Abstract
Roads and highways play an important function in the human-dominated Earth landscape and strongly affect the environment. Roadside ecosystems receive a number of pollutants, including deicing salt and inorganic nitrogen (N) from automobiles. We investigated how soil carbon (C) and N cycling were impacted by the application of salt (NaCl) and nitrate (NO3−) to experimental plots in a field that is adjacent to Interstate 81, in Binghamton, NY. Experimental plots were constructed on two parallel transects; one was adjacent to the highway (0-m) and the other 50 m away from the highway (50-m). We hypothesized that the 0-m transect was exposed to roadway-derived pollutants over a long-term period of time, while the 50-m transect was exposed to fewer pollutants due to its distance from the road. Soils were collected in July and November 2011 and June and October 2012. Salt significantly decreased the rates of soil C mineralization and in situ soil respiration in both 0- and 50-m transects (p < 0.001), though it did not discernibly affect the rates of N mineralization or nitrification. Applications of NO3− had no significant impact on soil C or N mineralization. The effects of roadway pollutants were reflected in higher soil conductivities and pH at the 0-m transect. Under experimental salt treatment, C mineralization was reduced by 75% in the 50-m transect, compared to 20% reduction in the 0-m transect. We conclude that microbial communities near roads might have evolved to better withstand the impacts of roadway pollutants. Nevertheless, roads and vehicle traffic have strong impacts on the environment, and the application of road salt has important environmental consequences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biogeochemical cycles in urbanized areas are impacted in part by alterations to land cover, such as roads, and changes to atmospheric chemistry, including chemicals released from motor vehicles and roadways (Kaye et al. 2006). Roadways and vehicles can impact the ecosystems adjacent to the roads. The impact of roadways and vehicles on biogeochemical cycling has been of particular interest, since roadway expansion has accompanied the growth of urban areas worldwide; in the USA alone, there are over four million kilometers of roads (United States Department of Transportation, 2013). Deicing salt, inorganic nitrogen (N) from vehicle fuel combustion, and metals released from vehicles and roads are common chemicals that may enter ecosystems adjacent to roadways (Bettez et al. 2013; Councell et al. 2004; Findlay and Kelly 2011; Johansson et al. 2009). Over time they accumulate in soils and can leach to water resources, becoming detrimental to ecosystem function and human health (Hjortenkrans et al. 2008). The impact of metals on roadside and urban soils has been well documented, but research on the effects of deicing salt and inorganic N on ecosystem processes is limited (Fay and Shi 2012; Green and Cresser 2008; Pouyat et al. 2010).
Deicing road salt is a common traffic-related pollutant in temperate climates, where it is critical to maintain road safety during winter months (Fay and Shi 2012). Commonly used deicing salt is sodium chloride (NaCl) because of its relatively low cost, and road salt use has increased dramatically since 1950 (Fay and Shi 2012). Salt is highly soluble and usually enters ecosystems in runoff (Davidson et al. 2009). Roadside soils have been found to have salt concentrations that correlate positively with the rate of salt application (Fay and Shi 2012; Findlay and Kelly 2011). In soils, high salt concentrations can impact microbial communities that mediate carbon (C) and N cycling, thus altering biogeochemical cycling in areas adjacent to roadways (Fay and Shi 2012; Green and Cresser 2008; McCormick and Wolfe 1980). Salt deposition can also affect plant growth, plant community structure, and animal habitat (Bryson and Barker 2002; Fay and Shi 2012; Green and Cresser 2008; Heintzman et al. 2015). Deicing road salt was widely thought to be quickly “flushed-out” of soils and groundwater, but this view has been challenged by a number of recent studies that suggest a large proportion of it is retained in watersheds (Cunningham et al. 2008; Kelly et al. 2008; Kincaid and Findlay 2009). For example, Kelly et al. (2008) showed a doubling in concentration of salt in a rural watershed in the Dutchess County, NY. This increase was not accompanied by an increase in salt loads, road density or population density, indicating salt had been retained within the watershed, in soils, groundwater or both (Kelly et al. 2008). Kaushal et al. (2005) found that impervious surface cover in Baltimore, MD, was strongly correlated to the chloride (Cl−) increases in urban and suburban streams. Elevated urban and suburban stream Cl− concentrations were observed in winter months, and persisted in the spring, summer and fall (Kaushal et al. 2005). Year-round elevation of Cl− suggested that salt contamination had spread to groundwater (Kaushal et al. 2005, Craig and Zhu unpublished data). Because road networks are widespread and road salt applications are yearly events, roadside ecosystems could be critically altered by deicing salt.
In addition to road salt, inorganic N is a major traffic-related pollutant. Fossil fuel combustion in motor vehicles releases inorganic N in the form of nitrogen oxides (NOx) and ammonia (NH3), where it enters precipitation and runs off to roadsides and streams. The high temperatures generated by combustion cause atmospheric N2 and O2 to split; N and O then react to form NO and NO2 (NOx) (Abdel-Rahman 1998). Three-way catalytic converters remove NOx by reduction to N2—however “over-reduction” also occurs, resulting in the release of NH3 (Heeb et al. 2006). These processes result in higher automobile-sourced fluxes of NOx and NH3 near roadways, which are highly reactive forms of N (Cape et al. 2004; Redling et al. 2013). Atmospheric NOx is converted to nitrate (NO3−), and combustion-derived atmospheric NOx concentrations have been found to correlate with higher NO3− concentrations in precipitation (Butler et al. 2003). Atmospheric NH3 is converted to ammonium (NH4+), which becomes an important component of wet deposition (Asman et al. 1998; Lovett et al. 2000). Consequently, roadsides have elevated NH4+ and NO3− inputs, making these areas “hot spots” of N deposition (Bettez et al. 2013; Padgett et al. 1999).
