Abstract
In this study, we constructed aquatic microcosms, including four experimental groups, to simulate eutrophic lakes. Using polyurethane sponges as artificial substrates to support periphyton, we conducted a systematic study of the effect of periphyton on the water quality of eutrophic lakes by monitoring water-quality indexes including TN, TP, and NTU. The results show that periphyton can effectively degrade N and P in these bodies of water, reduce NTU and the concentration of Chl-a, and restore the aquatic environment. Periphyton can also promote the formation of the DO-Chl-a-NTU-TN-TP synergistic system, which can provide a basis for the comprehensive management of eutrophic lakes. Periphyton can also effectively improve the water quality of eutrophic lakes, and its positive impact on lake ecosystems cannot be ignored during the treatment of eutrophic lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Periphyton is comprised of green algae, diatoms, bacteria, and some smaller invertebrates (Azim et al. 2005), and is an important part of lake ecosystems. It can remove nutrients, toxic substances, and metals from bodies of water (Hill et al. 2000; Vymazal 1988) and is also a good indicator of water quality (Wu 2016). Meanwhile, it also plays an important role in the bio-productivity of lake ecosystems, especially those with sufficient light (Nelson et al. 1999; Wolfstein et al. 2000). The eutrophication of lakes will increase the amount of planktonic algae in lake water and change nutrient structures and environmental conditions, especially light conditions. Therefore, planktonic algae will inhibit the growth of periphyton and replace periphyton to become the main primary producer in lake ecosystems, degrading lake water and ecosystem quality (Zhiqiang et al. 2017). Methods of controlling conditions in eutrophic lakes by reducing the amount of planktonic algae are becoming more effective, but inadequacies remain. Physical methods including ultrasound (Park et al. 2017), UV irradiation (Alam et al. 2001), and membrane filtration techniques (Zhao et al. 2017) are eco-friendly but are high-cost and complex. Chemical methods including the addition of metals (such as copper) (Magdaleno 2014), photosensitizers (such as hydrogen peroxide), and herbicides (such as diuron) are highly efficient and low-cost, but the associated toxicity can be detrimental to other species. Biological methods including the use of aquatic plant techniques (Zuo et al. 2014) are user friendly and economical but include the risk of additional eutrophication. Since the 1980s, ecological restoration, using the self-purification ability of ecosystems to improve environmental health and sustainability, has a place in the control of eutrophic lakes because of its green and efficient characteristics. Periphyton plays an important role in restoration activities (Gaiser 2009). There are many reports on the effect of periphyton on water quality: Szlauer’s study shows that periphyton can improve the self-purification ability of eutrophic lakes (Szlauer and Szlauer 1996); Mariñelarena found that periphyton can rapidly reduce the concentration of P (phosphorus) in an artificial stream containing agricultural sewage (Mariñelarena and Di Giorgi 2001); and Jöbgen’s result illustrates that periphyton can remove nutrients from water bodies and retain them in their tissues (Jöbgen et al. 2004). These results lay a foundation for the application of periphyton in the control of eutrophic lakes. After consulting the literature, we found that there are many studies on the removal of phosphorus, heavy metals, and other pollutants but less published work on the systematic study of the effect of periphyton on eutrophic aquatic systems. Lake water is an important biochemical reaction site in this aquatic ecosystem and provides a medium for its material flow. Therefore, there must be some synergy and antagonism within the aquatic environment. Careful study of the relationship between water and its components can provide reference and a decision basis for the management of eutrophic lakes. We simulated eutrophic lakes using aquatic microcosms and added artificial substrates to provide space for the growth of periphyton and record the change in water-quality indexes during the experimental period. We then explored the effect of periphyton on the water quality of eutrophic lakes and the interaction among the water-quality indexes in order to provide reference and a decision basis for the management of eutrophic lakes.
