Abstract
Pharmaceutical agents, like diclofenac and acetaminophen, are sold without prescription leading to excessive use. These agents may reach water bodies through various routes and attain significant concentrations, posing a risk to hydrobiont health. Diverse studies have shown that during the biotransformation of these compounds, reactive metabolites and reactive oxygen species are produced which induce oxidative stress and damage to diverse biomolecules. However, toxicity studies that assess the effects of a mixture of contaminants are scarce, being very important as this is how they are actually in the environment. The present study aimed to evaluate the oxidative stress induced by mixture of diclofenac and acetaminophen on Cyprinus carpio and compare with the effect produced by these pharmaceuticals in isolation. A 96-h sublethal toxicity assay of the tested pharmaceuticals (isolated and in mixture) was performed and the following biomarkers were evaluated: lipid peroxidation, protein carbonyl content, and activity of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase. The pharmaceuticals evaluated induce oxidative stress on C. carpio in isolated form and as a mixture, but the level of damage being dependent on the organ evaluated as well as the type of toxicant and form of exposure (in isolation or as a mixture).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water is a vital resource which is constantly threatened, among other things, by discharges of diverse contaminants including heavy metals, hydrocarbons, and pesticides. In recent years increasing attention has been given to a group of substances known as “contaminants of emerging concern,” which include pharmaceutical products.
Pharmaceuticals are substances of natural or synthetic origin that are used to prevent, diagnose, or restore the health of humans or animals. These substances may have similar effects (including adverse ones) on mammals and non-mammals, since both of these share many similar receptors and target molecules that were retained in the course of evolution. However, these compounds may elicit unexpected responses in lower organisms due to pharmacokinetic, pharmacodynamic, and physiological differences (Fent et al. 2006).
Pharmaceuticals enter the environment after their human and/or veterinary use (Heberer 2002). One of their main routes of entry is discharges of animal or human excreta that are contaminated with pharmaceutical products and/or their metabolites. More than 50 % of pharmaceuticals are known to be eliminated in the feces or urine either as metabolites or in the form of unaltered minute traces that may eventually become bioavailable to the biota, damaging the latter. These compounds are usually present at low concentrations throughout the life cycle of many aquatic organisms and are particularly important for those living in waters receiving wastewater discharges (e.g., rivers). In general, pharmaceuticals are biologically active compounds that are soluble in water and not easily biodegradable. They may therefore be present in wastewater and can easily end up in natural waters. After their incorporation into a water body, pharmaceuticals can be absorbed by any one of the biotic elements of the ecosystem (Zhou et al. 2009).
Detection of residues of diverse pharmaceutical products in surface water during the past two decades has raised concern over their potential adverse effects for aquatic organisms (Hoeger et al. 2005). Some pharmaceuticals, such as anti-inflammatories, antimicrobials, anticonvulsants, and hypercholesterolemia medications, have been detected in many rivers and lakes of Europe and North America at concentrations of up to 1750 ng/L, and persist in the environment for some time before being degraded (Borgmann et al. 2007). In Mexico, Siemens et al. (2008) found concentrations of >1 μg/L of diverse pharmaceutical products, including diclofenac (DCF), in water from the Mezquital Valley, this drug has been detected in water bodies worldwide in concentrations that reach up to 3.6 μg/L (Santos et al. 2010). Similarly, acetaminophen (ACT), also known as paracetamol, has been detected at concentrations ranging from 6 to 246 μg/L (Schwaiger et al. 2004; Santos et al. 2010).
In Mexico, the most commonly used group of pharmaceuticals are nonsteroidal anti-inflammatory drugs (NSAIDs), which can be obtained without prescription, favoring self-medication and excessive use of these products (Gómez Oliván et al. 2009). This group includes ACT and DCF, which are among the most commonly used NSAIDs and have been detected in aquatic environments (Schwaiger et al. 2004). Both have been shown to be toxic since they induce oxidative stress on diverse aquatic species such as Hyallela azteca (Oviedo-Gómez et al. 2010; Gómez-Oliván et al. 2012) and Cyprinus carpio (Islas-Flores et al. 2013).