Greater N deposition could increase N availability in soils, which can then affect the structure and function of plant and microbial communities (Compton et al. 2004; Magill et al. 1997). Nitrogen deposition is a contributing factor to higher rates of N mineralization and nitrification in urban soils (Pouyat and Turechek 2001; Zhu and Carreiro 2004). Magill et al. (1997) found greater rates of nitrification and N mineralization, along with greater fluxes of N2O, a potent greenhouse gas, in plots with experimental N deposition. Elevated inputs of inorganic N can also stimulate plant growth, and may change plant community structure (Angold, 1997; Bignal et al. 2007). Besides biological uptake and loss via denitrification, NO3− is highly soluble and is readily leached from soil, especially during storm events.
Metals released from roadway activities have been recognized as a major threat to ecosystem health, and much of the research on roadside biogeochemistry has been focused on metal contamination (Pouyat et al. 2010; Yesilonis et al. 2008). Less is known about the effects of road salt and N on roadside ecosystem processes. Since roadways are inescapable fixtures of urban and suburban landscapes, understanding the impacts of these pollutants is crucial to mitigate their potential problems. This study investigates the effects of common roadside pollutants in an ecosystem adjacent to a major highway. Salt and N were experimentally applied to plots during the growing seasons from 2010 to 2012 to determine whether they affected net N mineralization, nitrification, C mineralization, and soil respiration in roadside soils. We established two transects and compared soils adjacent to the highway (0-m) that had been exposed to road pollutants for decades with soils away from the road (50-m) that had received less exposure. We hypothesized that (1) soils receiving experimental salt input would have lower rates of nitrification, N mineralization, and C mineralization; and (2) soils receiving experimental N input would have higher rates of nitrification and N mineralization. Further, we predicted that (3) long-term exposure to pollutants at the transect adjacent to the road (0-m) would cause soils to have lower rates of nitrification, N mineralization and C mineralization compared to those away from the road (50-m); and (4) soils from the 0-m transect and 50-m transect would respond differently to the experimental salt and N inputs. Preliminary data from 2010 (after a 5-month manipulative experiment) have been reported in Scott et al. (2011). The 2011 and 2012 results (years 2 and 3 of this 3-year study) are presented here.
2 Material and Methods
2.1 Study Site
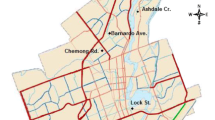
The study was conducted in Binghamton, NY (42.1′ N, 75.92′ W) at a field site alongside Interstate Highway 81 (I-81). Interstate-81 stretches from Tennessee to the Canadian border, passing through Binghamton, NY, a metropolitan area with a population of about 260,000. This portion of the interstate was built in the 1960’s and has an average daily traffic flow of approximately 70,000 automobiles (New York State Department of Transportation, 2010). The study site is an open field located southeast of the northbound lane of I-81 that is dominated by Solidago canadensis and Lythrum salicaria. The area has a temperate climate, receiving a yearly average rainfall of about 100 cm. The experiment was initiated in June, 2010 and continued through October 2012. In 2011, the average temperature was 8.83 °C, and the precipitation was 172.85 cm. Precipitation in 2011 was much higher than the average, partly due to the Tropical Storm Lee passing over Binghamton in September. In 2012, the average temperature was 9.67 °C and the precipitation was 99.92 cm, which is typical for the area.
2.2 Experimental Design
Experimental plots were constructed in a blocked factorial design on two 90-m-long transects that were parallel to I-81 (Fig. 1). One transect was at the base of the highway bank (0-m transect), and the other was 50 m away from the highway (50-m transect). Six positions were randomly selected every 15 m on each transect with distance between any two positions no less than 5 m; at each position, we made three adjacent 1 m2 sub-plots. Treatments of NaCl, NaNO3, and a water control were chosen randomly and applied to the sub-plots (Fig. 1). The 0-m transect has been exposed to roadside pollutants over a long period of time, while the 50-m transect is likely exposed to fewer pollutants. Thus, the experiment design allows us to test both the long-term effects of exposure to roadside pollutants and short-term effects of experimental salt and N inputs. The plots were treated on a bi-weekly (every other week) basis for 5 months of the growing season in 2010 (June–October) and 6 months in 2011 and 2012 (May–October). In 2011 and 2012, salt-treated plots received 337.2 g m−2 Na+ and 520.0 g m−2 Cl− for the year, while N-treated plots received 5.18 g m−2 N and 8.5 g m−2 Na+ for the year. The Na+ contained in the N treatment was a by-product unlikely to affect the N and C mineralization (it was just 2.5% of that in the salt treatment). Nitrogen deposition in the Northeast USA is approximately 1 g m−2 year−1 (Aber et al. 2003). The N concentrations for this study were therefore experimentally elevated over background N deposition levels, reflective of deposition in larger metropolitan areas and near roadways. The salt concentrations were similar to amounts reported in previous research on deicing salt deposition studies (Blomqvist and Johansson 1999; Lundmark and Olofsson 2007).