2 Methods and Materials
2.1 Experimental Design
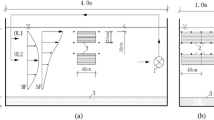
This experiment was carried out at Wuhan University, Wuhan, China (114° 21′ E, 30° 32′ N, 34 m above the sea level), from September 8, 2017 to October 8, 2017 (a total of 31 days). We built a medium-sized aquatic microcosm to simulate eutrophic lakes that comprised four experimental groups. Each group consisted of a concrete experiment pool (3 m × 1 m × 2 m), water pump, flow meter, and several PVC pipes (Fig. 1). We also put a deposition tank, geometrically similar to the experimental group tank, next to the aquatic microcosm (0.3 m × 0.1 m × 0.2 m) to measure atmospheric deposition of N (nitrogen) and P (phosphorus).
The artificial substrate in the experiment is a polyurethane sponge that was blasted with oxygen, with a density of 0.92 g/cm3 and a specific surface area of 40 cm2/cm3. The artificial substrate was in the form of a cube with 0.45 m edges, attached to a bar frame and placed into the experimental group. The placement is shown in Table 1. On September 7, 2017, we added pure water (1.5 m in depth, without nutrient) and clear lake water (0.3 m in depth) taken from Donghu Lake, Wuhan, which served as the microbial inoculant, into each experimental group. The total volume was 5.4 m3. A total 3 L of ultra-pure water was added to the deposition tank. To each experimental group, 100 g of glucose and 250 mL of nutrient solution containing KH2PO4 (119.5 g/L) and NH4Cl (104 g/L) was added to simulate the aqueous composition of eutrophic lakes. The water pump of each experimental group was turned on, and the initial value of TN (total nitrogen), TP (total phosphorus), NTU (nephelometric turbidity unit), and WCR (water circulation rate) of each experimental group was measured (Table 1).
By comparing the concentration of the initial values and the design values of TN and TP, we found that all of the experimental groups reached the requirements of the design. The WCR of each group exceeded 5.4 m3/day (5.4 m3 is the total water of each experimental group). The water of each group can be regarded as completely mixed. According to Ling Ding’s research on hydrodynamic conditions (Ling et al. 2005), we eliminated the effect of low fluidity on water-quality indexes. All initial NTUs were less than 1, so we eliminated the effect of impurities on initial water quality.
2.2 Sampling and Analyzing
This experiment began on September 8, 2017 (day 1). Every 2 days, we used 1.5-L glass samplers to collect water samples from 20 cm below the surface of each experimental group. At the time of sampling, the HACH HQ30D water-quality measuring instrument and the XIN RUI portable turbidimeter instrument were used to measure DO, water temperature, and NTU for each experimental group. The collected water samples were divided into three aliquots of 500 mL each. A portion of each was filtered using Whatman GF/F nucleopore filter paper, and then Chl-a (chlorophyll a) was leached from the filter paper by 90% acetone. Chl-a concentration was measured, and the filtered water samples were measured for TDP (total dissolved phosphorus), TDN (total dissolved nitrogen), SRP (soluble reactive phosphorus), NO3− (nitrate ion), and NH4+ (ammonium ion). Another sample was used to measure TN (total nitrogen) and TP (total phosphorus), and the last sample was retained as a spare. During this experiment, we used a spectrophotometric method to measure the concentration of Chl-a and NO3−. The alkaline potassium peroxodisulfate spectrophotometric method was used to measured TN and TDN, while the concentration of NH4+ was measured by the Nessler reagent spectrophotometric method. The concentration of TP, TDP, and SRP was measured using the ammonium molybdate spectrophotometric method.
In this experiment, the concentration of PP (particulate phosphorus) was calculated using Eq. (1), and the concentration of PN (particulate nitrogen) was calculated using Eq. (2):
This experiment was ended before a large-scale natural precipitation event in order to eliminate the effect of precipitation on water quality. There was no external runoff input in any of the experimental groups. To eliminate the effect of the deposition of atmospheric N and P on the water-quality indexes, the concentration of N and P in each group was corrected according to Eq. (3):
where Md is the average daily deposition of each experimental group, Vp is volume of water in each experimental group, n is the number of days of experiment, C′ is the concentration of the substance to be measured, and C is the corrected concentration.