In a general way, risk assessment takes into account only individual compounds. In the case of pharmaceutical products, however, consideration must be given to the fact that they are constantly being released into the aquatic ecosystem and are not present in isolated form, but as multiple-component mixtures that may additionally be transformed through physical and chemical environmental processes and/or absorbed, biotransformed, and excreted by one organism or other (abiotic and biotic transformations), so that diverse kinds of interactions can be expected (Kümmerer 2009). From an environmental perspective, even individual pharmaceutical products should be regarded as a mixture of several chemical components (the unaltered pharmaceutical, transformation products, and their metabolites).
On the other hand, some pharmaceuticals work by the same or a very similar mechanism of action, sharing the same receptors, which favors the occurrence of interactions. This is the case of DCF and ACT, whose mechanism of action is reduction of prostaglandin synthesis through inhibition of cyclooxygenase (Santos et al. 2010), and which have also been shown to induce oxidative stress.
Oxidative stress is defined as an imbalance between reactive oxygen species (ROS) and antioxidant defenses. ROS are produced during the normal metabolism of living organisms, and have diverse functions including the regulation of cellular events such as transcription factor activation, gene expression, and cell proliferation and differentiation. However, they are potent electrophilic agents able to remove electrons from nucleophilic agents, inducing oxidation of proteins, lipids, and nucleic acids; their regulation is therefore important for the maintenance of homeostasis in the organism (Valavanidis et al. 2006). Diverse toxicants, including heavy metals, hydrocarbons, and pharmaceuticals such as NSAIDs, have been shown to induce formation of these ROS (Sinha et al. 2007), making oxidative stress one of the major mechanisms of action of toxicants, and the assays used to evaluate it excellent biomarkers of damage.
The common carp, C. carpio, has a cosmopolitan distribution. In Mexico it has been introduced in 80 % of the freshwater bodies, and is an ecologically and economically important species. Only in 2012, about 7 t of this organism (growing and harvesting) were consumed, with an approximate value of US $6 million (Base de Datos del Registro Nacional de Pesca y Acuacultura 2013). Also, it is used in toxicity assays as a bioindicator due to its sensibility and easy maintenance under laboratory conditions.
Thus, the present study aimed to evaluate the oxidative stress induced by a mixture of DCF and ACT on C. carpio and compare with the effect produced by these pharmaceuticals in isolation.
2 Materials and Methods
2.1 Specimen Procurement and Maintenance
Common carp (C. carpio) with an average weight of 2.3 g were obtained from the carp culturing facility in Tezontepec de Aldama, Hidalgo (Mexico), transported to the laboratory, and acclimated for 2 weeks. During acclimation, specimens were maintained in 80-L glass tanks equipped with filtration systems, under 12:12-h light/dark conditions and constant aeration, and were fed Pedregal Silver Corp MR every third day. Tank water had the following physicochemical properties: dissolved oxygen 6.4 ± 0.5 mg/L, ammonia concentration 0.32 ± 0.2 mg/L, nitrates 0.26 ± 0.001 mg/L, temperature 20 ± 2 °C, oxygen saturation 90–100 % and pH 7.5–8.0.