Soils were collected on July 26 and November 8, 2011, and June 21 and October 25, 2012. There was a highway expansion project for I-81 starting in July 2012 which destroyed the 0-m transect, so subsequent data from October 2012 sampling were for 50-m transect only. Soil cores 5 cm in diameter and 15 cm in length were collected and immediately transported to the lab in a cooler. They were stored in a 4 °C cold room until processing, which occurred within 24–72 h of collection. Cores were separated into depths of 0–5 and 5–15 cm and were sieved through 4 mm sieves, removing roots and large debris.
2.3 Soil Chemistry, Net N Mineralization, Nitrification, and Carbon Mineralization
Soils were prepared for determining initial NH4+ and NO3− concentrations by extracting 15 g freshly sieved soil with 50 mL 1 M KCl solution. This was followed by analyses on a Lachat Analyzer. Soil extracts were filtered through Whatman 40 filter papers, acidified with 6 N HCl and stored in cold room (4 °C) until analysis. Concentrations of NH4+ and NO3− were quantified using a Lachat QuickChem 8000 Flow Injection Analyzer (Lachat Instruments, Milwaukee, WI). To determine percent soil moisture, soil samples were dried at 60 °C for 72 h. Percent organic matter was calculated using the loss-on-ignition method where samples were combusted in a muffle furnace at 550 °C for 2 h. Soil slurries (10 g air-dried soil mixed with 20 mL Nanopure water) were used to measure pH (Orion PerpHecT Log R Meter, Model 350) and conductivity (Omega Model CDH-42 Conductance/TDS Meter).
Rates of net N mineralization and net nitrification were measured using 28-day lab incubations. For the incubations, 15 g of freshly sieved soil were weighed into 125-mL glass Wheaton bottles. They were kept at constant room temperature (22 °C), and water was added weekly to maintain constant soil moisture. After the incubation, soils were extracted with 50 mL 1 M KCl solution. Soil concentrations of NH4+ and NO3− were also quantified before the incubation (see above) and the differences before and after the incubation were used to calculate the rates of net N mineralization and net nitrification.
Rates of carbon dioxide (CO2) flux from sieved 0–5 cm soils were measured in the lab in 2011. Soils were incubated in 125 mL glass Wheaton bottles. Using syringes with three-way stoppers, a 20 mL gas sample was collected from each bottle at time 0. After incubation for 4 h, another 20 mL gas sample was taken from the bottle. The difference of CO2 concentrations was used to calculate the rates of C mineralization. A Shimadzu Gas Chromatograph (GC 14-A) equipped with a thermal conductivity detector (TCD) was used for the CO2 measurement. Gas samples were analyzed within 10 h of collection. A pilot study determined that the syringes retained the gas samples for this period of time.
2.4 In Situ Soil Respiration
In 2012, we measured in situ rate of soil respiration. Collars of white PVC pipe were permanently installed in each of the sub-plots. Initial gas samples were taken immediately after the caps were secured and were followed with another sampling after 60 min to yield a 1-h incubation. Twenty milliliters of gas sample was taken using syringes with a three-way stopper. Gas samples were immediately transported back to the lab and analyzed using the Shimadzu GC as described above.
2.5 2.6 Data Analysis
The results of the experimental treatments at each of the transect (0- and 50-m) were analyzed using a blocked ANOVA looking at treatments (Control, N- and salt treatments). We also used a two-way ANOVA (not-blocked) to examine the (long-term) transect difference and the potential interactions with the (short-term) experimental treatments. Data normality and homoscedasticity were examined following each ANOVA analysis. Where there were differences between treatments, Tukey’s HSD (honest significant difference) test was used for posteriori analysis. Statistical analyses were computed using the R Statistical program (R Core Team 2016). Statistically significant results were reported when p < 0.05. Data means are presented ± one standard error (SE).
3 Results
3.1 Rates of Net N Mineralization, Nitrification, and C Mineralization
Experimental treatment with salt and NO3− did not discernably impact the rates of net N mineralization and nitrification in Year 2011 and 2012, at either 0- or 50-m transect. Net N mineralization and nitrification rates also did not differ significantly between the two transects in July or Nov 2011 (Table 1). In June 2012, the N rates were significantly elevated at the 0-m transect (p = 0.002); for the 0–5 cm soils, net N mineralization rates were about four times higher and nitrification rates were about three times higher compared to the soils from 50-m transect, although no difference was found in the 5–15 cm soils (Table 1). At the 0-m transect, nitrification and N mineralization rates were significantly lower in 5–15 cm soils compared to 0–5 cm soils in July 2011(p = 0.02) and June 2012 (p < 0.001). In November 2011, nitrification rates were significantly higher (p = 0.03) in 0–5 cm soils but mineralization rates did not differ between layers (p > 0.05) (Table 1). At the 50-m transect, rates of nitrification and N mineralization differed between two depths only in July 2011 (p < 0.001).