We used 0-stage and 1-stage dynamic equations to fit the change of nutrient concentration during the experiment as shown in Eqs. (4) and (5):
where C is the concentration of the substance to be measured, k is the degradation coefficient, t is time, and C0 is the initial concentration of the substance to be measured.
Equation (6) was used to standardize each monitored water-quality index. Equation 7 was used to standardize the water-quality index, calculate the correlation coefficient, and list the correlation coefficient matrix. Then, SPSS was used to analyze water-quality indexes.
where i = 1, 2, 3, …, n(n is number of samples), j = 1, 2, 3, …, m(mis number of variate), Xij is the initial data, \( \overline{X_j} \) is the average value of j variate in the sample, Sj is the standard deviation of variate, and Zij is standardized data \( {\overline{Z}}_i=\frac{1}{m}\sum \limits_k^m{Z}_{ik}\; and\;{\overline{Z}}_j=\frac{1}{m}\sum \limits_{k-1}^n{Z}_{jk} \).
All data and figures in this paper were processed using Excel (version 2016), Origin pro (version 8), Rhinoceros (version 5), and SPSS (version 19.0).
3 Results
On day 31, we took water samples from the deposition tank to measure atmospheric N and P settlement during the experiment. We determined that after conversion, the daily average settlement of N in each group was 19.7 mg/day and that of P was 0.34 mg/day. To eliminate the effect of atmospheric sedimentation on water quality, we used Eq. (3) to correct the N and P data. The average temperature of the water was 28.1 °C in this experiment, and there was no extreme weather in Wuhan that would have been bad for the growth of algae (in this paper, algae refers to all the microbial producers with chlorophyll). As a result, the influence of atmospheric settlement and meteorological factors in this experiment was eliminated.
3.1 Distributions of Algae
We took algae samples from the water bodies and the artificial substrates and observed them on day 31. The distributions of algae are shown in Fig. 2.
3.2 Changes of DO and NTU
The concentration of DO in each group decreased from day 1 and reached a minimum value on day 5. It then rose slowly and was slightly higher at the end of experiment than the initial value. The slopes of the fitted curves for DO concentration of each group increased with the number of artificial substrates in the early stage (after day 5, except TAG). The NTU of each group (except ZAG) was between 1 and 2 on day 1 and exhibited a downward trend up to day 23. It then rose slightly until the end of the experiment. The NTU of ZAG was different from that of other groups; it gradually rose starting on day 1 and began to increase sharply on day 25 (small pieces of water blooms were found in ZAG on day 25), with a peak of 16.42 on day 29 (Fig. 3).
3.3 Changes in Chl-a
During the experiment, the concentration of Chl-a of each group was as follows: ZAG = 92.6 mg/L, TAG = 2.53 mg/L, FAG = 1.98 mg/L, and EAG = 1.92 mg/L (Fig. 4a). To show the changes in Chl-a in each group more clearly, each group of data was plotted on a figure (Fig. 4b). We found that the concentration of Chl-a of ZAG and TAG changed dramatically in the late stage of the experiment (after day 21), similar to the planktonic algae.
3.4 Changes in Particulate Nutrient
The changes in PN and PP in each group can be calculated by Eqs. (1) and (2), as shown in Fig. 5.
The PP and PN of all the experimental groups decreased substantially prior to day 7 and then tended to be stable until the end of this experiment. Changes to the groups with artificial substrates were more subtle.
3.5 Changes in N Series Indexes
The TN concentration of each group (except EAG) reached the maximum value on day 1 and then decreased slowly. TAG, FAG, and EAG showed large fluctuations in TN concentration in the middle to late stages of the experiment (Fig. 6). The decrease in TN concentration of each group was 0.37 mg/L (ZAG), 0.40 mg/L (TAG), 0.55 mg/L (FAG), and 1.14 mg/L (EAG) at the end of the experiment. The plotted results of changes in TN concentration successively satisfied the 0-stage dynamic equation (R2 ≥ 0.71). The concentration of NH4+ in each group reached the maximum value on day 1 and then declined sharply and reached a lower value on day 3. The change in NO3− concentration in each group significantly lagged behind NH4+, by approximately 2 days (except EAG), increased rapidly from day 3, and reached a maximum on day 5 and then decreased slowly (Fig. 6).
