental approach was informative in delineating biological trends between sediments subject to varying levels of agricultural activity. Although an experiment of this nature was not able to identify a causal mechanism to explain the variation in invertebrate biological endpoint, it is still extremely useful as an exploratory approach to assess relative sediment toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Global freshwater systems are subject to a multitude of anthropogenic stressors (ex. urban development, introduction of invasive species) that can significantly impact the physical habitat and ecological functioning of these systems (Giller 2005). It has been increasingly recognized that anthropogenic influence at the landscape scale is a substantial threat to freshwater ecosystems and can impact habitat, water quality, and native biota, while ultimately altering ecological states (Allan et al. 1997; Søndergaard and Jeppesen 2007; Strayer et al. 2003; Townsend et al. 2003). Notably, habitat diversity of streams is strongly influenced by land use within the surrounding areas at multiple scales (Allan 2004). The widespread impacts caused by anthropogenic landscape-altering activity have been investigated from a multitude of viewpoints using numerous biological metrics and indices. However, land use alterations do not act in isolation; interactive effects with other anthropogenic drivers, such as climate change (Meyer et al. 1999), the presence of invasive species (Scott and Helfman 2001), and hydrological barriers (dams) (Nilsson and Berggren 2000) are able to affect stream health on various scales.
Agricultural land use and the vicinity in which these practices occur relative to aquatic systems pose serious risks and consequences for biodiversity and ecosystem function at all levels of organization (Allan 2004; Cooper 1993). Aquatic systems in particular have been shown to exhibit vulnerability to land use changes (Allan 2004), particularly, an increase in agricultural intensity (Herringshaw et al. 2011). Various ecosystem responses have been noted due to the presence of agricultural land use, such as changes in riparian vegetation, stream morphology, sedimentation levels, nutrient additions, organic enrichment, and pesticide contamination (Cooper 1993). The large number of ecosystem responses to agricultural land use can drastically impact the state of an ecosystem, in turn impacting the ability of organisms to survive and reproduce.
Agricultural development has been shown to decrease ecosystem diversity relative to forested and pasture areas (Egler et al. 2012). Specifically, the rapid growth of agriculture practices has introduced added stressors to freshwater systems, resulting in shifts in local abiotic conditions that have been shown to impact a number of organisms (Herringshaw et al. 2011; Pavlin et al. 2011). Interestingly, individuals of the taxa Oligochaeta were predominantly found in agricultural sites or sites determined to be of degraded status (Egler et al. 2012; Virbickas et al. 2011). As per Barbour et al. (1999), oligochaetes such as tubificid worms have a greater tolerance to measures of pollution relative to more sensitive species, e.g., Ephemeroptera, Trichoptera, and Plecoptera. This introduces the idea that while diversity and species richness may decrease, certain species are tolerant of agricultural input and, thus, may increase in abundance in the presence of agricultural land use (Lenat and Crawford 1994).
Agriculture, specifically nutrients and pesticides, was the primary factor associated with impairment in the Yakima River basin in Washington, USA, which caused community condition indices of fish, invertebrates, and algae to decline as agricultural intensity increased (Cuffney et al. 2000). Agricultural land use has been shown to correspond with a decline in invertebrate biological indices in response to increased agricultural intensity (Cuffney et al. 2000). The presence of agricultural land use in addition to other anthropogenic activities has clear impacts on invertebrate communities, creating the need for environmentally responsible land development and sustainable farming practices.
In a study performed in agricultural and urban streams in the Chesapeake Bay drainage basin, agricultural streams exhibited the highest macroinvertebrate diversity relative to both urban streams as well as other published estimates for agricultural streams (Moore and Palmer 2005). Moore and Palmer (2005) speculated that the higher richness values may be the result of “best management practices” undertaken by farmers in this area, leading to the alleviation of stress caused by agricultural activity. This introduces an important concept; certain agricultural practices, such as maintaining riparian forests or buffers, can facilitate stream biodiversity being conserved in areas of high agricultural activity, where macroinvertebrate subsistence would otherwise be limited (Moore and Palmer 2005). Similarly, riverine systems react differentially when certain supporting measures or modifications are present, such as forest belts (Virbickas et al. 2011) or riparian buffers (Storey and Cowley 1997; McTammany et al. 2007).
Streams and rivers provide multiple ecosystem services, including providing water for human uses, habitat provisioning for both aquatic and riparian organisms, and a trophic base for the same aquatic and riparian biota (Johnston et al. 2017). Land use impacts these ecosystem services by introducing a wide variety of inputs, most notably agricultural runoff (Johnston et al. 2017). This can, in turn, disrupt surrounding food webs through the deleterious impacts on surrounding benthic communities and, thus, severely decrease the ability of a system to provide these valuable ecosystem services to both humans and surrounding aquatic and terrestrial organisms. Macroinvertebrates are often utilized as indicators of stream health, due to their ability to respond to and represent habitat degradation (Herman and Najadhashemi 2015). A healthy benthic invertebrate community has widespread implications and is extremely relevant for both aquatic and terrestrial food webs as sources of energy input (Koop et al. 2011; Reynoldson and Metcalfe-Smith 1992). Both aquatic and terrestrial food webs are linked through invertebrate production, via particulate organic carbon input for zooplankton subsistence (Pace et al. 2004) and energy flow to a variety of fish species (Carpenter et al. 2005), and ultimately result in “reciprocal, across-habitat prey flux” (Nakano and Murakami 2001). This means that in situations when aquatic insect emergence is highest in the spring, terrestrial invertebrate biomass may be low and alternately, in the summer when terrestrial input is high, aquatic invertebrate biomass is low. Ultimately, this indicates that the aquatic and terrestrial food webs are being subsidized by the surrounding benthic invertebrate community in terms of energy input regardless of season through alternating cycles. This truly highlights the importance of a healthy benthic invertebrate community to provide for surrounding food webs and, subsequently, provide ecosystem services that are necessary for human activities and organism subsistence.
The sediment quality triad described by Chapman (1990) has been used to assess sediment quality, which is a reflection of the ability of stream to sustain a healthy benthic community. The sediment quality triad comprises three components: sediment chemistry, in situ invertebrate community composition, and bioassays conducted with collected sediment and laboratory-cultured organisms (Chapman 1990). Assessing these three components together can provide a comprehensive assessment of sediment quality. The one component of the sediment quality triad that is of particular interest for this study is the use of laboratory-cultured organisms to assess sediment quality. While many past studies have utilized laboratory-cultured species to assess sediment quality from sites impacted by industry, this approach has not been readily applied to assessing the impact of agriculture on sediment quality. Thus, there is a need to evaluate the ability of laboratory-cultured organisms to assess the quality of sediment that is potentially impacted by agriculture land use in the upstream catchment of a stream.