Potential C mineralization was quantified in July and November 2011 by incubating 0–5 cm soils in the lab. The salt-treated soils had significantly lower C mineralization rates (p < 0.001, Fig. 2), while NO3− treatment had no effect. Furthermore, the salt-treatment effect on C mineralization was more drastic in soils collected from the 50-m transect than from the 0-m transect. In July 2011, rates of C mineralization under salt treatment were 1.34 ± 0.08 μg CO2-C g−1 h−1 of the 0-m transect soil and 0.83 ± 0.08 μg CO2-C g−1 h−1 of the 50-m transect soil. In the control plots, they were 1.54 ± 0.08 and 1.58 ± 0.08 μg CO2-C g−1 h−1 of the 0- and 50-m transect soils, respectively, and in N-treated plots, 1.55 ± 0.10 and 1.52 ± 0.08 μg CO2-C g−1 h−1, respectively. Salt-treated soils from Nov 2011 had rates of C mineralization that were 1.72 ± 0.06 μg CO2-C g−1 h−1 at the 0-m transect, and 1.38 ± 0.07 μg CO2-C g−1 h−1 at the 50-m transect. Rates of C mineralization in control soils were 2.15 ± 0.15 and 1.93 ± 0.08 μg CO2-C g−1 h−1 at the 0-m transect and 50-m transect, respectively, and 1.92 ± 0.14 and 1.99 ± 0.10 μg CO2-C g−1 h−1 in N-treated soils. Compared to the controls, C mineralization rates in salt-treated soils were approximately 13% lower at the 0-m transect and 48% lower at the 50-m transect in July 2011, and were about 20% lower at the 0-m transect and 28% lower at the 50-m transect in Nov 2011 (Fig. 2).
3.2 Soil Chemistry
Neither soil NH4+ nor NO3− concentrations were impacted by salt and NO3− experimental treatments, with the exception of NO3− which was significantly higher in salt-treated plots compared to the controls in July 2011 (p = 0.02). There were also no differences in NH4+ or NO3− between the two transects in July and Nov 2011. In 2011, soil inorganic N was dominated by NH4+. In 0–5 cm soils, inorganic N were 4.12 ± 0.63 mg kg−1 for NH4+ and 1.92 ± 0.25 mg kg−1 for NO3− in July 2011, and 7.48 ± 0.45 mg kg−1 NH4+and 2.05 ± 0.30 mg kg−1 NO3− in Nov 2011 (Fig. 3a). In June 2012, concentrations of NO3− were considerably higher than NH4+, and higher than in other sampling times. In June 2012, soil NO3− concentrations in 0–5 cm soils were significantly higher at the 0-m (35.66 ± 2.20 mg kg−1) than at the 50-m (29.40 ± 3.47 mg kg−1) transect (p = 0.04), whereas NH4+ concentrations did not differ between the transects (0-m, 6.04 ± 0.524 mg kg−1; 50-m, 4.56 ± 1.02 mg kg−1; p > 0.05). The 5–15-cm layer had significantly lower NH4+ concentrations than in 0–5 cm soils in all four sampling dates (p < 0.001 except June 2012 when p = 0.003), while NO3− concentrations were significantly lower only in June 2012 (p = 0.005, Fig. 3b).
Extractable soil NH4+ and NO3− concentrations at 0- and 50-m transects in July and November 2011, and June and October 2012: a 0–5 cm layer, b 5–15 cm layer. Different capital letters indicate significant differences between the transects, while non-capital letters indicate differences among the treatments (Control, N, and Salt)
The electrical conductivities of soils were elevated under experimental salt treatment in both transects in July 2011 (p < 0.001), November 2011 (p < 0.001), and June 2012 (p = 0.004), and the only exception was in October 2012 (p = 0.66) when just the 50-m transect was sampled (Table 2). Between the transects, in sub-plots not receiving experimental salt input (control and N plots), conductivities were significantly higher at the 0-m than at 50-m transect in July 2011 and June 2012 (p = 0.003 and p = 0.001, respectively) for both 0–5 cm and 5–15 cm soils, and in November 2011 higher for 5–15 cm soils only (p < 0.001). Salt treatment also increased soil conductivities significantly more in the 0–5-cm layer than 5–15-cm layer at all sampling dates (p < 0.001), at both 0- and 50-m transects.
Soil pH was significantly lower in salt-treated soils compared to controls in July 2011 (p = 0.02) and June 2012 (p = 0.02), but not in Nov 2011 or Oct 2012 (p > 0.05). Soil pH was not measurably impacted by treatment with NO3− (p > 0.05) (Table 2). Soil pH was generally higher at the 0-m transect (Table 2). There was a significant transect difference in 0–5 cm soils from June 2012 (p < 0.001), and 5–15 cm soils from July 2011 (p < 0.001), and June 2012 (p < 0.001). Soil pH was somewhat higher in 5–15 cm soils than 0–5 cm soils, and the difference was significant in June 2012 at both the 0-m (p < 0.001) and 50-m (p = 0.003) transects.
Soil moisture was not affected by NO3− treatment (p > 0.05) but was significantly higher under experimental salt treatment compared to N and control sub-plots in July 2011, at the 0-m (p < 0.001) and 50-m transects (p < 0.001) (Table 2). In the 0–5-cm layer, soil was wetter at the 0-m transect than at the 50-m transect in July 2011 (p = 0.001), November 2011 (p = 0.02), and June 2012 (p = 0.001). In the 5–15-cm layer, soil moisture did not differ between the transects.
Soil organic matter content was not affected by the experimental treatments with salt and NO3− (Table 2). However, organic matter of 0–5 cm soils was significantly higher at the 0-m transect than at the 50-m transect in July 2011 (p = 0.001), November 2011 (p = 0.01), and June 2012 (p < 0.001). Soil organic matter content averaged 9.04 ± 0.20 and 8.10 ± 0.14% at the 0- and 50-m transects, respectively, in July 2011, 9.56 ± 0.31 and 8.65 ± 0.14% in November 2011, and 9.46 ± 0.23 and 8.10 ± 0.21% in June 2012. It was 8.07 ± 0.18% in October 2012 (at 50-m transect only). In the 5–15-cm layer, soil organic matter did not differ between two transects (p > 0.05), averaged 5.57 ± 0.39, 5.49 ± 0.10, and 5.35 ± 0.11% in July 2011, November 2011, and June 2012, respectively. It was 5.24 ± 0.18% in October 2012 (at the 50-m transect only).