3.6 Changes of P Series Indexes
The concentration of TP was at its maximum on day 1, declined irreversibly and sharply until day 12, and then continued to decline slowly (Fig. 7). The decrease in TP concentration of each group was 0.55 mg/L (ZAG), 0.58 mg/L (TAG), 0.75 mg/L (FAG), and 0.85 mg/L (EAG) at the end of the experiment. In this experiment, the trend of the changes in TP and SRP concentrations was the same and almost coincided at the late stage of experiment. The linear fitting result of TP successively satisfies the 1-stage dynamic equation (R2 ≥ 0.75).
3.7 Changes in N/P Ratio
The initial N/P ratio of each group was approximately 3 (Fig. 8a). As the experiment progressed, the N/P ratio of each group tended to increase. However, the final value of the N/P ratio of each group was less than 7, and the changes to the N/P ratio were ZAG = 0.95, TAG = 1.44, FAG = 2.72, and EAG = 3.54. To show the changes to the N/P ratio more clearly, data were plotted in a contour graph (Fig. 8b). We found that the N/P ratio of FAG and EAG increased dramatically in the late stage of this experiment (after day 21).
3.8 Cluster Analyze of Water-Quality Indexes
Based on the water-quality data of ZAG, we used that of EAG (the change is more obvious) to calculate the variations in water-quality indexes during this experiment (we did not include SRP because it is only related to TP). After cluster analysis, we found that the water-quality indexes can be divided into three categories: DO and NH4+; Chl-a and NTU; and TN, TP, and NO3− (Fig. 9).
4 Discussion
4.1 Inhibitory Effects of Periphyton on Planktonic Algae
At the start of this experiment (day 1), the concentration of DO in each group was between 6 and 8 mg/L (Fig. 3). According to Bensen’s research (Benson and Krause 1984), the concentration of DO in each group was at a high level and eliminated the influence of hypoxia on the growth of algae during the early stages of the experiment. In the first half of the experiment, the NTU of each group first showed a decline, fluctuated slowly, and then rose (except for ZAG, which showed a slight fluctuation on day 5). The concentration of DO in all groups reached a minimum on day 5. Because there was no sediment and runoff input in this experiment, consistent with Shou-bing Wang’s research (Shou-bing et al. 2014), we found that there was a positive correlation between NTU and the amount of planktonic algae, which fits well with the NTU changes in ZAG, while in the other three groups, the decline of NTU may have been related to the retention of nutrients in the periphyton (Adey et al. 1993). In combination with Figs. 3 and 4b, the changes in the concentration of Chl-a in each group coincided with the changes in NTU, which support the above viewpoint (NTU and the number of planktonic algae). In the middle stage of this experiment (about day 17), there was a moderate fluctuation of NTU in each group (except TAG) combined with a decrease in the concentration of Chl-a in each group in Fig. 4b (except TAG). According to Gejun Chen’s research (Chen et al. 2013), this phenomenon was related to the succession of the algal community caused by the change in nutrient concentration in the water body of the experimental groups. The NTU of each group reached a maximum value on day 25 and then decreased slowly. The concentration of Chl-a showed the same trend that was related to the decline of the algal community after the concentration of algae reached the environmental capacity. At the end of this experiment, the NTU and the concentration of Chl-a of ZAG were much greater than those of the other three groups. This showed that periphyton has the effect of inhibiting the growth of planktonic algae in the water bodies (both NTU and the concentration of Chl-a are closely related to the number of planktonic algae). The changes in NTU and the concentration of Chl-a in TAG, FAG, and EAG were more stable with the increase in the amount of periphyton (TAG<FAG<EAG), but the final value of NTU and the concentration of Chl-a was independent of the amount of periphyton.