The relationship between agricultural land use, benthic invertebrate abundance, and diversity is not explicitly clear; there are a wide range of practices and activities that may negatively impact stream ecosystems, ecology, and the invertebrates present. This study was designed to evaluate the effect of a gradient of agriculture activity in the upstream catchment on the response of laboratory-cultured Tubifex tubifex, Hyalella azteca, and Hexagenia spp. exposed to sediment collected from streams running through an agricultural landscape. These species were chosen as they occupy a gradient of microhabitats in freshwater ecosystems as well as a wide geographical distribution. By utilizing multiple species that subsist in varying parts of the sediment-water interface in freshwater systems, we attempt to facilitate extrapolation of trait-based assessment among freshwater macroinvertebrates (Rubach et al. 2011). Through the culturing of these three species of benthic invertebrates in accordance with standardized protocols in the laboratory, the differences in response of the species can be assumed to be due to the composition of the sediment and will thus, provide insight into the effects of agricultural input on multiple invertebrate species. We suspected that laboratory-cultured invertebrates would not perform well in sediment from sites with larger percentages of agricultural land use in the upstream catchment. The overall objectives of this study are to elucidate the impact of agricultural activity in the upstream catchment on the growth, mortality, and reproduction of three benthic invertebrate species. Additionally, we will evaluate the utility of using laboratory-cultured organisms to investigate the effects of upstream land use and provide information on its usefulness in contributing to the sediment quality triad.
2 Methods
2.1 Sediment Collection
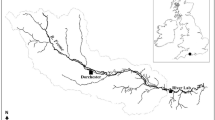
Sediment was collected according to the Ontario Ministry of the Environment, Conservation, and Parks (OMECP) sediment assessment protocol outlined in Jaagumagi and Persaud (1993) and Environment and Climate Change Canada (ECCC). An Ekman Grab was utilized to remove 500 mL to 1.5 L of sediment from chosen streams and immediately transferred to labeled glass mason jars or polypropylene buckets and stored at ~ 4.0 °C until used in testing. Sediment was collected from nine sites (A–I) in southwestern Ontario; five sites are part of the Alternative Land Use Service (ALUS), four conservation areas, and one provincial water quality monitoring station, which was considered our “high-impact” site (Fig. 1). ALUS is an example of an organization that is attempting to implement best management practices on agriculture lands to reduce the potential impact of agriculture to terrestrial and aquatic ecosystems (https://alus.ca/). Sites A–D were situated near varying agricultural inputs (cattle, squash, corn, corn and soy respectively). Site E was located in Lebo Drain, which is downstream of intensive field and greenhouse agriculture and near a provincial water quality monitoring station while sites F–H were within conservation areas. Sediment used as a control was obtained from Long Point Provincial Park (site I) in Lake Erie, Ontario, Canada (42.583683 N, 80.443726 W). The sediment is used by OMECP and ECCC as a control sediment in sediment toxicity testing and the culturing of T. tubifex and Hexagenia spp. (Prosser et al. 2017b). The sites were chosen in an attempt to include what were viewed as “good-quality” sites in terms of impact of the upstream catchment, such as conservation areas where there should be minimal harmful input, as well as “poor-quality” sites like Lebo Drain where there is extremely high chemical and agricultural input. The ALUS sites were ultimately utilized to see where they placed in terms of ability to support invertebrate growth and reproduction relative to the “good-quality and poor-quality” sites.
Map of southwestern Ontario, Canada, with field sites where sediment was collected. Sites A–D were situated near varying agricultural inputs (cattle, squash, corn, corn and soy respectively). Site E was located in Lebo Drain, which is downstream of intensive field and greenhouse agriculture as well as near a provincial water quality monitoring station, and sites F–H were conservation areas. Sediment used as a control was obtained from Long Point Provincial Park (site I) in Lake Erie, Ontario, Canada
2.2 Sediment Testing
Samples were sieved through a 250-μm stainless steel sieve to ensure that other living organisms and large particulate matter was removed from the sediment. The sediment was also sieved to allow for retrieval of test organisms and offspring at the conclusion of the tests. Three replicate test vessels (1-L glass beakers) were used in T. tubifex and Hexagenia spp. trials while four replicate vessels were used in H. azteca trials. Test vessels were composed of 200 g of sieved sediment with a moisture content of ~ 50% and 700 mL of overlying culture water. Culture water was city of Burlington (ON, Canada) tap water that had been UV sterilized and dechlorinated. Table S8 contains the physicochemical properties of the culture water. Aeration was achieved via a 60–100-gallon Stellar air pump (Toms Aquatics, CA, USA) split between 10 test vessels using two gang valves (Penn Plax, NY, USA) that allowed for five individual lines each, with a glass Pasteur pipette attached on the end as the inflow. Test vessels were aerated for a minimum 48 h prior to adding the organism to ensure full oxygen saturation and equilibration between sediment and overlying water. Water hardness, pH, conductivity, dissolved oxygen, and ammonia values were measured using an Orion VersaStar Pro Advanced Electrochemistry Meter (Thermo Scientific, MA, USA) prior to the beginning of each test, as well as at the conclusion to ensure no anomalous physicochemical changes occurred throughout the course of the experimental period (reported in the Supplementary Information (SI)). The sediment from each site was sub-sampled for physical and chemical analyses. The physical and chemical properties of sediment collected from each site are available in Tables 2 and 3. The physical and chemical properties were measured by the University of Guelph’s Agriculture and Food Laboratory (AFL) using standard methods (ISO/IEC 17025 accredited laboratory). Chemical measurements included pH, amount of carbon, and phosphorous, among additional properties. Physical properties, such as proportion of sand, clay, and silt, were measured, among additional properties. Metal and pesticides residue analyses using accredited methods for inductively coupled plasma mass spectrometry and gas and liquid chromatography coupled with mass spectrometry, respectively, were performed by the AFL at the University of Guelph (ISO/IEC 17025 accredited laboratory) to check for the presence and concentration of these substances. Table 3 lists the metal concentrations measured in each site that were quantified as over the minimum detection limit. Tables S9 and S10 list the metal and pesticides that were measured in sediment samples and their corresponding limits of detection (LOD) and limits of quantification (LOQ).
2.3 Tubifex tubifex
Tubifex tubifex used in this study were obtained from a permanent culture maintained at ECCC’s Canada Centre for Inland Waters in Burlington, ON, Canada. The worms were cultured in Long Point marsh sediment following procedures described in Milani et al. (2003). The culture has been in continuous production for over 20 years. Adult individuals were sieved out from the culture and placed into a petri dish with culture water. Mature adults with visibly developed gonads were identified using a SM-1 TX-PL stereo microscope (Amscope, CA, USA). Four mature adults were added to each replicate test vessel for a total of 27 test vessels and 12 individuals per site (Hulbert 1984). Test vessels were placed in a controlled I-36VL environmental chamber (Percival Scientific, Iowa, USA) maintained at 23 ± 2 °C (measured by both the chamber itself and an independent temperature probe). Test vessels were kept in 24 h of darkness, as T. tubifex are an infaunal species, which prefer darkness. Worms were not fed for the duration of the test as the sediment contained a sufficient source of organic carbon to allow for worm survival and reproduction.
Following the 28-day exposure, the sediment in each test vessel was passed through a 500-μm and 250-μm sieve sequentially to remove adult worms, juvenile worms, and cocoons. Worms and cocoons were transferred from each sieve to separate Petri dishes with culture water and observed under a dissecting microscope. Adult worms were counted, and observations were made on visibility of gonads and overall health of the worms. Juvenile worms greater than 500 μm and less than 500 μm in size, as well as full and empty cocoons were also quantified.