2.2 Toxicity Assays
Four test systems were set up in 80-L fish tanks with six carp each. Specimens in the first system were exposed to DCF (100 μg/L), those in the second to ACT (100 μg/L), and those in the third to a mixture of both pharmaceuticals (50 μg of each/L, 1:1 proportion according to Vanegas et al. 1997), while the fourth system was used as a toxicant-free control. The tests concentrations correspond to 1/10 of LC50 obtained from a previous lethality assay, where the median lethal concentration of both toxics on C. carpio was greater than 1,000 μg/L; also, the maximal environmental concentrations reported of both pharmaceuticals were considered (DCF 2.2 μg/L and ACT 246 μg/L, Santos et al. 2010). Tap water previously aerated during 24 h was used and solutions were not replaced during the experiment. Prior to this study, a toxicity assay was performed to ascertain that the test concentration was not lethal to C. carpio. Carp were exposed in a static system for 96 h under the same conditions used for maintenance of specimens. At the end of the exposure period, fish were euthanized and dissected in an ice bath to remove the liver, gills, and brain. Organs were weighed and individually homogenized in 2-mL phosphate buffered saline (PBS, pH 7.3) and oxidative stress was evaluated using the following biomarkers: the activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), as well as lipid peroxidation (LPX) and protein carbonyl content (PCC). Total protein content was used to express all biomarker results. The assay was performed in triplicate.
This protocol was reviewed and approved by the Bioethics Committee of the National School of Biological Sciences, IPN, ensuring that it was carried out in accordance with institutional standards for the care of animal test subjects. The specifications mentioned in the corresponding Official Mexican Standards were also considered (NOM-062-ZOO-1999, Technical specifications for the production, care and use of laboratory animals).
2.2.1 Determination of Superoxide Dismutase Activity
SOD activity was determined according to the Misra and Fridovich (1972) method. To 20 μL supernatant in a 1-cm cuvette, 150-μL carbonate buffer solution (50-mM sodium carbonate and 0.1 mM EDTA) pH 10.2 and 100-μL adrenaline (30 mM) was added. Absorbance was read at 480 nm, at 30 s and 5 min. SOD activity was determined by interpolating the data on a standard curve. Results were expressed as international units SOD per milligram protein.
2.2.2 Determination of Catalase Activity
CAT activity was determined as proposed by Radi et al. (1991). To 20-μL supernatant, 1-mL isolation buffer solution (0.3-M saccharose, 1-mM EDTA, 5-mM HEPES, and 5-mM KH2PO4) and 0.2 mL hydrogen peroxide (20 mM) was added. Absorbance was read at 240 nm, at 0 and 60 s. Results were obtained by substituting the absorbance value of each time in the formula: catalase activity = (A60 − A0)/molar extinction coefficient (MEC), where the MEC of H2O2 equals to 0.043 mM/cm. Results were expressed as millimeter H2O2 millimoles per milligram protein.
2.2.3 Determination of Glutathione Peroxidase Activity
GPx activity was determined using Paglia and Valentine’s (1967) methodology. To 100 μL supernatant, 900-μL buffer reagent solution (5-M K2HPO4, 5-M KH2PO4, 3.5-mM reduced glutathione, 1-mM sodium azide, 2-U glutathione reductase, and 0.12-mM NADPH, pH 7.0; Sigma) plus 200-μL H2O2 (20 M) was added. Absorbance was read at 340 nm, at 0 and 60 s. Activity was estimated using the MEC of NADPH (6.2 mM/cm). Results were expressed as millimeter NADPH per milligram protein.
2.2.4 Determination of Lipid Peroxidation
LPX was determined as in Büege and Aust (1978). The cell pellet was reconstituted with Tris–HCl buffer (pH 7.4) until a 5-mL volume was attained. Next, 1 mL of this solution was incubated at 37 °C for 30 min. Following this, 2-mL TBA-TCA reagent (0.375 % thiobarbituric acid in 15 % trichloroacetic acid) was added, and the sample was shaken in a vortex, placed in a bath of boiling water for 45 min, allowed to cool, and the precipitate removed by centrifugation at 3,000 rpm for 10 min. Absorbance was read at 535 nm using a reaction blank. Results were expressed as millimeter malondialdehyde per milligram protein using the MEC of 1.56 × 105 M/cm.