4.2 Degradation Effect of Periphyton on N and P
According to Fig. 5, the concentration of PN and PP in each group reached the minimum value (near 0) on day 5 and then showed a slight fluctuation until the end of this experiment. Thus, we eliminated the influence of particulate nutrients on water quality. The concentration of NH4+ of each group showed a large-scale decline in the early stage of the experiment (day 5) with the reduction in each group about equal to the increment of NO3− and with a time difference of 3 days between them (Fig. 6). Meanwhile, the DO concentration of each group showed a downward trend and reached its minimum value (Fig. 3). According to the research of Junzhuo Liu (Liu et al. 2017), we suggest that this phenomenon may be related to the oxidation of NH4+ and its incomplete oxidation products NO2− to NO3− associated with the nitrification by nitrifying bacteria. During the whole experiment, changes in the concentration of NO3− were not obvious. This condition may be associated with the dominant algae in the experimental group, which were associated with other forms of nitrogen as a nitrogen source. The concentration of NO3− of each group was independent of the amount of periphyton in this experiment. During the entire period of the experiment, the concentration of TN in each group decreased slowly, and the degree of TN fluctuation of the experimental group containing artificial substrates was larger (Fig. 6). According to Linlin Bao’s research, the fluctuation of TN is related to the N cycle in the water body (Bao et al. 2015) because when periphyton (the main producers of the experimental groups containing artificial substrates were periphyton) died, the remains were widely distributed on the artificial substrates, and the decomposer needed sufficient time to decompose the N containing organic matter. At the end of this experiment, the concentration of TN in the ZAG was higher than in the other three groups, indicating that periphyton could effectively degrade N in water bodies. Compared with the three experimental groups containing artificial substrates, we found that the amount of periphyton was positively correlated with the degradation ability of N in the water bodies. The concentration of TP and SRP in each group changed gradually during this experiment (Fig. 7). According to Xing-yong Xie’s research (Xie et al. 2011), this shows that all the other forms of P in each group were transformed into SRP for biochemical reaction during this experiment. The concentration of TP in each group showed an irreversible slow decline during this experiment that may be related to the properties of P being easily adsorbed onto iron hydroxide and organic particles (mainly algae in this paper) (Mortimer 1942). At the end of this experiment, the concentrations of TP and SRP in the ZAG were significantly greater than those of the other three groups, indicating that periphyton had a larger degradation effect on P in water bodies and that the degradation of P was sensitive to the amount of periphyton, increasing with an increase in the quantity of periphyton.
4.3 Restoration Effect of Periphyton to Water Environment
At the beginning of this experiment, we found that the initial N/P ratio of each group was very low (approximately 3) (Fig. 8a). According to Ling Sun’s research (Ling et al. 2006), the water body of each group was in a state of N restriction. Simultaneously, periphyton dominated the community, growing substantially because it is mainly composed of Cyanobacteria that has a nitrogen-fixing ability and is insensitive to the concentration of N in water bodies. During the whole experiment, the N/P ratio of all groups showed an upward trend (Fig. 8a). Because of the small changes in the concentration of TN during this experiment, and in consideration of the input of external N, mainly the nitrogen fixation by algae, we believe that the increase in N/P ratio mainly depends on the degradation of P by periphyton. This shows that periphyton can effectively improve the nutrient structure and repair an unbalanced aqueous environment, and this effect is positively correlated with the amount of periphyton (Fig. 8b). At the end of this experiment, the increment of the concentration of DO in the ZAG was 2.38 mg/L, and the other three groups were approximately 3.55 mg/L (Fig. 3). This shows that periphyton can significantly increase the DO concentration of a body of water by photosynthetic activity, and this process will increase the oxidation-reduction potential of water and further reduce the concentration of sulfur, methane, ammonia nitrogen, and other strong reducing substances, thereby improving water quality. Additionally, the amount of periphyton was only related to the change in rate of the concentration of DO and does not alter the final conditions associated with restoration of the aqueous environment.