2.4 Hyalella azteca
Hyalella azteca used in this study were also cultured at ECCC’s Centre for Inland Waters following procedures described in Borgmann et al. (1989). The culture has been in continuous production for ~ 15 years. Juvenile amphipods aged 5–11 days were exposed to each test sediment for 28 days. Vessels were placed in an environmental chamber at 23 ± 2 °C, measured by the chamber itself and with an independent temperature probe. A photoperiod of 16 h light/8 h dark regime was maintained for the 28-day period. Ten amphipods were added to each of the four replicate test vessels per site for a total of 36 test vessels and 40 individuals per site (Hulbert 1984). In addition, each vessel received 2.5 mg of ground TetraMin® fish food (Tetra, VA, USA) twice a week in the first 2 weeks, three times in the third week, and 5 mg of food three times in the final week (Prosser et al. 2017a). Following 28 days, surviving juvenile amphipods from each replicate test vessel per test site were counted and placed in a pre-weighed aluminum dish for drying. After being dried to constant weight at 60 °C, dishes were weighed to determine growth and production of biomass for each replicate.
Growth of amphipods for each replicate was determined by dividing the total dry mass of surviving amphipods by the total number of survival amphipods (i.e., g dw/amphipod). Production of biomass, which is a combined effect of survival and growth, was calculated for each replicate by dividing the total dry mass of surviving amphipods by the total number of amphipods in each replicate at the initiation of the experiment (i.e., 10).
2.5 Hexagenia spp.
Hexagenia spp. (larval mayfly; culture composed of Hexagenia rigida and Hexagenia limbata) were cultured at the University of Guelph according to standard method proposed by MOECC (2012). The eggs were collected in June 2017. The eggs were hatched and larvae grown to testing size in the fall of 2017. They were not from a continuous culture. Hexagenia spp. eggs were collected from adult female mayflies along the Detroit River (42.339272 N, 82.930815 W). Eggs were stored at 4 °C before being hatched in culture water at 23 ± 2 °C. After nymphs hatched, they were transferred to Long Point Marsh sediment. Nymphs were allowed to grow in sediment for a minimum of 7 weeks, to allow for adequate size among individuals to be used in testing. Mayflies removed after growing for 7–8 weeks were exposed to each test sediment for 28 days. Vessels were placed in an environmental chamber at 23 ± 2 °C, measured by the chamber itself and with an independent temperature probe. A photoperiod of 16 h light/8 h dark regime was maintained for the 28-day period. Fifteen mayflies were added to each of the three test vessels per site for a total of 27 test vessels and 45 individuals per site (Hulbert 1984). Each vessel was fed 0.45 mL of food three times per week. Feed consisted of 0.03 mL of deionized water per individual combined with 0.025 g of ground Nutrafin Max® fish food (Hagen INC., QC, Canada) and 0.0375 g chlorophyll alfalfa powder (Aldon Corp., NY, USA) per milliliter. A 150-μL aliquot of food was delivered to each test vessel. Following 28 days, surviving Hexagenia nymphs from each replicate test vessel were counted. An average mass was calculated from a random sample of 10 individuals and was 6.2 ± 1.2 mg.
2.6 In Situ Benthic Macroinvertebrate Community Sampling
In order to assess in situ community composition, benthic macroinvertebrate sampling was performed at six of the eight experimental sites. Two sites (F and H) were omitted from the sampling due to a lack of suitable sampling area. Sampling methods followed those laid out by the Ontario Benthos Biomonitoring Network (OBBN) protocol (Jones et al. 2007). Both Simpson’s diversity index and Shannon’s diversity index were also calculated to represent the extent of variation in the biotic assemblages.
2.7 Statistical Analysis
To address pseudoreplication, control treatments, randomized assignment of experimental units to treatments, as well as a completely randomized replicate design, and replication of treatments were utilized (Hulbert 1984). This facilitates observations that are as statistically independent as possible.
Shapiro–Wilk normality tests were used to check for data normality and in the case of non-normality, log and square root transformations were applied to data. Non-normality was still observed in the majority of response variables and was assumed to be due to large variability in biological data. Testing for homoscedasticity was achieved using the Levene’s test to ensure for homogeneity of variances.
A principal component analysis (PCA) was generated to reduce redundancy and dimensionality among predictor variables within the data set and explore trends among sites. Analysis of variance (ANOVA) was assumed to be robust to the assumption of normality (Glass et al. 1972; Harwell et al. 1992; Lix et al. 1996; Lantz 2013). One-way ANOVAs were utilized to elucidate significant differences in response variables between sites. Subsequent Tukey honest significant difference (HSD) tests were applied if significant differences occurred, to determine exactly where those significant differences existed.
Agricultural land usage data that was utilized as a proxy for agricultural intensity/activity was obtained via the Ontario Flow Assessment Tool (OMNRF 2017). A correlation matrix was built using R package “Hmisc” to determine correlation coefficients and associated p values to check for collinearity of variables. A Spearman rank correlation test (p < 0.05) was used for the correlation analysis. In the case of collinearity, variables were removed from subsequent analyses. The R package “corrplot” was subsequently utilized to allow for visualization of the correlation matrix. A multiple linear regression was created for each non-collinear response variable as outlined in Table 1. All multiple linear regressions utilized non-correlated predictor variables that were logically linked to differences in biological endpoints. We utilize non-correlated predictors to ensure that if a significant relationship is noted, it is not due to relationships between predictors. When using multiple linear regression, it is assumed that relationships between predictor variables and response variables are linear functions and that no interactions occur between explanatory variables (Yuan 2010). All statistical analyses were carried out in R studio version 3.4.4.
3 Results and Discussion
3.1 Metal Sediment Analysis
Arsenic, cadmium, chromium, copper, lead, mercury, nickel, and zinc concentrations measured in all test sediments were lower than sediment quality guidelines determined by MacDonald et al. (2000) and the Canadian Council of Ministers of the Environment (CCME (2001). The threshold effect level was defined as “the concentration below which adverse effects are expected to occur only rarely” (Smith et al. 1996), which indicated that the metal concentrations in sediment were not expected to significantly impact invertebrate subsistence. Sediment quality guidelines were not available for cobalt, molybdenum, or selenium; however, all measured values were less than soil quality guidelines for the protection of environmental and human health (CCME 2001). It was thus, probable that variation in biological endpoint was not significantly related to the concentrations of any of the aforementioned metals.
3.2 Pesticide Analysis
Only a few pesticides were detected in the relatively large pesticide screen (487 active ingredients) performed on sediments (Table S10). In sediment from site E (our high-impact site, Lebo Drain), boscalid and carbendazim were detected but could not be confidently quantified (i.e., above LOD but below LOQ) and aminomethylphosponic acid (AMPA) and glyphosate were present at 0.044 and 0.079 mg/L, respectively. DDD-p,p (0.0079 mg/L) (degradation product of DDT) was present in sediment from agricultural site C at 0.0079 mg/L. The greater quantity of pesticides detected in sediment from site E could be due to the greater organic matter content of the sediment. Many studies have shown that organic matter content can have a positive relationship with the quantity and magnitude of pesticides detected in sediments (Hung et al. 2007; Gan et al. 2005). These results for site E could also indicate that this site is exposed to agrochemical inputs to a greater extent than the other sampling sites. Site E also had the highest concentration of phosphorus in the sediment across the sites, which could also be an indicator of greater agrochemical exposure (Table 2). Overall, the lack of detection of pesticides in sediment across the sites provide little evidence that pesticide residues may be influencing any adverse effects observed in laboratory-cultured invertebrates observed in this study.