3.3 Soil Respiration
In situ soil respiration was measured from June through October 2012. The soil respiration data, which included both heterotrophic microbial respiration and plant root respiration, were from the 50-m transect only because the 0-m transect was lost due to highway expansion (see “Material and Methods”). Experimental salt input significantly lowed soil respiration (p < 0.001, Fig. 4). Soil respiration from salt-treated plots was 160 ± 25 mg CO2-C m−2 day−1, while it averaged 243 ± 39 mg CO2-C m−2 day−1 in N-treated plots, and 234 ± 38 mg CO2-C m−2 day−1 from the control. Soil respiration was generally higher when temperatures were warmer in the summer (June, July, and August, except the July 2 sampling) and lower in the fall (Fig. 4).
4 Discussion
We measured rates of C and N cycling, and common chemistry characteristics of soils from a roadside ecosystem. In this study, plots were experimentally treated with roadside pollutants of salt and NO3− to quantify the impacts of treatment exposure. Plots were treated on two transects: the 0-m transect immediately adjacent to the highway and the 50-m transect 50 m away from the highway, to explore the long-term road impacts. We did not find evidences of experimental nitrate treatment or long-term exposure to roadside pollutants on rates of N cycling, or extractable soil NH4+ or NO3−; however, there were measurable impacts of salt treatments on C mineralization, soil respiration and conductivities on soils in this roadside ecosystem.
4.1 Negative Impacts of Road Salt on Soil Carbon Mineralization, Soil Chemistry, and Soil Respiration
Road salt application can have major impact on roadside ecosystems. We found that rates of C mineralization were significantly lower in salt-treated experimental plots at both transects, which supported our hypothesis that salt inputs would negatively impact the soil microbial communities. However, in controls and N-treated plots, C mineralization rates did not differ between the 0-m and 50-m transects. We further found the reduction in C mineralization was more evident at the 50-m transect compared to the 0-m transect. Salt-treated soils had 38% less C mineralization at the 50-m transect than the 0-m transect in July 2011, while there was 19% less C mineralization at the 50-m transect in November 2011. This different impact could be due to different soil moisture and organic matter conditions between the two transects, which have been shown to affect soil microbial respiration (Bowden et al. 1998; Fierer et al. 2003). Or likely, soil microbial communities near the road may have evolved to be more resilient to the impacts of road salt, and other pollutants including inorganic N and metals.
In temperate climates where the use of deicing salt is common, it can accumulate in soils alongside roads (Bryson and Barker 2002; Cunningham et al. 2008; Findlay and Kelly 2011; Kincaid and Findlay 2009). Exposure to deicing salt alters the trajectories of roadside ecosystems by affecting soil processes and plant growth (Green and Cresser 2008; Green et al. 2008; Heintzman et al. 2015). Deicing salt has been shown to inhibit rates of nitrification and N mineralization. For example, McCormick and Wolfe (1980) showed that treatment of soils with salt inhibited rates of lab-measured nitrification and N mineralization. They also reported reduced CO2 emission from salt-treated soils, similar to what we reported in this paper. Contrarily, Green and Cresser (2008) found that salt-impacted roadsides had higher rates of nitrification and N mineralization. The soils from the Green and Cresser’s study also had higher pH measurements, possibly due to displacement of ions with Na+, and the higher pH might have stimulated rates of nitrification and N mineralization. In our study, we used both experimental salt treatment and compared transect difference related to the long-term road exposure, but we did not find any difference in nitrification or N mineralization rates (Table 1). Besides microbial communities, salt accumulation in soils negatively affects plant communities; another experiment at this site linked reduced plant growth in soils collected from the 0-m transect to plant tissue Na+ concentrations and soil Cl− concentrations, indicating impacts of deicing salt (Heintzman et al. 2015). Both soil microbial community and plant community can develop resistance to road pollutants; Brady et al. (2017) recently reviewed the evidence and called for more studies on the evolution of organism tolerance to the changing chemical environment.
The conductivities of salt-treated soils were all significantly elevated above control and N -treated soils, and were higher at the 0-m transect. Previous research on the fate of deicing salt has suggested that the majority of it ends up within 10 m of the roadside (Lundmark and Olofsson 2007). Conductivities were also significantly higher in 0–5 cm soils compared to 5–15 cm soils, suggesting retention of road salt in the upper layer of soil even after precipitation and leaching. Conductivities of salt-treated soils were generally higher in July 2011 and June 2012 compared to November 2011 and October 2012, which may have been due to less precipitation and infiltration of salt prior to the soil collections in summer months.
Soil pH did not significantly differ between treatments, and soil pH was found to be generally higher at the 0-m transect compared to the 50-m transect. Green and Cresser (2008) provide evidence that salt in roadside soil can raise pH due to Na+ occupying more exchange sites in the soil. Although in our study, we did not find higher pH in salt-treated soils, the long-term impacts of repeated road-salting could have caused Na+ to displace other cations in the soil, increasing pH. However, we do not currently have experimental data to support that notion.