4.4 Cluster Analysis Results of Water-Quality Indexes
According to Fig. 9, the analysis of the three categories is as follows. First, based on Figs. 3 and 6, the system of DO and NH4+ showed a dramatic decrease in the first 5 days of this experiment because in this period of time, periphyton was in a growth stage and consumed oxygen and nutrients. After day 5, the concentration of NH4+ reached its lowest value, while the concentration of DO showed a slight increase until the end of this experiment. Second, from the above analysis of the Chl-a and NTU system, we know that the changes in the concentration of Chl-a and NTU were the same during the whole experiment. This is because they both have a positive correlation to the concentration of the planktonic algae, and the growth of periphyton inhibits the amount of planktonic algae. Third, based on the results as shown in Figs. 6 and 7, the TN, TP, and NO3− system existed in a state of decline during the entire period of the experiment. We found that periphyton displays a variety of beneficial effects on the lake ecosystem at different stages of this experiment and exerted synergistic regulatory control on DO, NH4+, Chl-a, NTU, TN, TP, and NO3−. Combined, these factors establish a DO-Chl-a-NTU-TN-TP coordination regulation system (except for NO3− because it is one of the components in TN) in eutrophic lakes, which provides a basis for the comprehensive management of eutrophic lakes.
5 Conclusion
This experiment systematically evaluated the effect of periphyton on the water quality of eutrophic lakes. Based on our findings, periphyton can effectively degrade N and P and reduce NTU and the concentration of Chl-a in eutrophic lakes; however, the changes to water-quality indexes were not sensitive to the amount of periphyton (except for the degradation of P that was positively correlated with the amount of periphyton). Further, periphyton can promote the formation of a TN, TP, Chl-a, and NTU synergistic system in eutrophic lakes that provides a theoretical basis for the management of eutrophic lakes. Overall, periphyton increased the self-purification ability of eutrophic lakes, and its positive impact on lake water quality and lake ecosystem cannot be ignored during the treatment of eutrophic lakes.
References
Adey, W., Luckett, C., & Jensen, K. (1993). Phosphorus removal from natural waters using controlled algal production. Restoration Ecology, 1, 29–39.
Alam, M. Z. B., Otaki, M., Furumai, H., & Ohgaki, S. (2001). Direct and indirect inactivation of microcystis aeruginosa by UV-radiation. Water Research, 35, 1008–1014.
Azim, M.E., Asaeda, T., Azim, M.E., Verdegem, M.C.J., Van Dam, A.A. and Beveridge, M.C.M. 2005, Periphyton structure, diversity and colonization. 15–49.
Bao, L.L., Chen, Y.J. and Yan, W.X.: 2015, Ecological characteristics of nitrogen cycling microbes in river sediments, Microbiology China, 1141–1150.
Benson, B. B., & Krause, D. (1984). The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnology & Oceanography, 29, 620–632.
Chen, G., Zhou, B., Li, M., Le Tong and Hu, C.: 2013, Research on the of nitrogen and phosphorus on the phytoplankton community in Poyang Lake, China Rural Water and Hydropower, 48–52.
Gaiser, E. (2009). Periphyton as an indicator of restoration in the Florida Everglades. Ecological Indicators, 9, S37–S45.
Hill, B. H., Willingham, W. T., Parrish, L. P., & Mcfarland, B. H. (2000). Periphyton community responses to elevated metal concentrations in a Rocky Mountain stream. Hydrobiologia, 428, 161–169.
Jöbgen, A., Palm, A., & Melkonian, M. (2004). Phosphorus removal from eutrophic lakes using periphyton on submerged artificial substrata. Hydrobiologia, 528, 123–142.
Ling, D., Pang, Y. N., Li, L., & Gao, G. N. (2005). Simulation study on algal dynamics under different hydrodynamic conditions. Acta Ecologica Sinica, 1863–1868.