2.2.5 Determination of Protein Carbonyl Content
PCC was determined by the method of Levine et al. (1994). Soluble proteins were obtained by centrifugation of samples at 10,500 rpm for 30 min. To 100 μL of this supernatant, 150 μL of 10-mM dinitrophenylhydrazine in 2-M HCl (Sigma) was added prior to incubation at room temperature for 1 h in the dark. Next, 500 μL of 20 % trichloroacetic acid was added and the sample was allowed to rest for 15 min at 4 °C, then centrifuged at 16,000 rpm for 5 min. The bud was rinsed three times in 1:1 ethano/ethyl acetate (Baker), dissolved in 150 μL of 6-M guanidine (Sigma) pH 2.3, and incubated at 37 °C for 30 min. Absorbance was read at 366 nm and results were expressed as nanomole of reactive carbonyls formed per milligram protein based on their MEC of 21,000 M/cm.
2.2.6 Determination of Total Protein Content
To 25 μL of supernatant, 75 μL deionized water and 2.5 mL Bradford’s reagent (0.05 g Coommassie Blue dye, 25 mL of 96 % ethanol and 50-mL H3PO4, in 500-mL deionized water) was added. The test tubes were shaken and allowed to rest for 5 min prior to reading absorbance at 595 nm and interpolation on a bovine albumin curve (Bradford 1976).
2.2.7 Statistical Analysis
Results of oxidative stress determination were subjected to a one-way analysis of variance (ANOVA) (p < 0.05), while differences between means were analyzed using the Student-Newman-Keuls method. Sigma Stat v3.5 was used in these analyses.
3 Results
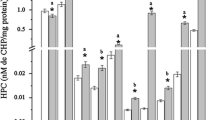
Figure 1 shows SOD activity in the various organs evaluated after exposure of C. carpio to DCF, ACT, and a 1:1 mixture of these pharmaceuticals. SOD activity increased relative to the control group in the brain of specimens exposed to ACT (230.7 %), while no significant difference was found with all other treatments. A significant reduction with respect to the control group was observed in liver of carp exposed to either DCF (74.2 %) or the mixture (43 %). In gill, a significant increase occurred in specimens exposed to DCF (203.7 %) and a significant reduction (60.4 %) in those exposed to the mixture (p < 0.05).
Superoxide dismutase (SOD) activity in Cyprinus carpio after exposure for 96 h to diclofenac, acetaminophen, and a 1:1 mixture of both pharmaceuticals. Values shown are the mean ± SE. *Significantly different from control group values, ANOVA, and S-N-K (p < 0.05). Lowercase letters indicate a significant difference relative to specimens exposed to a diclofenac, b acetaminophen, and c the pharmaceuticals mixture (p < 0.05)
CAT activity is shown in Fig. 2. A significant increase with respect to the control group was seen in the brain of specimens exposed to either the pharmaceuticals mixture (299.1 %) or DCF (311.7 %). In liver, CAT activity was significantly reduced in specimens exposed to either DCF (74.2 %) or ACT (53 %), while in gill, there was a significant reduction in fish exposed to DCF (34.3 %) and a significant increase in those exposed to the pharmaceuticals mixture (182 %) (p < 0.05).
Catalase (CAT) activity in Cyprinus carpio after exposure for 96 h to diclofenac, acetaminophen, and a 1:1 mixture of both pharmaceuticals. Values shown are the mean ± SE. *Significantly different from control group values, ANOVA, and S-N-K (p < 0.05). Lowercase letters indicate a significant difference relative to specimens exposed to a diclofenac, b acetaminophen, and c the pharmaceuticals mixture (p < 0.05)
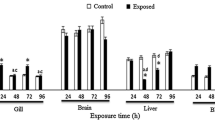
Figure 3 shows GPx activity results. Enzymatic activity increased significantly in brain and liver of specimens exposed to ACT (229 and 223.7 %, respectively) relative to the control group, while in gill significant increases were observed with all treatments (DCF 294.1 %, ACT 509.8 %, pharmaceuticals mixture 365.1 %) (p < 0.05).