We found significant experimental evidence that salt treatment negatively impacted soil in situ CO2 fluxes measured in 2012, a result in line with the lab-measured soil C mineralization in 2011. At the 50-m transect, emissions of CO2-C were 33% lower than emissions from N-treated and control soils. Only 2 days (June 22 and July 2) of gas sampling were completed before the 0-m transect was lost to demolition for expansion of I-81. From the 2 days of data we obtained, CO2-C emissions were 22% lower from salt-treated soils than from N-treated and control soils at the 0-m transect, a lesser reduction compared to the 50-m transect. Additionally, CO2-C fluxes were generally higher during summer months when ambient temperatures were higher; this was expected since microbial activity tends to be elevated by warmer temperatures (Bowden et al. 1998; Fierer et al. 2003; Mo et al. 2007).
4.2 Limited Impacts of Nitrate Addition on Soil Net Nitrification and N Mineralization Rates
We hypothesized that N treatments would increase rates of nitrification and N mineralization because it is typically a limiting nutrient in terrestrial ecosystems. We found no treatment effect in this study. Nitrogen applications have been reported as stimulating to soil microbial communities, increasing their rates of nitrification and N mineralization, and elevating available soil N (Magill et al. 2000; Phoenix et al. 2012; Pouyat and Turechek 2001). For example, in the Harvard Forest, MA, Magill et al. (2000) found that N additions of low (5 g m−2 year−1) and high (15 g m−2 year−1) contents increased rates of N mineralization, and high N input (15 g m−2 year−1) increased rates of nitrification of soils in pine and hardwood stands. Pouyat and Turechek (2001) compared rates of nitrification and N mineralization in urban, suburban and rural soils. They showed that with more mineralizable N in the top 10 cm of suburban and urban soils, rates of both nitrification and N mineralization were higher than in reference rural sites (Pouyat and Turechek 2001). However, in our experiment, we did not observe changes in rates of nitrification or N mineralization in N-treated plots, and our experimental treatments of N may have been too low (or for too short a time) to have a discernable impact.
Heavily trafficked roads have been reported to have high fluxes of N from automobiles (Bettez et al. 2013; Padgett et al. 1999). We assumed the plots at the 0-m transect to have exposure to more N inputs than at the 50-m transect, which could enhance nitrification and N mineralization. There were no transect differences in nitrification and N mineralization in July and November 2011, but rates of net nitrification and N mineralization were significantly elevated at the 0-m transect above the 50-m transect in June 2012. The warmer June temperature and significantly higher soil moisture at 0-m transect (Table 2) combined with less plant nutrient uptake (before the peak growing season) might have caused higher rates of nitrification and N mineralization here. There was also a significantly higher level of soil NO3− found in June 2012 (Fig. 3). Higher soil NO3− and nitrification rates in spring and early summer, before peak plant growth, were also reported in forests along an urban-to-rural gradient (Zhu and Carreiro 2004).
Extractable soil inorganic N concentrations were not impacted by the experimental treatments of N or salt (Fig. 3a, b). While the 0-m transect was likely exposed to more inorganic N due to atmospheric deposition derived from vehicles, we did not find differences in extractable N between the 0-m and 50-m transects except in June 2012 (Fig. 3a, b). During this study, June 2012 had the highest NO3− concentrations; extractable soil inorganic N was dominated by NO3−, with concentrations that were 10-15× higher than concentrations that were measured in other three sampling times. The drastic increase in extractable NO3− in June 2012 is probably a result of the time of the soil collection. The June 2012 sampling was earlier in the growing season than planned (we did that in compliance with unexpected road construction at the site, see “Material and Methods”). Vegetation was scant at this point. At the time of the June 2012 soil sampling, the vegetative cover of plots ranged from 0 to 5%. This would have resulted in less plant uptake of N, which along with the high rates of nitrification and N mineralization, explain the high concentrations of soil NO3− (Fig. 3a). In mid-summer, vegetation cover grown to 50–100%, which is probably why we measured lower inorganic N concentrations in soils from July 2011. Additionally, the June 2012 soil collection was the only time that NO3− was significantly higher in the 0–5 cm soils than the 5–15 cm soils. The June 2012 NH4+ concentrations were similar to the other three sampling times.
5 Conclusions
We did not find impacts of experimental treatments or long-term exposure to road pollutants on rates of N cycling, or extractable N. However, there were seasonal differences in rates of nitrification and N mineralization. Salt treatments induced measurable impacts on C mineralization, respiration and conductivities on soils in this roadside ecosystem. Salt treatments negatively impacted C mineralization and in situ soil respiration. This effect was less acute in soils from the 0-m transect, indicating that soil microbial communities near roads might have evolved to withstand the impacts of deicing salt. Road salt accumulation in soils was evident at the 0-m transect when compared to the 50-m transect, which could affect the long-term trajectory of the plant community and soil processes. The impacts of road salt have also been shown to extend beyond roadside ecosystems, including elevating the salt concentrations of freshwater resources (Kaushal et al. 2005). Best management practices (BMPs) are needed for the attenuation of the impacts of deicing salt on roadside environments, and should be implemented to maintain the health of roadside and other urban ecosystems (Fay and Shi 2012).
References
Abdel-Rahman, A. A. (1998). On the emissions from internal-combustion engines: A review. International Journal of Energy Research, 22, 483–513.