3.3 Tubifex tubifex
Adult mortality was not observed among replicates across sites (Table S5). This is likely due to the high tolerance of T. tubifex and their ability to survive harsh environmental conditions. As reported by Barbour et al. (1999), the tolerance value for T. tubifex individuals is 10, the highest possible tolerance value in the described scale. In a study done on spiked sediment, T. Tubifex individuals repeatedly demonstrated the lowest relative sensitivity when compared to H. azteca and Hexagenia spp. (Milani et al. 2003). Our results are consistent with this finding, in that both H. azteca and Hexagenia spp. individuals displayed mortality whereas no mortality was observed in mature adult T. tubifex individuals.
Although no variation in mortality occurred, there were significant differences noted between sites with respect to juveniles > 500 μm and < 500 μm, as well as both full and empty cocoons (Figs. 2 and 3). It can be expected that sites with larger levels of pollution or impairment would elicit lower reproductive rates in Tubifex tubifex as energy must be allocated to survival rather than reproduction (Reynoldson 1994; Gillis et al. 2002; Milani et al. 2003). While adult worm mortality has been shown to be a relatively insensitive effect endpoint, Prosser et al. (2017a) observed that Tubifex tubifex reproduction was one of the most sensitive endpoints when a variety of aquatic biota (H. azteca, mussels, fish) were exposed to sediment spiked with substituted phenylamine antioxidants. The number of juveniles and cocoons produced in sediment from site I (control sediment) was generally greater compared to the other field-collected sediments (Figs. 2 and 3), indicating that the worms were in good condition prior to entering the test sediments. There were clear differences in the amount of reproduction across the sediments collected from different sites (Figs. 2 and 3). Significant differences were noted in the reproductive endpoints among sites (p < 0.05) for each response variable (Figs. 2 and 3). In analyzing the total number of juveniles between sites, a significant relationship was noted (Fig. 2; F8,19 = 11.93, p = 6.04e−6). The number of total cocoons produced between sites was also significantly different (Fig. 3; F8,19 = 20.26, p = 9.3e−8). All associated significant pairwise comparisons as found using Tukey HSD tests are noted in Table S1. The number of juveniles produced was significantly greater (p < 0.05) in control sediment (site I) compared to two ALUS sites (B and D), the high-impact site (E), and three conservation areas (F, G, and H) (Fig. 2, Table S1). The number of cocoons produced was significantly greater in sediment from site I compared to two ALUS sites (B and D) and two conservation areas (sites G and H (Fig. 3, Table S1). All of the sites had lower quantities of organic carbon in the sediment compared to site I (Table 2) and other studies have shown that organic carbon content influences the capacity of T. Tubifex’s ability to survive and reproduce (Reynoldson et al. 1991). However, a clear relationship between organic carbon content and T. tubifex reproduction was not present (Figs. 2 and 3, Table 2). Significantly lower juvenile production was observed in sediment from the high-impact site (site E) relative to the control (site I), yet the organic carbon content of site E (2.61%) was second only to site I (7.70%) (Table 2). The largest number of pesticides was detected in sediment from site E. A greater exposure to agrochemicals in sediment from site E may explain the lower observed reproduction, despite the sediment containing a relatively high amount of organic carbon (Table 2). A clear relationship between the percentage of agriculture and undifferentiated rural land use in the upstream catchment of each site and T. tubifex reproduction was also not observed (Figs. 2 and 3, Table 4). Production of juveniles and cocoons was not significantly different from the control site (site I) at the two sites with the lowest community infrastructure in the upstream catchment (sites A and C) (Figs. 2 and 3, Table 4). A number of studies have observed a negative relationship between urban land use in the upstream catchment and the health of stream macroinvertebrate communities (Wang et al. 1997; Stepenuck et al. 2002).
The total number of Tubifex tubifex juveniles produced over 28 days of incubation in test sediments. If significant differences were present, bars denoted with the same letter (a–c) were not significantly different (p > 0.05). Significant differences were noted between sites via a one-way ANOVA (α = 0.05). Error bars are standard error of the mean
The total number of Tubifex tubifex cocoons produced over 28 days of incubation in test sediment. If significant differences were present, bars denoted with the same letter (a–b) were not significantly different (p > 0.05). Significant differences were noted between sites via a one-way ANOVA (α = 0.05). Error bars are standard error of the mean
T. tubifex are an endobenthic species, meaning all stages of their life cycle are contained within the sediment. Consequently, a number of studies have observed that the granulometric composition of sediment can influence the ability of oligochaetes to survive and reproduce. Casellato (1996) observed that variation in the presence of oligochaete species in the sediment of a lagoon varied with the granulometric composition of the sediment. Sediments with a greater composition of silt and clay, as opposed to sand, had lower densities of oligochaete species due to habitat preference. However, variation in granulometric composition of sediments from the sites in this study does not appear to explain the variation in oligochaete reproduction observed (Table 2, Figs. 2 and 3). The composition of sediment from the control (site I) is relatively high in silt and clay (> 70% and > 20%, respectively), while the composition of sediments from ALUS sites A and C, where reproduction was not significantly affected, are dominated by sand (> 90%) (Table 2). While mortality was not significantly different among the sites, reproduction was significantly different among the sites, but no variable clearly explained this variation in reproduction.
3.4 Hyalella azteca
There were no significant differences in mortality, growth, or biomass production in H. azteca exposed to sediment from the different sites when assessed using an ANOVA (Tables S1 and S6, Figs. S2–S4). The mean mortality across the sites was < 15%, except for ALUS sites C and D, which had a mean mortality of < 25% (Fig. S2). Unlike T. tubifex, mortality was observed in H. azteca, possibly due to a lower tolerance for adverse conditions relative to the oligochaetes; the tolerance values for H. azteca are between 7 and 8 (Barbour et al. 1999).
Hyalella azteca are epibenthic and do not interact with the sediment to the same extent as T. tubifex or Hexagenia spp. and thus are less likely to be impacted by the granulometric composition of the sediment. Suedel and Rodgers (1994) observed that H. azteca reproduction remained > 80% in response to sediments with varying granulometric compositions (sand 42.7–100%, silt 0.0–93.8%, clay 0.0–4.7%, organic matter 0.12–7.80%).