Ling, S., Jin, X., Zhong, Y., Zhang, D. E. I., Zhu, L., Dai, S. I., & Zhuang, Y. (2006). Changes of algal communities in water body with different proportions of nitrogen and phosphorus. Chinese Journal of Applied Ecology, 1218–1223.
Liu, J., Wu, Y., Wu, C., Muylaert, K., Vyverman, W., Yu, H., Muñoz, R., & Rittmann, B. (2017). Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: a review. Bioresource Technology, 241, 1127–1137.
Magdaleno, A. I. A. V. (2014). Effects of cadmium, copper and zinc on growth of four isolated algae from a highly polluted Argentina River. Bulletin of Environmental Contamination and Toxicology, 92, 202–207.
Mariñelarena, A. J., & Di Giorgi, H. D. (2001). Nitrogen and phosphorus removal by periphyton from agricultural wastes in artificial streams. Journal Freshwater Ecology, 16, 347–353.
Mortimer, C. H. (1942). The exchange of dissolved substances between mud and water in lakes. Journal Ecology, 30, 147–201.
Nelson, J. R., Eckman, J. E., Robertson, C. Y., Marinelli, R. L., & Jahnke, R. A. (1999). Benthic microalgal biomass and irradiance at the sea floor on the continental shelf of the South Atlantic bight: spatial and temporal variability and storm effects. Continental Shelf Research, 19, 477–505.
Park, J., Church, J., Son, Y., Kim, K., & Lee, W. H. (2017). Recent advances in ultrasonic treatment: challenges and field applications for controlling harmful algal blooms (HABs). Ultrasonics Sonochemistry, 38, 326–334.
Shou-bing, W., Xiao-xue, M., Yuan-an, W., Shu-qing, F. and Jian, H. 2014. A preliminary study on removal effects of a novel flocculant on algae and turbidity in algae-laden water samples from Dianchi Lake. Journal of Fudan University (Natural Science), 260–265.
Szlauer, L., & Szlauer, B. (1996). An attempt to manipulate a lake by deploying polyethylene sheets. Polskie Archiwum Hydrobiologii, 43, 311–321.
Vymazal, J. (1988). The use of periphyton communities for nutrient removal from polluted streams. Hydrobiologia, 166, 225–237.
Wolfstein, K., Colijn, F., & Doerffer, R. (2000). Seasonal dynamics of microphytobenthos biomass and photosynthetic characteristics in the northern German Wadden Sea, obtained by the photosynthetic light dispensation system. Estuarine Coastal & Shelf Science, 51, 651–662.
Wu, Y. (2016). Periphyton: functions and application in environmental. Remediation, 3–7.
Xie, X. Y., Zu, W., & Qian, X. (2011). Numerical study on the ecological dynamics of phosphorus circle in Taihu Lake. China Environmental Science, 858–862.
Zhao, F., Chu, H., Yu, Z., Jiang, S., Zhao, X., Zhou, X., & Zhang, Y. (2017). The filtration and fouling performance of membranes with different pore sizes in algae harvesting. Science of the Total Environment, 587-588, 87–93.
Zhiqiang, W., Aihua, C., Jianqun, M., Hai, W., & Guoqin, H. (2017). Research progress on the driving factors of freshwater lake ecosystem degradation and associated restoration techniques. Acta Ecologica Sinica, 6253–6264.
Zuo, S. P., Wan, K., Ma, S. M., & Ye, L. T. (2014). Combined allelopathic potential of aquatic plants species to control algae. Allelopathy Journal, 34, 315–324.
Acknowledgements
The authors thank Shu Chen and Yani Song for their help in the laboratory and Associate Professor Jing Lu for her advice. We also thank the anonymous reviewers for their relevant comments to improve this paper.
Funding
This research was supported and funded by the independent research project of Wuhan University (2042015kf1010, Associate Professor Jing Lu).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, D., Chen, S., Lu, J. et al. Study on the Effect of Periphyton on the Water Quality of Eutrophic Lakes. Water Air Soil Pollut 229, 209 (2018). https://doi.org/10.1007/s11270-018-3804-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3804-3