Aber, J. D., Goodale, C. L., Ollinger, S. V., Smith, M. L., Magill, A. H., Martin, M. E., Hallet, R. A., & Stoddard, J. L. (2003). Is nitrogen deposition altering the nitrogen status of northeastern forests? Bioscence, 53(4), 375–389.
Angold, P. G. (1997). The impact of a road upon adjacent heathland vegetation: Effects on plant species composition. Journal of Applied Ecology, 34, 409–417.
Asman, W. A. H., Sutton, M. A., & Schjorring, J. K. (1998). Ammonia: Emission, atmospheric transport and deposition. New Phytologist, 139, 27–48.
Bettez, N. D., Marino, R., Howarth, R. W., & Davidson, E. A. (2013). Roads as nitrogen deposition hot spots. Biogeochemistry, 114, 149–163.
Bignal, K. L., Ashmore M. R., Headley, A. D., Stewart, K., Weigert, K. (2007). Ecological impacts of air pollution from road transport on local vegetation. Applied Geochemistry, 22, 1265–1271.
Blomqvist, G., & Johansson, E. L. (1999). Airborne spreading and deposition of de-icing salt-a case study. Science of the Total Environment, 235, 161–168.
Bowden, R. D., Newkirk, K. M., & Rullo, G. M. (1998). Carbon dioxide and methane fluxes by a forest soil under laboratory-controlled moisture and temperature conditions. Soil Biology and Biochemistry, 30(12), 1591–1597.
Brady, S. P., Monosson, E., Matson, C., Bickham, L. (2017). Evolutionary toxicology: Toward a unified understanding of life’s response to toxic chemicals. Evolutionary Applications: Special Issue, in press.
Bryson, G. M. & Barker, A. V. (2002). Sodium accumulation in soils and plants along Massachusetts roadsides. Communications in Soil Science and Plant Analysis, 33(1&2), 67–78.
Butler, T. J., Likens, G. E., Vermeylen, F. M., & Stunder, B. J. B. (2003). The relation between NOx emissions and precipitation NO3 − in the eastern USA. Atmospheric Environment, 37, 2093–2104.
Cape, J. N., Tang, Y. S., van Dijk, N., Love, L., Sutton, M. A., & Palmer, S. C. F. (2004). Concentrations of ammonia and nitrogen dioxide at roadside verges, and their contribution to nitrogen deposition. Environmental Pollution, 132, 469–478.
Compton, J. E., Watrud, L. S., Porteous, L. A., & DeGrood, S. (2004). Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard forest. Forest Ecology and Management, 196, 143–158.
Councell, T. B., Duckenfield, K. U., Landa, E. R., & Callender, E. (2004). Tire-wear particles as a source of zinc to the environment. Environmental Science and Technology, 38, 4206–4214.
Cunningham, M. A., Snyder, E., Yonkin D., Ross, M., Elsen, T. (2008). Accumulation of deicing salts in soils in an urban environment. Urban Ecosystems, 11, 17–31.
Davidson, E. A., Savage, K. E., Bettez, N. D., Marino, R., & Howarth, R. W. (2009). Nitrogen in runoff from residential roads in a coastal area. Water, Air, and Soil Pollution. https://doi.org/10.1007/s11270-009-0218-2.
Fay, L., & Shi, X. (2012). Environmental impacts of chemicals for snow and ice control: State of the knowledge. Water, Air, and Soil Pollution, 223, 2751–2770.
Fierer, N., Allen, A. S., Schimel, J. P., & Holden, P. A. (2003). Controls on microbial CO2 production: A comparison of surface and subsurface soil horizons. Global Change Biology, 9, 1322–1332.
Findlay, S. E. G., & Kelly, V. R. (2011). Emerging indirect and long-term road salt effects on ecosystems. Annals of the New York Academy of Sciences, 1223, 58–68.
Green, S. M., & Cresser, M. S. (2008). Nitrogen cycle disruption through the application of de-icing salts on upland highways. Water, Air, and Soil Pollution, 188, 139–153.
Green, S. M., Machin, R., & Cresser, M. S. (2008). Effect of long-term changes in soil chemistry induced by road salt applications on N-transformations in roadside soils. Environmental Pollution, 152, 20–31.
Heeb, N. V., Forss, A. M., Bruhlmann, S., Luscher, R., Saxer, C. J., & Hug, P. (2006). Three-way catalyst induced formation of ammonia—Velocity- and acceleration-dependent emission factors. Atmospheric Environment, 40, 5986–5997.
Heintzman, R. L., Titus, J. E., & Zhu, W. X. (2015). Effects of roadside deposition on growth and pollutant accumulation by willow (Salix miyabeana). Water, Air, and Soil Pollution, 226, 11.
Hjortenkrans, D. S. T., Bergback, B. G., & Haggerud, A. V. (2008). Transversal immission patterns and leachability of heavy metals in road side soils. Journal of Environmental Monitoring, 10, 739–746.
Johansson, C., Norman, M., & Burman, L. (2009). Road traffic emission factors for heavy metals. Atmospheric Environment, 43, 4681–4688.
Kaushal, S. S., Groffman, P. M., Likens, G. E., Belt, K. T., Stack, W. P., Kelly, V. R., Band, L. E., & Fisher, G. T. (2005). Increased salinization of fresh water in the northeastern United States. Proceedings of the National Academy of Sciences, 102(38), 13517–13520.