3.5 Hexagenia spp.
Similar to the response of H. azteca, mortality was observed in Hexagenia spp. trials between sites (Fig. 4, Table S7). Relative to H. azteca and T. tubifex, Hexagenia spp. are the most sensitive to poor water quality, ranging on the tolerance scale from 3.6 to 6 (Barbour et al. 1999) and thus the most likely to be affected by poor sediment quality. Individuals of the genus Hexagenia burrow and create a U-shaped burrow in the top layer of sediment and have also shown a preference for fine sandy mud and adhesive mud relative to clay, gravel, and sandy clay sediments (Wright and Mattice 1981). Significant mortality, relative to the control (site I), was observed in sediment from two ALUS sites (A and B) as well as the high-impact site (E) and two conservation sites (F and H) (p < 0.001) (Table S1, Fig. 4). The sediment from ALUS sites A and B, as well as conservation areas F and H, had relatively low silt content (< 8.3%), which might explain the observed adverse effects, but sediment from the high-impact site (E), had a silt content of 38.7%, second only to sediment from our control site (I) (Table 2). Consequently, granulometric composition of the sediment does not appear to provide clear explanation of the adverse effects observed in Hexagenia spp. The potential greater exposure of sediment from site E to agrochemical inputs, as demonstrated by the quantity of pesticides detected, may explain the observed lower survival of Hexagenia spp. nymphs, despite the sediment having a granulometric composition that would be favorable to this mayfly species (Fig. 4, Table 2). A similar situation was observed with juvenile production for T. tubifex exposed to sediment from site E.
Mean number of Hexagenia spp. individuals that died out of the 15 individuals added to each test vessel over 28 days of incubation in test sediment. If significant differences were present, bars denoted with the same letter (a–c) were not significantly different (p > 0.05). Significant differences were noted between sites via a one-way ANOVA (α = 0.05). Error bars are standard error of the mean
3.6 In Situ Benthic Macroinvertebrate Community
The control site (I) had the greatest number of taxonomic orders among the sample collected, followed by two ALUS sites (B and C) (Table 5). Similarly, the Shannon diversity index was highest in site I, followed by sites B and C (Table 5), indicating that there is more macroinvertebrate diversity that is present in these sites. The communities at sites B and C also had the greatest proportion of Trichoptera relative to the other sites, site D had the greatest proportion of Ephemeroptera, and site I had the greatest proportion of Plecoptera (Table 5). In calculating the Simpson’s diversity index, a similar pattern emerged as well, as sites B, C, and I had relatively large values, as did sites D and G. Consequently, sites B, C, D, G, and I had the highest percentage of the community made up of these three orders (%EPT) at 48.6, 57.1, 36.9, 31.0, and 21.0%, respectively (Table 5). The proportion of the community that is composed of Ephemeroptera (mayflies), Plecoptera (stoneflies), and Tricoptera (caddisflies) is used as a metric for stream health, as these three species are viewed as being relatively intolerant to contaminants and stream disturbance (Barbour et al. 1999). The upstream catchments of ALUS sites C and D had a relatively large percent of land use assigned to agriculture relative to the other sites, 81.54 and 81.31%, respectively, and a relatively low percentage of treed land (i.e., < 5%) relative to other sites (e.g., sites B and F, 25.14 and 19.18%) (Table 4), which was not expected. It was hypothesized that catchments with lower agriculture land use and greater treed land would contain a greater diversity of benthic macroinvertebrates and a greater percentage of EPT species. It is important to note that there is variation in sensitivity among the species within the EPT orders (e.g., Beketov 2004). Consequently, it is possible that analyses of the benthic macroinvertebrate community with greater taxonomic resolution may have identified that the EPT species present at sites B and C were among the less sensitive species within these orders (Compin and Céréghino 2003).
3.7 Multiple Linear Regression
In order to assess redundancy in the data sets, a separate correlation matrix for both response and predictor variables was calculated. This allowed us to remove similarly correlated variables from subsequent analyses to avoid spurious relationships. Among the non-correlated response variables, a multiple linear regression was built for each of Hyalella growth, Hyalella mortality, Tubifex juvenile production, and Hexagenia spp. mortality. Tubifex cocoon production was correlated with Tubifex juvenile production and was thus removed. Similarly, Hyalella biomass was correlated with Hyallela growth and was also removed. Within predictor variables, most were removed due to correlations between variables and we were able to reduce the number of predictor variables down to four for subsequent models. Variance inflation factors confirmed that among predictor variables, none were collinear. Among uncorrelated predictor variables, pH, inorganic carbon, phosphorus, and percentage of land use for agriculture in the upstream catchment in the macroinvertebrate community were identified as biologically relevant variables for which to evaluate responses in Hyalella mortality, Hyalella growth, Tubifex juvenile production, and Hexagenia spp. mortality (Tables 1 and 6). Among the four multivariate linear regressions, R2 values ranged from 0 to 0.86 while p values ranged from 0.03–0.85. The regression evaluating Hexagenia mortality was the only significant model while the other three models had similar R2 and p values, indicating that among Hyalella and Tubifex models, we were not able to significantly explain the variation seen in biological endpoints (Table 6). The most significant predictors were pH and inorganic carbon as there was a large positive β value noted with pH and a highly negative value noted with inorganic carbon. This supports the general finding that decreased pH negatively impacts benthic macroinvertebrates (Courtney and Clements 1998) and that increased inorganic carbon also negatively impacts macroinvertebrates (Ometo et al. 2000).
3.8 Sediment Quality Triad
The sediment quality triad (i.e., chemical analysis, in situ macroinvertebrate community, and toxicity to laboratory-cultured macroinvertebrates) did not identify a clear relationship between the quantity of agricultural land use in the upstream catchment of streams and sediment quality in the stream. However, when comparing all predictor variables among sites in a PCA, it is clear that ALUS sites tend to cluster together, while control site I is a relative outlier (Fig. 5). It is also interesting to note that our high-impact site E (Lebo Drain) clusters with the majority of ALUS sites. Specifically, the response of laboratory-cultured macroinvertebrates did not correspond with the intensity of agriculture in the upstream catchment. One exception may be the laboratory-cultured macroinvertebrates adverse response to sediment from our high-impact site (E) (Figs. 2, 3, and 4), which had the greatest percentage of agricultural land use in the upstream catchment and was the only site at which pesticide residues were quantifiable in the sediment (Tables 4 and S10).
Numerous previous studies investigating the use of the sediment quality triad at sites impacted by industry and urbanization yielded clear trends between sediment quality and the level of exposure. For example, Tang et al. (2010) found that both benthic invertebrate abundance and diversity decreased as the level of organic pollution in sediment increased. It was also found that changes in invertebrate abundance and diversity in both Spanish and South African rivers also reflected a longitudinal industrial pollution gradient (López-Doval et al. 2010; Matlou et al. 2017). A number of studies have reported relatively low benthic invertebrate diversity and abundance of aquatic invertebrates where industrial and municipal effluent were present (Fils Mamert et al. 2016; Wright and Ryan 2016). Burt et al. (1991) observed a significant decrease in benthic invertebrate diversity and abundance in sediment from sites located near industrial and municipal sources along the St. Mary’s River in Ontario, Canada. Industrial and urban intensity within the upstream catchment may exert a greater influence on sediment quality and invertebrate community indices compared to agriculture, which may explain the sediment quality triad’s inability to identify a clear relationship between agricultural land use and sediment quality in this study. Herringshaw et al. (2011) reported that urban land use had a greater adverse effect on stream quality compared to agricultural land use in a watershed. Moore and Palmer (2005) observed a negative linear relationship between the quantity of impervious surface cover and richness of macroinvertebrate communities when comparing urban and agricultural land use.