Glutathione peroxidase (GPx) activity in Cyprinus carpio after exposure for 96 h to diclofenac, acetaminophen, and a 1:1 mixture of both pharmaceuticals. Values shown are the mean ± SE. *Significantly different from control group values, ANOVA, and S-N-K (p < 0.05). Lowercase letters indicate a significant difference relative to specimens exposed to a diclofenac, b acetaminophen, and c the pharmaceuticals mixture (p < 0.05)
Figure 4 shows LPX values in the various organs evaluated. In brain, no significant change was found with any of the treatments, while in liver, a significant increase with respect to the control group occurred in specimens exposed to either ACT (413.5 %) or the mixture of pharmaceuticals (166.4 %). In gill, significant increases were observed in carp exposed to either ACT (235.2 %) or the mixture (276.7 %). It is worth stressing that DCF induced no changes in LPX in any of the organs evaluated (p < 0.05).
Lipid peroxidation (LPX) in Cyprinus carpio after exposure for 96 h to diclofenac, acetaminophen, and a 1:1 mixture of both pharmaceuticals. Values shown are the mean ± SE. *Significantly different from control group values, ANOVA, and S-N-K (p < 0.05). Lowercase letters indicate a significant difference relative to specimens exposed to a diclofenac, b acetaminophen, and c the pharmaceuticals mixture (p < 0.05)
Figure 5 shows the PCC values obtained. No significant changes in PCC were found with respect to the control group in carp brain or liver with any of the treatments, while in gill, PCC increased significantly in specimens exposed to DCF (790.6 %), ACT (1120.3 %), or the pharmaceuticals mixture (1,643 %) (p < 0.05).
Protein carbonyl content (PCC) in Cyprinus carpio after exposure for 96 h to diclofenac, acetaminophen, and a 1:1 mixture of both pharmaceuticals. Values shown are the mean ± SE. *Significantly different from control group values, ANOVA, and S-N-K (p < 0.05). Lowercase letters indicate a significant difference relative to specimens exposed to a diclofenac, b acetaminophen, and c the pharmaceuticals mixture (p < 0.05)
4 Discussion
The sublethal toxicity assay results show that antioxidant status is modified in C. carpio by exposure to DCF, ACT, or a 1:1 mixture of these pharmaceuticals, the level of damage being dependent on the type of toxicant, form of exposure (in isolation or as a mixture), and organ evaluated. Thus, SOD activity increased in brain of specimens exposed to either of these pharmaceutical products in isolated form or as a mixture, this increase being higher and significant only in fish exposed to ACT. CAT and GPx activity also increased in this organ. In mammals, some NSAIDs, including DCF and ACT, can affect the mitochondrion and consequently oxidative phosphorylation, inducing increased ROS production, in particular of O2 •−, and as a result, increases occur in SOD activity and hydrogen peroxide levels (Valavanidis et al. 2006; Asensio et al. 2007; Li et al. 2010), which in turn, activate CAT and GPx, and may explain the values obtained in brain in the present study. Similar results were reported by Parolini et al. (2010), who found a slight increase in SOD activity in Dreissena polymorpha after exposure for 24 h to ACT and by Oviedo-Goméz et al. (2010) in H. azteca exposed to DCF.