Kaye, J. P., Groffman, P. M., Grimm, N. B., Baker, L. A., & Pouyat, R. V. (2006). A distinct urban biogeochemistry? Trends in Ecology and Evolution, 21(4), 192–199.
Kelly, V. R., Lovett, G. M., Weathers, K. C., Findlay, S. E. G., Strayer, D. L., Burns, D. J., & Likens, G. E. (2008). Long-term sodium chloride retention in a rural watershed: Legacy effects of road salt on streamwater concentration. Environmental Science and Technology, 42(2), 410–415.
Kincaid, D. W., & Findlay, S. E. G. (2009). Sources of elevated chloride in local streams: Groundwater and soils as potential reservoirs. Water, Air, and Soil Pollution, 203, 335–342.
Lovett, G. M., Traynor, M. M., Pouyat, R. V., Carreiro, M. M., Zhu, W.-X., & Baxter, J. W. (2000). Atmospheric deposition to oak forests along an urban-rural gradient. Environmental Science and Technology, 34, 4294–4300.
Lundmark, A., & Olofsson, B. (2007). Chloride deposition and distribution in soils along a deiced highway—Assessment using different methods of measurement. Water, Air, and Soil Pollution, 182, 173–185.
Magill, A. H., Aber, J. D., Hendricks, J. J., Bowden, R. D., Melillo, J. M., & Steudler, P. A. (1997). Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecological Applications, 7(2), 402–415.
Magill, A. H., Aber, J. D., Berntson, G. M., McDowell, W. H., Nadelhoffer, K. J., Melillo, J. M., & Steudler, P. (2000). Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosystems, 3, 238–253.
McCormick, R. W., & Wolfe, D. C. (1980). Effect of sodium chloride on CO2 evolution, ammonification and nitrification in a Sassafras sandy loam. Soil Biology and Biogeochemistry, 12, 153–157.
Mo, J., Zhang, W., Zhu, W. X., Gundersen, P., Fang, Y., Li, D., & Wang, H. (2007). Nitrogen addition reduces soil respiration in mature tropical forest in southern China. Global Change Biology, 14, 1–10.
New York State Department of Transportation (2010). 2010 Traffic volume report. https://www.dot.ny.gov/divisions/engineering/technical-services/hds-respository/Traffic%20Volume%20Report%202010.pdf. Accessed 30 August 2014.
Padgett, P. E., Allen, E. B., Bytnerowicz, A., & Minich, R. A. (1999). Changes in soil inorganic nitrogenous pollutants in southern California. Atmospheric Environment, 33, 769–781.
Phoenix, G., Emmett, B., Britton, A., Caporn, S., Dise, N., Helliwell, R., Jones, L., Leake, J., Leith, I., Sheppard, L., Sowerby, A., Pilkington, M., Rowe, E., Ashmore, M., & Power, S. (2012). Impacts of atmospheric nitrogen deposition: Responses of multiple plant and soil parameters across contrasting ecosystems in long-term field experiments. Global Change Biology, 18, 1197–1215.
Pouyat, R. V., & Turechek, W. W. (2001). Short- and long-term effect of site factors on net N-mineralization and nitrification rates along an urban-rural gradient. Urban Ecosystems, 5, 159–178.
Pouyat, R. V., Szlavecz, K., Yesilonis, I. D., Groffman, P. M., & Schwarz, K. (2010). Chemical, physical and biological characteristics of urban soils. In J. Aitkenhead-Peterson & A. Volder (Eds.), Urban ecosystem ecology (pp. 119–152). Madison: American Society of Agronomy.
R Core Team. 2016. R: A language and environment for statistical Computing R Foundation for Statistical Computing, Vienna.
Redling, K., Elliot, E., Bain, D., & Sherwell, J. (2013). Highway contributions to reactive nitrogen deposition: Tracing the fate of vehicular NOx using stable isotopes and plant biomonitors. Biogeochemistry, 116, 261–274.
Scott, T. J., Craig, S. C., & Zhu, W. X. (2011). The effects of simulated nitrogen and salt deposition on nitrogen mineralization and soil chemistry in a roadside ecosystem. Bulletin of the New Jersey Academy of Sciences, 56(2), 9–11.
United States Department of Transportation, Federal Highway Administration (2013). Highway statistics series: Highway statistics 2013, Table HM-12. http://www.fhwa.dot.gov/policyinformation/statistics.cfm. Accessed September 30, 2015.
Yesilonis, I. D., Pouyat, R. V., & Neerchal, N. K. (2008). Spatial distribution of metals in soils in Baltimore, Maryland: Role of native parent material, proximity to major roads, housing age and screening guidelines. Environmental Pollution, 156, 723–731.
Zhu, W. X., & Carreiro, M. M. (2004). Temporal and spatial variations in nitrogen cycling in deciduous forest ecosystems along and urban-rural gradient. Soil Biology and Biochemistry, 36, 267–278.
Acknowledgements
We would like to thank Tim Scott, Megan Larson, Jonathan Schmitkons, Joseph Graney, John Titus, and other students and faculty from the Center for Integrated Watershed Studies (CIWS) at Binghamton University for the help and support of this project, and funding support from the Wallace Research Foundation. We would also like to thank the highly professional review that had improved the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Craig, S., Zhu, W. Impacts of Deicing Salt and Nitrogen Addition on Soil Nitrogen and Carbon Cycling in a Roadside Ecosystem. Water Air Soil Pollut 229, 187 (2018). https://doi.org/10.1007/s11270-018-3838-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3838-6