A number of studies have shown that increasing agricultural intensity relates to a decrease in sediment quality and invertebrate community indices (Wood and Armitage 1997; Cuffney et al. 2000; Matthaei et al. 2006; Egler et al. 2012; Herringshaw et al. 2011; Pavlin et al. 2011; Virbickas et al. 2011). For example, Egler et al. (2012) observed significantly lower macroinvertebrate richness at stream sites surrounded by agriculture compared to sites surrounded by pasture and forest. In this study, the relationship between agricultural land use and sediment quality and invertebrate community indices was not clear.
There are a number of potential reasons why the sediment quality triad and the response of laboratory-cultured invertebrates did not identify a clear relationship between agricultural intensity and sediment quality. There is the potential that the gradient of intensity of agricultural land use in the upstream catchments of the chosen sites was not great enough to allow for the identification of variables that would explain the differences in response of the laboratory-cultured invertebrates. The agricultural land use in the upstream catchment across the sites where sediment was sampled ranged from 62.43 to 84.68% (Table 4). The variation in response of T. tubifex reproduction and Hexagenia spp. mortality across the sites that were sampled would indicate that variation in sediment quality across the sites was present (Figs. 2, 3, and 4). However, the magnitude of responses in T. tubifex and Hexagenia spp. among the sites did not correspond with agricultural intensity in the catchment. The inclusion of sites with absent or low agricultural land use in the catchment may have resulted in a consistent lack of adverse effects in laboratory-cultured invertebrates exposed to sediments from these sites. For example, Cuffney et al. (2000) observed a significant decline in biological indicators of stream health (fish, invertebrate, and algal indices) with increasing intensity of agriculture and observed a threshold response for invertebrate and algal communities. Invertebrate and algal communities exhibited rapid decline with a small increase in agricultural intensity, but the communities’ condition index changed very little once moderate to high levels of agricultural intensity were present (Cuffney et al. 2000). This illustrates the ability of a larger gradient of agricultural land use to allow for a clearer characterization of the relationship between agricultural intensity and sediment quality. The challenge with incorporating sites with lower percentages of agricultural land use in this study is that these types of sites are very difficult to locate in southwestern Ontario, where approximately 85% of the land use is related to agriculture (OMAFRA 2016).
It is also possible that the sediment quality triad does not incorporate environmental factors that explain the variation in sediment quality in an agroecosystem. For example, Virbickas et al. (2011) found that riverbed morphology and riparian vegetation were the environmental variables that best described the differentiation among macroinvertebrate communities at different stream sites. Richards et al. (1993) observed that substrate characteristics, instream cover, channel morphology, riparian zone and stream bank condition, riffle/run quality, and pool quality played an important role for stream macroinvertebrate communities in agricultural catchments. These physical characteristics of the stream are often not considered in the sediment quality triad approach, which may explain the outcome of our study.
4 Conclusions
The sediment quality triad approach did not identify a relationship between sediment quality and intensity of agriculture across stream sites. This result may be due to the triad needing a larger gradient of disturbance as a product of agricultural intensity before it can identify a significant effect on sediment quality. It is also important to consider that the sediment quality triad approach does not incorporate physical characteristics of the stream, which may be an important predictor variable for sediment quality and invertebrate community health. Additionally, measured abiotic variables were only significantly related to the biological endpoints of Hexagenia spp. This finding is likely due to the fact that this species is the least tolerant and most sensitive to changes away from an optimum, and thus, we were able to detect a significant relationship.
References
Allan, J. D. (2004). Influence of land use and landscape setting on the ecological status of rivers. Limnetica, 23, 187–198. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122.
Allan, J. D., Erickson, D. L., & Fay, J. (1997). The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biolology, 37, 149–161. https://doi.org/10.1046/j.1365-2427.1997.d01-546.x.
Barbour, M. T., Gerritsen, J., Snyder, B. D., Stribling, J. B. (1999). USEPA rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, Second edition. EPA 841-B-99-002. U.S. Environmental Protection Agency; Office of Water; Washington, D.C.
Beketov, M. (2004). Different sensitivity of mayflies (Insecta, Ephemeroptera) to ammonia, nitrite and nitrate: linkage between experimental and observational data. Hydrobiologia, 528, 209–216.
Borgmann, U., Ralph, K. M., & Norwood, W. P. (1989). Toxicity test procedures for Hyalella azteca, and chronic toxicity of cadmium and pentachlorophenol to H-azteca, Gammarus-fasciatus, and Daphnia magna. Archives of Environmental Contamination and Toxicology, 18, 756–764. https://doi.org/10.1007/BF01225013.
Burt, A. J., McKee, P. M., Hart, D. R., & Kauss, P. B. (1991). Effects of pollution on benthic invertebrate communities of the St. Marys River, 1985. Hydrobiologia, 219, 63–81. https://doi.org/10.1007/BF00024747.
Carpenter, S. R., Cole, J. J., Pace, M. L., Van de Bogert, M., Bade, D. L., Bastviken, D., Gille, C. M., Hodgson, J. R., Kitchell, J. F., & Kritzberg, E. S. (2005). Ecosystems subsidies: terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology, 86, 2737–2750.
Casellato, S. (1996). Oligochaetes in the southern basin of the Venetian Lagoon: community composition, species abundance and biomass. Hydrobiologia, 334, 103–114. https://doi.org/10.1007/BF00017359.
CCME. (2001). CCME protocol for the derivation of Canadian sediment quality guidelines for the protection of aquatic life. Transport, 1–4.
Chapman, P. M. (1990). The sediment quality triad approach to determining pollution-induced degradation. Science of the Total Environment, 97(98), 815–825. https://doi.org/10.1016/0048-9697(90)90277-2.
Compin, A., & Céréghino, R. (2003). Sensitivity of aquatic insect species richness to disturbance in the Adour-Garonne stream system (France). Ecological Indicators, 3, 135–142.
Cooper, C. M. (1993). Biological effects of agriculturally derived surface water pollutants on aquatic systems—a review. Journal of Environmental Quality, 22, 402. https://doi.org/10.2134/jeq1993.00472425002200030003x.
Courtney, L. A., & Clements, W. H. (1998). Effects of acidic pH on benthic macroinvertebrate communities in stream microcosms. Hydrobiologia, 379(1–3), 135–145.
Cuffney, T. F., Meador, M. R., Porter, S. D., & Gurtz, M. E. (2000). Responses of physical, chemical, and biological indicators of water quality to a gradient of agricultural land use in the Yakima river basin, Washington. Environmental Monitoring and Assessment, 64, 259–270. https://doi.org/10.1023/A:1006473106407.
Egler, M., Buss, D., Moreira, J., & Baptista, D. (2012). Influence of agricultural land-use and pesticides on benthic macroinvertebrate assemblages in an agricultural river basin in Southeast Brazil. Brazilian Journal of Biology, 72, 437–443. https://doi.org/10.1590/S1519-69842012000300004.
Fils Mamert, O., Serge Hubert, Z. T., Ernest, K., Nectaire Lie, N. T., & Simenon, T. (2016). Influence of municipal and industrial pollution on the diversity and the structure of benthic macro-invertebrates community of an urban stream in Douala, Cameroon. Journal of Biodiversity and Environmental Sciences, 8, 120–133.