In liver, antioxidant enzymes activity showed a different behavior from that in brain: SOD and CAT activity decreased with all treatments (DCF, ACT, and their combination) while GPx activity increased in specimens exposed to ACT in isolated form. In mammals, DCF is biotransformed to 4′-hydroxydiclofenac and 5′-hydroxydiclofenac (Scheurell et al. 2009) which have been shown to be highly reactive and induce ROS formation. Furthermore, ACT is biotransformed by cytochrome P450 in to various glucuronides and N-acetyl-p-benzoquinone imine (NAPQI), which interacts with proteins and nucleic acids due to its electrophilic nature, leading to ROS formation (Parolini et al. 2010). The liver is the primary biotransforming organ, so these kind of reactive metabolites (both of DCF and of ACT) are probably also formed in significant amounts in C. carpio. In principle, an increase in antioxidant enzymes activity is to be expected; however, given their proteinaceous and nucleophilic nature, they are direct targets of attack by ROS or any reactive metabolites formed (Oviedo-Gómez et al. 2010). This may explain reductions in SOD and CAT activity occurring with all treatments in our study and reductions in GPx activity evidenced in specimens exposed to either DCF or the pharmaceuticals mixture. On the other hand, ACT is a hepatotoxic agent (Jaeschke et al. 2003) in mammals while DCF induces apoptosis in rat and human hepatocytes (Gómez-Lechón et al. 2003), if this kind of changes occur in carp and the liver is damaged, hepatic function and enzyme amounts will decrease. However it is important to mention that Islas-Flores et al. (2013) found no changes in antioxidant enzyme activity in the liver of the same species exposed to DCF, probably due to the difference in the concentration employed.
In gill, SOD activity increased in fish exposed to DCF. However, the opposite occurred in those exposed to ACT and the pharmaceuticals mixture. CAT activity decreased in carp exposed to either of these pharmaceuticals in isolation, but increased with exposure to their combination, while GPx activity increased with all treatments. It is worth noting that antioxidant status impairment in the present study was much higher in gill than in brain or liver. In this respect, it is necessary to consider that the gills are an extremely important organ, since respiration, osmoregulation, acid–base balance, and nitrogen removal are carried out within them, and are also the first route of contact with the external environment in fish (Sepici-Dinçel et al. 2009), which makes them one of the main tissues damaged by xenobiotics.
In the present study, increased LPX was found in liver and gill of carp exposed to either ACT or the pharmaceuticals mixture, while no changes occurred in any of the organs evaluated in fish exposed to DCF alone. Similar results were obtained by Islas-Flores et al. (2013) in C. carpio exposed to 7.08 mg/L of DCF, nevertheless this toxic induces LPX on other aquatic species, particularly invertebrates such as Daphnia magna and H. azteca (Oviedo-Gómez et al. 2010).
In regards to LPX, our results show that neither DCF nor ACT induce LPX in brain of C. carpio, displaying organ-selective toxicity to the liver and gills, organs in which LPX increased with both ACT and the mixture of pharmaceuticals. Jaeschke et al. (2003) say that during ACT biotransformation, massive lipid peroxidation is unleashed in mammals, this event being responsible for liver damage. ACT biotransformation in the liver produces NAPQI that is initially conjugated by hepatic glutathione; however, when NAPQI concentrations increases, hepatic glutathione decreases and superoxide radical levels are induced, increasing LPX (Jaeschke et al. 2003).
Direct damage to proteins or chemical modification of their amino acids induced by oxidative stress may increase PCC (Parvez and Raisuddin 2005). In the present study, DCF, ACT, and their combination induced an increase in PCC in gill, but no significant changes in this biomarker occurred in brain or liver. DCF is transformed to 4′-hydroxydiclofenac and 5′-hydroxydiclofenac, and subsequently to benzoquinones (Stülten et al. 2008), an electrophilic metabolite capable of inducing ROS formation and combining with sulfhydryl groups at the cytosol level in proteins or enzymes containing these groups. On the other hand, during the biotransformation of ACT, NAPQI is produced and there is increased ROS formation (Parolini et al. 2010), which may explain the changes seen in gill of C. carpio. In brain, where neither pharmaceutical induced significant PCC changes, increased SOD and CAT activity occurred, probably protecting this organ against oxidative damage to proteins and even lipids, since no significant increase in LPX was observed either.
In conclusion, DCF and ACF produce oxidative stress in brain, liver, and gills of the common carp, this effect being modified when they are in mixture. Environmental contaminants, including pharmaceutical products, are not present in isolated form but as mixtures. Moreover, in water bodies, a single pharmaceutical may be present as a mixture consisting of the active ingredient, a number of its metabolites, and products derived from abiotic and biotic transformations, and will behave very differently from the compound in isolated form (Santos et al. 2010). That is why studies of the toxicity produced by mixtures of compounds are very important to understand the behavior of these toxics in the environment.
References
Asensio, C., Levoin, N., Guillaume, C., Guerquin, M., Rouguieg, K., Chrétien, F., Chapleur, Y., Netter, P., Minn, A., & Lapicque, F. (2007). Irreversible inhibition of glucose-6-phosphate dehydrogenase by the coenzyme A conjugate of ketoprofen: a key to oxidative stress induced by non-steroidal anti-inflammatory drugs? Biochemical Pharmacology, 73, 405–416.
Base de Datos del Registro Nacional de Pesca y Acuacultura (2013). (RNPA), Consejo Nacional de Pesca de la Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación SAGARPA, México.
Borgmann, U., Bennie, D. T., Ball, A. L., & Palabrica, V. (2007). Effect of a mixture of seven pharmaceuticals on Hyallela azteca over multiple generations. Chemosphere, 66, 1278–1283.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72, 248–254.
Büege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302–310.
Fent, K., Weston, A., & Caminada, D. (2006). Ecotoxicology of human pharmaceuticals. Aquatic Toxicology, 76, 122–159.
Gómez-Lechón, M. J., Ponsoda, X., O’Connor, E., Donato, T., Castell, J. V., & Jover, R. (2003). Diclofenac induces apoptosis in hepatocytes by alteration of mitochondrial function and generation of ROS. Biochemical Pharmacology, 66, 2155–2167.
Gómez-Oliván, L., Galar-Martínez, M., Téllez-Lopéz, A., Carmona-Zepeda, F., & Amaya-Chávez, A. (2009). Estudio de automedicación en una farmacia comunitaria de la Ciudad de Toluca. Revista Mexicana de Ciencias Farmacéuticas, 40, 5–11.
Gómez-Oliván, L. M., Neri-Cruz, N., Galar-Martínez, M., Vieyra-Reyes, P., & García-Medina, S. (2012). Assessing the oxidative stress induced by paracetamol spiked in artificial sediment on Hyalella azteca. Water Air and Soil Pollution, 223(8), 5097–5104.
Heberer, T. (2002). Ocurrence, fate and removal of pharmaceuticals residues in the aquatic environment: a review of recent research data. Toxicology Letters, 131, 5–17.
Hoeger, B., Köllner, B., Dietrich, D., & Hitzfeld, B. (2005). Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario). Aquatic Toxicology, 75, 53–64.
Islas-Flores, H., Gómez-Oliván, L. M., Galar-Martínez, M., Colín-Cruz, A., Neri-Cruz, N., & García-Medina, S. (2013). Diclofenac-induced oxidative stress in brain, liver, gill and blood of common carp (Cyprinus carpio). Ecotox Environmental Safe, 92, 32–38.
Jaeschke, H., Knight, T. R., & Bajt, M. L. (2003). The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicology Letters, 144, 279–288.
Kümmerer, K. (2009). The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. Journal of Environmental Management, 90, 2354–2366.
Levine, R., Williams, J., Stadtman, E., & Shacter, E. (1994). Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology, 233, 346–357.
Li, Z. H., Li, P., Rodina, M., & Randak, T. (2010). Effect of human pharmaceutical carbamazepine on the quality parameters and oxidative stress in common carp (Cyprinus carpio L.) spermatozoa. Chemosphere, 80, 530–534.
Misra, P., & Fridovich, I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry, 247, 3170–3175.
Oviedo-Gómez, D. G. C., Galar-Martínez, M., García-Medina, S., Razo-Estrada, C., & Goméz-Oliván, L. M. (2010). Diclofenac-enriched artificial sediment induces oxidative stress in Hyalella azteca. Environmental Toxicology and Pharmacology, 29, 39–43.