Gan, J., Lee, S. J., Liu, W. P., Haver, D. L., & Kabashima, J. N. (2005). Distribution and persistence of pyrethroids in runoff sediments. Journal of Environmental Quality, 34, 836–841.
Giller, P. S. (2005). River restoration: seeking ecological standards. Editor’s introduction. Journal of Applied Ecology, 42, 201–207. https://doi.org/10.1111/j.1365-2664.2005.01020.x.
Gillis, P. L., Diener, L. C., Reynoldson, T. F., & Dixon, D. G. (2002). Cadmium-induced production of a metallothionein-like protein in Tubifex tubifex (Oligochaeta) and Chironomus riparius (Diptera): correlation with reproduction and growth. Environmental Toxicology and Chemistry, 21, 1836–1844.
Glass, G. V., Peckham, P. D., & Sanders, J. R. (1972). Consequences of failure to meet assumptions underlying fixed effects analyses of variance and covariance. Review of Educational Research, 42, 237–288.
Harwell, M. R., Rubinstein, E. N., Hayes, W. S., & Olds, C. C. (1992). Summarizing Monte Carlo results in methodological research: the one- and two-factor fixed effects ANOVA cases. Journal of Statistics Education, 17, 315–339.
Herman, M. R., & Najadhashemi, A. P. (2015). A review of macroinvertebrates- and fish-based stream health indices. Ecohydrology and Hydrobiology, 15, 53–67.
Herringshaw, C. J., Stewart, T. W., Thompson, J. R., & Anderson, P. F. (2011). Land use, stream habitat and benthic invertebrate assemblages in a highly altered Iowa watershed. The American Midland Naturalist, 165, 274–293. https://doi.org/10.1674/0003-0031-165.2.274.
Hurlbert, S. H. (1984). Pseudoreplication and the design of ecological field experiments. Ecological Monographs, 54(2), 187–211.
Hung, C., Gong, G., Chen, H., Hsieh, H., Santschi, P. H., Wade, T. L., & Sericano, J. S. (2007). Relationship between pesticide and organic carbon fractions in sediments of the Danshui River estruary and adjacent coastal areas of Taiwan. Environmental Pollution, 148, 546–554.
Jaagumagi, R., & Persaud, D. (1993). Sediment assessment: a guide to study design, sampling, and laboratory analysis. Toronto: Queen’s Printer for Ontario.
Johnston, J. M., Barber, M. C., Wolfe, K., Galvin, M., Cyterski, M., & Parmar, R. (2017). An integrated ecological modeling system for assessing impacts of multiple stressors on stream and riverine ecosystem services within river basin. Ecological Modelling, 354, 104–114.
Jones, C., Somers, K. M., Craig, B., & Reynoldson, T. B. (2007). Ontario Benthos Biomonitoring Network: protocol manual. Toronto: Queens Printer for Ontario.
Koop, J. H. E., Winkelmann, C., Becker, J., Hellmann, C., & Ortmann, C. (2011). Physiological indicators of fitness in benthic invertebrates; a useful measure for ecological health assessment and experimental biology. Aquatic Ecology, 45, 547–559.
Lantz, B. (2013). The impact of sample non-normality on ANOVA and alternative methods. The British Journal of Mathematical and Statistical Psychology, 66(2), 224–244.
Lenat, D. R., & Crawford, J. K. (1994). Effects of land use on water quality and aquatic biota of three North Carolina Piedmont streams. Hydrobiologia, 294, 185–199.
Lix, L. M., Keselman, J. C., & Keselman, H. J. (1996). Consequences of assumption violations revisited: a quantitative review of alternatives to the one-way analysis of variance F test. Review of Educational Research, 66, 579–619.
López-Doval, J. C., Großschartner, M., Höss, S., Orendt, C., Traunspurger, W., Wolfram, G., & Muñoz, I. (2010). Invertebrate communities in soft sediments along a pollution gradient in a Mediterranean river (Llobregat, NE Spain). Limnetica, 29, 311–322. https://doi.org/10.1111/1467-9906.00002.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31. https://doi.org/10.1007/s002440010075.
Matlou, K., Addo-Bediako, A., & Jooste, A. (2017). Benthic macroinvertebrate assemblage along a pollution gradient in the Steelpoort River, Olifants River system. African Entomology, 25, 445–453. https://doi.org/10.4001/003.025.0445.
Matthaei, C. D., Weller, F., Kelly, D. W., & Townsend, C. R. (2006). Impacts of fine sediment addition to tussock, pasture, dairy and deer farming streams in New Zealand. Freshwater Biology, 51, 2154–2172. https://doi.org/10.1111/j.1365-2427.2006.01643.x.
McTammany, M. E., Benfield, E. F., & Webster, J. R. (2007). Recovery of stream ecosystem metabolism from historical agriculture. Journal of the North American Benthological Society, 26, 532–545. https://doi.org/10.1899/06-092.1.
Meyer, J. L., Sale, M. J., Mulholland, P. J., & Poff, N. L. (1999). Impacts of climate change on aquatic ecosystem functioning and health. Journal of the American Water Resources Association, 35, 1373–1386. https://doi.org/10.1111/j.1752-1688.1999.tb04222.x.
Milani, D., Reynoldson, T. B., Borgmann, U., & Kolasa, J. (2003). The relative sensitivity of four benthic invertebrates to metals in spiked-sediment exposures and application to contaminated field sediment. Environmental Toxicology and Chemistry, 22, 845–854. https://doi.org/10.1002/etc.5620220424.
MOECC. (2012). Standard operating procedure: Hexagenia spp. culturing. SOP HX1.v5. Ontario Ministry of the Environment; Laboratory Services Branch; Aquatic Toxicology Unit; Toronto, Ontario, Canada.
Moore, A. A., & Palmer, M. A. (2005). Invertebrate biodiversity in agricultural and urban headwater streams: implications for conservation and management. Ecological Applications, 15, 1169–1177. https://doi.org/10.1890/04-1484.
Nakano, S., & Murakami, M. (2001). Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Sciences of the United States of America, 98, 166–170.
Nilsson, C., & Berggren, K. (2000). Alterations of riparian ecosystems caused by river regulation. Bioscience, 50, 783–792. https://doi.org/10.1641/0006-3568(2000)050[0783:AORECB]2.0.CO;2.
OMAFRA. 2016. Area of census farm (acres) by county, Ontario: 2016. http://www.omafra.gov.on.ca/english/stats/census/cty30a.htm
Ometo, J. P. H. B., Martinelli, L. A., Ballester, M. V., Gessner, A., Krusche, A. V., Victoria, R. L., & Williams, M. (2000). Effects of land use on water chemistry and macroinvertebrates in two streams of the Piracicaba river basin, south-east Brazil. Freshwater Biology, 44, 327–337. https://doi.org/10.1046/j.1365-2427.2000.00557.x.
OMNRF (Ontario Ministry of Natural Resources and Forestry). (2017). User guide for Ontario Flow Assessment Tool (OFAT). Provincial Mapping Unit, Mapping and Information Resources Branch.
Pace, M. L., Cole, J. J., Carpenter, S. R., Kitchell, J. F., Hodgson, J. R., Van de Bogert, M. C., Bade, D. L., Kritzberg, E. S., & Bastviken, D. (2004). Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature, 427, 240–243.
Pavlin, M., Birk, S., Hering, D., & Urbanič, G. (2011). The role of land use, nutrients, and other stressors in shaping benthic invertebrate assemblages in Slovenian rivers. Hydrobiologia, 678, 137–153. https://doi.org/10.1007/s10750-011-0836-8.
Prosser, R. S., Bartlett, A. J., Milani, D., Holman, E. A. M., Ikert, H., Schissler, D., Toito, J., Parrott, J. L., Gillis, P. L., & Balakrishnan, V. K. (2017a). Variation in the toxicity of sediment-associated substituted phenylamine antioxidants to an epibenthic (Hyalella azteca) and endobenthic (Tubifex tubifex) invertebrate. Chemosphere, 181, 250–258. https://doi.org/10.1016/j.chemosphere.2017.04.066.
Prosser, R. S., Parrott, J. L., Galicia, M., Shires, K., Sullivan, C., Toito, J., Bartlett, A. J., Milani, D., Gillis, P. L., & Balakrishnan, V. K. (2017b). Toxicity of sediment-associated substituted phenylamine antioxidants on the early life stages of Pimephales promelas and a characterization of effects on freshwater organisms. Environmental Toxicology and Chemistry, 36, 2730–2738.
Reynoldson, T. B. (1994). A field test of a sediment bioassay with the oligochaete worm Tubifex tubifex (Müller, 1774). Hydrobiologia, 278, 223–230.
Reynoldson, T. B., & Metcalfe-Smith, J. L. (1992). An overview of the assessment of aquatic ecosystem health using benthic invertebrates. Aquatic Ecosystem Health, 1, 295–308.
Reynoldson, T. B., Thompson, S. P., & Bamsey, J. L. (1991). A sediment bioassay using the tubificid oligochaete worm Tubifex tubifex. Environmental Toxicology and Chemistry, 10, 1061–1072.
Richards, C., Host, G. E., & Arthur, J. W. (1993). Identification of predominant environmental factors structuring stream macroinvertebrate communities within a large agricultural catchment. Freshwater Biology, 29, 285–294. https://doi.org/10.1111/j.1365-2427.1993.tb00764.x.
Rubach, M. N., Ashauer, R., Buchwalter, D. B., De Lange, H. J., Hamer, M., Preuss, T. G., Töpke, K., & Maund, S. J. (2011). Framework for traits-based assessment in ecotoxicology. Integrated Environmental Assessment and Management, 7, 172–186. https://doi.org/10.1002/ieam.105.
Scott, M. C., & Helfman, G. S. (2001). Native invasions, homogenization, and the mismeasure of integrity of fish assemblages. Fisheries, 26, 6–15. https://doi.org/10.1577/1548-8446(2001)026<0006:NIHATM>2.0.CO;2.
Smith, S. L., MacDonald, D. D., Keenleyside, K. A., Ingersoll, C. G., & Field, L. J. (1996). A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. Journal of Great Lakes Research, 22, 624–638. https://doi.org/10.1016/S0380-1330(96)70985-1.
Søndergaard, M., & Jeppesen, E. (2007). Anthropogenic impacts on lake and stream ecosystems, and approaches to restoration. Journal of Applied Ecology, 44, 1089–1094. https://doi.org/10.1111/j.1365-2664.2007.01426.x.
Stepenuck, K. F., Crunkilton, R. L., & Wang, Z. (2002). Impacts of urban landuse on macroinvertebrate communities in southeastern Wisconsin streams. Journal of American Water Resources Association, 38, 1041–1051.
Storey, R. G., & Cowley, D. R. (1997). Recovery of three New Zealand rural streams as they pass through native forest remnants. Hydrobiologia, 353, 63–76. https://doi.org/10.1023/A:1003042425431.
Strayer, D. L., Beighley, R. E., Thompson, L. C., Brooks, S., Nilsson, C., Pinay, G., & Naiman, R. J. (2003). Effects of land cover on stream ecosystems: roles of empirical models and scaling issues. Ecosystems, 6, 407–423. https://doi.org/10.1007/s10021-002-0170-0.
Suedel, C. B., & Rodgers Jr., J. J. (1994). Responses of Hyalella Azteca and Chironomus Tentans to particle-size distribution and organic matter content of formulated and natural freshwater sediments. Environmental Toxicology and Chemistry, 13, 1639–1648. https://doi.org/10.1002/etc.5620131013.
Tang, H., Song, M.-Y., Cho, W.-S., Park, Y.-S., & Chon, T.-S. (2010). Species abundance distribution of benthic chironomids and other macroinvertebrates across different levels of pollution in streams. Annales de Limnologie—Internal Journal of Limnology, 46, 53–66. https://doi.org/10.1051/limn/2009031.
Townsend, C. R., Dolédec, S., Norris, R., Peacock, K., & Arbuckle, C. (2003). The influence of scale and geography on relationships between stream community composition and landscape variables: description and prediction. Freshwater Biology, 48, 768–785. https://doi.org/10.1046/j.1365-2427.2003.01043.x.
Virbickas, T., Pliuraite, V., & Kesminas, V. (2011). Impact of agricultural land use on macroinvertebrate fauna in Lithuania. Polish Journal of Environmental Studies, 20, 1327–1334.
Wang, L., Lyons, J., Kanehl, P., & Gatti, R. (1997). Influences of watershed land use on habitat quality and biotic integrity in Wisconsin streams. Fisheries Magazine, 22, 6–12.
Wood, P. J., & Armitage, P. D. (1997). Biological effects of fine sediment in the lotic environment. Environmental Management, 21, 203–217. https://doi.org/10.1002/hyp.7604.
Wright, L. L., & Mattice, J. S. (1981). Substrate selection as a factor in Hexagenia distribution. Aquatic Insects, 3, 13–24. https://doi.org/10.1080/01650428109361039.
Wright, I. A., & Ryan, M. M. (2016). Impact of mining and industrial pollution on stream macroinvertebrates: importance of taxonomic resolution, water geochemistry and EPT indices for impact detection. Hydrobiologia, 772, 103–115. https://doi.org/10.1007/s10750-016-2644-7.
Yuan, L. (2010). Estimating the effects of excess nutrients on invertebrates from observational data. Ecological Applications, 20(1), 110–125.
Acknowledgments
The authors would like to thank the Long Point Region Conservation Authority, the Kettle Creek Conservation Authority, the farmers who allowed us access to their land, and Emelia Myles-Gonzalez, Laura Johnson, Kathleen Lo, and Eric Johnson for their time and contributions to the study.
Funding
This work was supported by the Canada First Research Excellence Fund – Food For Thought Grant to K.S. McCann and Natural Science and Engineering Research Council Discovery Grant to R.S. Prosser. The funding source had no such involvement in the study design, collection, analysis, or interpretation of the data. There was also no involvement in the writing of the report or the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 1.91 mb)
Rights and permissions
About this article
Cite this article
Wolf, J.F., Prosser, R.S., Champagne, E.J. et al. Variation in Response of Laboratory-Cultured Freshwater Macroinvertebrates to Sediment from Streams with Differential Exposure to Agriculture. Water Air Soil Pollut 231, 13 (2020). https://doi.org/10.1007/s11270-019-4376-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4376-6