Paglia, D. E., & Valentine, W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine, 70, 158–169.
Parolini, M., Binelli, A., Cogni, D., & Provini, A. (2010). Multi-biomarker approach for the evaluation of the cyto-genotoxicity of paracetamol on the zebra mussel (Dreissena polymorpha). Chemosphere, 79, 489–498.
Parvez, S., & Raisuddin, S. (2005). Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environmental Toxicology and Pharmacology, 20, 112–117.
Radi, R., Turrens, J., Chang, Y., Bush, M., Capro, D., & Freeman, A. (1991). Detection of catalase in rat heart mitochondria. Journal of Biological Chemistry, 22, 20028–22034.
Santos, L. H. M. L. M., Araújo, A. N., Fachini, A., Pena, A., Delerue-Matos, C., & Montenegro, M. C. B. S. M. (2010). Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. Journal of Hazardous Materials, 175, 45–95.
Scheurell, M., Franke, S., Shah, R. M., & Hühnerfuss, H. (2009). Occurrence of diclofenac and its metabolites in surface water and effluent samples from Karachi, Pakistan. Chemosphere, 77, 870–876.
Schwaiger, J., Ferling, H., Mallowa, U., Wintermayr, H., & Negele, R. D. (2004). Toxic effects of the non-steroidal anti-inflammatory drug diclofenac Part I: histopathological alterations and bioaccumulation in rainbow trout. Aquatic Toxicology, 68, 141–150.
Sepici-Dinçel, A., Çağlan, K. B., Mahmut, S., Rabia, S., Duygu, S., Ayhan, I. O., & Figen, E. (2009). Sublethal cyfluthrin toxicity to carp (Cyprinus carpio L.) fingerlings: biochemical, hematological, histopathological alterations. Ecotox. Environ. Safe., 72, 1433–1439.
Siemens, J., Huscheka, G., Siebeb, C., & Kaupenjohann, M. (2008). Concentrations and mobility of human pharmaceuticals in the world’s largest wastewater irrigation system, Mexico City—Mezquital Valley. Water Research, 42, 2124–2134.
Sinha, S., Mallick, S., Misra, R. K., Singh, S., Basant, A., & Gupta, A. K. (2007). Uptake and translocation of metals in Spinacia oleracea L. grown on tannery sludge-amended and contaminated soils: effect on lipid peroxidation, morpho-anatomical changes and antioxidants. Chemosphere, 67, 176–187.
Stülten, D., Zühlke, S., Lamshöft, M., & Spiteller, M. (2008). Occurrence of diclofenac and selected metabolites in sewage effluents. Science of the Total Environment, 405, 310–316.
Valavanidis, A., Vlahogiannia, T., Dassenakis, M., & Scoullos, M. (2006). Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox. Environ. Safe., 64, 178–189.
Vanegas, C., Espina, S., Botello, A. V., & Villanueva, S. (1997). Acute toxicity and synergism of cadmium and zinc in white shrimp, Penaeus setiferus, juveniles. Bulletin of Environmental Contamination and Toxicology, 58, 87–92.
Zhou, J. L., Zhang, Z. L., Banks, E., Grover, D., & Jiang, J. Q. (2009). Pharmaceutical residues in wastewater treatment work effluents and their impact on receiving river water. Journal of Hazardous Materials, 166, 655–661.
Acknowledgments
This study was made possible through support from the National Science and Technology Council (CONACYT, project 151665) as well as the Research and Postgraduate Secretariat of the National Polytechnic Institute (SIP-IPN, projects 20100546 and 20121226).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nava-Álvarez, R., Razo-Estrada, A.C., García-Medina, S. et al. Oxidative Stress Induced by Mixture of Diclofenac and Acetaminophen on Common Carp (Cyprinus carpio). Water Air Soil Pollut 225, 1873 (2014). https://doi.org/10.1007/s11270-014-1873-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1873-5